Abstract
Although, several categories of nosocomial infections are presented during the recent years, urinary tract infections (UTIs) considered as one of the most important systemic infections. The presence of Candida species in the urinary tract system (Candiduria) is seen in only 10–15% of the cases with UTI, however candiduria has been considered as more problematic infection for patients, laboratory workers and physicians. Due to increasing numbers of several predisposing factors, such as antibacterial agents, urinary tract instrumentation, diabetes mellitus, invasive therapies, and prolonged hospital stay, candiduria develops among the hospitalized patients, especially hospitalized in intensive care units (ICUs) and neonatal intensive care units (NICUs). According to the epidemiological studies, Candida albicans is the most common isolated species from candiduric patients. However, during the recent years, due to increasing resistance to antifungal drugs, non-albicans Candida species including, C. glabrata, C. krusei, C. parapsilosis and C. tropicalis have been also implicated. We found that the mean prevalence of candiduria among Iranian patients was lower (16.5%) than worldwide ratio and also males were more frequently affected than females (M:F, 1.2:1). Similar to other countries, C. albicans was most common infectious agent followed by non-albicans Candida species including, C. glabrata, C. tropicalis and C. krusei.
Keywords: Candiduria, Candida albicans, Candida species, Iran, urinary tract infection
Introduction
Urinary tract infections (UTIs) are the most common nosocomial infections among the hospitalized patients and their incidence has considerably increased during the recent decades.[1–4] Several microorganisms are associated with UTIs including, bacteria, viruses, filamentous and yeasts fungi, however 10–15% of them are caused by Candida species, common fungal mycoflora.[5] Funguria is a general term for the presence of several species fungi in the urinary system, such as moulds (Aspergillus, Penicillium, Cladosporium, and Geotrichum species).[6–11] However, the presence of Candida species in urine are considered as candiduria and its severity varies from asymptomatic candiduria to clinical sepsis.[12,13] In addition, candiduria was defined as the presence of Candida >104 CFU/mL (colony forming unit/mL of urine) together with pyuria (>10 polymorphonuclear leukocytes/mm3).[14]
On the other hand, some urologists believe that there are no clear criteria or available recommendations for candiduria. So, candiduria may indicate contamination of the urine samples, lower urinary tract colonization with Candida, or true invasive infection of the upper and/or lower urinary tract caused by Candida species.[15] As a result, there are several responses by physicians considering the finding of organisms in the urine. The incidence rate of candiduria varies in different reports from different areas.[9,12,16,17]
Clinical and research consequences
Urinary tract infections due to fungi are occurring most commonly as a part of systemic mycosis in patients with predisposing factors including immunodeficiency, long-term hospital stays in intensive care unit (ICU) and neonatal intensive care units (NICU), long-term antibiotherapy and chemotherapy, indwelling catheters and elderly patients.[9,12,15,18–20] In addition, some researchers have believed that the incidence of candiduria in teaching hospitals increased due to use of broad-spectrum antibiotics.[18] The new invasive therapies are also raised the incidence of candiduria among patients with cancer and neutropenia.[4,21] In some studies, the mortality rate in candiduria was high and antifungal therapy could not make a significant difference in mortality rates (30%).[19,22] Furthermore, the virulence factors of Candida species have an important role in mortality rates.[20] During the last 2–3 decades increasing incidence rates in non-C. albicans species of Candida such as, Candida glabrata, C. tropicalis, C. krusei and C. parapsilosis have been observed that caused a major problem among hospitalized patients due to high resistance to antifungal drugs.[23,24]
Considering the above-mentioned facts, the increasing knowledge of clinicians, researchers and laboratory workers about candiduria, causative agents, laboratory identification methods, and antifungal susceptibility, have an important role in the prophylaxes and effective treatments as well as more accurate selection of antifungal agents for candiduric patients.
Search strategy and study selection
The searching terms were candiduria, UTI, funguria, and candiduric patients. All papers published in full text in English and/or Persian have been selected for review and suitable data have been extracted. Published papers were searched via PubMed, Scopus and Google Scholar. The search of the Iranian literatures was performed by using the international resources; Medline database through PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Scopus (http://www.scopus.com), Google Scholar (http://scholar.google.com), Google (http://www.google.com) and ISC (http://www.isc.gov.ir/). Moreover, local databases such as, Magiran (http://www.magiran.com) and SID (http://www.sid.ir) have been also searched.
Predisposing factors related to host
Although, Candida species, especially C. albicans are part of human normal mycoflora, candiduria is rarely present in healthy individuals. On the contrary, it is mostly found in hospitalized patients (ICU and NICU patients) and patients with predisposing factors.[9,25] The impairment of host defense mechanisms (cellular and humoral defenses), damage in anatomical human barriers (burns, invasive surgeries, skin maceration), and underlying diseases can cause an imbalance in the host defence mechanism.[21,23] As a result, the tissues of the hosts are invaded by normal commensal organisms. Patients in both ICU and NICU have often several predisposing factors, such as, diabetes mellitus, immunosuppressive therapy, prolonged antibiotherapy, advanced age, previous surgery (urological and non-urological), leukemia, chronic renal failure, renal transplantation, malignancy, neutropenia, genitourinary tuberculosis and bone marrow transplantation.[9,15,19,20,26–29] Usually, up to 20% of hospitalized patients (especially ICU, NICU patients) may expose to candiduria throughout their hospitalization due to invasive therapeutic and diagnostic procedures.[14,23] Corticosteroid therapy, and patients with hematologic malignancies may also contribute to the development of candiduria among patients.[14,30,31]
Female sex, nephrolithiasis, urinary tract obstruction, the presence of stones and benign prostatic hyperplasia are other predisposing factors for candiduria.[1,27,32] In addition, urinary tract instrumentation such as insertions of Foley catheters, double-J stenting, suprapubic catheters, nephrostomy tubes and hemodialysis were also noted as the predisposing factors for candiduria.[15–17,33] The role of duration of catheterization and antibiotic therapy in the increasing incidence of candiduria has been well discussed in the literature.[1,15,34] Catheters are biomaterials that cause biofilm formation and serve as a portal of entry for Candida into the urinary system. Some reports show that 78% of the patients carrying urinary devices have candiduria.[15] On the other hand, the dramatic increase in the prevalence of candidiasis was also observed among patients with acquired immunodeficiency syndrome (AIDS).[35,36] Also, such patients have a high risk of morbidity and mortality.[36]
In Paul et al.[20] study, the class of cephalosporins was the most commonly prescribed antimicrobial agents for patients in whom candiduria was detected. Other researchers believed that there was a strong correlation between candiduria and uncontrolled intake of broad-spectrum antibiotics[24], for example, use of antimicrobial agents as meropenem and ceftazidime.[18] Generally, Guler et al.[21] believed that incidence rates of candiduria increase due to urinary catheterization (12-fold), antibiotic use and urinary tract abnormalities (6-fold), abdominal surgeries (4-fold), diabetes mellitus (2-fold), corticosteroid and immune suppressive administration (1.4-fold).
Pathogenicity of Candida
Candida species, especially C. albicans are harmless commensal yeasts, and a lifelong resident of human body that cause several superficial, cutaneous, and systemic infections under suitable circumstances. Although the predisposing factors of host have an important role in the development of candidiasis, many pathogenicity factors are associated with microorganism for invading host tissues. Several factors are associated with the pathogenicity of Candida including; biofilm formation, extracellular enzymes, germ tube formation and phenotype switching phenomena.[20,37–44] Several studies have shown that the secretion of hydrolytic enzymes, such as proteases, phospholipases, and lipases have an important role in the pathogenesis of Candida species.[43,45] Adherence to the tissue surfaces, heat shock proteins, galvanotropism, and thigmotropism have also been demonstrated and may contribute to the subsequent UTI.[41,46] In addition, some of these factors such as biofilm formation can interfere with antifungal therapy.[5,44] On the other hand, routine antifungal prophylaxis may increase the pathogenicity of Candida and resistance to antifungal drugs.[47,48]
Microbiology of candiduria
A broad variety of fungi are associated with fungal UTIs including, filamentous fungi (Aspergillus and Penicillium species), Cryptococcus and Candida species.[2,6,7,9,16,49] Various ecosystems such as soils, foods and water are the main sources of Candida species. In addition, several species of Candida (especially, C. albicans) are the mycobiota of gastrointestinal tract, vaginal mucosa, urethra and lungs in humans and animals. They are also as transit saprophytes and colonizing skin and nail of human body. Several reports have shown that nearly 10–15% of nosocomial UTIs are usually caused by Candida species[5] and out of more than 200 species of the genus Candida, only 12 species are associated with different types of candidiasis.[2,15,35,48]
Candiduria is most commonly caused by C. albicans that are detected in 50–70% of urinary isolates, followed by C. glabrata (20% ) and C. tropicalis.[9,17,24,27,29,50] However, incidence rates of other non-albicans Candida species; such as C. parapsilosis, C. lusitaniae, C. guilliermondii and C. krusei have also increased during last decades.[16,22,37,51] In a study conducted by Paul et al.[20], in India C. tropicalis with an incidence rate of 30.5% was the most common microorganism isolated from candiduric patients followed by C. albicans. Although, coinfection with bacteria is more common among patients, polymicrobial infections with several yeast species were reported in 5–10% of Candida-related UTIs. Interestingly, in most cases, C. glabrata appears to be a frequent pathogen in combination with other species.[9,12,16] In addition Trichosporun and Geotrichum species are rarely isolated from urine samples.[8,9,52]
Epidemiology, sex and age distribution of candiduria
Naturally, due to anatomical and functional characteristics of the urinary tract system, the incidence of candiduria is higher among females than males.[1,21,27] In addition, some of the researchers have believed that this high incidence of candiduria among women may reflect vaginal candidiasis.[15,27] The incidence of candiduria was reported as 57.8% among women by Artiaga Kobayashi et al.[27] in Brazil, 61.9% by Hassaneen et al.[24] in Egypt and 76% by Dalen et al.[34] in Ottawa, Canada. Reports have shown that candiduria develops in different age groups ranging from three months to 81 years.[4,53–55] Several studies have shown that there is a significant association between female gender and candiduria among the HIV-infected patients and ICU patients.[31,36] In addition, in a report from Egypt Hassaneen et al.[24] found that candiduria was more prevalent among female gender, with a female/male ratio of 1.62. On the other hand, Paul et al.[20] indicated that the male:female ratio among candiduric patients was 1.08.
The study has shown that the the incidence of nosocomial UTIs due to Candida species increased 22–40% during 1992–1997 when compared with the time interval between 1986, and 1989.[2] Several reports from around the world show that the prevalence of candiduria varies between countries (Egypt 14%[24], and Brazil 22%[27]). In a study, 7.5% and 17.1% of diabetic patients had asymptomatic and symptomatic candiduria, respectively with overall prevalence of 8.3% in Ethiopia.[56]
Direct microscopic examination and urine culture
Suprapubic urine sample is the best urine sample to be used for the diagnosis of candiduria; however the second sterile urine samples are usually used. Asymptomatic catheter-associated candiduria is defined as the presence of a microorganism at a minimum concentration of 103 CFU/mL in a urine culture, in the absence of signs and symptoms of a UTI.[34] There are no sensitive/specific laboratory diagnostic tests to distinguish infection from colonization in urine samples. According to literature candiduria can be demonstrated in symptomatic or asymptomatic UTIs.
The presence of Candida species in both direct microscopic examinations and/or cultures of urine samples couldn’t specifically confirm the presence of UTI. In all candiduric conditions, including contamination, colonization of catheter and infection, Candida species may be isolated from urine samples. Although, routinely the second urine samples were collected into the sterile urine bottles (for adults) and disposable urine collection bags (for neonates and children), suprapubic specimens are the best urine samples to be used for diagnostic purposes.[15]
Routine mycology laboratory culture media, such as Sabouraud dextrose agar (SDA), potato dextrose agar (PDA), and nutrient agar with or without an antibacterial are usually used for culture. In addition, during the two last decades a relatively new culture medium (CHROMagar Candida) was incorporated in medical mycology laboratories.[9,24] One of the most important advantages of CHROMagar Candida is to detect polymicrobial growth in urine cultures (Figure 1).[9,54] Although, the best temperature for incubation under aerobic conditions is 35–37°C maintained for 24–48 hours, for slow growing species ambient temperature for one week has been reported.
Figure 1.
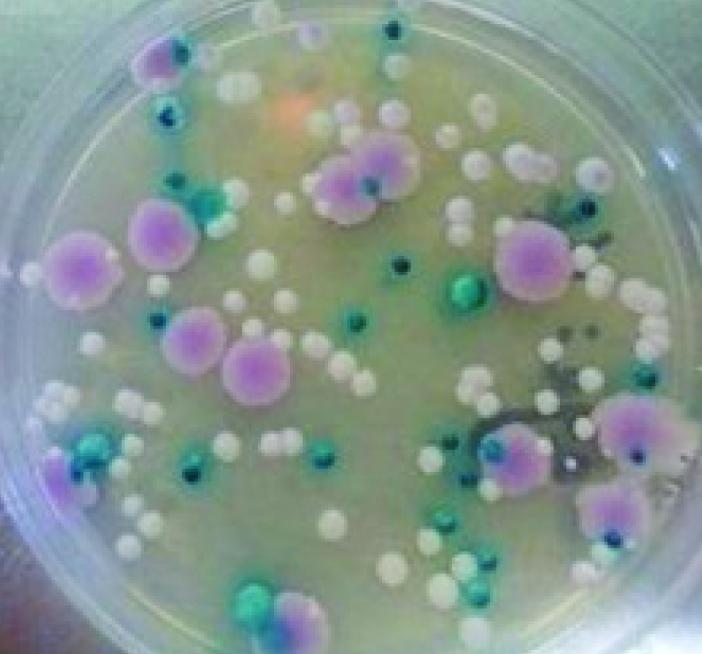
Growth of multi species Candida on CHROMagar Candida medium
Urine specimens were usually stained with Gram, Giemsa or methylene blue dyes. In addition, wet mount preparation from the urine sediment can be used for direct examination. Presence of ovoid shaped yeast cells, budding cells (3–15 μm) and pseudohyphae are identifying features of positive direct smears. Candidoma (fungus ball) or granular cyst containing yeast cells, budding cells and pseudohyphae of Candida are found in urine samples (Figure 2). The presence of renal fungal balls has been reported in 12% of the cases.[22] Fungal balls are the most frequent manifestation of renal candidiasis in hospitalized neonates in a NICU. Identification of species is usually applied in specific and research laboratories and general laboratories in hospitals can only identify yeast or C. albicans. Hence, clinicians always overlook non-albicans Candida species that are more resistant to antifungal therapy.
Figure 2.
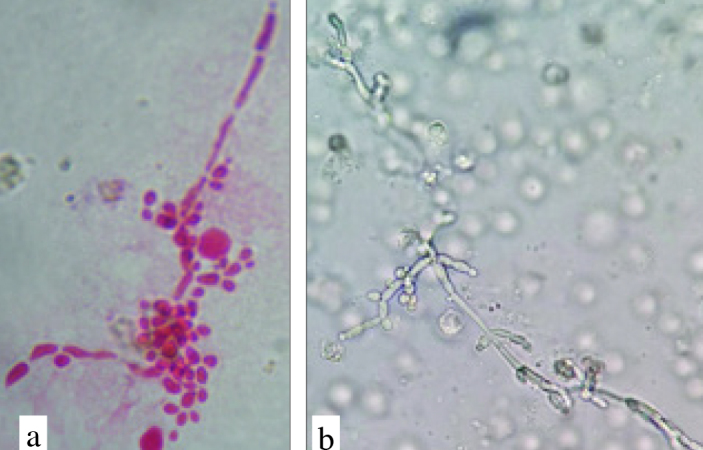
a, b. Yeast cells, budding cells and pseudohyphae of Candida albicans in urine sample (a: Fuchsin stained 100×; b: Wet smear)
The colony count of Candida species in urine samples is an important tool for clinicians in their decision-making process for either finding an effective antifungal for patients or removing urinary catheter. Furthermore, monitoring the treatment process is actually completed by checking the colony count during treatment. Although, candiduria has been defined as the presence of Candida species more than 104 CFU/mL in urine samples[14], Bukhary believed that clinically significant renal candidiasis might develop even with low colony counts (1000 CFU/mL).[15] Furthermore, the colony counts more than 105 CFU/mL, are usually associated with long-term indwelling urethral catheters.[1]
In vitro antifungal assay
Although, antimicrobial-surveillance programmes are providing useful information for empirical therapy, species assignment as well as in vitro antifungal assay are usually applied in the specific medical mycology laboratories. As a result, all clinicians have not a specific antifungal susceptibility profile against Candida isolates. On the other hand, widespread use of antifungal agents, and increased number of opportunistic fungal infections have increased resistance to available drugs. In the 1990s, azole-resistant isolates of C. albicans were found among HIV patients who were receiving long-term antifungal therapy, particularly fluconazole.[57] Literatures have shown that resistance against Candida species was first observed against fluconazole and increased progressively during several last decades.[58,59] Furthermore, resistance against other antifungals such as amphotericine B, caspofungin, posaconazole and itraconazole was also reported.[51,60–63]
Out of several antifungal identification tests, microdilution test is the most popular one among medical mycology researchers and CLSI and EUCAST defined specific breakpoints for some clinical important fungi.[25] Recently, modified microdilution technique using Resazurin (colorimetric method) was employed for antifungal susceptibility tests due to rapid measurement of minimum inhibitory concentration (MIC).[25,28,64] E-test and disk diffusion tests are simple and user friendly for laboratory technicians, however these tests were less frequently used by scientists due to their rare availability and lower accuracy. Agar well diffusion is another antifungal assay that is usually used for the evaluation of the in vitro antifungal activity with plant extracts.[65] Totally, MIC was measured as MIC ranges, MIC50, MIC90 and MICGM according to above tests for antifungal evaluation in vitro. These parameters are relatively associated with clinical outcomes of patients.
Interventions and outcomes
The treatment of candiduria is related to several key factors including presence of a confirmed candiduria using a second, clean-voided urine culture, physical examination, a detailed history to look for signs or symptoms and the presence of predisposing factors. Although, amphotericin B was traditionally the unique systemic antifungal for UTIs, its toxicity prevented its widespread use for all clinical forms of candiduria and Candida species.[66,67] Furthermore, new antifungals such as, fluconazole, flucytosine, voriconazole, itraconazole, posaconazole, isavuconazole and echinocandins (caspofungin) are introduced for therapy during last decades.[50,66–70]
Asymptomatic catheter-associated candiduria is usually a transient and benign condition, and does not associate with invasive candidiasis. In these cases, especially in adult patients hospitalized in ICUs, candiduria presents as fungal colonizations, and antifungal therapy is not required. Disease is resolved spontaneously when catheter is removed. Although asymptomatic candiduria does not need systemic antifungal therapy[18,34], physicians need to confirm the infection by a second sterile urine sample. On the other hand, some authors have believed that 103 CFU/mL of Candida in urine is sufficient for the diagnosis of infection and immunocompromised patients (diabetes mellitus, leukemia, organ transplants) should be treated with systemic antifungals.[15]
Voltan et al.[23], have believed that UTI due to non-C. albicans species such as, C. glabrata, C. tropicalis, C. krusei, and C. parapsilosis constitute major problems in the hospital environment. Incidence of urinary tract infections due to C. glabrata has increased during last decades.[27] Studies have demonstrated that prescribing oral fluconazole for a short-term was effective for the eradication of Candida from urine.[27] However, treating candiduria due to non-albicans Candida species can be difficult because of resistance to fluconazole.[50] Saha et al.[51], have believed that innate resistance of C. krusei to fluconazole may result from hospital practice of empirical administration of the drug which lead to selection of resistant species.
On the other hand some studies have shown that mortality rate of 26.2% was seen among candiduric patients and the most common predisposing factors associated with death are related to urinary tract instrumentation devices, antimicrobial therapy, ICU hospitalization, renal failure, and use of umbilical venous catheter.[20,25] However, candiduria among the critically ill newborn in NICUs can be a sign of a disseminated infection, especially candidemia. Although, spontaneous resolution occurred among patients with asymptomatic candiduria in several studies, treatment with fluconazole eradicated candiduria in 60.8%[27]-50% of the cases.[71]
Generally, fluconazole is the first choice antifungal drug for candiduria, however in resistant cases, flucytosine is a useful alternative.[72] During the last decades, new effective antifungals belonging to echinocandin class were introduced into clinical usage. In a case series study, Lagrotteria et al.[50] found that micafungin may be used for the treatment of candiduria due to less susceptible strains of non-albicans species.
Candiduria in Iran
The Islamic Republic of Iran is located in western Asia, north of Persian Gulf and Oman Sea. It is the second-largest country in the Middle East with 1,648,195 km2 and with over 81 million inhabitants. The first available documented data about candiduria in Iran was reported by Zaini et al.[73], in 1993 and more recently published papers have illustrated candiduria pattern in Iranian hospitals so far. Disease is approximately widespread in Iranian people, however, its frequency vary in different provinces. The mean prevalence of candiduria was approximately 16.5% in Iran, with highest rate (32.3%) in Qazvin and the lowest in Khuzestan (5.2%).[12,53]
Totally in Iran the most common etiologic agents of candiduria were C. albicans (58.53%), followed by C. glabrata (15.39%), C. tropicalis (5%), C. krusei (2.72%), C. parapsilosis (1.53%), C. kefyr (1.03%), C. lusitaniae (0.42%) and Candida species (14.72%). Furthermore, uncommon yeast/yeast-like microorganisms such as C. albidus (0.23%), C. laurentii (0.07%), Geotrichum (0.12%) and unidentified yeasts (0.22%) were also isolated from urine samples.[9,12,17,25,28,31,33,47,49,53,55,74–77] Furthermore, rarely Trichosporum and Saccharomyces were isolated from patients’ urine cultures.[73] In addition, recently Moslem et al.[77], isolated Rhodotorula species from a patient with candiduria in Ahvaz. Furthermore, co-cultures of different species of bacteria (Staphylococcus and Escherichia species) with Candida species were identified in several investigations.[25,78]
Although in some reports, candiduria was more commonly seen among Iranian female population[9,17,53,75], but overall, our review have indicated that candiduria was more prevalent among male population with a male:female ratio of 1.2:1. Furthermore, age range of the candiduric patients varied between 5 days and 81 years, however mostly middle aged patients were affected.[9,12,17,25,28,31,33,47,49,53,55,74–77]
In conclusion, candiduria due to nosocomial UTI is relatively more common among patients with specific predisposing factors. Host factors (genitourinary abnormality, diabetes mellitus, and immunodeficiency) as well as invasive therapy (indwelling urinary catheters, widespread systemic antibiotic use, surgery, and chemotherapy) have important roles in the prevalence and increasing rate of candiduria in patients. Moreover, pathogenicity factors of organisms and specific species, non-albicans Candida species (resistance to antifungals), are other factors that change the incidence of disease, antifungal susceptibility and mortality rates. We found that the mean prevalence of candiduria among Iranian patients was lower (16.5%) than worldwide prevalence rate and also males were more frequently affected than females (M:F, 1.2:1). Similar to other countries, C. albicans was the most common agent followed by non-albicans species including, C. glabrata, C. tropicalis and C. krusei.
Footnotes
Peer-review: This manuscript was prepared by the invitation of the Editorial Board and its scientific evaluation was carried out by the Editorial Board.
Author Contributions: Concept - A.Z.M.; Design - A.Z.M.; Data Collection and/or Processing - M.G., S.T., M.H.; Analysis and/or Interpretation - A.Z.M., S.T., M.G.; Writing Manuscript - A.Z.M., S.T., M.G.; Critical Review - A.Z.M.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that they haven’t received any financial support for this study.
References
- 1.Kauffman CA. Candiduria. Clin Infect Dis. 2005;41(Suppl 6):S371–6. doi: 10.1086/430918. [DOI] [PubMed] [Google Scholar]
- 2.Sobel JD, Fisher JF, Kauffman CA, Newman CA. Candida urinary tract infections-epidemiology. Clin Infect Dis. 2011;52(Suppl 6):S433–6. doi: 10.1093/cid/cir109. [DOI] [PubMed] [Google Scholar]
- 3.Esmailzadeh A, Zarrinfar H, Fata A, Sen T. High prevalence of candiduria due to non-albicans Candida species among diabetic patients: A matter of concern? J Clin Lab Anal. 2018;32:e22343. doi: 10.1002/jcla.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgiadou SP, Tarrand J, Sipsas NV, Kontoyiannis DP. Candiduria in haematologic malignancy patients without a urinary catheter: nothing more than a frailty marker? Mycoses. 2013;56:311–4. doi: 10.1111/myc.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain N, Kohli R, Cook E, Gialanella P, Chang T, Fries BC. Biofilm formation by and antifungal susceptibility of Candida isolates from urine. Appl Environ Microbiol. 2007;73:1697–703. doi: 10.1128/AEM.02439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal A, Grover C, Pandhi D, Das S, Jain BK. Nosocomial urinary tract aspergilloma in an immunocompetent host: An unusual occurrence. Indian J Dermatol. 2013;58:408. doi: 10.4103/0019-5154.117346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Pajares JD, Martinez-Ferriz M, Moreno-Perez D, Garcia-Ramirez M, Martin-Carballido S, Blanch-Iribarne P. Management of obstructive renal failure caused by bilateral renal aspergilloma in an immunocompetent newborn. J Med Microbiol. 2010;59:367–9. doi: 10.1099/jmm.0.012799-0. [DOI] [PubMed] [Google Scholar]
- 8.Trabelsi H, Néji S, Gargouri L, Sellami H, Guidara R, Cheikhrouhou F, et al. Geotrichum capitatum septicemia: case report and review of the literature. Mycopathologia. 2015;179:465–9. doi: 10.1007/s11046-015-9869-2. [DOI] [PubMed] [Google Scholar]
- 9.Zarei Mahmoudabadi A, Zarrin M, Ghanatir F, Vazirianzadeh B. Candiduria in hospitalized patients in teaching hospitals of Ahvaz. Iran J Microbiol. 2012;4:15–24. [PMC free article] [PubMed] [Google Scholar]
- 10.Lyratzopoulos G, Ellis M, Nerringer R, Denning D. Invasive infection due to Penicillium species other than P. marneffei. J Infect. 2002;45:184–95. doi: 10.1053/jinf.2002.1056. [DOI] [PubMed] [Google Scholar]
- 11.Kordbacheh P, Zaini F, Kamali P, Ansari K, Safara M. Study on the sources of nosocomial fungal infections at intensive care unit and transplant wards at a teaching hospital in Tehran. Iranian J Public Health. 2005;34:1–8. [Google Scholar]
- 12.Seifi Z, Azish M, Salehi Z, Zarei Mahmoudabadi A, Shamsizadeh A. Candiduria in children and susceptibility patterns of recovered Candida species to antifungal drugs in Ahvaz. J Nephropathol. 2013;2:122–8. doi: 10.5812/nephropathol.10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathor N, Khillan V, Sarin S. Nosocomial candiduria in chronic liver disease patients at a hepatobilliary center. Indian J Crit Care Med. 2014;18:234–7. doi: 10.4103/0972-5229.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trnka P, Kralik J, Pevalova I, Tuharsky J, Sagat T, Hudecova N, et al. Candiduria in critically ill children: risk factors and predictors of mortality. Infect Dis Clin Prac. 1998;7:234–9. doi: 10.1097/00019048-199806000-00007. [DOI] [Google Scholar]
- 15.Bukhary ZA. Candiduria: a review of clinical significance and management. Saudi J Kidney Dis Transpl. 2008;19:350–60. [PubMed] [Google Scholar]
- 16.Zarei Mahmoudabadi A, Keradmand AR, Enayatollahi N. Frequency of candiduria in inpatients and outpatients in department of urology, Golestan Hospital, Ahvaz, Iran. Iranian J Kidney Dis. 2009;3:114–5. [PubMed] [Google Scholar]
- 17.Pakshir K, Moghadami M, Emami M, Kord Bacheh P. Prevalence and identification of etiological agents of funguria in Foley catheterized patients. Med Res Shiraz Univ Med Sci. 2004;3:33–41. [Google Scholar]
- 18.Weinberger M, Sweet S, Leiboviciy l, Pitlik SD, Samraz Z. Correlation between candiduria and departmental antibiotic use. J Hosp Infect. 2003;53:183–6. doi: 10.1053/jhin.2002.1354. [DOI] [PubMed] [Google Scholar]
- 19.Fraisse T, Crouzet J, Lachaud L, Durand A, Charachon S, Lavigne JP, et al. Candiduria in those over 85 years old: a retrospective study of 73 patients. Intern Med. 2010;50:1935–40. doi: 10.2169/internalmedicine.50.5560. [DOI] [PubMed] [Google Scholar]
- 20.Paul N, Mathai E, Abraham OC, Michael JS, Mathai D. Factors associated with candiduria and related mortality. J Infect. 2007;55:450–5. doi: 10.1016/j.jinf.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Guler S, Ural O, Findik D, Arslan U. Risk factors for nosocomial candiduria. Saudi Med J. 2006;27:1706–10. [PubMed] [Google Scholar]
- 22.Robinson JL, Davies HD, Barton M, O’Brien K, Simpson K, Asztalos E. Characteristics and outcome of infants with candiduria in neonatal intensive care-a Paediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. BMC Infect Dis. 2009;9:183. doi: 10.1186/1471-2334-9-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voltan AR, Fusco-Almeida AM, Mendes-Giannini MJS. Candiduria: epidemiology, resistance, classical and alternative antifungals drugs. SOJ Microbiol Infect Dis. 2014;2:1–7. https://doi.org/10.15226/sojmid.2014.00116. [Google Scholar]
- 24.Hassaneen AM, Ghonaim RA, Hassanin HM, Salama NA, Elgohary T. Different aspects of candiduria as an important nosocomial infection. Med J Cairo Univ. 2014;82:199–204. [Google Scholar]
- 25.Kooshki P, Rezaei-Matehkolaei A, Mahmoudabadi AZ. The patterns of colonization and antifungal susceptibility of Candida, isolated from preterm neonates in Khorramabad, South West of Iran. J Mycol Med. 2018;28:340–4. doi: 10.1016/j.mycmed.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Jain M, Dogra V, Mishra B, Thakur A, Loomba PS, Bhargava A. Candiduria in catheterized intensive care unit patients: emerging microbiological trends. Indian J Pathol Microbiol. 2011;54:552–5. doi: 10.4103/0377-4929.85091. [DOI] [PubMed] [Google Scholar]
- 27.Artiaga Kobayashi CCB, Lisboa Fernandes DF, Miranda KC, de Sousa ED, Rodrigues Silva MR. Candiduria in hospital patients: A study prospective. Mycopathologia. 2004;158:49–52. doi: 10.1023/B:MYCO.0000038436.51918.d9. [DOI] [PubMed] [Google Scholar]
- 28.Gharaghani M, Rezaei-Matehkolaei A, Zarei Mahmoudabadi A, Keikhaei B. The frequency, antifungal susceptibility and enzymatic profiles of Candida species isolated from neutropenic patients. Jundishapur J Microbiol. 2016;9:e41446. doi: 10.5812/jjm.41446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmadzadeh A, Valavi E, Shamsizadeh A, Zarei Mahmoudabadi A, Hydari M, Ahmadzadeh A. Fungal urinary tract infection in an infant with posterior urethral valves. Jundishapur J Microbiol. 2011;4(Suppl 1):S71–6. [Google Scholar]
- 30.Marotta F, Naito Y, Bishier M, Jain S, Jadav H, Minelli E, et al. Subclinical candiduria in patients with gastrointestinal malignancies: a preliminary study on the protective effect of a natural phitocompound. J Biol Regul Homeost Agents. 2010;24:317–24. [PubMed] [Google Scholar]
- 31.Jozepanahi M, Mobaien AR, Karami A, Ahadi S. Frequency of candiduria in patients Hospitalized in Intensive Care Units. J Kerman Univ Med Sci. 2011;18:228–34. [Google Scholar]
- 32.Carvalho M, Guimarães CM, Mayer JR, Júnior, Fernandes Bordignon GP, Queiroz-Telles F. Hospital-associated funguria: analysis of risk factors, clinical presentation and outcome. Braz J Infect Dis. 2001;5:313–8. doi: 10.1590/S1413-86702001000600004. [DOI] [PubMed] [Google Scholar]
- 33.Zarei Mahmoudabadi A, Shahbazyan H, Zahiry M. Isolation of fungi from urine and dialysis filter in patients on hemodialysis in dialysis centers of Ahvaz, Iran. Iran J Kidney Dis. 2009;3:174–5. [PubMed] [Google Scholar]
- 34.Dalen DM, Zvonar RK, Jessamine PG. An evaluation of the management of asymptomatic catheter-associated bacteriuria and candiduria at The Ottawa Hospital. Can J Infect Dis Med Microbiol. 2005;16:166–70. doi: 10.1155/2005/868179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pignato S, Salvo S, Coniglio M, Marranzano M, Faro G, Giammanco G. Persistent oral and urinary Candida spp. carriage in Italian HIV-seropositive asymptomatic subjects. J Prev Med Hyg. 2009;50:232–5. [PubMed] [Google Scholar]
- 36.Esebelahie N, Enweani I, Newton-Esebelahie F, Omoregie R. Candiduria among HIV-infected patients attending a tertiary hospital in Benin vity. Afr J Clin Exp Microbiol. 2014;15:84–90. doi: 10.4314/ajcem.v15i2.5. [DOI] [Google Scholar]
- 37.Mohammadi P, Shoaie N, Roudbar Mohammadi S. Isolation and detection of yeast biofilms from urine catheters of infectious patients. Jundishapur J Microbiol. 2012;5:533–6. doi: 10.5812/jjm.2640. [DOI] [Google Scholar]
- 38.Zarei Mahmoudabadi A, Zarrin M, Miry S. Phospholipase activity of Candida albicans isolated from vagina and urine samples. Jundishapur J Microbiol. 2010;3:169–73. [Google Scholar]
- 39.Antony G, Saralaya V, Gopalkrishna Bhat K, Shalini Shenoy M, Shivananda PG. Effect of phenotypic switching on expression of virulence factors by Candida albicans causing candidiasis in diabetic patients. Rev Iberoam Micol. 2009;26:202–5. doi: 10.1016/j.riam.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Moslem M, Zarei Mahmoudabadi A. Extracellular enzymes in the different phenotypes of Candida albicans from different sources. IJAPBS. 2014;3:60–70. [Google Scholar]
- 41.Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–28. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seifi Z, Mahmoudabadi AZ. Extracellular esterase secretion by vaginal isolates of Candida albicans. Jentashapir J Health Res. 2014;5:e21881. [Google Scholar]
- 43.Seifi Z, Mahmoudabadi AZ, Zarrin M. Extracellular enzymes and susceptibility to fluconazole in Candida strains isolated from patients with vaginitis and healthy individuals. Jundishapur J Microbiol. 2015;8:e20162. doi: 10.5812/jjm.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarei Mahmoudabadi A, Zarrin M, Kiasat N. Biofilm formation and susceptibility to amphotericin B and fluconazole in Candida albicans. Jundishapur J Microbiol. 2014;7:e17105. doi: 10.5812/jjm.17105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trofa D, Gacser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606–25. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher JF, Kavanagh K, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infection: pathogenesis. Clin Infect Dis. 2011;52(Suppl 6):S437–51. doi: 10.1093/cid/cir110. [DOI] [PubMed] [Google Scholar]
- 47.Badiee P, Alborzi A. Susceptibility of clinical Candida species isolates to antifungal agents by E-test, Southern Iran: A five year study. Iran J Microbiol. 2011;3:183–8. [PMC free article] [PubMed] [Google Scholar]
- 48.Zarei Mahmoudabadi A, Zarrin M, Beheshti Fard M. Antifungal susceptibility of Candida species isolated from candidura. Jundishapur J Microbiol. 2013;6:24–8. doi: 10.5812/jjm.4633. [DOI] [Google Scholar]
- 49.Fakhri A, Navid M, Seifi Z, Zarei Mahmoudabadi A. The frequency of candiduria in hospitalized patients with depressive syndrome. J Renal Inj Prev. 2014;3:97–8. doi: 10.12861/jrip.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagrotteria D, Rotstein C, Lee CH. Treatment of candiduria with micafungin: A case series. Can J Infect Dis Med Microbiol. 2007;18:149–50. doi: 10.1155/2007/768734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saha R, Das Das S, Kumar A, Kaur IR. Pattern of Candida isolates in hospitalized children. Indian J Pediatr. 2008;75:858–60. doi: 10.1007/s12098-008-0159-6. [DOI] [PubMed] [Google Scholar]
- 52.Tomšíková A. Causative agents of nosocomial mycoses. Folia Microbiol. 2002;47:105–12. doi: 10.1007/BF02817666. [DOI] [PubMed] [Google Scholar]
- 53.Ghiasian SA, Aghamirian MR, Eshghi GR. Nosocomial candiduria in critically Ill patients admitted to intensive care units in Qazvin, Iran. Avicenna J Clin Microbiol Infec. 2014;1:e21622. [Google Scholar]
- 54.Caggiano G, Puntillo F, Coretti C, Giglio M, Alicino I, Manca F, et al. Candida colonization index in patients admitted to an ICU. Int J Mol Sci. 2011;12:7038–47. doi: 10.3390/ijms12107038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gholampour P, Mahmoudi S, Pourakbari B, Ashtiani MT, Sabouni F, Teymuri M, et al. Candiduria in children: a first reportfrom an Iranian referral pediatric hospital. J Prev Med Hyg. 2014;55:54–7. [PMC free article] [PubMed] [Google Scholar]
- 56.Yismaw G, Asrat D, Woldeamanuel Y, Unakal C. Prevalence of candiduria in diabetic patients attending Gondar University Hospital, Gondar, Ethiopia. Iran J Kidney Dis. 2013;7:102–7. [PubMed] [Google Scholar]
- 57.EL-Garhy OH. An overview of the azoles of interest. Int J Curr Pharm Res. 2015;7:1–6. [Google Scholar]
- 58.Bennett JE, Izumikawa K, Marr KA. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob Agents Chemother. 2004;48:1773–7. doi: 10.1128/AAC.48.5.1773-1777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jafari-nodoushan AA, Kazemi A, Mirzaii F, Dehghani M. Fluconazole susceptibility profile of Candida isolates recovered from patients specimens admitted to Yazd Central Laboratory. Iran J Pharmaceut Res. 2010:69–75. [Google Scholar]
- 60.Zarei Mahmoudabadi A, Rezaei-Matehkolaei A, Ghanavati F. The susceptibility patterns of Candida species isolated from urine samples to posaconazole and caspofungin. Jundishapur J Microbiol. 2015;8:e24298. doi: 10.5812/jjm.24298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zarei Mahmoudabadi A, Rezaei-Matehkolaei A, Navid M, Torabizadeh M, Mazdarani S. Colonization and antifungals susceptibility patterns of Candida species isolated from hospitalized patients in ICUs and NICUs. J Nephropathol. 2015;4:77–84. doi: 10.12860/jnp.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rezaei-Matehkolaei A, Shafiei S, Zarei-Mahmoudabadi A. Isolation, molecular identification, and antifungal susceptibility profiles of vaginal isolates of Candida species. Iran J Microbiol. 2016;8:410–7. [PMC free article] [PubMed] [Google Scholar]
- 63.Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin Infect Dis. 2006;42:938–44. doi: 10.1086/500939. [DOI] [PubMed] [Google Scholar]
- 64.Elshikh M, Ahmed S, Funston S, Dunlop P, McGaw M, Marchant R, et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett. 2016;38:1015–9. doi: 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abew B, Sahile S, Moges F. In vitro antibacterial activity of leaf extracts of Zehneria scabra and Ricinus communis against Escherichia coli and methicillin resistance Staphylococcus aureus. Asian Pacific J Tropic Biomed. 2014;4:816–20. https://doi.org/10.12980/APJTB.4.201414B16. [Google Scholar]
- 66.Fisher JF, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infections--treatment. Clin Infect Dis. 2011;52(Suppl 6):S457–66. doi: 10.1093/cid/cir112. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan KA, Caylor MM, Lin FC, Campbell-Bright S. Comparison of amphotericin B bladder irrigations versus fluconazole for the treatment of candiduria in intensive care unit patients. J Pharm Pract. 2017;30:347–52. doi: 10.1177/0897190016645032. [DOI] [PubMed] [Google Scholar]
- 68.Wood GC, Adamczyk K, Boucher BA, Croce MA, Kuhl DA, Rhea CL, et al. Short-term fluconazole therapy for the treatment of candiduria in ICU and ICU step-down patients. Clin Med Insights, Trauma Intensive Med. 2015;6:S20140. [Google Scholar]
- 69.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2015;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gabardi S, Martin S, Sura M, Mohammed A, Golan Y. Micafungin treatment and eradication of candiduria among hospitalized patients. Int Urol Nephrol. 2016;48:1881–5. doi: 10.1007/s11255-016-1410-0. [DOI] [PubMed] [Google Scholar]
- 71.Sobel JD, Kauffman CA, McKinsey D, Zervos M, Vazquez JA, Karchmer AW, et al. Candiduria: a randomized, double-blind study of treatment with fluconazole and placebo. The National Institute of Allergy and Infectious Diseases (NIAID) Mycoses Study Group. Clin Infect Dis. 2000;30:19–24. doi: 10.1086/313580. [DOI] [PubMed] [Google Scholar]
- 72.Fisher JF. Candiduria: When and How to Treat It. Curr Infect Dis Rep. 2000;2:523–30. doi: 10.1007/s11908-000-0056-2. [DOI] [PubMed] [Google Scholar]
- 73.Zaini F, Azordegan F, Chabavizadeh J. Study of fungal infection in urine. Iran J Public Health. 1993;22:13–31. [Google Scholar]
- 74.Farasat A, Ghahri M, Mirhendi H, Beiraghi S. Identification of candida species screened from catheter using patients with PCR-RFLP method. Eur J Experiment Biol. 2012;2:651–6. [Google Scholar]
- 75.Behzadi P, Behzadi E, Yazdanbod H, Aghapour R, Akbari Cheshmeh M, Salehian Omran D. Urinary tract infections associated with Candida albicans. Maedica. 2010;5:277–9. [PMC free article] [PubMed] [Google Scholar]
- 76.Mohammadi R, Mirhendi H, Rezaei-Matehkolaei A, Ghahri M, Shidfar MR, Jalalizand N, et al. Molecular identification and distribution profile of Candida species isolated from Iranian patients. Med Mycol. 2013;51:657–63. doi: 10.3109/13693786.2013.770603. [DOI] [PubMed] [Google Scholar]
- 77.Moslem M, Zarei Mahmoudabadi A, Fatahinia M, Kheradmand A. Mannose-binding lectin serum levels in patients with candiduria. Jundishapur J Microbiol. 2015;8:e29491. doi: 10.5812/jjm.29491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghoutaslou R, Yaghoubi AR, Sharifi S. Urinary tract infections in hospitalized patients during 2006 to 2009 in Madani heart center Tabriz, Iran. J Cardiovasc Thorac Res. 2010;2:39–42. [Google Scholar]


