Abstract
Abdominal compartment syndrome (ACS) is seen with increasing frequency in the critically-ill. Elevated intra-abdominal pressures interfere with vital organ function and contribute to mortality. Prevention, when possible and early recognition of occurrence with timely therapy will improve survival. Measurement of bladder pressures plays a critical role in diagnosis and guiding therapy. Treatment includes non-invasive and invasive methodologies designed to decrease the volume of abdominal contents and invasive methods to increase the compartment dimensions.
Introduction
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are recognized with increasing frequency. There has been a steady rise in the annual number of publications on the subject over the last 15 years.1 The awareness of this disease has evolved. Once an obscure and poorly-understood entity, it is now a well defined disease process known to be a significant cause of morbidity and mortality. But while IAH has been shown to be independently associated with mortality,2 there are persisting issues with prevention, recognition and intervention. A survey study published in 2006 reported that 17% of intensivists said they had not seen a case in the last year and 16% said they never checked intra-abdominal pressures.1 Although originally described in trauma patients as the result of recurrent hemorrhage and visceral edema, it is now apparent that intra-abdominal hypertension and abdominal compartment syndrome surface in surgical, medical and pediatric critical care units.
In response to the growing awareness of IAH and ACS, an international consensus group of multidisciplinary critical care specialists convened at the second World Congress on Abdominal Compartment Syndrome in 2004, to develop definitions and guidelines regarding IAH and ACS.3 The definitions include: IAH is an intra-abdominal pressure (IAP) at or above 12mmHg and ACS is an intra-abdominal pressure above 20mmHg with evidence of organ dysfunction.
The definition for intra-abdominal pressure takes into account that the abdomen and its contents behave in accordance to Pascal’s law. Relatively non-compressive and primarily fluid in character, abdominal pressure is uniform. The IAP measured at one point is an accurate measure of the IAP throughout the abdomen. Intra-abdominal pressure is affected by inspiration, the volume of solid organs, presence of space-occupying lesions, ascites and presence of conditions that limit expansion of the abdominal wall. The consensus conference developed a grading scheme for elevated IAP. More than that, they defined abdominal perfusion pressure (APP) as the mean arterial pressure minus the IAP, and recommended that the APP be kept above 50–60 mmHg. But even if the APP is kept high, it is important to understand that IAP in itself may inhibit flow in compressible structures, in particular the renal and mesenteric veins and the ureters.
The pathophysiology of ACS is similar to the pathogenesis of compartment syndromes in other body regions such as the extremities and cranium. Increased pressure in a relatively fixed space interferes with perfusion and organ function. Above a threshold of about 20 mmHg, there is a reduction in the capillary perfusion, resulting in ischemia, release of inflammatory mediators and a cascade of adverse events.4
Risk Factors
Risk factors for the development of intra-abdominal hypertension and abdominal compartment syndrome include diminished abdominal wall compliance (abdominal surgery, major burns and trauma), increased intra-luminal contents (gastroparesis, ileus, and intestinal edema), increased abdominal contents (hemoperitoneum/pneumoperitoneum, ascites), capillary leak (acidosis, hypotension, hypothermia, coagulopathy, sepsis, and oliguria) and massive fluid resuscitation (greater than 5L in 24 hours). Many of these conditions are seen in critically-ill patients. A 1-day prevalence study in 13 intensive care units in Europe and Australia noted that >50% of medical critical care patients had an IAP of >12mmHg.5
Diagnosis
The diagnosis of ACS begins with awareness of the patients at risk. It is important to recognize that there are primary causes, particularly intra-abdominal swelling from trauma or other conditions, and secondary causes due to the edema associated with fluid resuscitation of shock and the effects of other diseases. The clinical signs of ACS are related to the consequences of these events. Within the abdomen, these include decreased renal blood flow with a resultant decrease in urine output, diminished hepatic perfusion with liver dysfunction, and decreased mesenteric venous drainage with consequent intestinal swelling and ileus. Systemic consequences include increasing positive airway pressure in the ventilated patient and decreased cardiac output due to compression of the inferior vena cava, which lowers venous return to the heart. If a patient has a tense abdomen, low urine output, increased peak airway pressure and a history of large volume fluid resuscitation, the clinician should be very suspicious, and should actively consider intrabdominal hypertension and/or abdominal compartment syndrome. Unfortunately, these signs are not specific. Many critically-ill patients will have such findings, especially patients with major abdominal injuries. Fortunately, measurement of the IAP by determining the bladder pressure is definitive and diagnostic, easily accomplished, and readily available - if we think of it. The recommended technique is to instill 25–50 ml of sterile saline into the bladder via the Foley catheter, clamp the catheter, and insert a needle attached to a pressure transducer in the specimen port proximal to the clamp. There are commercially available kits that are helpful; IAP can even be measured continuously, using a pressure transducer equipped catheter. The pressure should be measured in the supine position at end inspiration with no abdominal muscle contractions and with the transducer zeroed at the mid axillary line.
Prevention
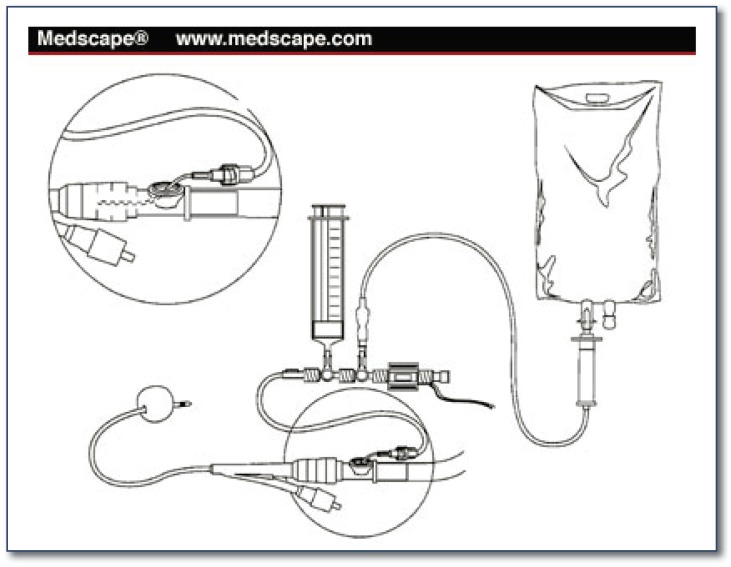
Prevention of abdominal compartment syndrome can be enhanced by avoidance of excessive fluid resuscitation, use of the open abdomen at the time of initial laparotomy and temporary abdominal closure with a Bogota bag (an open sterile saline bag) or vacuum assisted device (See Figure 1). The risk factors for developing ACS are numerous. That having been said, one might be more inclined to consider leaving the abdomen open in a patient with risk factors such as five liters or more of fluid resuscitation, acidosis, hypothermia, and polytransfusion. Elevated peak inspiratory pressure (PIP) is a risk factor for the development of ACS and a PIP that increases when the fascia is closed is an additional concern. If the closure is difficult and the abdomen is tense, one might be inclined to leave it open, and it’s important to recognize that inability to close the abdomen at the time of initial surgery is not a failure of judgment or technique, but instead may be the opposite. Closing the abdomen is not immediately necessary. Survival of the patient is the goal. One of the major advantages of “damage control surgery” for abdominal trauma is that it greatly reduces the risk of ACS.
Figure 1.
Bladder Pressure Monitor
Medical Treatment
Medical treatment can be helpful. The first step is body positioning. Elevating the head of the bed even 20 degrees6 can increase the IAP by 2mmHg or more. However, elevating the head of the bed is known to decrease ventilator-associated pneumonia, and is part of the “ventilator bundle” being used in many critical care units. One must balance the positive effects of the preventive measure (head of bed elevation) with the effects of the therapeutic measure (supine positioning). Other maneuvers to lower IAP by decreasing the intra-abdominal volume are intuitive. They include removal of gastric contents with a nasogastric tube and colonic evacuation stimulated by prokinetic agents and enemas. Removal of excess fluid with diuretics or even ultrafiltration may be of value, but it is critical to maintain adequate intravascular volume. Invasive monitoring may be needed to assure adequate perfusion during these interventions.
Mechanical decompression is a mainstay of treatment. It may be accomplished in several ways with varying degrees of invasiveness. Percutaneous catheter decompression7 is a less invasive method of treating IAH and may be more palatable, especially for the hesitant. It can be effective in the presence of tense ascites. Performed with ultrasound or CT guidance, it can be effective in draining abscesses and other localized fluid collections.
Surgical Treatment
Surgical techniques are much more invasive. Subcutaneous anterior abdominal fasciotomy can be done at the bedside to decompress the abdomen. This involves minimally invasive techniques to engage and incise the linea alba allowing fascial diastasis and relief of IAH.8, 9 Open abdominal decompression is the definitive treatment for fully manifested ACS. Various techniques such as suturing sterile plastic or using a vacuum wound closure devise can be employed in the management of the open abdomen. It is important to allow for additional bowel swelling in this situation. Utilization of an evidence-based, algorithmic approach to recognize, define and treat IAH and ACS with early and aggressive therapy has been shown to improve survival.10
Conclusion
Intraabdominal hypertension and abdominal compartment syndrome are objectively definable abnormalities that are much more common than previously recognized, and may threaten the survival of the critically-ill patient. Recognition and acceptance of their existence and impact, coupled with known therapies to decrease intraabdominal pressure, has been shown to improve survival.
Biography
Gerald L. Early, MD, MA, MSMA member since 1982, (top) is the Medical Director of Patient Safety and Associate Professor in the Department of Surgery at the University of Missouri - Kansas City School of Medicine and Truman Medical Center in Kansas City, Mo. Stanley Augustin, MD, FACS, (bottom left) is the Medical Director for Centerpoint Trauma and Surgical Services. Julie Wesp, MD, MSMA member since 2011, (bottom right) is in the General Surgery Department at the University of Missouri - Kansas City School of Medicine.
Contact: gerald.early@tmcmed.org



Footnotes
Disclosure
None reported.
References
- 1.Kimball EJ, Rollins MD, Mone MC, et al. Survey of intensive care physicians on the recognition and management of intra-abdominal hypertension and abdominal compartment syndrome. Crit Care Med. 2006;34(9):2340–2348. doi: 10.1097/01.CCM.0000233874.88032.1C. [DOI] [PubMed] [Google Scholar]
- 2.Vidal GV, Weisser JR, Gonzalez F, et al. Incidence and clinical effects of intra-abdominal hypertension in critically-ill patients. Crit Care Med. 2008;36(6):1823–1831. doi: 10.1097/CCM.0b013e31817c7a4d. [DOI] [PubMed] [Google Scholar]
- 3.Malbain MLNG, Cheatham ML, Kirkpatrick A, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I Definitions. Intensive Care Med. 2006;32:1722–1732. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 4.An G, West MA. Abdominal compartment syndrome: A concise clinical review. Crit Care Med. 2008;36:1304–1310. doi: 10.1097/CCM.0b013e31816929f4. [DOI] [PubMed] [Google Scholar]
- 5.Ivatury RR. Abdominal compartment syndrome: A century later, isn’t it time to accept and promulgate? Crit Car Med. 2008;34:2494–2495. doi: 10.1097/01.CCM.0000235680.83667.EE. [DOI] [PubMed] [Google Scholar]
- 6.Cheatham ML, Malbrain MLNG, Kirkpatrick A, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II Recommendations. Intensive Care Med. 2007;33:951–962. doi: 10.1007/s00134-007-0592-4. [DOI] [PubMed] [Google Scholar]
- 7.Cheatham ML, Safcsak K. Percutaneous catheter decompression in the treatment of elevated intraabdominal pressure. Chest. 2011;140:128–1435. doi: 10.1378/chest.10-2789. [DOI] [PubMed] [Google Scholar]
- 8.Lepaniemi AK, Hienonen PA, Siren JE, et al. Treatment of abdominal compartment syndrome with subcutaneous anterior abdominal fasciotomy in severe acute pancreatitis. World J Surg. 2006;30:1922–1924. doi: 10.1007/s00268-006-0024-6. [DOI] [PubMed] [Google Scholar]
- 9.Cheatham ML, Fowler J, Pappas P. subcutaneous linea alba fasciotomy: A less morbid treatment for abdominal compartment syndrome. The American Surgeon. 2008;74:746–749. [PubMed] [Google Scholar]
- 10.Cheatham ML, Safcsak K. Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit Care Med. 2010;38:402–407. doi: 10.1097/ccm.0b013e3181b9e9b1. [DOI] [PubMed] [Google Scholar]