Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia, resulting in significant morbidity and mortality, and enormous socio-economic consequences. Though many surgical procedures exist for the treatment of AF, the Cox-Maze IV procedure developed at Washington University has shown excellent long-term results in diverse patient populations. Furthermore, advances in preoperative diagnostic technology currently under investigation at our institution may allow for further refinement and individualization of the surgical treatment of AF in the future.
Introduction
Atrial fibrillation (AF) is the most common of all cardiac arrhythmias, affecting nearly 4.5 million people in the European Union and 2.2 million people in the United States alone. The risk of thromboembolism with resultant stroke, the most serious complication of AF, is increased three- to five-fold in these patients. Significant morbidity and mortality also result from hemodynamic compromise due to loss of atrial contraction, exacerbations of congestive heart failure from atrio-ventricular asynchrony, and tachycardia-induced cardiomyopathy. As a result, AF has an enormous socio-economic impact. Because the incidence of AF increases with age, AF is expected to become an even larger public health burden as the country’s general population ages.
Classification of Atrial Fibrillation
The most commonly used classification system for atrial fibrillation is that published jointly by the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society (HRS).1 In this system, AF is defined as either paroxysmal or persistent, and it is considered recurrent if a patient has had two or more episodes. If recurrent AF terminates spontaneously, it is designated paroxysmal. If it is sustained beyond seven days, it is termed persistent. Patients with AF that has lasted longer than one year are labeled as having long-standing AF. Importantly, the definitions of paroxysmal, persistent and long-standing AF do not imply a specific mechanism, and human mapping data from our laboratory and others have not shown significant differences in mechanism between paroxysmal and persistent AF.
Electrophysiology of Atrial Fibrillation
Atrial fibrillation is characterized by the irregular activation of the atria and an accompanying irregular ventricular response. Several activation patterns may underlie this pathology, including focal drivers and single reentrant circuits acting on vulnerable substrates.2 Perpetuation of AF may also be maintained in a disorganized fashion by “multiple wavelets,” representing numerous simultaneous functional reentrant circuits.2
In order for reentry to be maintained, an impulse must traverse the entire circuit slowly enough for the early areas of activation to regain excitability. The area of tissue required to support a particular reentrant circuit is known as the “critical mass.” The critical mass hypothesis states that a certain minimal amount of atrial tissue is required for the induction and maintenance of AF. In vitro and in vivo studies from our laboratory support this model and have shown that the probability of sustained AF is dependent on atrial tissue width and weight, conduction velocity (CV), the length of the refractory period (RP), and increasing atrial surface area.3
Further aberrancies in atrial conduction that help maintain AF may result from electrical and structural remodeling. Such changes include alterations in ion channels, gap junction channels (connexins) and tissue structure. Increased atrial fibrosis from remodeling, which occurs with congestive heart failure and with aging, results in inhomogeneities in conduction and enhanced tissue anisotropy, which can markedly slow CV.
Specific mechanisms responsible for AF in individual patients change over time, and intraoperative mapping data from our laboratory have shown that the source of AF is variable in almost half of patients, even moving from one atrium to the other.4 Surgical treatment of AF is intended to alter the geometry and anatomy needed to support fibrillation, regardless of the mechanism.
Indications for Surgical Ablation of Atrial Fibrillation
While there remains controversy over the relative roles of catheter-based and surgical ablation in patients with medically-refractory lone AF, a recent consensus statement endorses the indication for surgical ablation of AF in the following patient subgroups:1
all symptomatic AF patients undergoing other cardiac surgery;
selected asymptomatic AF patients undergoing cardiac surgery in which the ablation can be performed with minimal additional risk; and
symptomatic AF patients who prefer a surgical approach, have failed one or more attempts at catheter ablation, or are not candidates for catheter ablation. Thus, surgery is a complimentary, rather than a competitive, approach to catheter ablation.
There are also relative indications for surgery that were not included in the consensus statement. The first is the presence of a contraindication to long-term anticoagulation in patients with persistent AF and a high risk for stroke. The risk of stroke with a CHADS score ≥2 is 4% per year off anticoagulation.5 In contrast, the stroke rate following the Cox-Maze procedure off anticoagulation has been remarkably low (annual risk = 0.2%).
Finally, surgical treatment for AF with amputation of the left atrial appendage should also be considered in high-risk patients with persistent AF and a documented cerebrovascular event while on therapeutic anticoagulation. Anticoagulation with coumadin reduces the risk of ischemic and hemorrhagic strokes by more than 60% in patients with AF but does not completely eliminate this serious complication. At our institution, 20% of patients who underwent the Cox-Maze III procedure had experienced at least one episode of significant cerebral thromboembolism preoperatively. Less than 1% of patients (2 of 306) had a late stroke after a mean follow-up of 3.8 ± 3.0 years, even with 90% of patients off anticoagulation at last follow-up.6
Development of the Cox-Maze Procedure
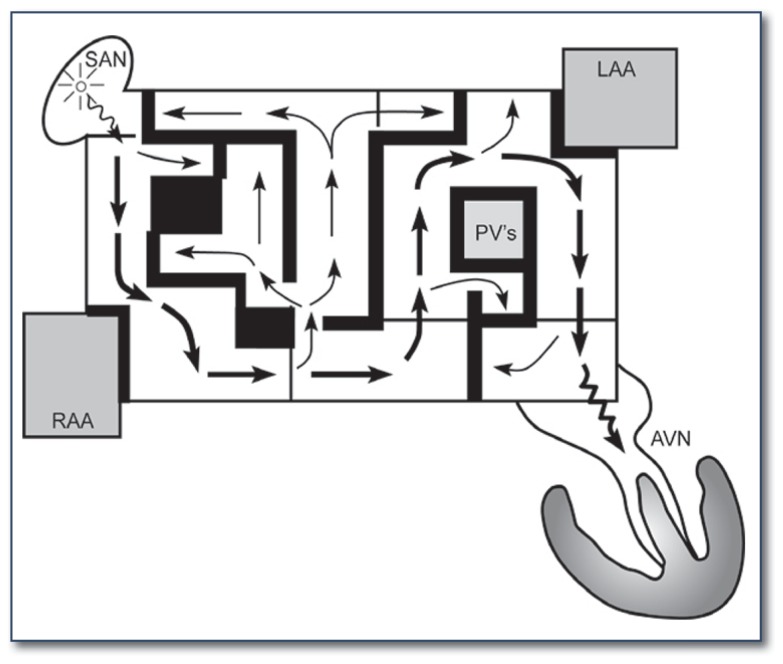
James Cox, MD, introduced the Cox-Maze procedure at Washington University in 1987. It was developed to interrupt the multiple macro-reentrant circuits that were felt to cause AF. Unlike previous procedures, the Cox-Maze procedure successfully restored both AV synchrony and sinus rhythm, thereby significantly reducing the risk of thromboembolism, stroke, and hemodynamic compromise.7 The operation consisted of biatrial surgical incisions placed so that the sinoatrial node could still direct the propagation of the sinus impulse, allowing most of the atrial myocardium to be activated and resulting in preservation of atrial transport function in most patients (See Figure 1).8
Figure 1.
The original maze operation was conceptualized as a pattern of surgical incisions that would prevent atrial fibrillation by blocking macro re-entrant circuits while still allowing propagation of a sinus impulse. Both atrial appendages were excised, and the pulmonary veins were isolated. AVN = Atrioventricular node; LAA = left atrial appendage; PVs = pulmonary veins; RAA = right atrial appendage; SAN = sinoatrial node.
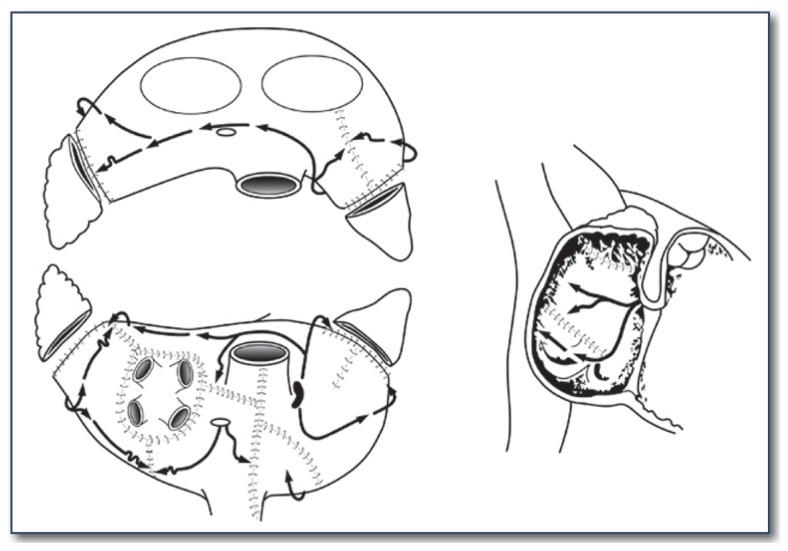
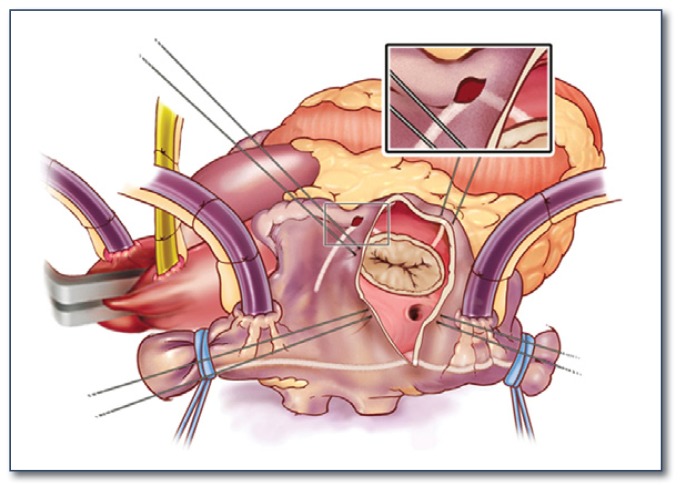
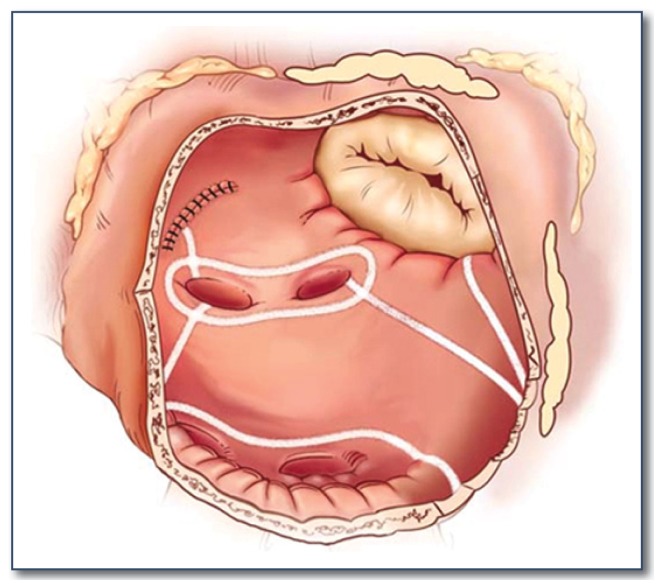
The first versions of the Cox-Maze procedure were complicated by late chronotropic incompetence resulting in a high incidence of pacemaker implantation, as well as significant surgical complexity. The third iteration, the Cox-Maze III procedure, became the gold standard for the surgical treatment of AF (See Figure 2). However, it failed to gain widespread acceptance due to technical difficulty, and significantly prolonged time on cardiopulmonary bypass. During the last decade, most groups have replaced the traditional “cut-and-sew” lesions with ablation lines created using various energy sources in an attempt to make the operation simpler and faster to perform. In 2002, our group introduced the Cox-Maze IV operation, which uses a combination of bipolar radiofrequency ablation and cryoablation to effectively replace the majority of incisions of the Cox-Maze III (See Figures 3 and 4). These ablation-assisted procedures have resulted in widespread adoption of the Cox-Maze and a significant increase in the number of AF operations performed annually. 9
Figure 2.
The lesion set of the traditional cut-and-sew Cox-Maze III operation.
Figure 3.
Illustration of the right atrial lesion set of the Cox-Maze IV procedure. Bipolar radiofrequency ablation is indicated by the white lines. Cryoablation is used to complete the ablation lines at the tricuspid valve annulus.
Figure 4.
Illustration of the left atrial lesion set of the Cox-Maze IV procedure. Bipolar radiofrequency ablation is indicated by the white lines. Cryoablation is used to complete the ablation line at the mitral valve annulus.
Surgical Results: Cox-Maze Procedure
The Cox-Maze III procedure has had excellent long-term results. In our series, 97% of 198 consecutive patients who underwent the procedure were free from symptomatic AF at a mean follow-up of 5.4 years. There was no difference in the cure rates between patients undergoing a stand-alone Cox-Maze procedure and those undergoing concomitant procedures.6
Our results with the Cox-Maze IV procedure have been encouraging as well. A recent prospective trial from our institution followed 100 consecutive patients undergoing a stand-alone procedure between January 2002 and May 2010.10 The mean follow-up was 17 ±10 months, and enrolled patients had paroxysmal (31%), persistent (6%) and longstanding persistent (63%) AF. This study demonstrated postoperative freedom from atrial tachyarrhythmias (ATAs) of 93%, 90%, and 90% at six, twelve, and twenty-four months, respectively. Freedom from ATAs off antiarrhythmic drugs was 82%, 82% and 84% at the same time points. In a group of 282 patients at our institution, the majority of whom had a Cox-Maze IV procedure with concomitant cardiac surgery, the results were similar with a freedom from ATAs of 89%, 93% and 89% at three, six, and twelve months, respectively.11 These studies are difficult to compare to the prior Cox-Maze III results due to more stringent follow-up, though a propensity analysis demonstrated no significant difference in the freedom from ATAs at three, six, and twelve months between the Cox-Maze III and IV groups.12
The Cox-Maze IV procedure is performed on cardiopulmonary bypass using either a median sternotomy, often in combination with other cardiac surgery, or a less-invasive right minithoracotomy (See Figure 5). The Cox-Maze IV procedure had significantly shorter mean cross-clamp times for a lone Cox-Maze (41 ± 13 minutes) compared to a Cox-Maze III (93 ± 34 minutes, p < 0.001).10 In those undergoing the Cox-Maze procedure concomitantly with another cardiac operation, cross clamp time was reduced from 122 ± 37 to 92 ± 37 minutes (p < 0.005).13
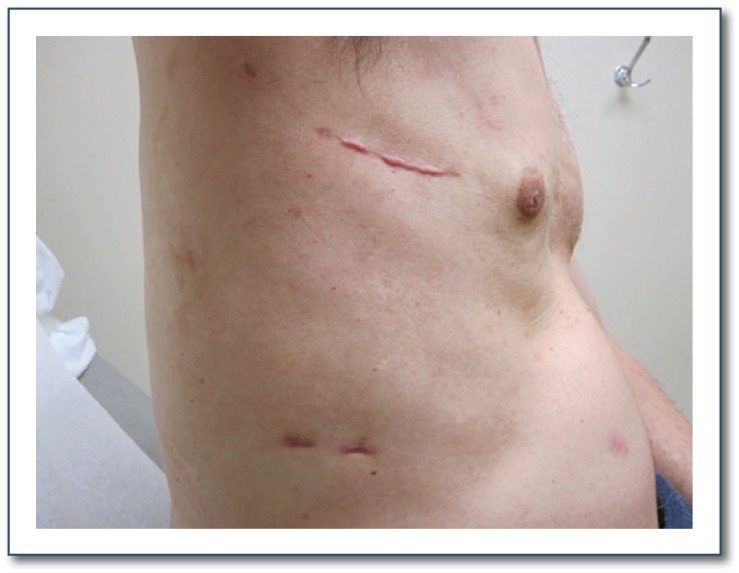
Figure 5.
Picture demonstrating the healed incision sites of the minimally-invasive right minithoracotomy approach used when performing a lone Cox-Maze IV procedure.
In our experience, risk factors for late recurrence of AF at one year have included enlarged left atrial diameter, failure to isolate the entire posterior left atrium, and early atrial tachyarrhythmias.11 Increasing left atrial size has been related to operative failure in several studies, and our group has clearly demonstrated that the probability of recurrence exceeds 50% once left atrial diameter is greater than 8 cm.11 Early atrial tachyarrhythmias were also associated with recurrence, and it is thought that these might be a marker of more advanced pathology of the atrial substrate in patients with AF of long duration.
Surgical Results: Other Atrial Fibrillation Procedures Left Atrial Lesion Sets
There are a wide range of procedures that incorporate only left-sided atrial lesions. Left atrial lesion sets have been performed from both an endocardial and epicardial approach, and have utilized many different ablation technologies. All of these procedures have incorporated at least some subset of the left atrial lesion set of the Cox- Maze procedure, and most have attempted to electrically isolate the pulmonary veins.
Although results have been variable, the value of left atrial lesion sets in maintaining sinus rhythm has been documented in the literature. While studies generally have demonstrated inferior results to those previously reported for the complete biatrial Cox-Maze lesion set, it is important to note that even a more limited left atrial lesion set proved superior to no intervention for patients with AF in a prospective, randomized study.14
There have been no randomized trials comparing biatrial versus left atrial ablation in the surgical population. As a result, the importance of the right atrial lesions of the Cox-Maze procedure is difficult to define. Although some evidence suggests a left atrial lesion set is as effective as a biatrial lesion set in patients with chronic AF undergoing concomitant open-heart procedures,15 other studies have not been as promising.16 In one large metaanalysis of over 5,000 patients, patients who underwent biatrial ablation demonstrated superior freedom from atrial fibrillation at all time points.17 These results are not surprising, as our intraoperative mapping experience in patients referred for surgery showed a stable origin of AF in the left atrium only 30% of the time. AF originated in the right atrium 12% of the time, and moved during the recording period in almost half of all patients.4
Of the specific left atrial lesions of the Cox-Maze procedure, it is difficult to determine the precise importance of each particular ablation. Several studies have shown the importance of the left atrial isthmus lesion in maintaining sinus rhythm.18, 19 Moreover, we have demonstrated the importance of a “box” lesion around the pulmonary veins, with failure to isolate the entire posterior left atrium resulting in higher recurrence rates of AF.11 Therefore, most of the left atrial Cox-Maze lesion set is likely needed to ensure a high success rate. It must also be kept in mind that recurrent right atrial flutter or tachycardia is a well-known complication of performing only the left atrial lesions. If the left atrial isthmus line is incomplete or omitted, patients are also at risk for late left atrial flutter.
Pulmonary Vein Isolation
Pulmonary Vein Isolation (PVI) is an attractive treatment option, since it can be performed quickly and does not require cardiopulmonary bypass. In lone AF, PVI can be executed via a minithoracotomy or a totally thoracoscopic approach. Electrical isolation of the pulmonary veins can be performed around the right and left pulmonary veins individually, with or without a connecting lesion between the two individual lesions, or as a box isolation of the entire posterior left atrium. Although a variety of energy sources have been used successfully in PVI, our institution favors bipolar RF clamps for this procedure. The problem with the unipolar energy sources has been their inability to reliably create transmural lesions on the beating heart.20
Although this approach has its benefits, the results of PVI have been variable. While most of the triggers for paroxysmal AF originate around the pulmonary veins, over 30% of triggers originate elsewhere.21 Thus, the success of PVI has proven to be highly dependent on patient selection. Although some studies have reported success rates ranging anywhere from 65–91% in mixed patient populations, these studies are limited by small sample size and limited follow-up.22,23 Encouraging results have been demonstrated in patients with paroxysmal AF, with one study reporting 82% freedom from AF at six months with 74% off antiarrhythmic drugs, and another reporting 88% freedom from AF at one year without antiarrhythmic drugs when PVI was combined with GP ablation.24 In patients with longstanding persistent AF, PVI has been less successful, with success rates as low as 35% freedom from AF and antiarrhythmic drugs at six months in one study.25 With concomitant procedures, the success rate of PVI is even lower, with one study reporting 61% freedom from AF, and only 17% freedom from antiarrhythmic drugs in the setting of mitral valve disease.26 These results highlight the need to fully understand the electrophysiological substrate of AF in order to perform an optimal operation for any individual patient.
Ganglionated Plexus Ablation
Electrophysiologic studies have demonstrated that local autonomic ganglia in ganglionated plexi clustered in the epicardial fat pads play a role in the initiation and maintenance of AF.27 Both pulmonary vein myocardial sleeves and adjacent atrial muscle are innervated by these plexi. As a result, some surgeons have added GP ablation to pulmonary vein isolation in hopes of increasing procedural efficacy. Some of the initial surgical results have been encouraging. One study of GP ablation combined with catheter PVI in 74 patients with lone AF demonstrated 91% freedom from AF after a relatively short median follow-up of five months.27 However, there have not been any direct comparisons as part of randomized clinical trials, and other groups have demonstrated much worse efficacy.
Moreover, the effects of vagal denervation and the long-term efficacy of GP ablation have not been clearly defined. Experimental evidence in our laboratory and others has demonstrated recovery of autonomic function in as few as four weeks after GP ablation. It is worrisome that reinnervation may not be homogeneous and could result in a more arrhythmogenic substrate. In another report using left atrial GP ablation alone to treat 19 patients with paroxysmal AF, fourteen of these patients (74%) had recurrent AF during one-year follow-up.28 Due to these suboptimal results and the lack of any long-term follow-up demonstrating the efficacy of GP ablation, our practice is not to perform GP ablation to treat AF. GP ablation should be reserved for centers participating in clinical trials.
Future Directions in Atrial Fibrillation Surgery
Although it has become less invasive, the Cox-Maze IV still requires cardiopulmonary bypass. Additionally, there are certain populations, such as those with enlarged atria, that have unacceptably high postoperative failure rates. Novel developments in diagnostic and surgical techniques are addressing these issues using patient-specific and minimally-invasive approaches. Ideally, such refinements will preserve normal atrial physiology, incur minimal morbidity, and achieve high success rates in curing AF.
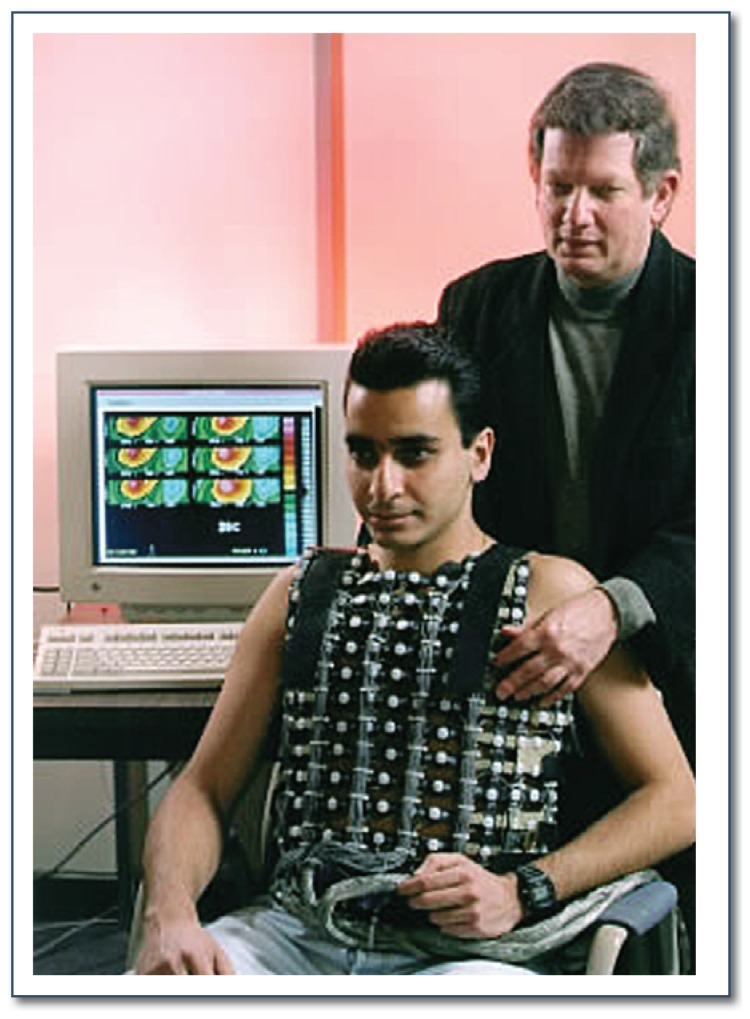
In the decades since the introduction of the Cox-Maze procedure, it has been proposed that a “critical mass” of tissue is requisite for fibrillation. However, deducing the electrophysiologic parameters necessary to define the “critical mass” in any given patient has presented a challenge. Because epicardial activation mapping, the traditional gold standard for mapping of AF, is both invasive and time-consuming, a new method of multipoint mapping known as electrocardiographic imaging (ECGI) is currently being evaluated. ECGI uses body surface potential mapping to obtain patient-specific heart-torso geometry and create maps of individual cardiac electrophysiologic activation (See Figure 6).29 With this information, the critical mass of tissue needed to sustain AF in an individual patient could be calculated, allowing a surgeon to create a novel patient-specific procedure to theoretically cure AF.
Figure 6.
Picture of a patient undergoing ECGI. The ECGI vest uses 250 body-surface electrodes to represent over 800 epicardial sites.
The development of new ablation technologies has introduced the possibility of novel surgical techniques that can be performed through small incisions without the need for cardiopulmonary bypass. As discussed previously, there is strong evidence that PVI performed epicardially on the beating heart may be effective in a subset of patients with paroxysmal AF. Using minimal access techniques, a novel linear lesion set that uses RF ablation to mimic most of the left atrial lesions of the Cox-Maze procedure on the epicardial surface has been described, and has shown promising results at both six and twelve months.30 Although current literature is scarce, two-stage hybrid procedures that combine PVI and resection of the left atrial appendage via a minimally invasive approach with either concomitant or delayed endocardial catheter-based ablation in the area of the biatrial isthmus lines are being performed at some institutions. However, present ablation technology remains unreliable at creating transmural linear lesions on the beating heart.20 This is a major limitation of these new procedures.
Conclusion
Surgical treatment of AF has been performed for over two decades, and has excellent efficacy. Recent advances in ablation technology have revolutionized the field and allowed for the development of simpler and less invasive approaches. The results of the Cox-Maze procedure done with ablation technology have been excellent in all patient subgroups. Success rates in other centers for more limited left atrial lesion sets, pulmonary vein isolation, and ganglionated plexus ablation have shown some promise, but have demonstrated suboptimal results in many patient populations. Future advances may be anticipated with improved devices, or by new techniques that allow surgeons and electrophysiologists to obtain reproducible complete lines of block. Moreover, the use of more sophisticated preoperative diagnostic technology may allow for a better definition of the underlying mechanisms of AF, and allow for a more rational selection of surgical ablation procedure for each patient.
Biography
Lindsey L. Saint, MD, and Ralph J. Damiano, Jr., MD, who is the John M. Shoenberg Professor of Surgery, practice in the Division of Cardiothoracic Surgery, Department of Surgery, Washington University School of Medicine, St. Louis.
Contact: damianor@wudosis.wust.edu


Footnotes
Disclosure
R. Damiano: Grant/Research Support from Atricure, Medtronic and Edwards Lifesciences. Consultant for Atricure and Medtronic.
References
- 1.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. Hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 3.Byrd GD, Prasad SM, Ripplinger CM, Cassilly TR, Schuessler RB, Boineau JP, Damiano RJ., Jr Importance of geometry and refractory period in sustaining atrial fibrillation: Testing the critical mass hypothesis. Circulation. 2005;112:I7–13. doi: 10.1161/CIRCULATIONAHA.104.526210. [DOI] [PubMed] [Google Scholar]
- 4.Schuessler RB, Kay MW, Melby SJ, Branham BH, Boineau JP, Damiano RJ., Jr Spatial and temporal stability of the dominant frequency of activation in human atrial fibrillation. J Electrocardiol. 2006;39:S7–12. doi: 10.1016/j.jelectrocard.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. Acc/aha/esc 2006 guidelines for the management of patients with atrial fibrillation: Full text: A report of the american college of cardiology/american heart association task force on practice guidelines and the european society of cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the european heart rhythm association and the heart rhythm society. Europace. 2006;8:651–745. doi: 10.1093/europace/eul097. [DOI] [PubMed] [Google Scholar]
- 6.Prasad SM, Maniar HS, Camillo CJ, Schuessler RB, Boineau JP, Sundt TM, 3rd, Cox JL, Damiano RJ., Jr The cox maze iii procedure for atrial fibrillation: Long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 7.Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg. 1999;118:833–840. doi: 10.1016/s0022-5223(99)70052-8. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg MS, Waggoner AD, Kater KM, Cox JL, Lindsay BD, Perez JE. Restoration of atrial function after the maze procedure for patients with atrial fibrillation. Assessment by doppler echocardiography. Circulation. 1994;90:II285–292. [PubMed] [Google Scholar]
- 9.Gammie JS, Haddad M, Milford-Beland S, Welke KF, Ferguson TB, Jr, O’Brien SM, Griffith BP, Peterson ED. Atrial fibrillation correction surgery: Lessons from the society of thoracic surgeons national cardiac database. The Annals of thoracic surgery. 2008;85:909–914. doi: 10.1016/j.athoracsur.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 10.Weimar T, Bailey MS, Watanabe Y, Marin D, Maniar HS, Schuessler RB, Damiano RJ., Jr The cox-maze iv procedure for lone atrial fibrillation: A single center experience in 100 consecutive patients. J Interv Card Electrophysiol. 2011;31:47–54. doi: 10.1007/s10840-011-9547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damiano RJ, Jr, Schwartz FH, Bailey MS, Maniar HS, Munfakh NA, Moon MR, Schuessler RB. The cox maze iv procedure: Predictors of late recurrence. J Thorac Cardiovasc Surg. 2011;141:113–121. doi: 10.1016/j.jtcvs.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lall SC, Melby SJ, Voeller RK, Zierer A, Bailey MS, Guthrie TJ, Moon MR, Moazami N, Lawton JS, Damiano RJ., Jr The effect of ablation technology on surgical outcomes after the cox-maze procedure: A propensity analysis. J Thorac Cardiovasc Surg. 2007;133:389–396. doi: 10.1016/j.jtcvs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MS, Damiano NR, Bloch JB, Moon MR, Damiano RJ., Jr A prospective, single-center clinical trial of a modified cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–542. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Doukas G, Samani NJ, Alexiou C, Oc M, Chin DT, Stafford PG, Ng LL, Spyt TJ. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: A randomized controlled trial. Jama. 2005;294:2323–2329. doi: 10.1001/jama.294.18.2323. [DOI] [PubMed] [Google Scholar]
- 15.Deneke T, Khargi K, Grewe PH, von Dryander S, Kuschkowitz F, Lawo T, Muller KM, Laczkovics A, Lemke B. Left atrial versus bi-atrial maze operation using intraoperatively cooled-tip radiofrequency ablation in patients undergoing open-heart surgery: Safety and efficacy. J Am Coll Cardiol. 2002;39:1644–1650. doi: 10.1016/s0735-1097(02)01836-3. [DOI] [PubMed] [Google Scholar]
- 16.Man KC, Knight B, Tse HF, Pelosi F, Michaud GF, Flemming M, Strickberger SA, Morady F. Radiofrequency catheter ablation of inappropriate sinus tachycardia guided by activation mapping. J Am Coll Cardiol. 2000;35:451–457. doi: 10.1016/s0735-1097(99)00546-x. [DOI] [PubMed] [Google Scholar]
- 17.Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: A meta-analysis. The Journal of thoracic and cardiovascular surgery. 2006;131:1029–1035. doi: 10.1016/j.jtcvs.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Gillinov AM, McCarthy PM, Blackstone EH, Rajeswaran J, Pettersson G, Sabik JF, Svensson LG, Cosgrove DM, Hill KM, Gonzalez-Stawinski GV, Marrouche N, Natale A. Surgical ablation of atrial fibrillation with bipolar radiofrequency as the primary modality. J Thorac Cardiovasc Surg. 2005;129:1322–1329. doi: 10.1016/j.jtcvs.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Gaita F, Riccardi R, Caponi D, Shah D, Garberoglio L, Vivalda L, Dulio A, Chiecchio A, Manasse E, Gallotti R. Linear cryoablation of the left atrium versus pulmonary vein cryoisolation in patients with permanent atrial fibrillation and valvular heart disease: Correlation of electroanatomic mapping and long-term clinical results. Circulation. 2005;111:136–142. doi: 10.1161/01.CIR.0000151310.00337.FA. [DOI] [PubMed] [Google Scholar]
- 20.Schuessler RB, Lee AM, Melby SJ, Voeller RK, Gaynor SL, Sakamoto S, Damiano RJ., Jr Animal studies of epicardial atrial ablation. Heart rhythm: the official journal of the Heart Rhythm Society. 2009;6:S41–45. doi: 10.1016/j.hrthm.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Tai CT, Hsieh MH, Tsao HM, Lin YJ, Chang SL, Huang JL, Lee KT, Chen YJ, Cheng JJ, Chen SA. Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: Implication for catheter ablation. J Am Coll Cardiol. 2005;46:1054–1059. doi: 10.1016/j.jacc.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Wolf RK, Schneeberger EW, Osterday R, Miller D, Merrill W, Flege JB, Jr, Gillinov AM. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg. 2005;130:797–802. doi: 10.1016/j.jtcvs.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 23.Beyer E, Lee R, Lam BK. Point: Minimally invasive bipolar radiofrequency ablation of lone atrial fibrillation: Early multicenter results. J Thorac Cardiovasc Surg. 2009;137:521–526. doi: 10.1016/j.jtcvs.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Edgerton JR, Jackman WM, Mack MJ. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. J Interv Card Electrophysiol. 2007;20:89–93. doi: 10.1007/s10840-007-9177-y. [DOI] [PubMed] [Google Scholar]
- 25.Edgerton JR, Edgerton ZJ, Weaver T, Reed K, Prince S, Herbert MA, Mack MJ. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. Ann Thorac Surg. 2008;86:35–38. doi: 10.1016/j.athoracsur.2008.03.071. discussion 39. [DOI] [PubMed] [Google Scholar]
- 26.Tada H, Ito S, Naito S, Hasegawa Y, Kurosaki K, Ezure M, Kaneko T, Oshima S, Taniguchi K, Nogami A. Long-term results of cryoablation with a new cryoprobe to eliminate chronic atrial fibrillation associated with mitral valve disease. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S73–77. doi: 10.1111/j.1540-8159.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- 27.Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: Their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13(Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 28.Katritsis D, Giazitzoglou E, Sougiannis D, Goumas N, Paxinos G, Camm AJ. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am J Cardiol. 2008;102:330–334. doi: 10.1016/j.amjcard.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 29.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr, Li L, Rudy Y. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgerton JR, Jackman WM, Mack MJ. A new epicardial lesion set for minimal access left atrial maze: The dallas lesion set. The Annals of thoracic surgery. 2009;88:1655–1657. doi: 10.1016/j.athoracsur.2009.05.046. [DOI] [PubMed] [Google Scholar]