Abstract
Pathogens generate molecules, or virulence factors, that enable them to colonize host tissues through several mechanisms, including adhesion to host tissues, or superior invasive capability. Some allow the pathogen to evade the host’s immune system. Many of these molecules are proteins that are exported to the cell’s surface or secreted. Curiously, GAPDH, which is a glycolytic enzyme, is also a virulence factor that has been shown to contribute to Streptococcus pyogenes pathogenicity by each of these mechanisms.
Introduction
Group A streptococci (conveniently abbreviated as GAS) represent a class of pathogenic microorganisms that cause a tremendous amount of human affliction. Importantly, GAS infections are considered highly communicable and a public health concern. The clinical presentations of GAS infections range from mild cases, such as pharyngitis and pyoderma, to more severe cases, such as rheumatic fever, rheumatic heart disease, and other invasive infections. Rheumatic fever occurs as a consequence of these mild GAS infections and represents an inflammatory disease in which antibodies created in response to the microorganism cross-react to one’s own tissues. Rheumatic fever may lead to rheumatic heart disease through repeated bouts of carditis (i.e. inflammation of the heart muscle) that resolve with fibrosis. It is thought that immune response factors, which are under genetic control, may account for pathogenesis of rheumatic heart disease.
More than 700 million people have mild cases of GAS infection worldwide.1 Severe cases, such as rheumatic fever and rheumatic heart disease, are responsible for an estimated 517,000 deaths per year globally. Rheumatic heart disease, which by far has the greatest impact on mortality and morbidity of GAS-related infections, contributes more than 282,000 new cases per year globally. Developing countries have the highest prevalence rate of rheumatic heart disease (i.e. 2 per 100 inhabitants).2 The prevalence rate in the United States is approximately 3 per 10,000 children ages 5–14.1 The annual number of deaths in the U.S. due to rheumatic heart disease, has fallen from 15,000 in 1950 to 3,100 in 2005–2008.2 Furthermore, from 1996 to 2006, the rate of mortality due to either rheumatic fever or rheumatic heart disease dropped about 8%. Despite these encouraging statistics GAS-related infections remain a major public health concern.
Diagnosis
A reference manual (i.e. Communicable Disease Investigation Reference Manual) that was released by the Missouri Department of Health and Senior Services in 2002 states that invasive GAS infections may develop into any of the following clinical manifestations: pneumonia, bacteremia in association with cutaneous infection (e.g. cellulitis, erysipelas, or infection of a surgical or nonsurgical wound), deep soft-tissue infection (e.g. myositis or necrotizing fasciitis), meningitis, peritonitis, osteomyelitis, septic arthritis, postpartum sepsis (i.e. puerperal fever), neonatal sepsis, and nonfocal bacteremia. GAS infections in some instances may result in shock and multi-organ failure. The laboratory criterion for diagnosis is identification of Streptococcus pyogenes by culture. Penicillin is the antibiotic of choice for GAS infections. There are rapid ‘strep’ tests available that take approximately 15 minutes, utilizing proprietary antibodies to detect specific GAS polysaccharide antigens.
The primary care physician typically encounters ‘strep throat’ (the colloquial term for streptococcal pharyngitis). This condition, which may also involve the larynx, results in fever as well as sore throat. This infection aggravates the immune system; therefore, enlargement of lymph nodes is also common. Thirty-seven percent of children under the age of 18 that present with sore throat contain positive strep cultures.3 Interestingly, 12% of children that do not exhibit any symptoms appear to have streptococcal microorganisms as part of their flora. In another study,4 it is estimated that approximately 20% of asymptomatic children are positive for streptococcal microorganisms. These observations suggest that streptococcal microorganisms may in some way be avoiding immune surveillance.
Streptococcus represents a genus classification of bacteria. This genus is described as spherical Gram positive bacteria that can be seen as round cells grouped together in a chain like fashion (see Figure 1). The various species within the genus of Streptococcus are divided into three subcategories (i.e. α-, β-, and γ-hemolytic), based on the growth properties in culture. Streptococcus pyogenes is a β-hemolytic bacterium, meaning that in blood agar cultures, it completely lyses red blood cells. The lysis of red blood cells is due to the exotoxin, streptolysin (i.e. two types: streptolysin O and streptolysin S), that is also thought to participate in pathogenesis. Streptococcus pyogenes belongs to the Lancefield serogroup A, which defines this streptococcal microorganism as a Group A member.
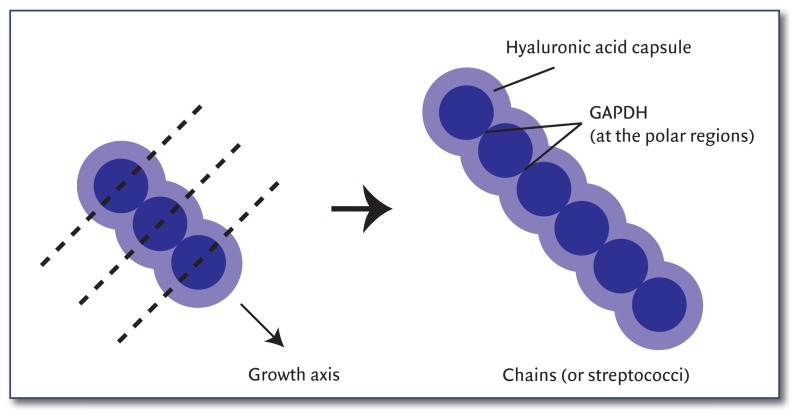
Figure 1. Morphology of Streptococcus pyogenes.
The cells are spherical, asymmetrical, and exhibit polarity. Parallel dashed lines represent the equatorial axes of the cells. The direction of growth is indicated by the diagonal arrow and cell division takes place along a single plane. During cell division, the cells remain attached, resulting in chains of cells. GAPDH is concentrated at the polar regions of the cells15 along the growth axis likely contributing to the polarization of the cell.
What is GADPH?
GlycerAldehyde 3-Phosphate DeHydrogenase (GADPH) is a highly conserved glycolytic enzyme found in all living cells. It is an enzyme that is indispensable, meaning that its loss by genetic mutation is incompatible with life. The enzyme catalyzes the oxidative phosphorylation of the glycolytic substrate, D-glyceraldehyde 3-phosphate, to 1,3-bis-phospho-D-glycerate with the concomitant reduction of NAD+ to NADH. This reaction is a pivotal step in glycolysis, which generates cellular energy.
While GAPDH has been known to function as a glycolytic enzyme since before 1950,5 it was not until the last 25 years that the enzyme’s amazing multifunctionality has come to light.6 GAPDH plays a role in the structural organization of the cell, transmission of genetic information, and cellular signal transduction networks. And all of these functions are relevant to the life of human cells. The mechanism by which GAPDH affects these various cellular processes is associated with its ability to form functional complexes with diverse cellular components, ranging from soluble actin to trans-membrane proteins. GAPDH in human cells is now known to be found throughout the cytoplasm as well as bound to cytoskeletal structures, each of the organelles, and the inner leaflet of the plasma membrane. These newly discovered functions of GAPDH include some fascinating findings about this protein in pathogenic microorganisms.
The microorganism Streptococcus pyogenes, like all cells, also contains GAPDH. In fact, the sequence of the streptococcal GAPDH is very homologous to the human GAPDH. Streptococcal GAPDH (UniProtKB/Swiss-Prot P0C0G7) is 59% similar and 49% identical to human GAPDH (UniProtKB/Swiss-Prot P04406) according to BLAST analysis (ncbi. nlm.nih.gov/blast). Curiously, streptococcal GAPDH is not only found in the cytoplasm, but it is found tightly bound to the external surface of this microorganism.7,8,9 While this phenomenon (i.e. surface-localized GAPDH) occurs in many pathogenic microorganisms,10,11,12,13 this article will only discuss the surface-localized GAPDH of Streptococcus pyogenes.
With the exception of a recent 2012 article that identified a surface-localized GAPDH on human macrophages that behaves as a transferrin receptor,14 a surface-localized or secreted GAPDH has not been documented in human cells. The appearance of surface-localized GAPDH is a puzzling phenomenon. Streptococcal GAPDH does not contain a secretion signal sequence, nor a hydrophobic trans-membrane domain, nor a hexapeptide sorting motif8. It is considered an anchorless surface protein, though it is tightly bound. The manner by which the GAPDH is trafficked to the outer surface, and the way that it is attached to the membrane are still mysteries to be resolved.
In 1991, a streptococcal surface protein was first isolated that interacted with plasmin with high affinity and therefore the gene for this protein was designated, plr (for PLasmin Receptor). The following year this plasmin-binding protein was identified as the streptococcal GAPDH.7
GAS contains several virulence factors. Some of the more well characterized virulence factors include the M protein, lipoteichoic acid (i.e. used for adherence), hyaluronic acid capsule (i.e. used as an immunological disguise), and invasins, for example, streptokinase and streptolysins (i.e. used to promote tissue invasion).
Cell Surface Structure
Pancholi and Fishetti,7 in 1992, identified the GAPDH protein as a major surface protein on this pathogen. GAPDH is tightly bound to the outside surface of the cell. Layers of peptidoglycan surround the plasma membrane and lie just below the outer hyaluronic acid capsule (see Figure 2). The surface-localized GAPDH was found in all of the serotypes that were tested and the protein appeared at similar levels. The authors7 purified the protein and identified it as being 39kD by SDS-PAGE (i.e. denaturing gel electrophoresis). The protein eluted from a gel filtration column as a tetrameric 150kD protein that exhibited enzymatic activity.
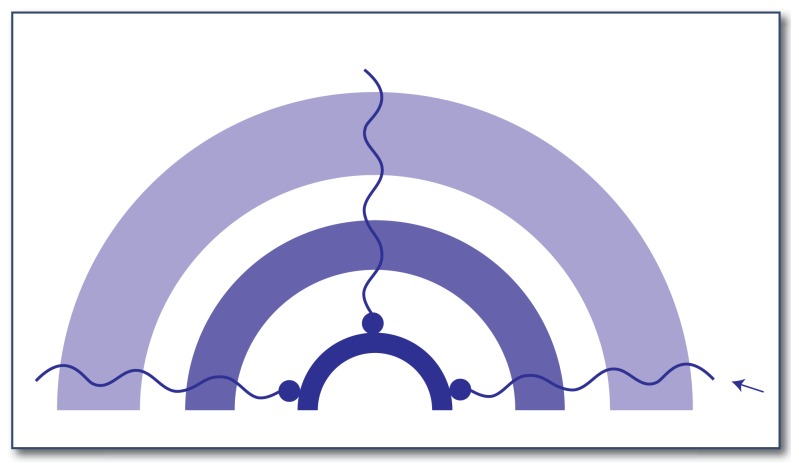
Figure 2. Cell surface structure of Streptococcus pyogenes.
The peptidoglycan cell wall (shown as a arc) surrounds the plasma membrane (shown as the innermost arc). The hyaluronic acid-containing capsule (shown as the outermost arc) covers the cell wall with a gel-like coat. The peptidoglycan layer typically consists of repeating units of N-acetylglucosamine and N-acetylmuramic acid via 1,4 β-glycosidic linkages. Group A polysaccharide antigen contains N-acetylglucosamine and rhamnose. GAPDH binds to the M-protein attached to the pili (shown by the arrow).
The authors7 designated the purified cell surface GAPDH as streptococcal SDH (for Surface DeHydrogenase). Streptococcal SDH was observed to bind host fibronectin, which supports the hypothesis that GAPDH plays a role in the colonization of the pathogen. Fibronectin resides in the dermis and is an important adhesive protein. Streptococcus pyogenes would have to penetrate the skin epidermis in order to reach the dermal layer where it would be able to bind to fibronectin. The binding of surface-localized GAPDH to an adhesive host protein would secure the microorganism to the site of infection, allowing colonization. Damaged skin becomes particularly susceptible to infection by Streptococcus pyogenes due to the ability of surface-localizing GAPDH to bind to the underlying dermal layer by adhering to fibronectin. Another complementary observation is that pathogenic GAPDH, which is surface localized, binds to host actin and myosin. These host proteins are major constituents of human cells, particularly myofibers that are found in both smooth and skeletal muscle. Hence, this property of streptococcal GAPDH binding to host cellular muscle proteins would enable the streptococcal microorganism to adhere to bruised or damaged tissue, enhancing streptococcal colonization particularly in damaged tissue, where the integrity of underlying myofibers is compromised. Therefore, these observations provide compelling evidence for GAPDH acting as a virulence factor.
Additionally, surface-localized GAPDH (or, so-called streptococcal SDH) binds to host lysozyme.7 The host enzyme, lysozyme, provides innate immunity. The lysozyme is a protease that is abundant in human secretions, including tears, salvia, and mucus. The host lysozyme hydrolyzes 1,4-β glycosidic-linkages found in the peptidoglycan layer of the cell wall of invading microorganisms. Surface-localized GAPDH of Streptococcus pyogenes may act as a virulence factor by suppression of the innate immune system through the binding to and inhibition of the host lysozyme.
A plasmin(ogen)-binding protein was found on the surface of Streptococcus pyogenes in 1987 by Lottenberg and his coworkers,16 well before it was identified as being GAPDH. Lottenberg and coworkers17 later recognized that the surface protein was an ortholog of human GAPDH. The surface-localized GAPDH binds to both the host plasmin, as well as host plasminogen proteins. This interaction isolates the host fibrinolytic system at the surface of the microorganism. The bacterium-bound plasmin is still catalytically active and capable of proteolysis. It is conceivable that GAPDH-mediated binding to plasmin allows the bacterium to use the host’s proteolytic action against the host, contributing to the pathogen’s invasiveness. Streptococci that were designed to have less surface-localized GAPDH (i.e. five-fold less) exhibited substantially decreased plasminogen-binding properties.18
A high and low affinity plasmin-binding site exists on the surface-localized pathogenic GAPDH. The C-terminal end of the protein has an α-helix, which contains amino acid residues 306–336 (particularly important is the lysine residue at position 336), that is the high affinity site. A lower affinity site was identified in regions that flank the S-loop (i.e. located in the middle of the protein).19 It is quite apparent that the GAS microorganism utilizes host plasmin-binding as a major strategy for virulence. The system for binding of the microorganisms to host plasmin is overbuilt in that it contains other plasmin-binding surface proteins besides the surface-localized GAPDH. This ensures that the mechanism of infection continues despite GAPDH interacting with other host proteins, as described above. Therefore, we see that GAPDH plays many roles as a bacterial surface protein, exhibiting numerous binding partners that contribute to diverse mechanisms of virulence.
Study Results
The study of the surface-localized GAPDH from Streptococcus pyogenes resulted in the breakthrough of still yet more potential mechanisms of virulence by this misconceived protein.18–22 Nitric oxide (or, so-called endothelial-derived relaxation factor) is important for human cardiovascular function. This compound acts on vascular smooth muscle resulting in vasodilation. Nitric oxide readily reacts with GAPDH. Nitric oxide-modified surface GAPDH is mono-ADP-ribosylated in the presence of the coenzyme, NAD+.20 A single ADP-ribose is auto-catalytically attached to the active site cysteine residue of GAPDH and then transferred to a target protein. This catalytic property is poorly understood, however, recent evidence suggests that it may inhibit macrophage activity,23 providing a growth advantage for this streptococcal microorganism.
The C-terminal α-helix and the flanking regions of the S-loop of GAPDH, binds to host pharyngeals cells via the host urokinase plasminogen activator receptor, mediating adhesion to human pharyngeal cells. The cell bound bacterium via interaction between surface GAPDH and the urokinase plasminogen activator receptor, enhances the genetic expression of more urokinase plasminogen activator receptor, populating the pharyngeal surface. This mechanism would increase the binding of the host urokinase to pharyngeal cells, promoting localized invasion to the infected tissue.
An additional mechanism involves bypassing the host immune surveillance due to the strong homology of pathogenic GAPDH to human GAPDH, host immune cells may not identify it as foreign.
Streptococcal surface GAPDH may also have anti-phagocytic activity as evidence by engineered bacteria that show diminished levels of surface GAPDH also exhibit decreased anti-phagocytosis.18 Interestingly, the re-distribution of GAPDH in these mutants results in the simultaneous loss of the surface M protein, which has known anti-phagocytic activity. The M-protein is found on pili (see Figure 2). It is thought that surface GAPDH may be attached via its association with the M protein. D’Costa and coworkers24 provide evidence that Streptococcus pyogenes GAPDH is anchored to the cell surface by means of the M-protein.
Summary
In summary, Streptococcus pyogenes GAPDH not only acts as an important cytoplasmic, glycolytic energy generating enzyme, this misconceived protein is an important virulence factor. It becomes surface-localized by a currently unknown mechanism and on the surface of the pathogen it can wreak havoc. By binding host plasmin(ogen) it contributes to the microorganisms invasive properties. Pathogenic GAPDH can bind to intact host pharyngeal cells as well as to damaged host tissues via fibronectin, actin, and myosin. Additionally, GAPDH can affect the host immune system by directly inhibiting phagocytosis, as well as escaping the immune-surveillance. Despite the strong homology between pathogenic and human GAPDH there still exists differences in the protein sequence, which may allow for production of specific small molecular weight compounds as selective antibiotics. The fact that pathogenic GAPDH is so close in structure to the human GAPDH may provide insight into molecular mechanisms of autoimmune diseases.
Biography
Kirsten A. Seidler (left) recently graduated magna cum laude (Class of 2013) from the University of Missouri with a Bachelor of Science degree in Biochemistry. Norbert W. Seidler, PhD, serves as professor and chair of biochemistry at Kansas City University of Medicine and Biosciences in Kansas City, Mo.
Contact: nseidler@kcumb.edu


Footnotes
Disclosure
None reported.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126(3):e557–564. doi: 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 4.Bisno AL, Gerber MA, Gwaltney JM, Jr, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis. 2002;35(2):113–125. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 5.Cori GT, Slein MW, Cori CF. Cr ystalline d-glyceraldehyde-3-phosphate dehydrogenase from rabbit muscle. J Biol Chem. 1948;173(2):605–618. [PubMed] [Google Scholar]
- 6.Sirover MA. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: biochemical mechanisms and regulator y control. Biochim Biophys Acta. 2011;1810(8):741–751. doi: 10.1016/j.bbagen.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Pancholi V, Fischetti VA. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992;176(2):415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pancholi V, Chhatwal GS. Housekeeping enzymes as virulence factors for pathogens. Int J Med Microbiol. 2003;293:1–11. doi: 10.1078/1438-4221-00283. [DOI] [PubMed] [Google Scholar]
- 9.Broder CC, Lottenberg R, von Mering GO, Johnston KH, Boyle MD. Isolation of a prokar yotic plasmin receptor. Relationship to a plasminogen activator produced by the same micro-organism. J Biol Chem. 1991;266(8):4922–4928. [PubMed] [Google Scholar]
- 10.Seifert KN, McArthur WP, Bleiweis AS, Brady LJ. Characterization of group B streptococcal glyceraldehyde-3-phosphate dehydrogenase: surface localization, enzymatic activity, and protein-protein interactions. Can J Microbiol. 2003;49(5):350–356. doi: 10.1139/w03-042. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann S, Rohde M, Hammerschmidt S. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect Immun. 2004;72(4):2416–2419. doi: 10.1128/IAI.72.4.2416-2419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amano A, Fujiwara T, Nagata H, et al. Porphyromonas gingivalis fimbriae mediate coaggregation with Streptococcus oralis through specific domains. J Dent Res. 1997;76:852–857. doi: 10.1177/00220345970760040601. [DOI] [PubMed] [Google Scholar]
- 13.Winram SB, Richardson LC, Lottenberg R. Mutational analysis of a plasmin receptor protein expressed by group A streptococci. Dev Biol Stand. 1995;85:199–202. [PubMed] [Google Scholar]
- 14.Rawat P, Kumar S, Sheokand N, Raje CI, Raje M. The multifunctional glycolytic protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a novel macrophage lactoferrin receptor. Biochem Cell Biol. 2012 Jan 31; doi: 10.1139/o11-058. [DOI] [PubMed] [Google Scholar]
- 15.Terao Y, Yamaguchi M, Hamada S, Kawabata S. Multifunctional glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pyogenes is essential for evasion from neutrophils. J Biol Chem. 2006;281(20):14215–14223. doi: 10.1074/jbc.M513408200. [DOI] [PubMed] [Google Scholar]
- 16.Lottenberg R, Broder CC, Boyle MD. Identification of a specific receptor for plasmin on a group A streptococcus. Infect Immun. 1987;55(8):1914–1918. doi: 10.1128/iai.55.8.1914-1918.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lottenberg R, Broder CC, Boyle MD, Kain SJ, Schroeder BL, Curtiss R., 3rd Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J Bacteriol. 1992;174(16):5204–5210. doi: 10.1128/jb.174.16.5204-5210.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boël G, Jin H, Pancholi V. Inhibition of cell surface export of group A streptococcal anchorless surface dehydrogenase affects bacterial adherence and antiphagocytic properties. Infect Immun. 2005;73(10):6237–6248. doi: 10.1128/IAI.73.10.6237-6248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin H, Song YP, Boel G, Kochar J, Pancholi V. Group A streptococcal surface GAPDH, SDH, recognizes uPAR/CD87 as its receptor on the human pharyngeal cell and mediates bacterial adherence to host cells. J Mol Biol. 2005;350(1):27–41. doi: 10.1016/j.jmb.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 20.Pancholi V, Fischetti VA. Glyceraldehyde-3-phosphate dehydrogenase on the surface of group A streptococci is also an ADP-ribosylating enzyme. Proc Natl Acad Sci USA. 1993;90(17):8154–8158. doi: 10.1073/pnas.90.17.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pancholi V, Fischetti VA. Regulation of the phosphor ylation of human pharyngeal cell proteins by group A streptococcal surface dehydrogenase: signal transduction between streptococci and pharyngeal cells. J Exp Med. 1997;186(10):1633–1643. doi: 10.1084/jem.186.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin H, Agarwal S, Agarwal S, Pancholi V. Surface export of GAPDH/SDH, a glycolytic enzyme, is essential for Streptococcus pyogenes virulence. MBio. 2011;2(3):e00068–11. doi: 10.1128/mBio.00068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerfaoui M, Davis K, Banerjee S, Friggeri A, Bell C, Abraham E. Poly ADP-ribosylation of HMGB1 enhances inhibition of efferocytosis. Mol Med. 2011 Dec 22; doi: 10.2119/molmed.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Costa SS, Romer TG, Boyle MD. Analysis of expression of a cytosolic enzyme on the surface of Streptococcus pyogenes. Biochem Biophys Res Commun. 2000;278(3):826–832. doi: 10.1006/bbrc.2000.3884. [DOI] [PubMed] [Google Scholar]