Abstract
Motor Neuron Diseases (MNDs) are neurological disorders characterized by the selective and progressive degeneration of motor neurons. Amyotrophic Lateral Sclerosis (ALS), commonly known as Lou Gehrig’s disease, is the most common. ALS causes diffuse muscle weakness and death secondary to respiratory failure. The diagnosis is made clinically, supported by electrodiagnostic testing. Although medications are limited, careful attention to breathing, nutrition, and patient mobility can have a major, positive impact on the course of the disease.
Introduction
Motor Neuron Diseases (MNDs) are a group of progressive disorders that are invariably fatal and affect the motor system, involving the upper, lower motor neurons, or both. The most common entity under the umbrella of MNDs is Amyotrophic Lateral Sclerosis (ALS). ALS is a progressive neurodegenerative disease that affects both upper and lower motor neurons, with pathology spanning the primary motor cortex, corticospinal tracts, brainstem and spinal cord, and causing diffuse muscle weakness, atrophy, spasticity and eventually death, typically due to respiratory failure. Other, less common subtypes of MND include Progressive Muscular Atrophy (PMA), which affects only the lower motor neurons; Primary Lateral Sclerosis (PLS), which affects only the upper motor neurons; and Progressive Bulbar Palsy (PBP), which involves both upper and lower motor neurons but remains confined to bulbar muscles (i.e. brainstem centers responsible for facial movements, swallowing, speech, chewing and breathing). MNDs also include hereditary diseases such as Spinal Muscular Atrophy (SMA) and Spinal-Bulbar Muscular Atrophy (SBMA), etc. This review will focus mainly on ALS.1
The incidence of ALS is estimated to be 1.7 cases per 100,000 per year,2 with the cumulative lifetime risk of developing the disease by age 75 being approximately 1:1000.3 The median age of onset is approximately 64 years for men and 67 for women with the male to female ratio estimated to be 1.8:1.4 ALS is associated with a family history in approximately 10–20% of cases. Familial ALS (FALS) is defined as the presence of a first degree relative also affected by the disease and most commonly follows an autosomal dominant inheritance pattern. As many as one-third of familial ALS cases are caused by a recently discovered repeat expansion that is also linked to Frontotemporal Dementia (FTD), and hence, a careful family history exploring the presence of a dementia syndrome and/or other neurodegenerative disorders in any family members should always be performed.5
Clinical Presentation
The hallmark of ALS is progressive development of motor symptoms and signs, with no accompanying sensory involvement.
Symptoms
Onset typically is gradual, occurring over a period of months, and is characterized by speech, breathing, or swallowing difficulty and weakness or loss of dexterity in one limb. Symptoms inevitably progress to involve other regions. Other features may include muscle wasting, muscle twitching (fasciculations), cramps, stiffness, and slowness. Fasciculations that are unaccompanied by wasting or weakness generally are due to benign causes rather than ALS. Bulbar onset with dysarthria and dysphagia occurs in one quarter to one third of cases.6,7 Bulbar disease can also manifest with voice hoarseness, sialorrhea, or “pseudobulbar affect,” i.e. mood instability characterized by insuppressible crying or laughter in response to emotional stimuli. Weight loss can occur with bulbar dysfunction or respiratory involvement.
Signs
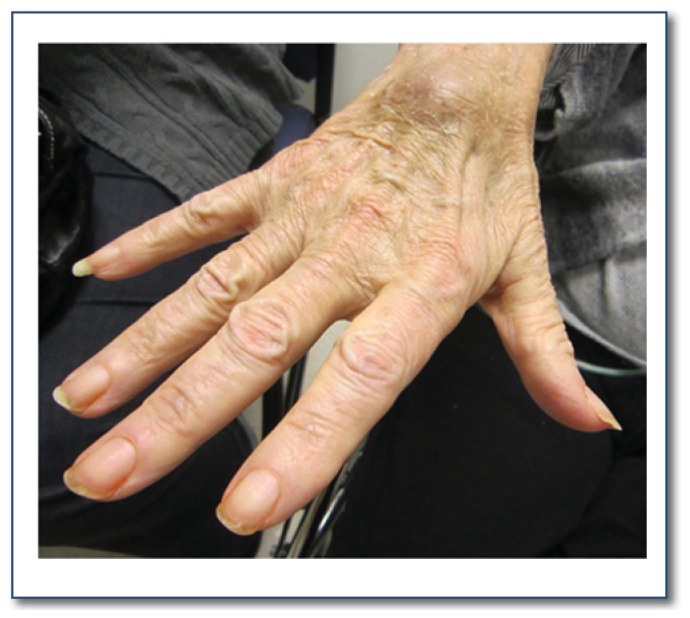
The neurological exam for the diagnosis of ALS should focus on testing for upper motor neuron signs (UMN) and lower motor neuron signs (LMN). UMN signs are spasticity, hyperreflexia, and slowed movements of arms or legs. LMN signs include weakness, muscle atrophy (See Figure 1) and fasciculations.
Figure 1.
Intrinsic hand muscle atrophy commonly seen in ALS patients.
Symptoms/Signs that Typically Do Not Appear in ALS and Suggest Another or Additional Diagnosis
Although symptomatic stabilization in a certain affected body region is occasionally observed, remission of symptoms and signs is rare, and when present should cast doubt over the ALS diagnosis. Similarly, bowel and bladder sphincter function, extraocular muscles and the sensory system are all typically spared, and when involved should trigger further investigation into other potential causes of the patient’s illness.
Disease Course
Typical survival time from diagnosis is three to five years, although there is wide variation with some patients surviving for as little as six months and others for up to 20 years. For most patients, the pace of the disease remains relatively constant. A rapid increase in the rate of disease progression should provoke examination of other possible causes of worsening (e.g. infection).
Disease Variants
While ALS is typified by the combination of both upper and lower motor dysfunction without sensory or cognitive disturbances, there are distinct variants of ALS that do not follow this pattern. Progressive muscular atrophy (PMA) is a lower motor neuron only variant of ALS. These patients have progressive muscle atrophy and weakness, but not the hyperreflexia, spasticity, and slowness typical of ALS. Many, though not all, patients with PMA eventually develop the UMN signs and symptoms. It is this variant that symptomatically overlaps most closely with multifocal motor neuropathy, a treatable ALS mimic.
Primary lateral sclerosis (PLS) is an upper motor neuron variant. These patients have severe spasticity, hyperreflexia, slowness, but do not have the muscle atrophy and weakness. Central nervous system disorders such as multiple sclerosis, strokes, and other structural lesions can give a similar presentation and are typically excluded by MRI imaging. Some of these patients do develop LMN signs and symptoms and more typical ALS.
Frontotemporal Dementia with ALS (FTD/ALS): A variant of ALS that suggests a hereditary disease is Frontotemporal Dementia with ALS (FTD/ALS). Patients develop a dementia syndrome distinguished by prominent deterioration in language and behavior, concomitant with, and occasionally years preceding the typical ALS motor symptoms (see “Unravelling the Mysteries of Frontotemporal Dementia” by Ghoshal and Cairns).7 Unlike Alzheimer’s disease, memory is typically preserved early in the course of dementia. While up to 40% of individuals with ALS may have some evidence of FTD on careful neuropsychiatric testing, only about 5–10% have fully developed FTD that manifests with behavioral changes noticed by the physician or caregivers. As noted previously, a recently reported autosomal dominant inherited expansion of a region of chromosome 9 underlies most of recognized FTD/ALS cases.5
When To Refer
Since ALS is rare and many physicians are unfamiliar with its presentation, the diagnosis is often delayed. Common clinical features that should prompt early referral to a neuromuscular specialist include: atrophy/weakness of muscles in a limb with increased deep tendon reflexes in that same limb, atrophy/weakness of muscles in one area with complaint of more widespread fasciculations and cramps, and progressively slurred speech with an unremarkable MRI of the brain.
Diagnostic Testing
The disease ALS is diagnosed clinically based on the presence of UMN signs and LMN signs while excluding other possible causes. Formal criteria for ALS have been established (the El Escorial Criteria).8 Laboratory investigation is focused on defining other causes (possible ALS mimic syndromes) which may be amenable to therapy. Testing for patients with suspected ALS typically consists of electrodiagnostic tests (electromyography [EMG] and nerve conduction studies [NCS]), MRI brain imaging, and laboratory testing (See Table 1).
Table 1.
Tests indicated in the evaluation of patients suspected to have ALS
AChR acetylcholine receptor; EMG/NCS: Electromyogram and nerve conduction studies; HSP: Hereditary Spastic Paraplegia; MuSk: muscle specific kinase; OPMD: oculopharyngeal muscular dystrophy; SBMA: Spinal-Bulbar Muscular Atrophy; SMA: Spinal Muscular Atrophy; SOD1: Superoxide dismutase 1; RNS: Repetitive nerve stimulation; VGCC: voltage-gated calcium channel.
| TESTS THAT SHOULD BE OBTAINED FOR ALL PATIENTS |
| MRI imaging (of brain, spine, and occasionally brachial or lumbosacral plexi) |
| EMG/NCS |
| Anti-GM1 and anti-NS6S antibodies Serum immunofixation, quantitative immunoglobulins |
| Copper, B12, TSH |
| TESTS THAT SHOULD BE CONSIDERED IN CERTAIN CLINICAL SCENARIOS |
| CSF studies (protein, glucose, cell count and differentiation, inflammation markers. West Nile serology) |
| Muscle biopsy |
| CK, aldolase (only mildly elevated in ALS) |
| Myasthenic syndromes work up (RNS, anti-AChR antibodies, anti-MuSK antibodies, anti-VGCC antibodies) |
| Myelopathy work up (RPR, HIV, HTLV, Lyme serology, rheumatologic labs) |
| Paraneoplastic antibodies |
| Heavy metal panel |
| Genetic studies to rule out other MNDs or MND-like diseases when suspected (SMA, SBMA, OPMD, HSP, etc) |
| Genetic studies to work up familial ALS (SOD1, C9orf72, TDP-43, FUS) |
Imaging
Magnetic resonance (MR) imaging is used to exclude any structural lesions that could potentially account for the patient’s presentation. Cervical spine disease with wasting and weakness in the arms or hands at the level of the lesion and spasticity in the legs typically is the most important condition to exclude. For presentations with speech abnormalities, excluding structural lesions (e.g., stroke) with brain MRI is important.
Electrodiagnostic Testing and Lab Work
EMG and NCS evidence of denervation in three body regions with normal sensory responses is the hallmark of ALS. Treatable motor neuropathies that mimic ALS such as multifocal motor neuropathy (MMN) are the most important diagnoses to exclude and can often be recognized on these tests.
Laboratory tests are focused on excluding autoimmune-mediated neuropathy, such as Multifocal Motor Neuropathy (MMN). Anti-GM1 or NS6S antibodies are present in up to 64% of MMN patients9 and combined with characteristic changes on EMG/NCS strongly suggest MMN, a disorder that often is responsive to immunomodulatory therapy. A low vitamin B12 level or thyroid disorders may complicate management and thus should be identified and corrected. Low copper can present with motor greater than sensory disturbances, although the sensory complaints provide a clue that ALS is unlikely.
Genetic Testing
Genetic testing is recommended for patients with a family history of ALS. The most common genetic cause for familial ALS known to date is the hexanucleotide repeat expansion in the C9ORF72 gene, which is also known to be associated with FTD. A family history of FTD, even without an associated motor syndrome might spark testing for a C9ORF72 mutation.5 Genetic counseling before testing is appropriate for patients and is strongly recommended before asymptomatic family members are tested.
Management
Following a diagnosis of ALS, the patient and care providers should receive regular support from a multidisciplinary care team. While the medications available to slow the progression of ALS are limited, careful attention to breathing, nutrition, and patient mobility can have a positive impact on the course of the disease.
Riluzole
Riluzole (Rilutek®) is the only FDA approved drug for ALS, and has been shown to prolong survival in ALS patients by three to six months. The modest benefit and high cost of the medication has limited enthusiasm for this treatment among some neurologists and patients, but the medication should be discussed with each patient. Riluzole is well tolerated overall, but patients should be monitored for liver toxicity. Elevation of hepatic enzymes to three to five times the upper limit of normal can occur but is typically self-limited and transient.10 It is generally recommended to monitor liver enzymes at one week, one month and every six months after the initiation of riluzole, and to stop the drug if liver enzymes are elevated to more than five times the upper limit of normal. Riluzole typically is given at 50 mg twice per day.
Respiration
Respiratory function should be assessed by measuring forced vital capacity (FVC)11 and maximal inspiratory pressure (MIP; a.k.a. negative inspiratory force (NIF)), and nocturnal oximetry12. The potential need for non-invasive ventilation (NIV) should be discussed with patients early in disease, and offered when FVC is less than 50%.13 Patients using NIV have been shown in a randomized controlled study to have a median survival benefit of 205 days.14
Nutrition
Dysphagia is a common problem for ALS patients, leading to choking, aspiration, poor nutrition, and weight loss. At the first signs of dysphagia, a swallowing study should be obtained by a speech pathologist. A change in diet to soft consistency, high caloric foods may initially ameliorate the problem, but in many ALS cases, oral intake becomes insufficient. Supplementation, via percutaneous endoscopic gastrostomy (PEG), is often required. PEG placement in ALS patients results in the stabilization, and occasionally partial regain, of weight15 and may prolong survival 16. In discussing PEG tube it is important to emphasize that that oral feeding does not have to completely stop. The decision for a PEG placement should be made early in the course of the disease as restricted pulmonary function (FVC<50%) increases the chances of procedure-related complications.17
Sialorrhea
Sialorrhea and drooling in ALS is not related to saliva overproduction, but results from an inability to adequately handle and swallow saliva. In some cases, a suction machine is sufficient to alleviate the problem. Traditional pharmacologic treatment of sialorrhea involves the use of anticholinergic agents, including glycopyrrolate (Robinul®), amitriptyline (Elavil®), benztropinemesylate (Cogentin®), trihexyphenidyl (Artane®), transdermal hyoscine (Scopolamine®), and atropine drops in mouth13. Common side effects include excessive drying of the nasopharynx, constipation, urinary retention, confusion, and sedation, particularly in the elderly. Other treatments include Botox injections and low dose radiation therapy to the salivary glands.
Pseudobulbar Affact
Antidepressants have been historically the mainstay of treatment for management of pseudobulbar affect. Both selective serotonin reuptake inhibitors (SSRIs) and tricylic antidepressants (TCAs) may be helpful, with TCAs superior in treating concomitant sialorrhea when present18. More recently, a fixed-dose combination of dextromethorphan (DM)/quinidine (Q) (30 mg DM/30 mg Q BID) has been shown to be effective in a randomized trial.19 Possible side effects include dizziness, nausea, and somnolence.
Emotional Support
The diagnosis of ALS can be emotionally difficult for patients and their caregivers. Many patients find antidepressants (SSRI) medications helpful for mood even when not meeting the full definition of depression. Support groups, available in most areas, can be very important for families and caregivers (see web resources at end of article).
Web Resources for ALS.
End of Life Issues
A discussion regarding end of life planning should be initiated relatively early in disease. For example, code status should be clarified. Most patients do not inquire about assisted suicide, though physicians should be prepared to discuss this issue.
Other Problems
Patients with ALS may develop other complaints related to the disease pathology, directly or indirectly, such as pain, spasticity, cramps, fatigue, depression and sleep difficulty. There are limited data focused on treatment of these problems in ALS patients, therefore standard management as with other conditions is recommended. Spasticity is generally treated with baclofen or tizanidine as first line agents. Agents of choice for treatment of cramps are gabapentin and phenytoin,20 while quinine has been recently restricted to treatment of malaria by the Food and Drug Administration (FDA) given its serious cardiac side effects profile.
Summary
Amyotrophic Lateral Sclerosis is a fatal neurodegenerative disease that currently remains incurable, but can be managed symptomatically over the disease duration to maintain the highest quality of life possible. Management is focused on controlling the symptoms, improving patient safety and maintaining autonomy to the extent possible. Multidisciplinary care in a coordinated ALS clinic setting is recommended. A careful family history is important to determine the presence of disease in other family members, and increase the likelihood of finding a genetic cause of the patient’s ALS syndrome. Vigorous clinical and basic research in ALS may ultimately bring about treatments to successfully alter the disease course.1
Biography
Taha Bali, MD, (left), is a Fellow, Neuromuscular Disease Division, and Timothy M. Miller, MD, PhD, is an Assistant Professor of Neurology at the Washington University School of Medicine in St. Louis.
Contact: millert@neuro.wustl.edu. http://millerlab.wustl.edu/


Footnotes
Disclosures
T Bali has no disclosures. T Miller receives research support from the following: NIH/NIA, NIH/NINDS, Project 5 for ALS, Tau Consortium, ALS Association, Muscular Dystrophy Association, Mallinckrodt Foundation, Hope Center for Neurological Disorders at Washington University, Washington University ICTS, Cure PSP, Isis Pharmaceuticals, Regulus Therapeutics and has filed patents in conjunction with Washington University regarding use of antisense oligonucleotides based therapeutic strategies for neurodegenerative diseases.
References
- 1.Bedlack RS, Mitsumoto H. Amyotrophic lateral sclerosis : a patient care guide for clinicians [Google Scholar]
- 2.Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology. 2002 Jul 23;59(2):280–282. doi: 10.1212/wnl.59.2.280. [DOI] [PubMed] [Google Scholar]
- 3.Johnston CA, Stanton BR, Turner MR, et al. Amyotrophic lateral sclerosis in an urban setting: a population based study of inner city London. J Neurol. 2006 Dec;253(12):1642–1643. doi: 10.1007/s00415-006-0195-y. [DOI] [PubMed] [Google Scholar]
- 4.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Incidence and prevalence of ALS in Ireland, 1995–1997: a population-based study. Neurology. 1999 Feb;52(3):504–509. doi: 10.1212/wnl.52.3.504. [DOI] [PubMed] [Google Scholar]
- 5.Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011 Oct 20;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chio A, Calvo A, Moglia C, Mazzini L, Mora G. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011 Jul;82(7):740–746. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- 7.Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002 Oct 8;59(7):1077–1079. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- 8.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994 Jul;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 9.Pestronk A, Chuquilin M, Choksi R. Motor neuropathies and serum IgM binding to NS6S heparin disaccharide or GM1 ganglioside. J Neurol Neurosurg Psychiatry. 2010 Jul;81(7):726–730. doi: 10.1136/jnnp.2009.202796. [DOI] [PubMed] [Google Scholar]
- 10.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996 May 25;347(9013):1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 11.Czaplinski A, Yen AA, Appel SH. Forced vital capacity (FVC) as an indicator of survival and disease progression in an ALS clinic population. J Neurol Neurosurg Psychiatry. 2006 Mar;77(3):390–392. doi: 10.1136/jnnp.2005.072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson CE, Rosenfeld J, Moore DH, et al. A preliminary evaluation of a prospective study of pulmonary function studies and symptoms of hypoventilation in ALS/MND patients. J Neurol Sci. 2001 Oct 15;191(1–2):75–78. doi: 10.1016/s0022-510x(01)00617-7. [DOI] [PubMed] [Google Scholar]
- 13.Miller RG, Rosenberg JA, Gelinas DF, et al. Practice parameter: The care of the patient with amyotrophic lateral sclerosis (An evidence-based review) Muscle Nerve. 1999 Aug;22(8):1104–1118. doi: 10.1002/(sici)1097-4598(199908)22:8<1104::aid-mus15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006 Feb;5(2):140–147. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 15.Kasarskis EJ, Scarlata D, Hill R, Fuller C, Stambler N, Cedarbaum JM. A retrospective study of percutaneous endoscopic gastrostomy in ALS patients during the BDNF and CNTF trials. J Neurol Sci. 1999 Oct 31;169(1–2):118–125. doi: 10.1016/s0022-510x(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 16.Mazzini L, Corra T, Zaccala M, Mora G, Del Piano M, Galante M. Percutaneous endoscopic gastrostomy and enteral nutrition in amyotrophic lateral sclerosis. J Neurol. 1995 Oct;242(10):695–698. doi: 10.1007/BF00866922. [DOI] [PubMed] [Google Scholar]
- 17.Mathus-Vliegen LM, Louwerse LS, Merkus MP, Tytgat GN, Vianney de Jong JM.Percutaneous endoscopic gastrostomy in patients with amyotrophic lateral sclerosis and impaired pulmonary function Gastrointest Endosc July-August1994404463–469. [DOI] [PubMed] [Google Scholar]
- 18.Miller RG, Rosenberg JA, Gelinas DF, et al. Practice parameter: the care of the patient with amyotrophic lateral sclerosis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology ALS Practice Parameters Task Force. Neurology. 1999 Apr 22;52(7):1311–1323. doi: 10.1212/wnl.52.7.1311. [DOI] [PubMed] [Google Scholar]
- 19.Brooks BR, Thisted RA, Appel SH, et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial. Neurology. 2004 Oct 26;63(8):1364–1370. doi: 10.1212/01.wnl.0000142042.50528.2f. [DOI] [PubMed] [Google Scholar]
- 20.Miller TM, Layzer RB. Muscle cramps. Muscle Nerve. 2005 Oct;32(4):431–442. doi: 10.1002/mus.20341. [DOI] [PubMed] [Google Scholar]