Abstract
Alzheimer disease (AD) is the most common cause of dementia in individuals over age 65, and is expected to cause a major public health crisis as the number of older Americans rapidly expands in the next three decades. Herein, we review current strategies for diagnosis and management of AD, and discuss ongoing clinical research and future therapeutic directions in the battle against this devastating disease.
Introduction and Epidemiology
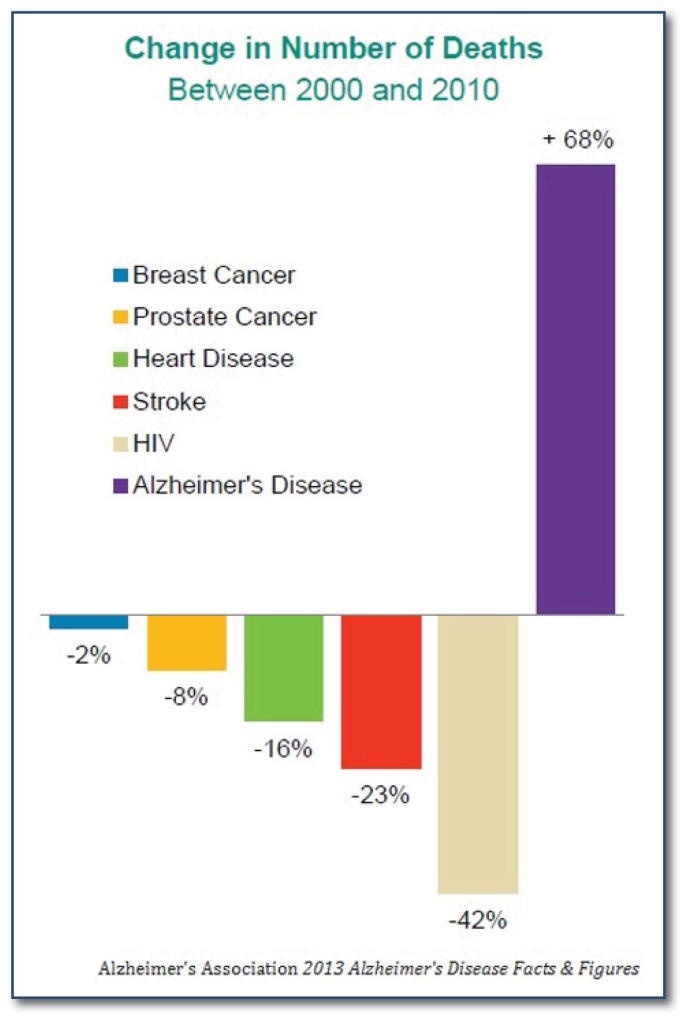
Alzheimer disease (AD) is a major public health problem in the United States and throughout the world. AD is the most common cause of dementia in people over the age of 65 and affects well over five million people in the United States (U.S.), including 110,000 people in Missouri1. As the population ages, the number of American AD cases is projected to explode to 16 million by 2050, with an estimated annual cost of 1 trillion dollars.1 Aside from its devastating effect on affected individuals, AD also takes an enormous toll on caregivers, as caring for an Alzheimer’s patient has been associated with financial stress, depression, and increased risk for other medical problems. Moreover, while the numbers of deaths caused by the other major killer diseases in the U.S., such as heart disease, cancer, and HIV, have declined in the past decade, deaths caused by AD continue to increase (See Figure 1). While no curative therapies have yet been developed, diagnosis of AD early in the disease course is important in that it allows for optimal initiation of symptomatic therapy and lifestyle modification, provides the opportunity for the patient to make plans for her own future, and may someday facilitate the preservation of cognition through disease-modifying therapy.
Figure 1.
Percent change in number of deaths caused by major diseases in the US, 2000–2008. Source: The Alzheimer’s Association.
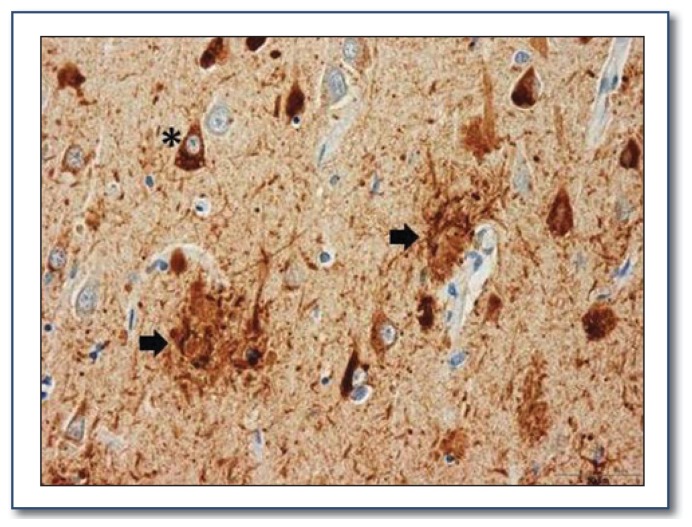
Alzheimer disease is characterized clinically by an insidious onset and progressive decline of cognitive function, usually beginning with impairment of short term memory. The classic neuropathologic hallmarks of AD are amyloid plaques, which are formed by aggregation of the amyloid-beta (Aβ) peptide, and neurofibrillary tangles, which consist of misfolded tau protein (See Figure 2). Autopsy data demonstrate that amyloid plaques begin forming in the brain many years before the onset of any symptoms. Amyloid plaques likely initiate a pathological cascade of events, including tau misfolding, oxidative stress and synaptic injury that eventually lead to neuronal death and brain dysfunction.2 The medial temporal lobe, which plays a critical role in short term memory, is particularly affected in AD, explaining the early characteristic deficits in short term memory.
Figure 2.
Amyloid plaques and neurofibrillary tangles within neurons in a silver-stained brain section from an Alzheimer’s disease patient (see asterisk and black arrows below).
Age is the strongest risk factor for the development of AD. At age 65, 13% of Americans have AD and by age 85 over 45% are affected.1 Family history impacts AD risk, as individuals with an affected first degree relative (parent or sibling) have a two-three fold increased risk of developing AD. The vast majority of AD cases (99%) are sporadic, following no clear Mendelian genetic inheritance pattern. For sporadic AD, Apolipoprotein E (APOE) genotype is the major genetic susceptibility factor. APOE ɛ3 is the most common genotype in the overall population. In comparison to ɛ3, the ɛ2 allele imparts a decreased risk of AD, while the ɛ4 allele is associated with an increased risk. Two copies of the ɛ4 allele increase the risk of AD by 12-fold, and about half of all AD patients harbor at least one APOE ɛ4 allele.3 Recent evidence suggests that APOE is important in Aβ metabolism and amyloid plaque formation.3 Several other genes have recently been identified which modulate the risk of sporadic AD, and ongoing research is investigating their functions. In less than 1% of cases, a dominantly inherited familial form of AD is caused by mutations in amyloid precursor protein (APP), presenilin 1 (PSEN1), or presenilin 2 (PSEN2). Disease-causing mutations have been shown to influence production of Aβ peptide.4 Most patients with dominantly inherited AD present with symptoms between age 30–50.
Proposed environmental risk factors for AD include traumatic brain injury, low educational attainment, diabetes, obesity, and cardiovascular risk factors such as high cholesterol and coronary artery disease.1 Women have a significantly higher lifetime risk of AD than men, due in part to their longer average lifespan. Caucasians have lower rates of AD than do African Americans or Hispanic Americans, though the reason for this is unknown. Exercise, healthy diet (including high intake of fish, fruits, and vegetables), and remaining cognitively involved and stimulated are all associated with lower risk of AD, although it has not been proven that any specific food or activity can prevent AD or slow its course. While a variety of over the counter agents have been purported to bolster cognition or prevent AD, most of these have not been rigorously tested, and none have been proven efficacious in randomized controlled clinical trials in humans.
Phenotype
The typical clinical course of AD dementia is characterized by an insidious onset and slow progression of symptoms over years.5 The initial symptoms are often related to short term memory deficits, such as repeating questions or statements, forgetting appointments, misplacing items, or forgetting important details about events. In the early stages, these symptoms can be very subtle, and can manifest simply as slight decline in the patient’s ability to perform complex tasks. Problems with orientation, including confusion about the date or getting lost, are often seen early in AD dementia. As the disease progresses, patients often require assistance managing finances, shopping, driving, and keeping track of their schedule. In moderate AD dementia, patients depend heavily upon a caregiver and often require assistance maintaining their hygiene. At the end stages of AD dementia, patients are completely dependent on others, may forget the identities of their closest friends and family members, and may become bed-bound. The proximate cause of death is usually aspiration or infection.
Diagnostic Methods
The goals of the initial history, exam, laboratory work-up and imaging are 1) to rule-out causes of cognitive dysfunction not related to neurodegenerative disease and 2) to diagnose the presence or absence of a neurodegenerative disease based on the history, exam, any available psychometric data, labs and imaging data.
History and exam
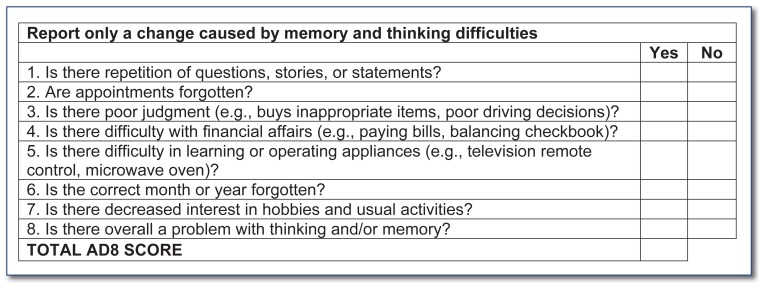
The majority of the history should be obtained from a collateral source familiar with the patient’s daily life, as patients with cognitive impairment often have poor insight into their condition. The focus of the history should be on changes in the patient’s memory and thinking over time, as individuals vary greatly in their baseline abilities and habits. For example, are there certain abilities the patient has lost because of a decline in memory or thinking? Although most people can describe occasional lapses in memory, the history should probe for problems that are consistent and worsening over time. The AD-8 Dementia Screening Interview can be helpful, as it is sensitive to early cognitive changes associated with dementia6 and is brief, reliable, and available at no cost. The AD-8 does not definitively diagnose dementia, but it can be helpful in identifying patients who require further evaluation (See Figure 3).
Figure 3. 8-item Informant Interview to Differentiate Aging and Dementia*.
(Positive Predictive Value = 87% for CDR 0 vs CDR ≥ 0.5)
*Adapted from Galvin et al, “The AD8: A Brief Informant-Interview to Detect Dementia”, Neurology. 2005;65:559–564.
Common causes of cognitive dysfunction, particularly in the elderly, include depression and cognitive side effects of medications. It is important to directly ask the collateral source and patient about symptoms of a possible mood disorder because this information is often not immediately offered. The patient’s medication list should be scrutinized for psychoactive medications, such as benzodiazepines, anti-cholinergics, pain medications, and sleep aids that can cause or exacerbate cognitive dysfunction. Any association between the patient’s symptoms and alcohol consumption should be assessed. A family history of dementia can suggest the patient is at higher risk, especially in cases of early onset dementia. Finally, it is helpful to screen for obstructive sleep apnea (OSA), as OSA is associated with a higher rate of developing dementia and is often treatable.7 In patients with dementia, disrupted sleep-wake cycles are common and may be a major cause of caregiver stress.
The patient should undergo a screening neurological examination. Verbal fluency should be noted, as certain dementias are associated with prominent language difficulties. A neurologic exam should be performed to exclude focal signs, which might suggest stroke, demyelination, or a mass lesion. The examiner should also evaluate for increased muscle tone, tremor, slowness, abnormal gait, or poor balance, which may indicate a Parkinsonian syndrome. Most patients presenting with AD dementia have normal neurological exams except for evidence of cognitive impairment.
Psychometric testing can be helpful in some circumstances: 1) Quantifying a subtle deficit 2) Identifying which cognitive domains are affected 3) Providing a baseline against which future testing can be compared. In addition to the informant-based AD8, short instruments that can be administered to the patient in an office setting include the Mini Mental State Exam (MMSE),8 the Short Blessed Test9 and the Montreal Cognitive Assessment (MOCA, available at www.mocatest.org).10 Interpretation of the tests must take into account educational level and any other factors that could affect the result, including decreased vision or hearing. A poor result on a test does not definitively diagnose dementia, but may lend support to the diagnosis. Complete evaluation performed by a licensed clinical neuropsychologist can be helpful in complex or atypical cases.
Laboratory work-up and imaging
Since cognitive dysfunction may be caused by a large number of medical and neurological causes, a thorough evaluation to rule-out potentially treatable causes of dementia is needed. Blood tests to be ordered include blood chemistries (including liver function test and creatinine), complete blood cell count, thyroid stimulating hormone (TSH) and vitamin B12 level. In patients with early onset dementia, atypical dementia, or risk factors for sexually transmitted infections, it is also appropriate to check a rapid plasma reagin (RPR) and HIV antibody. At this time, it is not recommended that patients routinely be tested for APOE isoform or mutations associated with autosomal dominant Alzheimer’s disease.11
Practice parameters developed by the American Academy of Neurology recommend that brain imaging be performed on all patients with cognitive decline.11 A brain MRI both with and without contrast and including diffusion sequences is the most sensitive test. In patients with renal dysfunction the MRI can be performed without contrast. In patients who cannot undergo an MRI because of contraindications such as an implanted pacemaker, a head CT is acceptable but may not visualize more subtle findings. In cases of early onset dementia, atypical dementia or dementia of uncertain diagnosis, cerebrospinal fluid (CSF) and advanced imaging techniques may help clarify the diagnosis. These methods, including recently-developed tests for CSF Aβ and tau and PET-based imaging of brain amyloid plaques (amyloid imaging) allow for more specific and earlier diagnosis of AD. These tests are described further in the article entitled, “Advances in Diagnostic Testing for Alzheimer Disease.”
Patients with onset of dementia prior to age 60, rapid progression of dementia over months, or atypical forms of dementia may benefit from seeing a dementia specialist for further evaluation. Symptoms of atypical dementias may include early and prominent changes in behavior, language, visuospatial function or movement.
Management
Two classes of medications are FDA approved for the treatment of AD. Acetylcholinesterase inhibitors, including donepezil (Aricept®), rivastigmine (Exelon®), and galantamine (Razadyne®) are approved for symptomatic treatment of mild to moderate AD, and are intended to support memory by increasing the amount of available acetylcholine in the synaptic cleft by preventing its breakdown. All of these agents are available in generic forms for oral administration. These drugs can cause gastrointestinal side effects including nausea, vomiting, and diarrhea, as well as cardiovascular effects such as bradycardia. Donepezil is the most commonly prescribed, and is usually initiated at 5 mg daily for four to six weeks, then increased to 10 mg daily if tolerated. A 23mg dosage has been approved that may impart slight increases in efficacy, but it is associated with significantly higher risks of gastrointestinal side effects12. Rivastigmine is available as a patch (not yet available as a generic), which may have lower incidence of nausea and can be helpful in more advanced AD patients who have difficulty remembering to take their pills.
The second class of FDA approved AD medication is N-methyl-D-aspartate (NMDA) glutamate receptor antagonists. Memantine (Namenda®) has been approved for use in moderate to severe AD. The initial postulated mechanism of action was reduction in glutamatergic excitotoxicity, but several studies have shown this is not the case. Memantine is also a non-competitive antagonist of serotonin 5HT3 and nicotinic acetylcholine receptors, and activates dopamine D2 receptors, though it is unclear if these actions mediate its effects. Side effects from memantine are quite rare, but can include confusion, drowsiness, headache, agitation, insomnia, and hallucinations.13 Generic memantine is not yet available in the U.S. Generally, memantine is added to the regimen after the patient has already reached a stable dose of an acetylcholinesterase inhibitor, though it can be used as monotherapy in patients who do not tolerate acetylcholinesterase inhibitors.
In more advanced disease, patients can become aggressive and violent. Atypical antipsychotic medications are sometimes indicated, but should be used carefully because of studies showing that antipsychotics increase mortality in elderly dementia patients. Benzodiazepines should also be used with great caution, as they can cause paradoxical increases in agitation, as well as dependence and withdrawal. Non-pharmacologic strategies for behavioral management include keeping the environment consistent, non-threatening, and calm, avoiding cues which may precipitate agitation, and simplifying the daily routine. Depression and anxiety are very common in AD patients, and physicians and caretakers must be vigilant for these conditions and treat them appropriately. The Alzheimer’s Association (www.alz.org) provides a wealth of valuable resources for both patients and caregivers, and in many areas provides a variety of informational sessions, support groups, and referral services.
Regarding safety, assessment of driving skills by a professional is recommended for any individuals with notable cognitive impairment, and restricting or eliminating driving is encouraged if there is substantial concern. Firearms in the home should be secured. Patients may need oversight with meals and with their medications, as well as with personal finances, and in some cases access to banking and investment accounts must be restricted. Patients with moderate disease may not be safe to leave unattended at home, as dangerous situations such as leaving water or a gas stove on are quite common. As the disease progresses, patients are likely to wander, and may need constant supervision, as well as a SafeReturn® bracelet, available through the Alzheimer’s Association.
Future Directions
It is an exciting time for research in AD and other dementias, as over 70 clinical trials of experimental therapies are ongoing. Large-scale clinical studies of dementia and healthy aging, including the Memory and Aging Project at Washington University and the nationwide Alzheimer’s Disease Neuroimaging Initiative, have provided critical insights into how AD begins and progresses, and have shown that the pathological process leading to clinical AD begins at least a decade prior to the onset of any cognitive symptoms.2 With the advent of new biomarkers, very early and even presymptomatic diagnosis is now possible. Unfortunately, at this point in time, all efforts at therapeutic intervention in the symptomatic disease course have failed. Thus, it appears that treatment strategies for AD must be initiated as early in the disease course as possible, to prevent ongoing neurodegeneration.2 Ideally, asymptomatic individuals could be screened and treated presymptomatically, thereby preventing or delaying dementia. At present, there is no such therapeutic “prevention” option and thus presymptomatic screening cannot be encouraged.
The majority of current experimental therapeutic strategies for AD focus on eliminating Aβ, as Aβ accumulation appears to precede neurodegeneration and symptom onset by years, and likely initiates the pathogenic cascade in AD. Passive immunization with antibodies that bind Aβ is an attractive paradigm, and a number of monoclonal antibodies are in various stages of clinical trials in humans.1. Small molecule inhibitors of beta-and gamma-secretases, enzymes which play critical roles in the generation of Aβ, have also been developed, and several are in late stage clinical trials.15, 16 As mentioned above, there have been several well-publicized failures of anti-amyloid therapies in phase III clinical trials in the past few years, including both Aβ antibodies and gamma secretase inhibitors. It is likely that these failures were due to multiple issues including poor efficacy of the drug in reducing Aβ levels, treatment too late in the disease process, and dilution of the trial population with non-AD dementias. A second generation of therapeutics is now entering phase III trials, and clinical trial methodology is being refined, so there is hope that an effective Aβ-targeted therapy will be identified. The first clinical trials providing presymptomatic therapy for rare early onset familial AD patients are beginning this year, including the Dominantly Inherited Alzheimer’s Network (DIAN) treatment trial, and the Alzheimer’s Prevention Initiative (API). The DIAN trials will employ two distinct anti-Aβ antibodies and API will use another Aβ antibody.17, 18 While they focus on rare autosomal dominant AD, success of these trials may set the stage for future preventative trials in sporadic (late onset, non-familial) AD. Indeed, a third trial, entitled the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) study, will evaluate presymptomatic therapy with anti-Aβ antibodies in 70+ year old participants with no cognitive symptoms, but with amyloid imaging evidence of presymptomatic AD. Thus, the era of presymptomatic anti-amyloid experimental therapy is at hand (but far from ready for clinical use), and these important trials likely will have major impact on the AD field for years to come.
While these initial trials all employ therapies targeting Aβ, non-Aβ therapies are also in development, including agents which aim to reduce tau aggregation, suppress neuroinflammation, prevent oxidative injury, augment neuronal metabolism, and modulate APOE levels. Small molecule activators of α7 nicotinic acetylcholine receptor and nasally-inhaled insulin have entered clinical trials, both of which have been show to enhance cognition in AD in smaller studies, providing new possible avenues of symptomatic therapy.19, 20 Intravenous immunoglobulin (IVIG) has shown promise in stabilizing cognition in small cohorts of symptomatic AD patients, though the mechanisms are unclear, and a larger trial has reportedly failed. Preclinical studies in mouse models have identified dozens more potential therapeutic targets, which await validation in humans but will hopefully keep the drug development pipeline stocked for years to come.
Conclusion
Alzheimer disease is a devastating neurodegenerative disease which affects millions of people, and threatens to become a public health crisis in coming years. Great strides have been made in our understanding of the underlying disease mechanisms and in early diagnosis, and the first experimental efforts to treat AD presymptomatically are beginning, potentially heralding a new era of AD management.
Biography
Erik S. Musiek, MD, PhD, (left) is a an Assistant Professor of Neurology, and Suzanne E. Schindler, MD, PhD, is a Clinical and Postdoctoral Research Fellow in Neurology. Both are with the Knight Alzheimer’s Disease Research Center at the Washington University School of Medicine in St. Louis.
Contact: musieke@neuro.wustl.edu


Footnotes
Disclosure
None reported.
References
- 1.Alzheimer’s Association. Alzheimer’s Disease Facts and Figures, Alzheimer’s & Dementia. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurology. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayeux R. Clinical practice. Early Alzheimer’s disease. N Engl J Med. 2010;362:2194–2201. doi: 10.1056/NEJMcp0910236. [DOI] [PubMed] [Google Scholar]
- 5.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant inter view in dementia. Neurology. 2006;67:1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Katzman R, Brown T, Fukd P, Peck A, Schechter R, Shimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiat. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 10.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment (MoCA©): A Brief Screening Tool For Mild Cognitive Impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 11.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 12.Farlow MR, Salloway S, Tariot PN, Yardley J, Moline ML, Wang Q, Brand-Schieber E, Zou H, Hsu T, Satlin A. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: A 24-week, randomized, double-blind study. Clin Therapy. 2010;32:1234–1251. doi: 10.1016/j.clinthera.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Areosa Sastre A, Sherriff F, McShane R. Memantine for dementia. The Cochrane Database of Systematic Reviews. 2005;(3) doi: 10.1002/14651858.CD003154.pub4. Art. No.: CD003154. [DOI] [PubMed] [Google Scholar]
- 14.Panza F, Frisardi V, Imbimbo BP, Seripa D, Solfrizzi V, Pilotto A. Monoclonal antibodies against β-amyloid (Aβ) for the treatment of Alzheimer’s disease: the Aβ target at a crossroads. Expert Opin Biol Ther. 2011;11:679–686. doi: 10.1517/14712598.2011.579099. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh AK, Brindisi M, Tang J. Developing β-secretase inhibitors for treatment of Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):71–83. doi: 10.1111/j.1471-4159.2011.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe MS. γ-Secretase inhibitors and modulators for Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):89–98. doi: 10.1111/j.1471-4159.2011.07501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JC, Aisen PS, Bateman RJ, Benzinger TL, Cairns NJ, et al. Developing an international network for Alzheimer research: The Dominantly Inherited Alzheimer Network. Clin Investig (Lond) 2012;2:975–984. doi: 10.4155/cli.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, Quiroz YT, Kosik KS, Lopera F, Tariot PN. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(Suppl 3):321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haydar SN, Dunlop J. Neuronal nicotinic acetylcholine receptors - targets for the development of drugs to treat cognitive impairment associated with schizophrenia and Alzheimer’s disease. Curr Top Med Chem. 2010;10:144–152. doi: 10.2174/156802610790410983. [DOI] [PubMed] [Google Scholar]
- 20.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]