Abstract
Parkinson disease is the second most neurodegenerative disease, after Alzheimer disease, that affects up to two million Americans, the overwhelming majority of whom are aged 60 and older. The changing demographics of the country place more Americans at risk for Parkinson disease (PD) than ever before. Primary care physicians treat the majority of PD patients in the United States. Here I review diagnosis and treatment strategies for idiopathic Parkinson disease in the elderly adult.
Parkinson Disease Epidemiology
Parkinson disease (PD) is a common neurodegenerative disease of older adulthood. Population-based data on 36 million Medicare beneficiaries over the age of 65 suggests that 1.6% of Americans are treated yearly for PD, approaching the rate of stroke or migraine. The cardinal clinical symptoms of Parkinson disease - limb tremor, shuffling gait, slowness, stiffness, and postural instability - can be accompanied by autonomic nervous system dysfunction, depression, dementia and psychosis. In addition to physical disability, PD is associated with an increased likelihood of death.1–3 The shifting demographics of the U.S. population due to the aging of the Baby Boomers has placed more adults at risk of PD than ever before, underscoring the need for increased understanding of PD diagnosis, clinical course and management.
Demographic and Geographical Variation in PD Diagnosis
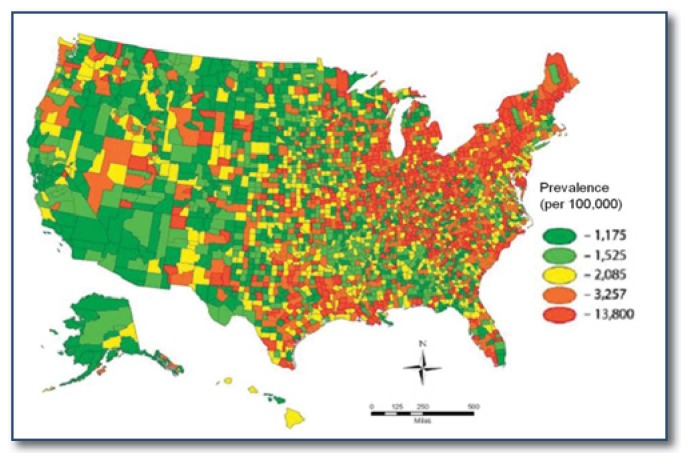
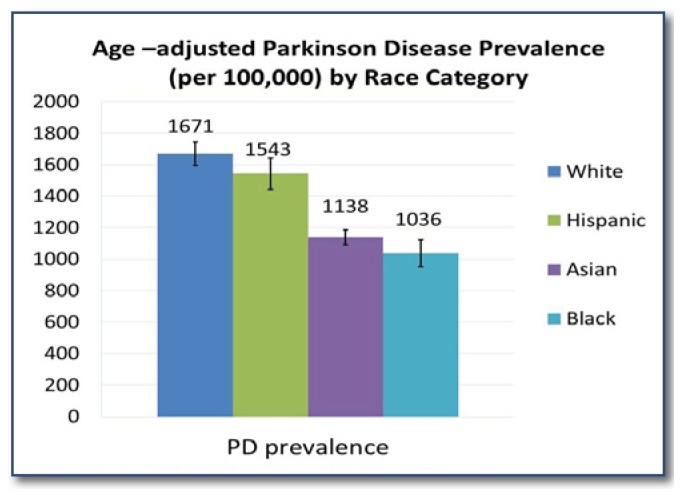
Although the cause of PD remains unknown, environmental factors are suspected.4–7 PD diagnoses cluster in the Midwest and Northeast United States, even after adjustment for population characteristics and physician density (See Figure 1).8 PD risk also varies across demographic groups, suggesting differential susceptibility to PD-type neurodegeneration. Parkinson disease has been consistently diagnosed more often in men compared to women, and the neuroprotective effects of estrogen have been evidenced by increased risk of PD in women with early menopause.9–11 In a nationwide Medicare beneficiary study, Asian and African Americans had almost half the risk of PD as whites (See Figure 2), and an excess of diagnoses among whites has been found in most mixed race populations worldwide.8 Proposed biological mechanisms for this include increased baseline neuromelanin, the antioxidant that protects from free radical damage in people of color, increased resilience to age-related degeneration and lower prevalence of common PD-causing genes.12–15
Figure 1.
Predicted Age, Race, and Sex Adjusted Prevalence of Parkinson Disease among U.S. Medicare Beneficiaries, 2003.
Figure 2.
Age-Adjusted Prevalence of Parkinson Disease Diagnoses among Medicare Beneficiaries ≥ 65 by Race/Ethnic Category, 2003.
Parkinson Disease Symptoms and Diagnosis
Despite recent efforts to develop predictive imaging tests, PD remains a clinical diagnosis. Motor-related complaints or symptoms include tremor, decreased arm swing, shuffling walk, stopped posture, shortened steps, decreased facial expressiveness, stiffness, overall slowing of movement compared to peers, difficulty in rolling over in bed or in rising from a seated position. Autonomic symptoms include constipation, urinary disturbance (frequency, urgency), hyperhidrosis, nausea, flushing, orthostasis. Non-motor symptoms are highly prevalent, may predate motor symptoms, and may be under-assessed and undertreated.16 These include anxiety, depression, difficulty thinking or concentrating, fatigue, anosmia, and sleep disturbance.
Diagnostic Criteria
A clinical PD diagnosis requires a minimum of two of the following four cardinal signs of parkinsonism:
Tremor: distal resting tremor of 3 to 6HZ or higher frequency. Postural/kinetic tremors may also occur.
Muscle rigidity (a velocity independent resistance to passive movement).
Bradykinesia (slowness and poverty of movement).
Postural instability (not caused by primary visual, cerebellar or proprioceptive dysfunction).
Although bilateral motor findings often are present by the time a diagnosis is made, presentations with asymmetric motor signs (one side being worse than the other) are supportive of PD. A history of gradual onset of symptoms is also supportive, but this relies heavily on patient perception.
The final criterion is a sustained and robust response to a dopaminergic challenge. The equivalent of levodopa titrated up to 1,000–1,500 mg/day is considered an adequate challenge.
Exclusion Criteria for PD
Parkinsonism (including resting tremor and mild asymmetry of motor findings) can be seen in essential tremor, tardive dyskinesia, and Parkinson plus syndromes. Therefore, assessment for the following historical and clinical features is part of the standard initial evaluation for PD, and their presence suggests an alternative diagnosis:
Secondary Parkinsonism
History of dopamine receptor blockade drug exposure (metoclopramide, haloperidol, atypical antipsychotics, promethazine, tricyclic antidepressants)
Repeated strokes with stepwise progression of symptoms
Repeated head injury with loss of consciousness
History of preceding encephalitis
Transient symptoms or remission in absence of treatment
Parkinson-Plus Syndrome (Dementia with Lewy Bodies, Multiple Systems Atrophy, Progressive Supranuclear Palsy, Corticobasal Degeneration), ALS with Parkinsonism, Alzheimer-Type Dementia with Parkinsonism
Lack of subjective and objective response to levodopa (when titrated to at least 1200mg/day-1500mg/day with no evidence of gastrointestinal malabsorption)
Prominent hallucinations, psychosis, bizarre personality changes early in the disease course
Dementia on presentation or early in the disease course
Early or prominent dysautonomia (orthostatic hypotension, incontinence, erectile dysfunction, inorgasmia, syncope)
Double vision, supranuclear gaze palsy, eyelid opening apraxia
Early or prominent gait impairment or postural instability
Cerebellar signs, spasticity, Babinski sign
Spasticity, fatigability, shortness of breath, fasciculations
Limb apraxia, “alien limb” phenomenon, aphasia
Parkinson Disease Management
Multilevel engagement of the medical community by industry has been necessary to evaluate the multitude of PD treatments that have come to market in the past two decades. However, this practice has also resulted in conflicting information regarding treatment strategies. Below are principles of treatment for PD in the elderly, based on the least biased data:
1. No drugs have been proven to slow PD progression: In spite of many clinical trials, there is no convincing evidence that any currently available medications (rasagiline) or supplements (Co-Q 10) alter disease progression.
-
2. In the elderly, immediate release levodopa is the best initial treatment choice for PD symptoms that result in functional impairment. Levodopa is the most effective treatment for the motor symptoms of PD, and may also improve mood and bradyphrenia. Carbidopa/levodopa (Sinemet®) is the most widely used levodopa preparation as it is well tolerated and inexpensive, with several available generic preparations. Levodopa needs to be taken on an empty stomach (one hour before or two hours after meals) for maximum uptake. Carbidopa prevents peripheral metabolism of dopamine, and is included in all formulations of levodopa to minimize gastrointestinal and peripheral vasculature effects. However, flushing, sweating, and most commonly, nausea can occur with use of carbidopa/levodopa. Levodopa-induced nausea should be treated with supplemental carbidopa to allow titration to best motor benefit to continue. Entacapone (Comtan®) is peripherally acting catechol-O-methyltransferase (COMT) inhibitor that is taken with levodopa to extend its clinical benefit (it has no effect on its own). Entacapone is most useful for patients who have optimal/near optimal peak motor benefit, but wearing off between doses in spite of q.i.d. levodopa dosing. Branded combinations of Sinemet® and entacapone (Stalevo®) make individualized titration of levodopa and carbidopa to maximum benefit impossible, and are associated with higher patient out of pocket costs. The extended and controlled release forms of Sinemet® have unpredictable bio-availability that limits their daytime use as a primary therapy, but may be useful for overnight wearing off, generally described as awakening due to the return of PD symptoms.
Dopamine agonists are less potent than levodopa, and are associated with compulsive behaviors (gambling, shopping, hypersexuality, fetishism), paranoia, cognitive impairment that resembles dementia and sleep attacks.17 These side effects are worse among the elderly. In our practice, dopamine agonist use is the most common cause of reversible cognitive impairment in the elderly PD patient. Low dose dopamine agonists are useful as an add-on therapy for patients on a stable dose of levodopa who are experiencing wearing off that is resistant to frequent dosing and a COMT inhibitor (entacapone).
Anticholinergic medications (trihexyphenidyl, benztropine) provide the least amount of motor benefit and are among the most harmful medications one can use in an elderly person with PD. Total anticholinergic burden is associated with a dose–response decline in cognitive function in the elderly.18,19
Monoamine oxidase B (MAO-B) inhibitors (rasagiline, selegiline) block the breakdown of dopamine in the brain, and can therefore provide a modest dopaminergic effect. However, this chemical class interferes with many common medications, including almost all commonly used antidepressant classes, narcotics, muscle relaxants, over-the-counter cold medications, and general and local anesthetics, thus limiting their usefulness in a group of people with common comorbid diseases.
4. Some medications should be avoided in PD patients: Common causes of hospitalizations, emergency department visits, and mortality in PD are polypharmacy, drug-drug interactions, and “drug-disease” interactions. For example, dysautonomia is almost universal in PD, and can be present before motor symptoms are manifest. Continuing or failing to reduce blood pressure medications when initiating or increasing levodopa may cause syncope. Sitting and standing blood pressure measurements should be obtained at all office visits, with reduction of antihypertensive medications (and in severe cases, addition of a hypertensive agent to prevent syncope) as needed. Hallucinations may be part of the disease in later stages, but may also be levodopainduced or a sign of infection. Narcotic, anticholinergic, hypnotic and benzodiazepine associated falls and confusion are also common triggers of an emergency department visit or hospitalization. Table 1 lists common medications that should be avoided in PD.
5. Motor fluctuations such as the loss of sustained benefit from levodopa (“wearing off ”), dystonia, and dyskinesias, occur at some point in nearly all PD patients due to disease progression, not because of initial medication choice. Despite past suggestions made by industry-sponsored studies, there is no benefit to “saving” or storing motor benefit by treating PD patients with a dopamine agonist instead of levodopa. This usually results in under-treatment and risk of complications, including lower mood, cognitive slowing, falls, and more rapid functional disability, which may not be reversible once proper treatment begins. Motor fluctuations in the elderly can be managed by frequent dosing and the use of the following adjunct therapies: COMT inhibitors, extended release carbidopa/levodopa combinations, dopamine agonists, and MAO-B inhibitors.
6. Neurology referral and Deep Brain Stimulation. Levodopa associated side effects, most commonly chorea, psychosis and symptomatic orthostasis, may occur rarely in newly diagnosed patients on low dose therapy, but more generally occur in advanced disease and indicate advanced neurodegeneration. They are treated by dose adjustment, use of atypical antipsychotics (clozapine, quetiapine), and hypertensive agents (caffeine, midodrine, fludrocortisone). Referral for neurologist care is appropriate at any time the diagnosis is considered, but especially when symptoms and signs substantially improve after treatment with levodopa (suggesting undertreatment or an alternative diagnosis). Deep Brain Stimulation (DBS) is an effective surgical treatment for motor fluctuations in PD only when strict inclusion exclusion criteria are followed, as not all clinical forms of PD respond to DBS. Referral for DBS is appropriate when significant motor fluctuations persist in spite of optimal medical management and is best performed at an experienced multidisciplinary center that incorporates neurosurgical, neurological, neuropsychological testing in the evaluation and treatment process.
7. Cognitive impairment is common. Cognitive impairment or dementia is diagnosed in nearly seventy percent of elderly diagnosed PD patients.3, 20 Dementia phenotype varies, depending on the primary cause (progression of PD, concomitant Alzheimer or vascular dementia). However, iatrogenic cognitive impairment in neurodegenerative populations is increasingly recognized. Limiting exposure to centrally acting medications known to cause confusion in the elderly (narcotics, antihistamines, sedatives, hypnotics, anticholinergics), and adequate dopaminergic therapy is prudent to retain as much cognitive function as possible. Acute cognitive impairment or sudden change in motor benefit in a PD patient may signal an occult infection or metabolic disturbance.
8. Exercise may be neuroprotective. If the cardio-protective, cancer-fighting, mood-elevating, and dementia-preventing effects of regular exercise are not sufficient, yet another reason to treat PD motor symptoms is to allow for exercise. A decreased risk of PD has been demonstrated among those who exercise, and several specific types of exercise have associated with better function and motor preservation in person with PD symptoms.21–23
Table 1.
Medications to Avoid in Patients with Parkinson Disease
| Medication Class | Examples | Interaction/Binding Site | Clinical Result |
|---|---|---|---|
| Typical Antipsychotics | Chlorpromazine, Haloperidol, Fluphenazine, Loaxapine, Thiothixene, Pimozide, Thioridizine | Dopaminergic blockade | PD symptoms worsen |
|
| |||
| Antiemetic | Chlorpomazine, Metoclopramide, Droperidol Promethazine, Prochlorperazine | Dopaminergic blockade | PD symptoms worsen |
|
| |||
| Antihypertensive | All | Dopaminergic blockage (reserpine, methyldopa) | PD symptoms worsen |
| Increased orthostasis (all) | Syncope, Hypotension-associated organ ischemia | ||
|
| |||
| Antihistamine Anticholinergic | Diphenhydramine, hydroxyzine oxybutynin, trihexyphenidyl, bupropnon, dextromethorophan, | Cholinergic blockade | Confusion, falls, dry mouth, blurred vision, incontinence |
|
| |||
| Benzodiazepine Hypnotic | Alprazolam, clonazepam, diazepam, lorazepam, triazolam, eszopiclone, zolpidem | Gamma-amlnobutyrlc acid (GABA) receptor | Confusion, falls, excess sedation, paradoxical agitation |
|
| |||
| Narcotic | Codeine, morphine, hydromorphone | Opioid receptor | Confusion, falls, paradoxical agitation |
Exercise booklets and tutorial videos, and iphone/ipad applications are wide available for download on the world wide web for PD.
Partnership with a Specialist
Neurologists, particularly those who specialize in movement disorders, can be helpful at all stages of PD patient care. They have clinical experience with the various causes of parkinsonism and phenotypes of PD. As the disease progresses, partnering with a specialist is helpful for both the increased complexity of PD management, consideration of DBS, and to assure that proper management of non-PD medical illnesses can continue.
Where To Go For Help
Several national PD outreach groups exist and provide education, community support, research funding, patient and caregiver programs. Clinical trials of experimental therapies for PD are listed at www.clinicaltrials.gov. The organizations in Table 2 have many patient booklets available for download, educational DVDs, physician information, local support groups, dance and exercise classes, caregiver respite care, fundraising opportunities, and movement disorder specialist referral lists.
Table 2.
Patient, Family and Physician Resources for Parkinson Disease
| Organization Name | Web address |
|---|---|
| American Parkinson Disease Foundation | www.apdaparkinson.org |
| National Parkinson Foundation | www.parkinson.org |
| Movement Disorders Society | www.movementdisorders.org |
| Michael J. Fox Foundation | www.michaeljfox.org |
| Parkinson Disease Foundation | www.pdf.org |
Biography
Allison W. Willis, MD, MSCI, is an Assistant Professor of Neurology, in the Movement Disorders Division, at the Knight Alzheimer’s Disease Research Center at the Washington University School of Medicine in St. Louis.Contact: willisa@neuro.wustl.edu

Footnotes
Disclosure
None reported.
References
- 1.CL CE. Mortality from Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;68:254–255. doi: 10.1136/jnnp.68.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Survival of Parkinson’s disease patients in a large prospective cohort of male health professionals. Mov Disord. 2006;21:1002–1007. doi: 10.1002/mds.20881. [DOI] [PubMed] [Google Scholar]
- 3.Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of survival in patients with Parkinson disease. Arch Neurol. 2012;69:601–607. doi: 10.1001/archneurol.2011.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 5.Tanner CM, Ottman R, Goldman SM, et al. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 6.Uversky VN, Li J, Bower K, Fink AL. Synergistic effects of pesticides and metals on the fibrillation of alpha-synuclein: implications for Parkinson’s disease. Neurotoxicology. 2002;23:527–536. doi: 10.1016/s0161-813x(02)00067-0. [DOI] [PubMed] [Google Scholar]
- 7.Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol. 2011;26:547–555. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright WA, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34:143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav R, Shukla G, Goyal V, Singh S, Behari M. A case control study of women with Parkinson’s disease and their fertility characteristics. J Neurol Sci. 2012;319:135–138. doi: 10.1016/j.jns.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 11.Shulman LM. Gender differences in Parkinson’s disease. Gend Med. 2007;4:8–18. doi: 10.1016/s1550-8579(07)80003-9. [DOI] [PubMed] [Google Scholar]
- 12.Ross OA, Wilhoite GJ, Bacon JA, et al. LRRK2 variation and Parkinson’s disease in African Americans. Mov Disord. 2010;25:1973–1976. doi: 10.1002/mds.23163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alladi PA, Mahadevan A, Yasha TC, Raju TR, Shankar SK, Muthane U. Absence of age-related changes in nigral dopaminergic neurons of Asian Indians: relevance to lower incidence of Parkinson’s disease. Neuroscience. 2009;159:236–245. doi: 10.1016/j.neuroscience.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 14.Muthane UB, Chickabasaviah YT, Henderson J, et al. Melanized nigral neuronal numbers in Nigerian and British individuals. Mov Disord. 2006;21:1239–1241. doi: 10.1002/mds.20917. [DOI] [PubMed] [Google Scholar]
- 15.Muthane U, Yasha TC, Shankar SK. Low numbers and no loss of melanized nigral neurons with increasing age in normal human brains from India. Ann Neurol. 1998;43:283–287. doi: 10.1002/ana.410430304. [DOI] [PubMed] [Google Scholar]
- 16.Shearer J, Green C, Counsell CE, Zajicek JP. The impact of motor and non motor symptoms on health state values in newly diagnosed idiopathic Parkinson’s disease. J Neurol. 2012;259:462–468. doi: 10.1007/s00415-011-6202-y. [DOI] [PubMed] [Google Scholar]
- 17.Bastiaens J, Dorfman BJ, Christos PJ, Nirenberg MJ. Prospective cohort study of impulse control disorders in Parkinson’s disease. Mov Disord. 2013 doi: 10.1002/mds.25291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson’s disease: a cohort study. J Neurol Neurosurg Psychiatry. 2010;81:160–165. doi: 10.1136/jnnp.2009.186239. [DOI] [PubMed] [Google Scholar]
- 19.Kolanowski A, Fick DM, Campbell J, Litaker M, Boustani M. A preliminary study of anticholinergic burden and relationship to a quality of life indicator, engagement in activities, in nursing home residents with dementia. J Am Med Dir Assoc. 2009;10:252–257. doi: 10.1016/j.jamda.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70:1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 21.Shulman LM, Katzel LI, Ivey FM, et al. Randomized Clinical Trial of 3 Types of Physical Exercise for Patients With Parkinson Disease. Arch Neurol. 2012:1–8. doi: 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Lao L. Tai chi for patients with Parkinson’s disease. N Engl J Med. 2012;366:1737. doi: 10.1056/NEJMc1202921. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366:511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]