Abstract
Many times, when a human genetic disease is mapped to mutations in a specific gene, little is known about the biological functions of the affected gene. Development of new therapeutic methods is facilitated by understanding the gene’s biological roles. Such information can often be obtained in animal models, such as the fruit fly. Here we describe how understanding a gene’s function in fruit flies has illuminated the etiology of Cornelia de Lange syndrome.
Introduction: Cornelia de Lange Syndrome
A few years ago, on a sweltering summer day in a park not far from Saint Louis, I met a 15-year-old boy and his mother. He only came up to his mother’s waist. As I gazed down at his shining face, the features characteristic of Cornelia de Lange Syndrome (CdLS), a rare genetic disorder, were obvious even to me, trained as a fruit fly, not a human, dysmorphologist. Talking with his mother, I was surprised to learn that he had actually just been diagnosed only the week before by his aunt, who had read about CdLS online. The boy, with verbal skills that were unusually good for someone with this syndrome, was quite excited to explain why he was different. Although CdLS can be easily suspected based on highly characteristic facial features, including arched eyebrows, thin lips, and long philtrum, there’s more to it. Individuals with this syndrome suffer a raft of more serious problems, including intellectual impairment, autism, slow growth, and limb and multiple organ abnormalities (See Table 1).1
Table 1.
Common features of Cornelia de Lange Syndrome
| Feature | Percentage of individuals affecteda |
|---|---|
| Development and behavior | |
| Growth retardation | 100 |
| Intellectual disability | mean IQ 53, range <30 to 102 |
| Autism spectrum disorder | 65 |
| Speech disorders | + |
| Self injurious behavior | 70 |
| Physical aggression | 40 |
| Facies | |
| Microcephaly | 93 |
| Low posterior hairline | 92 |
| Bushy eyebrows, synophrys | 98 |
| Ocular abnormalities (ptosis, myopia, nystagmus) | 57 |
| Long curly eyelashes | 99 |
| Depressed, wide nasal bridge | 83 |
| Anteverted nares | 85 |
| Long philtrum, thin upper, downturned mouth | 94 |
| High arched palate | 86 |
| Widely spaced teeth, late eruption | 86 |
| Micrognathia | 84 |
| Skin | |
| Hirsutism | 78 |
| Cutis marmorata | 56 |
| Hypoplastic nipples, navel | 50 |
| Extremities | |
| Micromelia | 93 |
| Reduction defects, oligodactyly | 27 |
| Clinodactyly (5th finger) | 74 |
| Simian crease | 51 |
| Proximally placed thumbs | 72 |
| Elbow flexion contractures | 64 |
| Syndactyly (toes 2 and 3) | 86 |
| Genitalia | |
| Hypoplasia | 57 |
| Undescended testes | 73 |
| Hypospadias | 33 |
| Gastrointestinal | |
| GE reflux | + |
| Gut duplication, malrotation | + |
| Volvulus | + |
| Other | |
| Hearing loss | 60 |
| Structural heart defects | 33 |
| Kidney, urinary tract defects | 36 |
| Thrombocytopenia | + |
It is not always recognized initially by physicians or other caretakers. In fact, it is not uncommon, particularly in more rural areas, for CdLS to go undiagnosed, as it was in this 15-year-old. Cornelia de Lange first described this condition, which is also called Brachmann de Lange Syndrome, in the 1930s based on two unrelated pediatric patients she saw in Amsterdam.2 Today, a CdLS-likely diagnosis is made relatively easily by a physician who has seen a few cases and recognizes the pattern of abnormalities. Early diagnosis makes a difference. It can lead to substantial improvement in management, as many of the diverse health risks can be anticipated.1
Feeding is a very common problem. On the summer day I met this patient, I also met many caring family members of other patients, including a grandfather who had used techniques similar to those he used to handle young livestock on his farm to develop a diet that was palatable to his granddaughter. I also met a young mother who refused to accept being told by her pediatrician that poor mothering was responsible for the failure of her beautiful daughter to thrive, and who ferociously sought out other opinions until the correct diagnosis was made.
Six Degrees of Separation: Making the Connection
You might wonder why a fruit fly molecular geneticist was attending a CdLS family picnic? There is a connection! The answer goes back to the mid-1990s, when our laboratory was investigating how genes are turned on and off during the development of a fruit fly called Drosophila melanogaster. This insect is a “model organism” that offers powerful genetic and molecular tools. It has long been a tenet of the Drosophila research community that for most biological functions, ranging from metabolism and endocrinology to learning and memory, the fruit fly is equivalent to a simple small model of a human, but with a more compact genome and wings. We were looking for genes that control limb development and discovered one that we named Nipped-B, based on the moth-eaten appearance of the mutant wings.3 Nipped-B, it turned out, controls development of several tissues.
It also soon became apparent, first from genetic studies with the equivalent genes in yeasts, that Nipped-B is also important in ensuring that chromosomes are dealt out equally to the two daughter cells when a cell divides. Nipped-B protein helps a protein ring called cohesin snap like a bracelet around chromosomes and hold sister chromosomes together, so they can be separated and sent in opposite directions at the moment of division (See Figure 1). It actually doesn’t take much Nipped-B or cohesin, less than 20% of normal, to ensure proper chromosome segregation. Even a small reduction on the order of 30% or less can dramatically alter growth and development. This is because many other genes that are controlled by Nipped-B and cohesin produce either too little or too much of their products when Nipped-B is reduced.
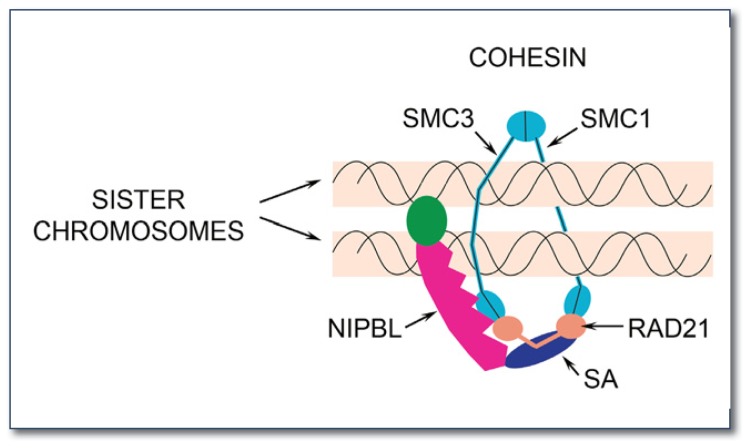
Figure 1.
The NIPBL and cohesin protein complexes. The cohesin protein ring topologically encircles DNA, thereby holding replicated chromosomes together until cell division, to ensure proper chromosome segregation20. The cohesin complex has four subunits: Smc1, Smc3, Rad21, and SA. Smc1 and Smc3 have long arms, and Rad21 bridges the enzymatic head domains of Smc1 and Smc3. The cohesin ring is opened by NIPBL to snap it around chromosomes, and Rad21 is either removed or destroyed to reopen the ring and allow chromosomes to separate when a cell divides. As described in the article, cohesin and NIBPL bind to many genes on chromosomes and control their activities. Changes in expression of these genes are the underlying cause of the many physical and intellectual deficits in Cornelia de Lange syndrome.
Making the Human Connection
The connection to human development, which we were sure would happen sooner or later (we could see the same gene in the DNA sequence of the human genome), came earlier than expected. In 2004, I received separate phone calls from Ian Krantz at the Children’s Hospital of Philadelphia and Tom Strachan at the University of Newcastle, who had both recently discovered that mutations in the human Nipped-B gene, which they named Nipped-B-Like (NIPBL), caused most cases of CdLS.4, 5 Both were seeking any insights we might have into how NIPBL might be involved in the diverse deficits associated with CdLS. Since that time, Ian and others have found that mutations affecting portions of the cohesin ring can cause milder forms of CdLS, or a similar syndrome.6–8 Most recently, mutations in a gene encoding an enzyme that chemically modifies the cohesin ring were also found to cause CdLS.9
Mapping the first CdLS gene was not for the faint of heart. The NIPBL mutations that cause CdLS are genetically dominant and it is extremely rare for the disorder to be inherited – virtually all cases are caused by sporadic mutations. As a consequence, the usual method of following family lineages could not be used. The key was that beginning in the 1970’s, Laird Jackson of Drexel University, responding to some questions from some concerned CdLS mothers, began organizing CdLS family picnics in the US, and traveling around the world to meet other CdLS families. Laird had the foresight to collect blood samples from the CdLS children, parents, and siblings, so that a large number had been collected by the time the human genome sequence became available. This collection allowed linkage exclusion analysis to narrow things down to five regions that might harbor the critical gene. Coupled with some inspired guesswork, and a balanced de novo chromosomal translocation affecting one of the five suspect regions, this analysis led to NIPBL.
Identification of NIPBL led to the first CdLS Scientific Symposium in June 2004, held in Lincolnshire, Illinois, in conjunction of the biennial family meeting organized by the CdLS Foundation that had grown out of the picnics organized by Laird many years before. I was invited to talk about our work with fruit flies, which began my association with the CdLS Foundation, as a member of the Board of Directors from 2004 to 2010, and currently as Chair of their Research Council.
One of the responsibilities that I have enjoyed is meeting CdLS families at occasional family picnics along the I-70 corridor from western Illinois to eastern Kansas. It is gratifying to reassure parents that it is nothing they did wrong that caused their child’s condition. They find it a relief to have the genetics and causes of CdLS explained to them, and also to learn that there are dedicated physicians and scientists who care about this rare condition, and are working hard to improve the methods of diagnosis and treatment. They are excited to learn that members of the Clinical Advisory Board of the CdLS Foundation, who have diverse expertise, can be approached to address their many medical questions. They are also thrilled to learn that teams of scientists are exploring the mechanism of the disease in search for clues to treatments that could improve their children’s lives.
So, why do we think there is hope for developing effective treatments for CdLS? The problems with growth and development begin in utero, and CdLS has been diagnosed by ultrasound (e.g., reference 10). It is not beyond imagining that in the near future, CdLS, like many other rare disorders, can be diagnosed early in pregnancy with a combination of ultrasound, DNA sequencing, and molecular markers. As a rare, non-inherited genetic disorder, however, it would not become routine to check for CdLS until a safe method that can detect many genetic problems with early development is available.
If in utero diagnoses can be made, it would then be feasible to think about very early therapeutic intervention that could ameliorate many of the physical deformities. I also believe that therapeutic intervention that begins within a few weeks of birth could be very helpful. Surgery can be used to correct the occasional severe congenital heart defects, which are a leading cause of early death.11 We can even envision the possibility that we can improve growth and intellectual development.
Mental retardation, poor speech, and autism are major impediments that keep most individuals with CdLS reliant on constant care and from becoming independent. There is good reason to believe that brain development is highly plastic, and that early intervention may greatly improve intellectual development of children with CdLS. For instance, using a mouse model of Rett syndrome, a leading cause of mental retardation in girls, Adrian Bird’s laboratory in Edinburgh showed that turning the affected gene back on even in young adults led to substantial improvements in learning, memory, and behavior.12 Similarly, flies with Nipped-B and cohesin deficits show delays in axon pruning and problems with dendrite branching,13 two key processes in neural development, which have the potential to be corrected later in development. That’s the long-range hope.
Research ‘Flies’ By
Where do we start, and what strategies might lead to therapeutic discoveries? First we need to know how NIPBL and cohesin control genes, what genes they control, and when they control them, in order delineate what is possible. Our laboratory has exploited the fruit fly to gain some of this knowledge. Using techniques that allow us to examine the entire genome, we mapped where the Nipped-B and cohesin proteins bind to chromosomes in multiple types of cells and tissues, and which genes produce too little or too much product when the amount of Nipped-B or cohesin is reduced. These studies have provided important clues.
We discovered that Nipped-B and cohesin bind selectively to hundreds of active genes that control growth and development, and that the products of many of these genes and the genes they control are often altered when there is too little Nipped-B or cohesion.14–18 Strikingly, one of the key genes that binds Nipped-B and cohesin and whose output decreases when there is too little Nipped-B or cohesion, is myc, a key growth promoter in both flies and humans. The myc gene is perhaps best known for causing cancer when it is too active, leading to unchecked growth. In Drosophila, myc is called diminutive, because the first mutation, isolated back in the 1930’s, only partially reduces gene function, giving rise to tiny flies.19 It turns out that the zebrafish, mouse, and human myc genes are also down-regulated when the NIPBL activity is reduced, providing one potential explanation for why CdLS was once known as “Amsterdam dwarfism.”
The overriding message of our Drosophila studies, and parallel work by other laboratories in cultured mammalian cells, zebrafish, and NIPBL mutant mice, is that NIPBL and cohesin directly control the activity of hundreds of genes important for growth and development.20 Many of the physical defects and other medical problems that are apparent upon birth can be handled with existing medical procedures. The diversity of the effects on gene function argue, however, that we will likely need to increase the activity of the remaining good NIPBL gene if we are to improve future development. It is easier to control a river at its source than at the delta.
The reality is that drug companies would go out of business if they poured buckets of money into developing treatments for disorders that occur on the order of once per ten thousand births. Different strategies have to be developed for identifying and developing effective drugs for orphan diseases. A model that we are testing for CdLS is to use fruit flies to screen libraries of chemical compounds that have already been approved for human use for the ability to enhance expression of Nipped-B. The idea is that this is a rich source of bioactive compounds, and it is not nearly as expensive to repurpose a drug as it is to develop a new one.
In collaboration with the laboratory of Justin Fay at Washington University, we screened a library of FDA-approved drugs for those that improve the growth of yeasts that have modest defects in their NIPBL and cohesin genes, and then tested the dozen or so that had the desired effect for their ability to improve some of the mutant phenotypes displayed by Nipped-B mutant flies. This analysis identified a few chemically-related compounds that were able to normalize the levels of NIPBL RNA in cell lines derived from individuals with CdLS. One of these compounds is used to treat other pediatric conditions. That’s hopeful! We are now seeking funding to test these compounds in a mouse model of CdLS, and consulting with physicians, allied health professionals, and CdLS families to decide what biomarkers can be used to conduct a meaningful clinical study in CdLS patients.
Conclusion
My take-home lesson is fruit flies and other non-vertebrate organisms can prove to be useful models for human maladies. They can provide relatively rapid and inexpensive methods, both to understanding the molecular basis of the disease and to developing potential therapies, particularly for rare conditions for which standard methods for drug development are not economically feasible.
Acknowledgments
The author is grateful to the CdLS families for their willingness to share their experiences, the CdLS Foundation, and Ian Krantz, Matt Deardorff, Laird Jackson, and Antonio Musio for many illuminating discussions. Work in the Dorsett laboratory is supported by grants from the National Institutes of Health (R01 GM055683, P01 HD052860).
Biography
Dale Dorsett, PhD, is a Professor in the Edward A. Doisy Biochemistry and Molecular Biology Department at Saint Louis University School of Medicine.
Contact: dorsett d@slu.edu

Footnotes
Disclsoure
NIH GM055683, HD052860 - not a commercial organization - government
References
- 1.Kline AD, Krantz ID, Sommer A, Kliewer M, Jackson LG, FitzPatrick DR, Levin AV, Selicorni A. Cornelia de Lange syndrome: clinical review, diagnostic and scoring systems, and anticipatory guidance. Am J Med Genet A. 2007;143A:1287–1296. doi: 10.1002/ajmg.a.31757. [DOI] [PubMed] [Google Scholar]
- 2.de Lange C. Sur un type nouveau de dégénération (typus Amstelodamensis) Arch Méd Enfants. 1933;36:713–719. [Google Scholar]
- 3.Rollins RA, Morcillo P, Dorsett D. Nipped-B, a Drosophila homologue of chromosomal adherins, participates in activation by remote enhancers in the cut and Ultrabithorax genes. Genetics. 1999;152:577–593. doi: 10.1093/genetics/152.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, Nowaczyk MJ, Toriello H, Bamshad MJ, Carey JC, Rappaport E, Kawauchi S, Lander AD, Calof AL, Li HH, Devoto M, Jackson LG. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet. 2004;36:631–635. doi: 10.1038/ng1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet. 2004;36:636–641. doi: 10.1038/ng1363. [DOI] [PubMed] [Google Scholar]
- 6.Musio A, Selicorni A, Focarelli ML, Gervasini C, Milani D, Russo S, Vezzoni P, Larizza L. X-linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat Genet. 2006;38:528–530. doi: 10.1038/ng1779. [DOI] [PubMed] [Google Scholar]
- 7.Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, Gil-Rodríguez C, Arnedo M, Loeys B, Kline AD, Wilson M, Lillquist K, Siu V, Ramos FJ, Musio A, Jackson LS, Dorsett D, Krantz ID. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of Cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet. 2007;80:485–494. doi: 10.1086/511888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deardorff MA, Wilde JJ, Albrecht M, Dickinson E, Tennstedt S, Braunholz D, Mönnich M, Yan Y, Xu W, Gil-Rodríguez MC, Clark D, Hakonarson H, Halbach S, Michelis LD, Rampuria A, Rossier E, Spranger S, Van Maldergem L, Lynch SA, Gillessen-Kaesbach G, Lüdecke HJ, Ramsay RG, McKay MJ, Krantz ID, Xu H, Horsfield JA, Kaiser FJ. RAD21 mutations cause a human cohesinopathy. Am J Hum Genet. 2012;90:1014–1027. doi: 10.1016/j.ajhg.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deardorff MA, Bando M, Nakato R, Watrin E, Itoh T, Minamino M, Saitoh K, Komata M, Katou Y, Clark D, Cole KE, De Baere E, Decroos C, Di Donato N, Ernst S, Francey LJ, Gyftodimou Y, Hirashima K, Hullings M, Ishikawa Y, Jaulin C, Kaur M, Kiyono T, Lombardi PM, Magnaghi-Jaulin L, Mortier GR, Nozaki N, Petersen MB, Seimiya H, Siu VM, Suzuki Y, Takagaki K, Wilde JJ, Willems PJ, Prigent C, Gillessen-Kaesbach G, Christianson DW, Kaiser FJ, Jackson LG, Hirota T, Krantz ID, Shirahige K. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature. 2012;489:313–317. doi: 10.1038/nature11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weichert J, Schröer A, Beyer DA, Gillessen-Kaesbach G, Stefanova I. Cornelia de Lange syndrome: antenatal diagnosis in two consecutive pregnancies due to rare gonadal mosaicism of NIPBL gene mutation. J Matern Fetal Neonatal Med. 2011;24:978–982. doi: 10.3109/14767058.2010.531312. [DOI] [PubMed] [Google Scholar]
- 11.Schrier SA, Sherer I, Deardorff MA, Clark D, Audette L, Gillis L, Kline AD, Ernst L, Loomes K, Krantz ID, Jackson LG. Causes of death and autopsy findings in a large study cohort of individuals with Cornelia de Lange syndrome and review of the literature. Am J Med Genet A. 2011;155A:3007–3024. doi: 10.1002/ajmg.a.34329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuldiner O, Berdnik D, Levy JM, Wu JS, Luginbuhl D, Gontang AC, Luo L. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev Cell. 2008;14:227–238. doi: 10.1016/j.devcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misulovin Z, Schwartz YB, Li XY, Kahn TG, Gause M, MacArthur S, Fay JC, Eisen MB, Pirrotta V, Biggin MD, Dorsett D. Association of cohesin and Nipped-B with transcriptionally active regions of the Drosophila melanogaster genome. Chromosoma. 2008;117:89–102. doi: 10.1007/s00412-007-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaaf CA, Misulovin Z, Sahota G, Siddiqui AM, Schwartz YB, Kahn TG, Pirrotta V, Gause M, Dorsett D. Regulation of the Drosophila Enhancer of split and invected-engrailed gene complexes by sister chromatid cohesion proteins. PLoS One. 2009;4:e6202. doi: 10.1371/journal.pone.0006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes JM, Bentley FK, Print CG, Dorsett D, Misulovin Z, Dickinson EJ, Crosier KE, Crosier PS, Horsfield JA. Positive regulation of c-Myc by cohesin is direct, and evolutionarily conserved. Dev Biol. 2010;344:637–649. doi: 10.1016/j.ydbio.2010.05.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fay A, Misulovin Z, Li J, Schaaf CA, Gause M, Gilmour DS, Dorsett D. Cohesin selectively binds and regulates genes with paused RNA polymerase. Curr Biol. 2011;21:1624–1634. doi: 10.1016/j.cub.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaaf CA, Kwak H, Koenig A, Misulovin Z, Gohara DW, Watson A, Zhou Y, Lis JT, Dorsett D. Genome-wide control of RNA polymerase II activity by cohesin. PLoS Genetics. 2013;9 doi: 10.1371/journal.pgen.1003382. e1003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridges C. Drosophila melanogaster: Legend for symbols, mutants, valuations. D I S. 1935;3:5–19. [Google Scholar]
- 20.Dorsett D. Cohesin: genomic insights into controlling gene transcription and development. Curr Opin Genet Dev. 2011;21:199–206. doi: 10.1016/j.gde.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson L, Kline AD, Barr MA, Koch S. de Lange syndrome: a clinical review of 310 individuals. Am J Med Genet. 1993;47:940–946. doi: 10.1002/ajmg.1320470703. [DOI] [PubMed] [Google Scholar]
- 22.Selicorni A, Sforzini C, Milani D, Cagnoli G, Fossali E, Bianchetti MG. Anomalies of the kidney and urinary tract are common in de Lange syndrome. Am J Med Genet A. 2005;132:395–397. doi: 10.1002/ajmg.a.30445. [DOI] [PubMed] [Google Scholar]
- 23.Selicorni A, Colli AM, Passarini A, Milani D, Cereda A, Cerutti M, Maitz S, Alloni V, Salvini L, Galli MA, Ghiglia S, Salice P, Danzi GB. Analysis of congenital heart defects in 87 consecutive patients with Brachmann-de Lange syndrome. Am J Med Genet A. 2009;149A:1268–1272. doi: 10.1002/ajmg.a.32838. [DOI] [PubMed] [Google Scholar]
- 24.Arron K, Oliver C, Moss J, Berg K, Burbidge C. The prevalence and phenomenology of self-injurious and aggressive behaviour in genetic syndromes. J Intellect Disabil Res. 2011;55:109–120. doi: 10.1111/j.1365-2788.2010.01337.x. [DOI] [PubMed] [Google Scholar]
- 25.Moss J, Howlin P, Magiati I, Oliver C. Characteristics of autism spectrum disorder in Cornelia de Lange syndrome. J Child Psychol Psychiatry. 2012;53:883–891. doi: 10.1111/j.1469-7610.2012.02540.x. [DOI] [PubMed] [Google Scholar]