Abstract
Background
Rates of sexually transmitted infections (STI) and HIV are highest in the southern U.S., but vary widely by gender, age and risk behavior. Current guidelines recommend annual screening for chlamydia, gonorrhea, syphilis and trichomoniasis in all sexually active women with HIV.
Methods
Screening rates and test positivity for chlamydia, gonorrhea, syphilis and trichomoniasis were determined per calendar year in this retrospective cohort study of women in care at an urban HIV clinic in Birmingham, Alabama, 2013–2015. Chlamydia, gonorrhea, and trichomonas infection were detected by molecular diagnostics and syphilis by serology. A combined endpoint for CT/GC/syphilis (STI-3) was created based on similar test positivity and predictors. Predictors of STI-3 were identified using logistic regression and generalized estimating equations.
Results
Among 745 women with HIV, median age was 46.8 years, 78.8% were Black and 61% were sexually active. In 2015, 83.7% of women were tested for STI. Test positivity was 1.0% for chlamydia, 0.5% for gonorrhea, 1.6% for syphilis, and 13.3% for trichomoniasis. Independent predictors of STI-3 were recent chlamydia or gonorrhea (OR 3.7, 95% CI 1–13.4, p=0.047), public insurance compared to private (OR 3.5, CI 1–11.8, p=0.048) and sex after drugs/alcohol (OR 3.0, CI 1.2–8.0, p=0.025). Women age ≥50 were less likely to have STI (OR 0.3, CI 0.1–1, p=0.040).
Conclusions
In a cohort of women engaged in HIV care in the southern United States, detection of chlamydia, gonorrhea and syphilis was infrequent but trichomoniasis was common. Many women screened for STI were low-risk and universal testing strategies warrant evaluation.
Keywords: chlamydia, gonorrhea, HIV in Women, STI screening, syphilis, trichomoniasis
Introduction
Women living in the southern region of the United States are at the epicenter of overlapping HIV and sexually transmitted infection (STI) epidemics. 1,2 More than two million cases of chlamydia (CT), gonorrhea (GC) and syphilis were reported to US Centers for Disease Control and Prevention (CDC) in 2016 - the highest number in history.3 Further, chlamydia and gonorrhea infection rates are highest in southern states, where an estimated 43% of people living with HIV reside.2,3 In the setting of finite resources for STI control, infection prevalence is a key determinant of cost-effective screening practice. STI rates differ significantly by gender, age and risk behavior and these factors incorporated into national STI screening guidelines for adolescents and adults, but not for adults living with HIV. This lack of specificity is increasingly relevant since the cohort of HIV-infected adults in the US is aging (42% were ≥50 years old in 2013), and older age is associated with lower STI risk. 4
STI screening of asymptomatic individuals is one cornerstone of an effective STI public health response. The rationale for annual STI screening in women with HIV is to prevent adverse outcomes of HIV-STI co-infection. These include a 2–3 fold increased risk of HIV transmission and negative birth outcomes.5–7 Since 2006, CDC has recommended universal STI screening in all HIV-infected women and men at entry to care and every year if sexually active.8 Routine testing includes the three most common, curable, reportable, bacterial STIs: chlamydia (Chlamydia trachomatis), gonorrhea (Neisseria gonorrhoeae) and syphilis (Treponema pallidum). Annual screening for trichomoniasis in women with HIV has been recommended since 2010.9 The HIV Medical Association of the Infectious Diseases Society of America recommends STI screening in adults with HIV at baseline and annually if “at risk”, although risk is not defined. The US Preventive Services Task Force (USPSTF) recommends annual syphilis screening with more frequent testing based on “individual risk behaviors and local epidemiology”.10 USPSTF has set a research priority to determine which subgroups benefit most from STI screening since poorly defined “risk” is a barrier to implementation. 11 In contrast to guidelines for persons living with HIV, national STI screening guidelines for the general population are age and gender-specific: annual screening is recommended for chlamydia and gonorrhea in sexually active females age <25 or age ≥25 if additional risk factors are present.12 This recommendation is based on models that estimate annual chlamydia screening in women (age 15–29) to be cost effective when infection prevalence is >3%.13,14 Since STI rates vary by gender, predictors of STI may also vary by gender and tailored screening guidelines for women living with HIV could reduce unnecessary testing.
Our hypothesis was that annual, universal screening for chlamydia, gonorrhea and syphilis for women engaged in HIV care in the current era is low yield. We undertook a retrospective cohort study to document annual STI testing rates and detection rates in this group. We also sought to identify independent predictors of bacterial STI to inform future screening practice.
Methods
Study Overview
We performed a retrospective cohort study at the 1917 clinic at the University of Alabama at Birmingham (UAB) where primary and specialty HIV care is provided. Study participants completed a series of patient-reported outcomes (PRO) on touch-screen computers during routine visits. Surveys included validated questions about sexual practices and behaviors, alcohol intake (AUDIT-C questionnaire) and drug use (ASSIST questionnaire).15,16 All HIV-positive, female clinic patients, age ≥19, in care during 2013–2015 were included in the cohort. We calculated the proportion of women in care screened for each STI (chlamydia, gonorrhea, syphilis and trichomoniasis) during each calendar year and at least once over the study period. We determined annual test positivity for individual and composite reportable STI measures (chlamydia, gonorrhea, and/or syphilis, called STI-3). We did not include trichomoniasis in the final model for two reasons: trichomoniasis was significantly more common which skewed the model and predictors of trichomoniasis in our study population have previously been published.17 We sought to identify unique predictors of chlamydia, gonorrhea and syphilis among demographic, clinical and risk behaviors using univariate and multivariable models. Finally, we tested the performance characteristics of CDC STI screening recommendations for sexually active adults by comparing screening rates and test positivity according to whether or not women reported sexual activity in the past 6 months.
Definitions
The analysis was restricted to women who were engaged in HIV care for at least one calendar year during 2013–2015. Engagement was defined using the standard Health Resources and Services Administration HIV AIDS Bureau (HRSA-HAB) measure: at least two clinic visits separated by 90 days in a calendar year.18 Clinic visits included routine visits, women’s health visits and sick call visits (where some women presented with an unrelated complaint but were screened for STI). STI screen was defined as having at least one STI test during the calendar year. Since information about the presence or absence of STI symptoms at the time of sample collection was not available, the measure of “test positivity” was used. The time of positivity for STI-3 was the first occurrence of chlamydia, gonorrhea or syphilis during the study period. An anchor date for each year in care was created for time-dependent variables (age, CD4, HIV viral load, and patient reported outcomes). The hierarchy for this anchor date was the date of the positive STI test, or the date of the STI screening test (if negative) or July 1st (if no STI testing was performed). Variables were captured closest to the anchor date and within +/− 180 days for age, CD4 and HIV viral load and within +/− 365 days for patient reported outcomes about sexual behaviors and substance use.
Participant Characteristics
Demographics and comorbidity data were collected from the electronic medical record: age (categorized 19–24, 25–29, 30–39, 40–49, 50+), race (White, Black, other/unknown), insurance (private, public, none), time since HIV diagnosis (<2 years, 2–9 years, >10 years), CD4 count (<200, 200–350, >350 cells/mm3), undetectable HIV viral load (<50 copies/mL) and active hepatitis B infection (hepatitis B surface antigen (HBsAg) test positive). Recent history of chlamydia or gonorrhea was defined as laboratory-confirmed nucleic acid amplification testing (NAAT) for CT or GC during the previous calendar year. Information about recent STI in sex partners was not available. Cervical dysplasia was defined as atypical squamous cells of undetermined significance (ASCUS), cervical intra-epithelial neoplasia (CIN) I-III or cervical cancer on the problem list. Patient-reported outcomes included: active problem alcohol use, number of sexual partners (0, 1, 2+), anal sex (ever), sex with an HIV-infected partner, sex with a partner with unknown HIV status, condom use “always” (yes/no/not applicable if no sex partners), sex after drugs or alcohol, and history or current drug abuse with cocaine, heroin, methamphetamine, or opiates in the past 3 months. Except for drug abuse, all patient reported outcomes referred to the preceding six months.
Diagnostic Testing
NAAT for chlamydia, gonorrhea or trichomoniasis was performed on vaginal or cervical swabs, or on urine samples. C. trachomatis and N. gonorrhoeae NAAT were performed on the DNA-based BD Viper system (BD Diagnostics, Sparks, MD) until 2014 when the lab switched to the RNA-based Aptima Hologic system (San Diego, CA). T. vaginalis was diagnosed with the InPouch system (BioMed Diagnostics. Santa Clara, CA), until August 2014, when it was replaced by RNA-based Aptima testing. For syphilis, RPR was used as the initial screening test until the reverse testing algorithm (starting with syphilis IgG EIA) was adopted in March 2015. Treponemal antibody IgG testing was performed with the Trep-Sure qualitative enzyme immunoassay (Trinity Biotech, Jamestown, NY). Positive treponemal and non-treponemal tests (any titer) were required for syphilis cases and chart review was conducted for disease staging.
Statistical Analysis
Descriptive statistics were used to summarize cohort characteristics. Findings were stratified by women with a positive test for chlamydia, gonorrhea, and/or syphilis (STI-3). Chi-square or Fisher’s exact tests were used to compare categorical variables and Wilcoxon rank-sum tests were used for continuous measures. Unadjusted and multivariable logistic regression models were created to identify predictors of STI-3. Variables for the multivariable model were chosen from literature review and significance and effect size in the UV models. Because individual study participants could contribute up to three separate years of time, generalized estimating equations (GEE) with an exchangeable correlation structure was used to account for repeated measures. The sensitivity, specificity, negative predictive value and positive predictive value of CDC STI screening criteria were determined for women based on self-reported sexual activity. A c-statistic was calculated to measure goodness of fit. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Ethics
The study was approved by the University of Alabama at Birmingham Institutional Review Board.
Results
Of 834 women with HIV infection who received care between January 1st, 2013 and December 31st, 2015, 745 (89.3%) were engaged in care during at least one calendar year. These 745 women comprise our study population. Baseline participant characteristics stratified by STI test results are shown in Table 1. Median age was 46.8 years (range 19.9–78.1 years), 70.4% of women were ≥40 years old and older women were less likely to have STI (p=0.02). A majority of study participants were Black (78.8%) and more than half (54.0%) were diagnosed with HIV at least a decade earlier. The median CD4 was 581 cells/mm3 (IQR 366–867 cells/mm3) and 65.7% of women had an undetectable HIV viral load (<50 copies/mL). Nearly one in five (19.7%) had documentation of cervical dysplasia. Although 26% had history of STI per the record, only 0.9% had laboratory confirmed CT/GC infection in the past year.
Table 1.
Baseline Characteristics of 745 Women in HIV Care during 2013–2015*
| Characteristic | Total (n=745) | Tested for Chlamydia, Gonorrhea and/or Syphilis (STI-3) (n=684) | ||
|---|---|---|---|---|
|
| ||||
| Positive n=19 |
Negative n=665 |
p value** | ||
|
| ||||
| Sociodemographic | ||||
|
| ||||
| Median age, years (IQR) | 46.8 (38.3–53.7) | 40.9 (32.0–44.9) | 46.7 (38.1–53.4) | <0.01 |
|
| ||||
| Age (years) | 0.02 | |||
| 19–24 | 14 (1.9) | 2 (10.5) | 12 (1.8) | |
| 25–29 | 28 (3.8) | 1 (5.3) | 26 (3.9) | |
| 30–39 | 178 (23.9) | 6 (31.6) | 160 (24.1) | |
| 40–49 | 235 (31.5) | 8 (42.1) | 215 (32.3) | |
| ≥50 | 290 (38.9) | 2 (10.5) | 252 (37.9) | |
|
| ||||
| Race | 0.48 | |||
| White | 144 (19.3) | 3 (15.8) | 124 (18.7) | |
| Black | 587 (78.8) | 15 (79.0) | 528 (79.4) | |
| Other/Unknown | 14 (1.9) | 1 (5.2) | 13 (1.9) | |
|
| ||||
| Insurance Status | <0.01 | |||
| Private | 204 (27.4) | 2 (10.5) | 177 (26.6) | |
| Public | 293 (39.3) | 3 (15.8) | 276 (41.5) | |
| None | 248 (33.3) | 14 (73.7) | 212 (31.9) | |
|
| ||||
| Clinical | ||||
|
| ||||
| Timing of HIV Diagnosis | <0.01 | |||
| 0–2 years ago | 113 (15.5) | 8 (44.4) | 95 (14.6) | |
| 3–9 years ago | 223 (30.5) | 7 (38.9) | 196 (30.0) | |
| 10+ years ago | 395 (54.0) | 3 (16.7) | 361 (55.4) | |
|
| ||||
| Median CD4 Count (IQR) | 581 (366–867) | 540 (290–726) | 579 (366–867) | 0.53 |
|
| ||||
| CD4 Count (cells/mm3) | 0.77 | |||
| <200 | 89 (12.1) | 3 (15.8) | 80 (12.2) | |
| 200–350 | 79 (10.8) | 2 (10.5) | 66 (10.1) | |
| >350 | 565 (77.1) | 14 (73.7) | 508 (77.7) | |
|
| ||||
| HIV Viral Load (copies/mL) | 0.03 | |||
| <50 | 486 (65.7) | 8 (42.1) | 434 (65.7) | |
| ≥50 | 254 (34.3) | 11 (57.9) | 227 (34.3) | |
|
| ||||
| CT/GC Infection in the Previous 12 mo | 0.01 | |||
| Yes | 7 (0.9) | 2 (10.5) | 5 (0.8) | |
| No | 738 (99.1) | 17 (89.5) | 660 (99.2) | |
|
| ||||
| Active Hepatitis B Infection | 1.0 | |||
| Yes | 15 (2.0) | 0 (0) | 13 (2.0) | |
| No | 730 (98.0) | 19 (100) | 652 (98.0) | |
|
| ||||
| Cervical Dysplasia (ever) | 0.03 | |||
| Yes | 147 (19.7) | 0 (0) | 133 (20.0) | |
| No | 598 (80.3) | 19 (100) | 532 (80.0) | |
|
| ||||
| Patient Reported Outcomes*** | ||||
|
| ||||
| Problem Alcohol Intake | 0.44 | |||
| Yes | 137 (29.1) | 3 (15.8) | 69 (10.4) | |
| No | 334 (70.9) | 16 (84.2) | 596 (89.6) | |
|
| ||||
| Drug Abuse (cocaine, heroin, meth, opiates) | 0.09 | |||
| Current (in the past 3 months) | 35 (7.4) | 3 (23.1) | 29 (6.9) | |
| History (ever) | 112 (23.8) | 3 (23.1) | 100 (23.6) | |
| No | 324 (68.8) | 7 (53.9) | 294 (69.5) | |
|
| ||||
| Number of Sex Partners | 0.01 | |||
| 0 | 184 (39.0) | 2 (15.4) | 156 (36.8) | |
| 1 | 255 (54.0) | 7 (53.9) | 240 (56.6) | |
| 2+ | 33 (7.0) | 4 (30.8) | 28 (6.6) | |
|
| ||||
| Sex with HIV+ Partner | 0.15 | |||
| Yes | 87 (18.9) | 5 (38.5) | 79 (19.1) | |
| No | 374 (81.1) | 8 (61.5) | 334 (80.9) | |
|
| ||||
| Sex with partner of unknown HIV status | 0.09 | |||
| Yes | 41 (8.7) | 3 (23.1) | 38 (9.0) | |
| No | 431 (91.3) | 10 (76.9) | 386 (91.0) | |
|
| ||||
| Condom Use “always” | 0.19 | |||
| Yes | 157 (33.1) | 7 (53.9) | 145 (34.0) | |
| No | 128 (27.0) | 4 (30.8) | 120 (28.2) | |
| Not Applicable | 189 (39.9) | 2 (15.4) | 161 (37.8) | |
|
| ||||
| Sex after drugs/alcohol | 0.01 | |||
| Yes | 53 (11.3) | 5 (38.5) | 47 (11.1) | |
| No | 417 (88.7) | 8 (61.5) | 375 (88.9) | |
|
| ||||
| Anal sex (ever) | 0.12 | |||
| Yes | 144 (30.4) | 7 (53.9) | 129 (30.4) | |
| No | 329 (69.6) | 6 (46.1) | 296 (69.7) | |
Characteristics during the 1st full year engaged in HIV care at the time of STI diagnosis, STI screening test or July 1st (if not screened). First year in care 2013 (N=542), 2014 (N=158), 2015 (N=45).
P-value from Chi-square tests or Fisher’s exact tests
Refers to behavior during the past six months unless specified
Missing data for each variable: Timing of HIV infection – 14; CD4 – 12; HIV VL – 5; drug use – 274; alcohol intake – 274; number of sex partners 273; sex with HIV+ partner – 284; sex with partner with unknown HIV status – 273; condom use – 271, sex after drugs or alcohol – 275; anal sex – 272. STI-3 – 61,
PRO data were available for 66% of women in 2013 (n=359), 71% in 2014 (n=437) and 71% in 2015 (n=421). Study participants with and without PRO data had similar baseline characteristics. Nearly four in 10 women (39.0%; 184/472) with PRO data reported no sexual activity in the past 6 months. Among women who were sexually active, most were practicing safer sex: 88.5% (255/288) reported monogamy and 55.1% (157/285) always used condoms. Some other risk behaviors were relatively infrequent: 7.4% reported active drug use, 8.7% had sex with a partner of unknown HIV status, and 11.3% had sex after drugs or alcohol. (Table 1)
STI screening rates per person-year are shown in Table 2. Between 67–72% of study participants were screened for chlamydia and gonorrhea in any given year and 94% of women were screened at least once if they were in care for three years. Of 1919 chlamydia tests performed, 1221 samples (63.6%) were from cervical or vaginal sites, and 698 (36.4%) were urine; dual testing for gonorrhea was performed in 100%. Annual syphilis screening rates decreased from 87.3% in 2013 to 64.8% in 2015 but 99.0% of participants in care for three years were tested at least once. The 2015 screening rate for trichomoniasis by NAAT was only 58.4%. Women who reported sexual activity on PROs were more likely to be screened for STI (annual rate of 75–83% for CT/GC) compared to women who reported no sex partners (annual rate of 57–59% for CT/GC) (all p<.001) (data not shown). One in three (33.2%; 239/720) chlamydia and gonorrhea tests performed in 2015 were in women who reported not having sex in the past six months.
Table 2.
STI Screening among Women with HIV
| Chlamydia | Gonorrhea | Syphilis | Trichomoniasis | |
|---|---|---|---|---|
| 2013* (n=542) | 69.4% | 68.8% | 87.3% | 0% |
| 2014* (n=613) | 71.5% | 71.5% | 74.4% | 33.6% |
| 2015* (n=594) | 67.2% | 67.0% | 64.8% | 58.4 % |
| At least once 2013–2015** (n=745) | 71.4% | 71.0% | 82.7% | 12.1% |
| In Care all of 2013–2015 (n=391) | 93.9% | 93.9% | 99.0% | 71.6% |
At least 1 STI test during the calendar year
At least 1 STI test during 1st full year engaged in HIV care
At least 1 STI test during 3 years in care
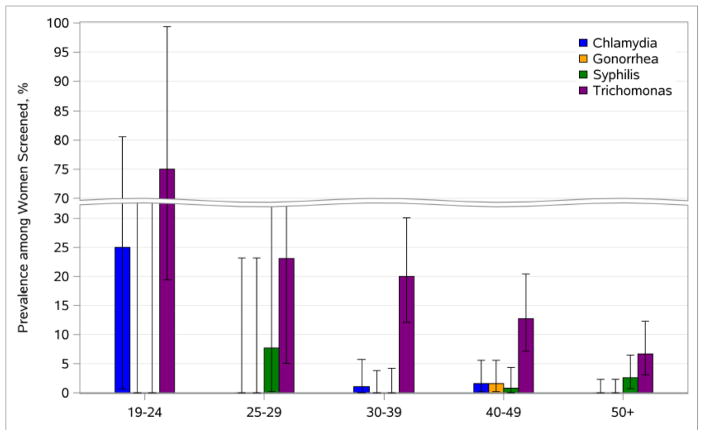
Annual test positivity for individual and composite STI outcomes are shown in Table 3 with findings stratified by sexual activity for women with PRO data available. Rates of chlamydia (1.0–2.1%), gonorrhea (0–0.8%) and syphilis (0.4–1.6%) were low but trichomoniasis was common (11.2–13.3%). A graph of 2015 STI test positivity by age category demonstrates the inverse association between STI and age (Figure 1). Twelve cases of syphilis were detected over three years: all had low titer RPR (eight 1:1, two 1:2, two 1:4) and positive treponemal testing. Upon medical record review, ten cases were categorized as possible early or latent syphilis and two had decreasing RPR titers but timing of therapy and historical test results were not available to allow for accurate disease staging. Table 3 also compares STI rates based on self-reported sexual activity. On average, sexually active women were more likely to have STI. Using sexual activity alone to direct bacterial STI screening yielded sensitivity 85.7% (95% CI 42.1–99.6), specificity 38.7% (CI 33.5–44.1), positive predictive value 2.8% (CI 1.0–6.0) and negative predictive value 99.3% (CI 95.5–100.0). The c-statistic value for the area under the ROC curve was 0.62, indicating poor performance of using self-reported sexual activity as the sole criteria to guide STI screening.
Table 3.
Annual STI Detection in Women with HIV: Overall and by Sexual Activity Status*
| Chlamydia n (%) |
Gonorrhea n (%) |
Syphilis n (%) |
Trichomoniasis n (%) |
STI 3** (CT/GC/Syphilis) n (%) |
|
|---|---|---|---|---|---|
|
| |||||
| 2013 | |||||
| Overall | 8/376 (2.1) | 3/373 (0.8) | 2/473 (0.4) | N/A | 13/506 (2.6) |
| Sexually Active | 7/166 (4.2) | 1/164 (0.6) | 0/195 (0) | N/A | 8/205 (3.9) |
| Not Sexually Active | 0/86 (0) | 1/86 (1.2) | 0/112 (0) | N/A | 1/126 (0.8) |
|
| |||||
| 2014 | |||||
| Overall | 7/438 (1.6) | 0/438 (0) | 4/456 (0.9) | 23/206 (11.2) | 11/539 (2.0) |
| Sexually Active | 3/210 (1.4) | 0/210 (0) | 4/206 (1.9) | 11/109 (10.1) | 7/237 (3.0) |
| Not Sexually Active | 3/102 (2.9) | 0/102 (0) | 0/124 (0) | 2/37 (5.4) | 3/149 (2.0) |
|
| |||||
| 2015 | |||||
| Overall | 4/399 (1.0) | 2/398 (0.5) | 6/385 (1.6) | 46/347 (13.3) | 12/493 (2.4) |
| Sexually Active | 3/184 (1.6) | 2/184 (1.1) | 1/169 (0.6) | 26/152 (17.1) | 6/215 (2.8) |
| Not Sexually Active | 0/102 (0) | 0/101 (0) | 1/108 (0.9) | 3/89 (3.4) | 1/133 (0.8) |
Sexual activity status among women with patient reported outcome data available. Active if 1+ sex partners in past 6 months. Not active if reported 0 sex partners in past 6 months.
Among women screened for at least one STI during the calendar year.
Figure 1.
(Alternative Version) – STI Positivity among Women with HIV by Age Group, 2015
Table 4 shows the STI-3 prediction model based on 1055 observations and 26 positive tests for chlamydia, gonorrhea, or syphilis. In the unadjusted model, age 19–24 was highly predictive of bacterial STI and women age ≥50 were less likely to have STI, (both compared to age 25–49). Other predictors in the unadjusted model were: CT/GC infection in the past 12 months, multiple sex partners, lack of medical insurance, sex after drugs or alcohol, sex with an HIV-infected partner, and detectable HIV viral load. In the multivariable model, older age (≥50) had a significant and inverse association with STI (aOR 0.3, 95% CI 0.1–1.0, p=0.040). Factors positively associated with STI were: CT/GC infection in the past 12 months (aOR 3.7, 95% CI 1.0–13.4, p=0.047), public insurance compared to private insurance (aOR 3.5, 95% CI 1.01–11.8, p=0.048), and sex after drugs or alcohol (aOR 3.0, 95% CI 1.2–8.0, p=0.025).
Table 4.
Predictors of Bacterial STI (CT/GC/Syphilis) in Women with HIV *
| Characteristic at time of STI | Unadjusted OR, 95% CI | p value | Adjusted OR**, 95% CI | p value |
|---|---|---|---|---|
|
| ||||
| Sociodemographics | ||||
|
| ||||
| Age | ||||
| <50 | Ref | Ref | ||
| 50+ | 0.3 (0.1–0.7) | 0.005 | 0.3 (0.1–1) | 0.040 |
|
| ||||
| Race | ||||
| White | Ref | |||
| Black | 0.9 (0.4–2.1) | 0.843 | ||
|
| ||||
| Insurance | ||||
| Private | Ref | Ref | ||
| Public | 2.1 (0.7–6.5) | 0.188 | 3.5 (1–11.8) | 0.048 |
| None | 3.9 (1.3–11.8) | 0.015 | 2.8 (0.8–9.9) | 0.108 |
|
| ||||
| Clinical | ||||
|
| ||||
| Years since HIV Diagnosis | ||||
| 0–2 | 1.8 (0.8–4.0) | 0.188 | ||
| 3–9 | Ref | |||
| >10 | 0.2 (0.1–0.5) | <0.001 | ||
|
| ||||
| CD4 (cells/mm3) | ||||
| <200 | 1.9 (0.8–4.8) | 0.162 | ||
| 200–350 | 1.4 (0.5–4.1) | 0.490 | ||
| >350 | Ref | |||
|
| ||||
| HIV Viral Load (copies/mL) | ||||
| <50 | Ref | |||
| >50 | 2.4 (1.2–4.7) | <0.001 | ||
|
| ||||
| CT/GC Infection in the Previous 12 mo | ||||
| No | Ref | Ref | ||
| Yes | 16.3 (5.9–44.9) | <0.001 | 3.7 (1–13.4) | 0.047 |
|
| ||||
| Cervical Dysplasia (ever) | ||||
| No | Ref | |||
| Yes | 0.6 (0.3–1.6) | 0.353 | ||
|
| ||||
| Chronic Hepatitis B Infection | ||||
| No | Ref | |||
| Yes | 1.4 (0.2–9.7) | 0.735 | ||
|
| ||||
| Patient Reported Outcomes | ||||
|
| ||||
| Problem Alcohol Intake in past 6 months | ||||
| No | Ref | |||
| Yes | 3.0 (1.3–6.8) | 0.008 | ||
|
| ||||
| Drug Abuse | ||||
| Never | Ref | |||
| Active | 2.8 (1.0–7.9) | 0.054 | ||
| History | 1.2 (0.5–3.1) | 0.735 | ||
|
| ||||
| Sex Partner # in past 6 months | ||||
| 0 | Ref | Ref | ||
| 1 | 2.1 (0.8–5.9) | 0.157 | 1.3 (0.5–3.6) | 0.658 |
| 2+ | 7.1 (2.2–22.9) | 0.001 | 2.8 (0.7–10.9) | 0.142 |
|
| ||||
| Sex with HIV+ Partner in past 6 months | ||||
| No | Ref | |||
| Yes | 2.7 (1.1–6.6) | 0.027 | ||
|
| ||||
| Sex with partner of unknown HIV status in past 6 months | ||||
| No | Ref | |||
| Yes | 1.4 (0.4–5.0) | 0.570 | ||
|
| ||||
| Condom Use “always” in past 6 months | ||||
| No | Ref | |||
| Yes | 1.0 (0.4–2.4) | 0.932 | ||
| N/A | 0.3 (0.1–1) | 0.056 | ||
|
| ||||
| Sex after drugs/alcohol in past 6 months | ||||
| No | Ref | Ref | ||
| Yes | 3.8 (1.6–9.1) | 0.003 | 3.0 (1.2–8.0) | 0.025 |
|
| ||||
| Anal sex (ever) | ||||
| No | Ref | |||
| Yes | 1.5 (0.7–3.3) | 0.272 | ||
Odds ratios, resulting 95% confidence intervals, and p-values from logistic regression models with a positive STI result during the calendar year as the event. Individual women contribute a separate observation for each year screened for at least 1 STI (up to 3). GEE with an exchangeable correlation structure used to account for possible correlation due to women contributing multiple observations.
Adjusted OR’s from single multivariable model including the variables with results shown in the table. Based on 1055 observations, 26 positive STI tests.
Discussion
Women in HIV care in urban Alabama had low annual detection rates of chlamydia, gonorrhea and syphilis (<2.2%) despite residence in a region of the United States where STI is endemic. Older age (>50) was associated with lower STI prevalence, and three factors were identified as independent predictors of STI: chlamydia or gonorrhea infection in the past 12 months, sex after drugs or alcohol and public health insurance. Trichomoniasis was common across all age categories.
Our findings add to accumulating evidence that show comparable STI rates among women in the United States, irrespective of HIV status. The most recent data from the National Health and Nutrition Examination Survey (NHANES), showed a 2% annual population prevalence of chlamydia in sexually active females age 14–39, compared to 1–2.1% in our older HIV cohort. 19 Two US studies of young, sexually active women documented a 0.3% gonorrhea detection rate, compared to 0–0.8% in our study.20,21 Finally, national T. vaginalis prevalence in NHANES was 3.1% among women age 14–49, but 10-fold higher in Black women which approximated the 11.2–13.3% positivity rate seen in our study. 21 If STI rates in HIV-infected women are comparable to national STI rates, it may no longer be appropriate to consider all women living with HIV as “high-risk” in terms of STI acquisition and STI/HIV transmission. It is not clear whether women with HIV with STI have higher rates of negative infection outcomes, but better predictors of STI risk than HIV status alone are needed to guide screening in this key population.
Age is one of the most consistent predictors of bacterial STI acquisition. Peak rates of chlamydia, gonorrhea and syphilis among women in the 2016 CDC STD Surveillance Report occurred in the 20–24 year old age group while age 15–24 is consistently associated with high risk of incident STI. 3 Yet, the median age of female study participants living with HIV in Alabama was 47 years. Although safer sex counseling is important for women of all ages, one recent multicenter study of women living with HIV in the US showed that only 1 in 3 women above age 50 were sexually active.22 Most participants in our cohort reported few (if any) traditional STI risk behaviors yet many were routinely screened for STI. Annual CT/GC screening rates of 65–70% increased to 75–80% among study participants who reported sexual activity. These rates are suboptimal, but similar to CT/GC screening rates in HIV-infected MSM and significantly higher than other US women in HIV care during 2009–2013 (27–45%). 23,24 In terms of sexual behaviors, nearly four in 10 (39%) women had not been sexually active in the past six months and 54% had acquired HIV more than 10 years ago. The vast majority of sexually active women were practicing safer sex with a single male partner. These risk behaviors in study participants differ compared to 890 men who have sex with men (MSM) surveyed in the same HIV clinic; 64% of MSM reported multiple partners in the past six months, 56% had unprotected sex and 58% had a history of STI. 25 While bacterial STI rates have increased to 20 million new infections each year, rates stratified by gender show that much of the trend is explained by increasing incidence among men 2,26 For example, according to the 2016 CDC surveillance report, the gonorrhea case-rate was 171 cases per 100,000 males compared to 121 cases per 100,000 females and in 2015, the rate of primary and secondary syphilis was 309 cases per 100,000 MSM compared to 1.4 cases per 100,000 females. 3,27
Most cost-effective screening strategies target populations based on individual risk. STI screening recommendations are limited by a paucity of risk factor data, particularly for women living with HIV. 28 Also, risk-based STI screening is less useful when the provider or patient is unaware of the risk behavior. Predictors of chlamydia infection among HIV-uninfected women include: younger age (<20), new or multiple sex partners, and having a partner with STI symptoms. 29 Predictors of gonorrhea among young women are: a new partner in the past 60 days and Black or Native American race.20 STI predictors among incarcerated Black women are: partner concurrency, inconsistent condom use, sex work, prior STI and drug abuse.30 One of few studies to identify predictors of incident STI in women with HIV was conducted in Africa where age <25 years and recent STI were predictive.31 Published predictors of trichomoniasis among women with HIV in our clinic include Black race, cocaine use and age ≤40. 17 Since trichomoniasis was common, molecular testing for trichomonas alone may be more cost-reasonable than combination testing for CT/GC/trichomonas.
In sum, in our model, younger age and recent STI are the best predictors of STI acquisition among women engaged in HIV care. The development and validation of a simple prediction model to allow for targeted bacterial STI screening in women with HIV could advance clinical care and reduce cost. The most useful predictors to include in a model would be easily ascertained factors, such as age and gender. Findings from this study suggest additional risk factors to test: recent CT/GC in the past year, sex after drugs/alcohol and socioeconomic status. CDC recommends repeat chlamydia and gonorrhea testing 3 months after diagnosis due to high reinfection rates - our data supports this practice in women with HIV.8 Additional data from a national, longitudinal HIV cohort study with validated STI outcomes and risk behaviors would provide the best opportunity to assess demographic and behavioral factors in a predictive model that could be subsequently validated for performance characteristics. Such an analysis within the Center for AIDS Research Network of Integrated Clinical Systems (CNICS) is ongoing. It is also important for future studies to explore STI outcomes in women who are not engaged in HIV care since higher infection rates and missed opportunities are likely. In the meantime, the high negative predictive value of using sexual activity in screening criteria indicates that it is reasonable for HIV providers to follow CDC guidelines and defer routine STI screening for women who are not having sex.
Our study had limitations. Low STI positivity rates limited our ability to assess all potential predictors. Study findings are not generalizable to pregnant women with HIV or women not engaged in care. Findings likely generalize to women with HIV in care living in urban areas in the US South but may not extend to communities with a larger proportion of young women with newly diagnosed HIV. STI screening in regions with lower infection rates may be even less useful than screening in Alabama. Also, risk behavior data were not available for all study participants and information about recent STI in sex partners was not captured. Since we did not capture symptoms, some testing was performed for diagnostic purposes but had we excluded testing among symptomatic women, we expect that STI detection rates would have been even lower. STI screening was not universal but comparable to other US HIV clinics.24 Finally, although our composite STI measure included 12 cases of syphilis, we were unable to stage cases which may have overestimated infectious syphilis. Strengths of this study include the size of the HIV cohort with the provision of primary care in a setting with frequent follow up, routine STI testing and high levels of HIV care engagement and retention. High quality information about sensitive risk behaviors was collected with validated computerized questionnaires.
The major implication of this study is that current CDC STI screening recommendations based solely on sexual activity for women living with HIV may be inadequate. Older women with few or no STI risk factors may be overscreened for chlamydia, gonorrhea and syphilis but insufficiently screened for trichomoniasis. Study findings support CDC guidelines to screen women with HIV annually for trichomoniasis, but we documented an annual chlamydia rate well below the <3% threshold that dictates cost-effective screening practices. HIV clinics and public health services must prioritize high-impact activities and STI molecular diagnostic testing is highly sensitive but costly. Moreover, many STI outcomes built into screening models are adverse pregnancy and fertility outcomes with less relevance in determining screening efficacy in postmenopausal women. 13,14
In conclusion, many women engaged in HIV care in Alabama are older, have few STI risk factors and infrequent infection with chlamydia, gonorrhea and syphilis. Current guidelines that recommend universal annual STI screening in women with HIV should be refined based on an improved understanding of risk. A simple STI prediction model based on age and recent STI may be effective.
Summary.
Women with HIV in Alabama were frequently screened for chlamydia, gonorrhea and syphilis but infection rates were low and women had few risk factors for sexually transmitted infection.
Acknowledgments
Source of Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23 HD090993 to JDO) of the National Institutes of Health and the University of Alabama at Birmingham Center for AIDS Research (CFAR P30 AI027767).
Footnotes
Conflicts of Interest: None of the authors have a conflict of interest to declare.
This work was presented, in part, at the 2017 Conference of the Infectious Diseases Society for OB/GYN.
References
- 1.Centers for Disease Control and Prevention N, editor. Atlas Plus. 2015. [Google Scholar]
- 2.Centers for Disease Control DoSP. STD Surveillance Report, 2015. 2016. [Google Scholar]
- 3.CDC. STD Surveillance Report, 2016. 2017. [Google Scholar]
- 4.Datta SD, Torrone E, Kruszon-Moran D, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999–2008. Sexually transmitted diseases. 2012;39(2):92–96. doi: 10.1097/OLQ.0b013e31823e2ff7. [DOI] [PubMed] [Google Scholar]
- 5.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Micro. 2004;2(1):33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 6.Adachi K, Klausner JD, Bristow CC, et al. Chlamydia and Gonorrhea in HIV-Infected Pregnant Women and Infant HIV Transmission. Sexually transmitted diseases. 2015;42(10):554–565. doi: 10.1097/OLQ.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeganeh N, Watts HD, Camarca M, et al. Syphilis in HIV-infected mothers and infants: results from the NICHD/HPTN 040 study. The Pediatric infectious disease journal. 2015;34(3):e52–57. doi: 10.1097/INF.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Control CfD. STD Treatment Guidelines. 2015. [Google Scholar]
- 9.Mavedzenge SN, Pol BV, Cheng H, et al. Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sexually transmitted diseases. 2010;37(7):460–466. doi: 10.1097/OLQ.0b013e3181cfcc4b. [DOI] [PubMed] [Google Scholar]
- 10.Force USPST. Screening for syphilis infection in nonpregnant adults and adolescents: Us preventive services task force recommendation statement. Jama. 2016;315(21):2321–2327. doi: 10.1001/jama.2016.5824. [DOI] [PubMed] [Google Scholar]
- 11.LeFevre ML. Screening for Chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2014;161(12):902–910. doi: 10.7326/M14-1981. [DOI] [PubMed] [Google Scholar]
- 12.Lee KC, Ngo-Metzger Q, Wolff T, Chowdhury J, LeFevre ML, Meyers DS. Sexually Transmitted Infections: Recommendations from the U.S. Preventive Services Task Force. American family physician. 2016;94(11):907–915. [PubMed] [Google Scholar]
- 13.Owusu-Edusei K, Jr, Hoover KW, Gift TL. Cost-Effectiveness of Opt-Out Chlamydia Testing for High-Risk Young Women in the U.S. American Journal of Preventive Medicine. 2016;51(2):216–224. doi: 10.1016/j.amepre.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu D, Hook EW, III, Goldie SJ. Screening for chlamydia trachomatis in women 15 to 29 years of age: A cost-effectiveness analysis. Annals of internal medicine. 2004;141(7):501–513. doi: 10.7326/0003-4819-141-7-200410050-00006. [DOI] [PubMed] [Google Scholar]
- 15.Fujii H, Nishimoto N, Yamaguchi S, et al. The Alcohol Use Disorders Identification Test for Consumption (AUDIT-C) is more useful than pre-existing laboratory tests for predicting hazardous drinking: a cross-sectional study. BMC public health. 2016;16:379. doi: 10.1186/s12889-016-3053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction (Abingdon, England) 2008;103(6):1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 17.Muzny CA, Burkholder GA, Fry KR, Austin EL, Schwebke JR. Trichomonas vaginalis Nucleic Acid Amplification Testing at an Urban HIV Clinic. Sexually transmitted diseases. 2016;43(8):483–488. doi: 10.1097/OLQ.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 18.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(8):1164–1171. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 19.Torrone E, Papp J, Weinstock H. Prevalence of Chlamydia trachomatis genital infection among persons aged 14–39 years--United States, 2007–2012. MMWR Morbidity and mortality weekly report. 2014;63(38):834–838. [PMC free article] [PubMed] [Google Scholar]
- 20.Manhart LE, Marrazzo JM, Fine DN, Kerani RP, Golden MR. Selective testing criteria for gonorrhea among young women screened for Chlamydial infection: contribution of race and geographic prevalence. The Journal of infectious diseases. 2007;196(5):731–737. doi: 10.1086/520517. [DOI] [PubMed] [Google Scholar]
- 21.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(10):1319–1326. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 22.Frazier EL, Sutton MY, Tie Y, Collison M, Do A. Clinical Characteristics and Outcomes Among Older Women with HIV. Journal of women’s health (2002) 2017 doi: 10.1089/jwh.2017.6380. [DOI] [PubMed] [Google Scholar]
- 23.Dean BB, Scott M, Hart R, et al. Sexually Transmitted Disease Testing of Human Immunodeficiency Virus-Infected Men Who Have Sex With Men: Room for Improvement. Sexually transmitted diseases. 2017;44(11):678–684. doi: 10.1097/OLQ.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 24.Mattson CL, Bradley H, Beer L, Johnson C, Pearson WS, Shouse RL. Increased STD testing among sexually active persons receiving medical care for HIV infection in the United States, 2009 - 2013. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 doi: 10.1093/cid/ciw834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong CMHJ, Tamhane AR, Hook EW, Van Wagoner N, Dionne-Odom J, Raper JL, Burkholder GA. Gonorrhea and Chlamydia Testing in Routine Clinical Care of HIV Positive Men who have Sex with Men. IDSA. 2014 [Google Scholar]
- 26.Raifman JR, Gebo KA, Mathews WC, et al. Gonorrhea and Chlamydia Case Detection Increased When Testing Increased in a Multisite US HIV Cohort, 2004–2014. Journal of acquired immune deficiency syndromes (1999) 2017;76(4):409–416. doi: 10.1097/QAI.0000000000001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Voux A, Kidd S, Grey JA, et al. State-Specific Rates of Primary and Secondary Syphilis Among Men Who Have Sex with Men - United States, 2015. MMWR Morbidity and mortality weekly report. 2017;66(13):349–354. doi: 10.15585/mmwr.mm6613a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falasinnu T, Gustafson P, Hottes TS, Gilbert M, Ogilvie G, Shoveller J. A critical appraisal of risk models for predicting sexually transmitted infections. Sexually transmitted diseases. 2014;41(5):321–330. doi: 10.1097/OLQ.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 29.Marrazzo JM, Celum CL, Hillis SD, Fine D, DeLisle S, Handsfield HH. Performance and cost-effectiveness of selective screening criteria for Chlamydia trachomatis infection in women. Implications for a national Chlamydia control strategy. Sexually transmitted diseases. 1997;24(3):131–141. doi: 10.1097/00007435-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Pelligrino N, Zaitzow BH, Sothern M, Scribner R, Phillippi S. Incarcerated Black Women in the Southern USA: A Narrative Review of STI and HIV Risk and Implications for Future Public Health Research, Practice, and Policy. Journal of racial and ethnic health disparities. 2017;4(1):9–18. doi: 10.1007/s40615-015-0194-8. [DOI] [PubMed] [Google Scholar]
- 31.Chirenje ZM, Gundacker HM, Richardson B, et al. Risk Factors for Incidence of Sexually Transmitted Infections Among Women in a Human Immunodeficiency Virus Chemoprevention Trial: VOICE (MTN-003) Sexually transmitted diseases. 2017;44(3):135–140. doi: 10.1097/OLQ.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]