Abstract
Background:
Breast cancer is most commonly managed with a combination of tumor ablation, radiation, and/or chemotherapy. Despite the oncologic benefit of these treatments, the detrimental effect of radiation on surrounding tissue challenges the attainment of ideal breast reconstruction outcomes. The purpose of this study is to determine the ability of topical deferoxamine to reduce cutaneous ulceration and collagen disorganization following radiotherapy in a murine model of expander-based breast reconstruction.
Methods:
Female Sprague-Dawley rats (n=15) were divided into three groups: Control (expander), XRT (expander + radiation), and DFO (expander + radiation + deferoxamine). Expanders were placed in a sub-musculocutaneous plane in the right upper back and ultimately filled to 15 cc. Radiation was administered via a fractionated dose of 28 Gy. Deferoxamine was delivered topically for 10 days following radiation. After a 20-day recovery period, skin ulceration and dermal type I collagen organization was analyzed.
Results:
Compared to Control, the XRT group demonstrated a significant increase in skin ulceration (3.7% vs. 43.3%, p=0.00) and collagen fibril disorganization (26.3% vs. 81.8%, p=0.00). Compared to the XRT group, treatment with topical deferoxamine resulted in a significant reduction in ulceration (43.3% vs. 7.0%, p=0.00) and fibril disorganization (81.8% vs. 15.3%, p=0.00). There were no statistical differences between the Control and DFO groups in skin ulceration or collagen disorganization.
Conclusions:
This study suggests topical deferoxamine is capable of reducing skin ulceration and type I collagen fibril disorganization following radiotherapy. This novel application of deferoxamine has potential to enhance expander-based breast reconstruction outcomes and improve quality of life for women suffering the devastating effects of breast cancer.
Keywords: Skin ulceration, fibrosis, type I collagen, breast expander, cancer
INTRODUCTION
Breast cancer is the most frequently diagnosed malignancy among women in the United States.1 Throughout the past several decades, advancements in clinical screening protocols have enhanced the precision and timeliness of diagnoses; moreover, refinements in oncologic management, including surgery, radiation (XRT), and chemotherapy, have improved prognoses.2–5 Notwithstanding, progress surrounding reconstructive outcomes for breast cancer survivors has been relatively inadequate in comparison to modern developments in breast cancer treatment.6
Breast reconstruction marks a critical component in the road to recovery for many women who elect to undergo mastectomy.7 Implant-based reconstruction is the most commonly utilized technique worldwide, as this method is relatively well tolerated by patients due to reduced operative times, hospital stay durations, and at-home recovery periods compared to autologous breast reconstruction.8 However, radiotherapy induces a persistent inflammatory response in skin and soft tissue that often results in wound breakdown, expander exposure, and skin necrosis.9 Overall aesthetic results and patient satisfaction are thus diminished when radiotherapy precedes reconstruction.10,11 On a cellular and sub-cellular level, XRT is known to disrupt healthy cell function and structure resulting in substantial skin ulceration, destruction of vascular networks, and severe inhibition of angiogenesis.12 Skin atrophy also reflects collagen reabsorption and disorganization following radiotherapy.12,13 These apparent clinical presentations and underlying physiological changes represent a critical barrier to achieving a satisfactory aesthetic result. Plastic and reconstructive surgeons must continually work to overcome this challenge, considering that current breast cancer survivorship population is 3.1 million in the United States alone.14
Deferoxamine (DFO) is an FDA-approved iron chelator currently utilized for the systemic treatment of hemochromatosis. Interestingly, this pharmacologic agent has also been found to increase VEGF production through the HIF-1α pathway when administered locally. Increasing VEGF in turn promotes the formation of healthy vascular networks.15 Previous studies support the ability of DFO to improve wound healing by recruiting hemotactic cells, augmenting epithelialization, and increasing vascular permeability and collagen deposition.16,17 DFO is thus a therapeutic agent with immense potential to reestablish underlying cellular structures such as collagen organization and restore the health of affected skin and soft tissue in breast cancer patients following XRT.
Moreover, DFO is a highly translatable adjuvant pharmaceutical considering its previous approval by the Food and Drug Administration for systemic intravenous delivery in patients with iron overload. However, the requirement for local injections into the breast is not clinically feasible given the risk of prosthetic puncture, clinical burden to physicians, and considerable discomfort for patients receiving treatment. Gurtner and colleagues previously introduced a topical DFO formulation for diabetic wound healing that eliminates the aforementioned disadvantages of repeated injection.18 The purpose of this study is to determine the ability of topically administered DFO to mitigate radiation-induced skin ulceration and type I collagen fiber disorganization in order to enhance the aesthetic outcomes of breast reconstruction following XRT and, ultimately, improve the quality of life of breast cancer survivors.
MATERIALS AND METHODS
Study Design
The animal protocol was approved by the University of Michigan’s Institutional Animal Care and Use Committee prior to implementation. All animal experimentation was conducted in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals: Eighth Edition. Adult female Sprague Dawley rats (n=15) weighing approximately 350 g were acclimated for 7 days, and given food and water ad libitum. Animals were then randomly assigned to three groups: Control (expander placement), XRT (expander placement + radiation therapy), or DFO (expander placement + radiation therapy + topical DFO) (Figure 1). The sample size for this study was selected in order to optimize study strength and resource utilization. Topical DFO, and not intravenously administered DFO, was selected as the experimental group given the superior clinical feasibility and efficiency of a topical therapeutic.
Figure 1:
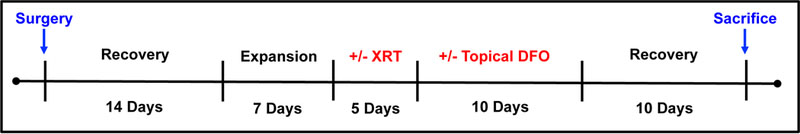
Experimental timeline. Expanders were placed on POD 0 and filled on POD 15, 18, and 21. Radiation was delivered via 5 fractionated doses, and followed by a 10-day topical DFO treatment period for select groups.
Surgical Procedure
Our surgical procedure has been previously described and published.19,20 Briefly, a 3-cm longitudinal incision was made 1 cm to the right of dorsal midline. Blunt dissection was used to generate a sub-musculocutaneous pocket of compatible size to accommodate the tissue expander. A sterile, silicon based, smooth-textured mini-expander (Allergan, Inc., Santa Barbara, CA) measuring 3 cm in diameter was placed in the tissue pocket with its port (2 cm distal, 1.5cm diameter, 0.6cm height) placed caudally and secured with a single 4.0 monocryl suture. The muscle and skin were re-approximated over the expander utilizing interrupted 4.0 vicryl sutures. The animals recovered in a warmed cage under continuous supervision.
Expansion and Recovery
Animals received daily operative site monitoring for 14-days postoperatively. Analgesia with buprenorphine was continued every 12 hours through post-operative day (POD) 4 and daily weights were obtained to monitor nutritional status until POD 10. Tissue expansion took place under isoflurane drop anesthesia on postoperative days 15, 18, and 21, with 5 cc 0.9% normal saline injected during each session to achieve a total fill volume of 15 cc (Figure 2). This total fill volume was determined based on the tension and compliance of the expanded tissue overlying the implant as previously described.19,20
Figure 2:
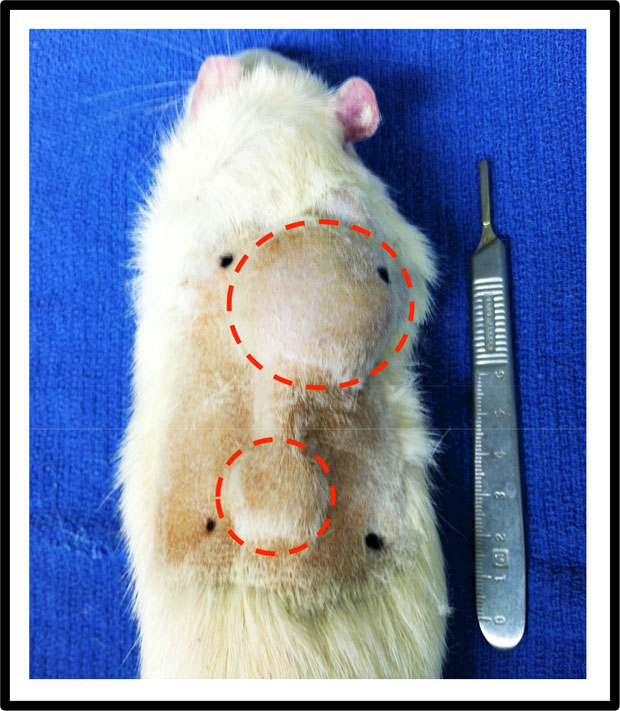
Animal on POD 21 with fill volume of 15 cc. Expanders were placed right of the midline, with the port extending caudally.
Radiation Procedure
All radiation procedures were conducted in the Irradiation Core at the University of Michigan Cancer Center. Radiation Oncology at the University of Michigan provided guidance on developing a human equivalent dose radiation of 28 Gy delivered as 5.6 Gy per day over 5 days beginning on POD 22. This dosage was calculated based upon a comparable 60 Gy dose of radiotherapy most commonly administered following mastectomy.21 After transient induction of anesthesia with an oxygen/isoflurane mixture, select rats were radiated using a Philips RT250 orthovoltage unit (250 kV, 15 mA) (Kimtron, Inc., Oxford, CT). A lead shield with a 3.5 cm diameter circular aperture was used to ensure localized delivery of radiation to our selected region of interest (ROI) corresponding with the area of expanded tissue.
Deferoxamine Treatment
In order to develop a translationally useable formulation of this therapeutic, we collaborated with the Bioengineering and Materials Science Laboratory at Stanford University for construction of a tailor-made topical DFO patch.18 DFO was subject to reverse micelle encapsulation with surfactants and incorporated into a polymer matrix transdermal drug delivery system (TDDS). This formulation permitted transfer of DFO through the stratum corneum with final release of the active pharmacologic within the dermis. DFO patches (1 × 2 cm) were dosed at 2 mg (1 mg/cm3) in order to imitate the dose utilized previously by our laboratory in other models of irradiated tissue healing.22–25 Prior to patch application, all animals underwent hair removal to maximize dermal delivery. Full adhesion of the DFO patches directly to the irradiated and expanded tissue was facilitated with a non-traumatic transpore tape. DFO treatment was initiated on the day following the final dose of radiation and was carried out over 10 days. Elizabethan collars (E collars) were utilized to reduce grooming of the expander and retain DFO patch treatment on the area of expanded tissue. Of note, the DFO group was compared to a non-sham Control group, as previous studies have demonstrated no significant variances between sham and non-sham controls.18
Analysis of Skin Ulceration
Animals were shaved and depilatory cream was applied 7 days prior to image acquisition. Digital images were collected at 20 days post-radiation. The ROI was defined as a 112 × 112 pixel area overlying the apex of the expander, consistent with the location of XRT and topical DFO treatment delivery. The area of skin ulceration identified in the ROI was measured via Image J (National Institutes of Health, Bethesda MD) and subsequently divided by the total area within the ROI to quantify the percentage of skin and soft tissue damage present.
Analysis of Collagen Organization
Twenty days following radiation, animals underwent euthanasia on POD 46 via systemic paraformaldehyde perfusion.19,20 Skin tissue sections of 25 µm thickness were prepared parallel to the dermal horizontal plane. Atomic force microscopy (AFM) analysis was utilized to identify collagen fibril sheet organization (micron scale architecture).26 All AFM imaging was carried out in air-dry conditions on a nanoIR2 (Anasys Instruments, Santa Barbara, CA). Imaging was performed in contact mode using Anasys Instruments contact mode nanoIR2 probes (silicon cantilever with gold coating, nominal radius 25 nm, resonance frequency 13 ± 4 kHz, spring constant 0.07–0.4 N/m, length 225 nm). All images used for assessment of type I collagen fibril sheet organization were 10µm x 10µm with a line scan rate of 1.0 Hz and 512 pixels per line (~19.5 nm/pixel). Regions of type I collagen organization were defined as 2 or more collagen fibrils organized in parallel within collagen sheets. Two independent, blinded reviewers conducted micro architecture analysis through quantification of the percentage of disorganized fibrils per unit area within the AFM images using Image J and the average of the two reviewers was used for statistical analysis.
Statistical Analysis
A power analysis was performed using SPSS 24.0 for Windows (SPSS, Inc, Chicago, IL) in consultation with the Center for Statistical Consultation and Research (CSCAR) at the University of Michigan. Analysis of skin ulceration and collagen disorganization metrics was conducted using ANOVA with statistical significance set at p < 0.05. Post-hoc Tukey HSD or Games-Howell tests were run depending on homogeneity of variances as per Levene’s Test.
RESULTS
Two rats were ultimately excluded from analysis. Despite E collar usage, one control group rat exhibited compulsive grooming with resultant severe wounding patterns in the ROI. A second rat from the XRT group experienced pre-emergence from anesthesia, interrupting delivery of a full radiation dose and therefore was excluded. All rats from the DFO patch group were retained for analysis.
Skin Ulceration
The administration of XRT and topical DFO significantly impacted the degree of skin ulceration within the ROI overlying the tissue expander. Compared to the Control group, the XRT group exhibited both a clinically and statistically significant increase in cutaneous ulceration (3.7% vs. 43.3%, p=0.00). This result typifies the corrosive and damaging effects of XRT on soft tissue. Treatment with topical DFO following radiotherapy achieved an extraordinary six-fold reduction in ulceration (43.3% vs. 7.0%, p=0.00). Furthermore, ulceration in Control and DFO groups showed no statistical difference, demonstrating the potential for topical DFO to remediate cutaneous ulceration incurred by XRT (3.7% vs. 7.0%, p=0.90) (Figure 3).
Figure 3:
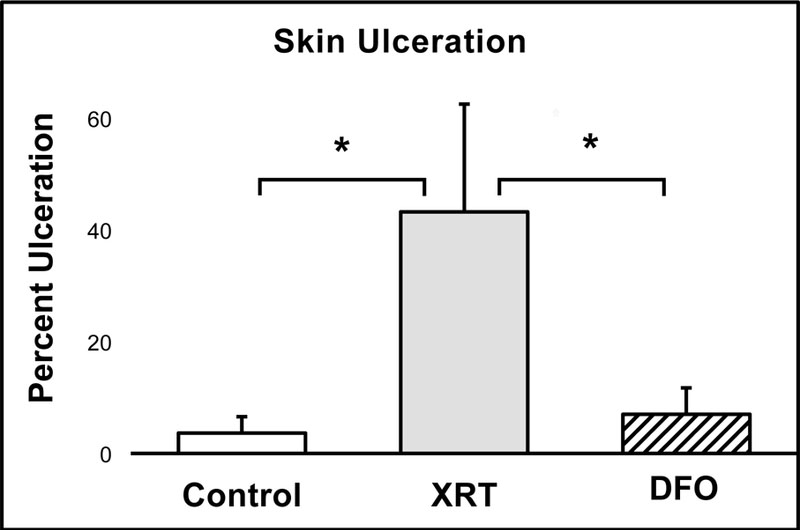
The XRT group demonstrated significantly greater skin ulceration compared to Control, which was completely remediated by topical DFO treatment.
Collagen Disorganization
The majority of patients undergoing radiotherapy experience acute skin reactions in exposed areas, including disruption of healthy type I collagen organization.27 In this investigation, XRT-induced skin injury was evidenced by a three-fold increase in type I collagen disorganization within the XRT group in comparison to non-irradiated controls (26.3% vs. 81.8%, p=0.00). Compared to the XRT group, treatment with topical DFO significantly reduced fibril disorganization by five-fold (81.8% vs. 15.3%, p=0.00). Moreover, no statistical differences were observed between the non-irradiated controls and the DFO group, again indicating a restoration of fibril organization and the capacity of topical DFO to mitigate the destructive effects of radiotherapy on soft tissue (26.3% vs. 15.3%, p=0.53) (Figure 4).
Figure 4:
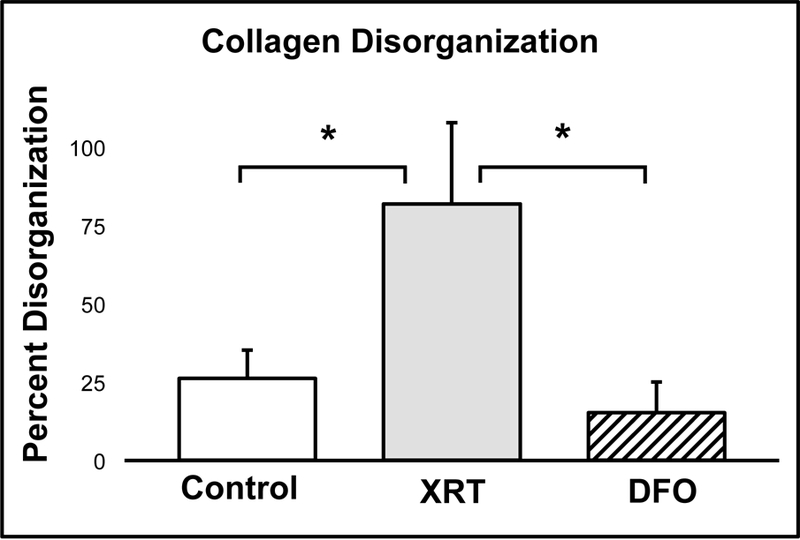
Based on atomic force microscopy analysis, the XRT group exhibited significantly greater collagen fibril disorganization compared to Control. This was successfully mitigated by topical DFO treatment.
DISCUSSION
Radiotherapy reduces the risk of breast cancer recurrence by destroying residual cancer cells post-surgery.4 This therapy is therefore essential for curative intent, but has devastating consequences on the reconstructive process. XRT treatment is associated with reconstructive complications including wound breakdown, skin necrosis, and expander exposure, which collectively result in high rates of operative failure.10 In the setting of radiotherapy, 33% of patients require re-operation and additional reconstructions.28,29 Moreover, for patients undergoing delayed reconstruction, the rate of required re-operations due to wound breakdown, flap failure, or contracture is five times greater among irradiated patients.11,30 Determining the mechanisms of XRT-induced injury to the expanded skin and soft tissue, and the subsequent innovation of therapeutic agents capable of mitigating such injury, would offer substantial benefits to both reconstructive surgeons and breast cancer survivors alike.
Our laboratory has extensive experience utilizing locally injected DFO as a method of tissue restoration and healing in irradiated fields.22–25 Our selection of DFO for remediation of XRT-induced skin injury was based on several studies demonstrating its capacity to impact key components of the wound healing process.15–17 Deferoxamine functions as an iron chelator that enhances VEGF production via the HIF-1α pathway, thereby increasing angiogenesis and aiding in the restoration of vascular networks depleted by radiation. Notably, previous investigations demonstrate that topical DFO enhances neovascularization and increases dermal thickness according to CD31 immunohistochemistry studies and picrosirius red histological examinations, respectively.18 The present study first aims to analyze cutaneous manifestations of radiation injury, which represent an important clinical feature that significantly impacts both the functional and aesthetic outcomes of breast reconstruction. Approximately three weeks following radiotherapy administration, we observed XRT-induced ulceration that was nearly 12-fold greater than in non-irradiated controls. Grossly, the radiated tissue exhibited central necrosis with hyperemic edges, with the brunt of injury occurring at the apex of the expanded tissue. The addition of DFO patches in this setting remediated those observations, as evidenced by a six-fold reduction in ulceration. In addition, the irradiated skin treated with DFO appeared grossly normal, and lacked the associated alopecia observed in the XRT group (Figure 5).
Figure 5:
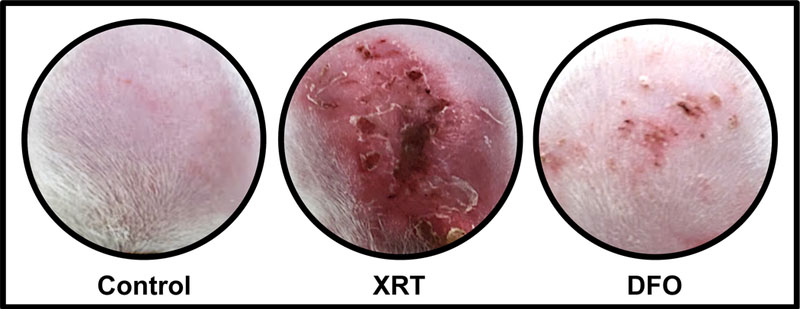
Skin overlying expander devices. Radiation resulted in grossly evident skin ulceration and necrosis. Such injury was assuaged by topical deferoxamine treatment.
Dermal collagen is a key constituent of the skin that impacts both fibrosis and wound healing.12,13 As a method of examining the dermis, we selected atomic force microscopy to identify structural changes in collagen sheets that could account for the known physical changes characteristic of radiotherapy-associated fibrosis. Ultimately, our AFM analysis yielded consequential revelations with regards to dermal type I collagen organization. In our study, irradiated collagen fibrils were found to display a disordered pattern consistent with previous studies using other surrogate metrics of collagen fibril order such as through histological evaluation utilizing Picrosirius red staining.13 Dermal samples from the Control group contained more highly organized, parallel, straight fibril alignment within collagen sheets when compared to the XRT group, which exhibited highly disorganized, irregular, and curved fibril patterns. These qualitative observations were corroborated by a quantitative three-fold increase in fibril disorganization in the irradiated group. DFO patch treatment was associated with a significant improvement in fibril alignment in comparison to XRT, as demonstrated by an associated five-fold decrease in fibril disorganization (Figure 6).
Figure 6:
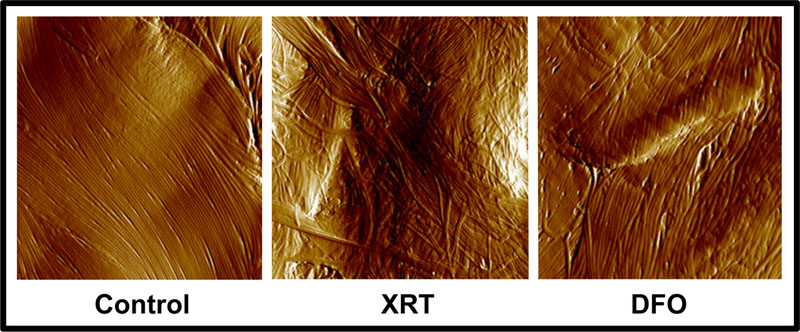
Atomic force microscopy images of type I collagen fibril organization in skin overlying expander devices. A significantly greater degree of fibril disorganization was seen in XRT compared to Control. Topical deferoxamine restored collagen organization to control levels.
In this study, metrics of cutaneous ulceration and type I collagen fibril organization were significantly improved in the setting of radiotherapy with the use of a topical DFO treatment in a murine model of expander-based breast reconstruction. The promising nature of this study encourages the performance of additional investigations to delineate the optimal dose of DFO per patch, quantify the impact of topical DFO on tissue vascularity following XRT, and identify any potential roles of deferoxamine in capsular contracture. A full analysis of the impact of DFO on breast cancer cell proliferation in both the presence and absence of XRT is also warranted.15 Interestingly, current studies are being performed to investigate the capacity of iron chelation to be utilized as a chemotherapeutic strategy due to its potential to disrupt the rapid metabolic processes associated with tumor growth.31 These investigations will help to rule out any potential safety concerns before the full consideration of fast-track clinical adoption of topical DFO treatment is realized in an attempt to improve surgical, aesthetic, and quality of life outcomes for breast cancer survivors.
ACKNOWLEDGEMENTS
The authors would like to thank Rachel L. Mertzel, MS for technical help with atomic force microscopy analysis. This work was supported by RO1 grants from the National Institutes of Health (CA12587–01, CA12587–06) awarded to Steven R. Buchman and from the National Institutes of Health (GM 8616–17) awarded to the University of Michigan, Department of Surgery, Section of Plastic Surgery.
Sources of Support: This work was supported by RO1 grants from the National Institutes of Health (CA12587–01, CA12587–06) awarded to Steven R. Buchman and from the National Institutes of Health (GM 8616–17) awarded to the University of Michigan, Department of Surgery, Section of Plastic Surgery.
Footnotes
CONFLICT OF INTEREST
The following patent is assigned to Dr. Geoffrey C. Gurtner, MD and Stanford University: Topical and Transdermal Delivery of HIF-1 Modulators to Prevent and Treat Chronic Wounds (20100092546). All authors state that they have no conflicts of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359–386 [DOI] [PubMed] [Google Scholar]
- 2.Marmot MG, Altman DG, Cameron DA, et al. The benefits and harms of breast cancer screening: an independent review. Br J Cancer 2013;108(11):2205–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med 2015;372(24):2353–2358 [DOI] [PubMed] [Google Scholar]
- 4.Gebski V, Lagleva M, Keech A, et al. Adjuvant radiation therapy using biologically equivalent doses: a clinical perspective. J Natl Cancer Inst 2006;98(1):26–38 [DOI] [PubMed] [Google Scholar]
- 5.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 2010;28(1):92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson JA, Disa JJ. Breast reconstruction and radiation therapy: an update. Plast Reconstr Surg 2017;140(5S):60S–68S [DOI] [PubMed] [Google Scholar]
- 7.Cordeiro PG. Breast reconstruction after surgery for breast cancer. N Engl J Med 2008;359:1590–1601 [DOI] [PubMed] [Google Scholar]
- 8.Ho AY, Hu ZI, Mehrara BJ, et al. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol 2017;18(12):e742–753 [DOI] [PubMed] [Google Scholar]
- 9.Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: a claims-based analysis. Ann Surg 2016;263(2):219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg 2004;113(3):877–881 [DOI] [PubMed] [Google Scholar]
- 11.Kronowitz SJ, Robb GL. Breast reconstruction with postmastectomy radiation therapy: current issues. Plast Reconstr Surg 2004;114(4):950–960 [DOI] [PubMed] [Google Scholar]
- 12.Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys 1995;31(5):1171–1185 [DOI] [PubMed] [Google Scholar]
- 13.Sassi ML, Jukkola A, Riekki R, et al. Type I collagen turnover and cross-linking are increased in irradiated skin of breast cancer patients. Radiother Oncol 2001;58(3):317–323 [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society. Global Cancer facts & figures 2nd edition. Available at: http://www.cancer.org/index. Accessed 20.02.18.
- 15.Beerepoot LV, Shima DT, Kuroki M, et al. Up-regulation of vascular endothelial growth factor production by iron chelators. Cancer Res 1996;56(16):3747–3751 [PubMed] [Google Scholar]
- 16.Duscher D, Januszyk M, Maan ZN, et al. Comparison of the hydroxylase inhibitor dimethyloxalylglycine and the iron chelator deferoxamine in diabetic and aged wound healing. Plast Reconstr Surg 2017;139(3):695e–706e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ram M, Singh V, Kumawat S, et al. Deferoxamine modulates cytokines and growth factors to accelerate cutaneous wound healing in diabetic rats. Eur J Pharmacol 2015;764:9–21 [DOI] [PubMed] [Google Scholar]
- 18.Duscher D, Neofytou E, Wong VW, et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc Natl Acad Sci U S A 2015;112(1):94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felice PA, Nelson NS, Page EE, et al. Amifostine reduces radiation-induced complications in a murine model of expander-based breast reconstruction. Plast Reconstr Surg 2014;134(4):551e–560e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polyatskaya Y, Nelson NS, Rodriguez JJ, et al. Prophylactic amifostine prevents a pathologic vascular response in a murine model of expander-based reconstruction. J Plast Reconstr Aesthet Surg 2016;69(2):234–240 [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network. National comprehensive cancer network (web site) Available at: http://www.NCCN.org. Accessed 20.10.2013
- 22.Felice PA, Ahsan S, Donneys A, et al. Deferoxamine administration delivers translational optimization of distraction osteogenesis in the irradiated mandible. Plast Reconstr Surg 2013;132(4):542e–548e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farberg AS, Jing XL, Monson LA, et al. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone 2012;50(5):1184–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farberg AS, Sarhaddi D, Donneys A, et al. Deferoxamine enhances bone regeneration in mandibular distraction osteogenesis. Plast Reconstr Surg 2014;133(3):666–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donneys A, Farberg AS, Tchanque-Fossuo CN, et al. Deferoxamine enhances the vascular response of bone regeneration in mandibular distraction osteogenesis. Plast Reconstr Surg 2012;129(4):850–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauble MA, Rothman E, Welch M, et al. Alteration of type I collagen microstructure induced by estrogen depletion can be prevented with drug treatment. BoneKey Rep 2015;4:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bray FN, Simmons BJ, Wolfson AH, et al. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther 2016;6(2):185–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys 2001;49(3):713–721 [DOI] [PubMed] [Google Scholar]
- 29.Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg 2002;109(6):1919–1924 [DOI] [PubMed] [Google Scholar]
- 30.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg 2013;131(1):15–23 [DOI] [PubMed] [Google Scholar]
- 31.Mertens C, Akam EA, Rehwald C, et al. Intracellular iron chelation modulates the macrophage iron phenotype with consequences on tumor progression. PLoS One 2016;11(11):e0166164. [DOI] [PMC free article] [PubMed] [Google Scholar]


