Abstract
Clinical and experimental observations suggest that chronic lung disease is linked to respiratory viral infection. However, the long-term aspect of this relationship is not yet defined using a virus that replicates at properly high levels in humans and a corresponding animal model. Here we show that influenza A virus infection achieves 1×106-fold increases in viral load in the lung and dose-dependent severity of acute illness in mice. Moreover, these events are followed by persistence of negative- and positive-strand viral-RNA remnants for 15 weeks and chronic lung disease for at least 26 weeks after infection. The disease is manifested by focal areas of bronchiolization and mucus production that contain increased levels of viral-RNA remnants along with mucin Muc5ac and Il13 mRNA compared to uninvolved areas of the lung. Excess mucus production and associated airway hyper-reactivity (but not fibrosis or emphysema) are partially attenuated with loss of IL-13 production or signaling (using mice with IL-13- or STAT6-deficiency). These deficiencies cause reciprocal increases in l17a mRNA and neutrophils in the lung, however, none of these disease endpoints are changed with IL-13–IL-17a-compared to IL-13-deficiency or STAT6-IL-17a-compared to STAT6-deficiency. The results establish the capacity of a potent human respiratory virus to produce chronic lung disease focally at sites of active viral-RNA remnants, likely reflecting locations of viral replication that reprogram the region. Viral dose-dependency of disease also implicates high-level viral replication and severity of acute infection as determinants of chronic lung diseases such as asthma and COPD with IL-13-dependent and IL-13/IL-17-independent mechanisms.
Introduction
Respiratory viral infections are linked to chronic lung diseases, both in childhood asthma and in adults with severe asthma and chronic obstructive pulmonary disease (COPD) (1). In humans, the link between viral infection and chronic lung disease was initially associated with severe infections due to respiratory syncytial virus (RSV) (2, 3) but later extended to human metapneumovirus and human rhinovirus (HRV) (4–7). Moreover, some reports suggest that the type of virus is not critical to the subsequent development of post-viral disease (8). In experimental mouse models, chronic lung disease can develop after a severe infection with mouse parainfluenza virus also known as Sendai virus (SeV) (9). In this case, the mechanism for chronic lung disease is based on long-term activation of a type 2 immune response that includes IL-13-driven mucus production and airway hyper-reactivity (10–12). The same disease traits are characteristic of patients with chronic lung disease due to asthma and COPD (10, 11, 13–15) and thereby help to validate the proposal that transient viral infections might trigger long-term consequences for initiation, exacerbation, or progression of chronic lung disease. However, the SeV-driven model relies on a virus that infects but does not cause significant disease in humans (16) and therefore might not be representative of more pathogenic human respiratory viruses.
In the present study, we address the need for a model of post-viral lung disease based on a potent human pathogen, focusing on the proposal that the severity of acute infection might determine the subsequent development of chronic lung disease. In a search for other viral pathogens responsible for respiratory illness, we recognized that some of the most common human pathogens linked to chronic lung disease do not replicate efficiently in mice. For example, viral loads in the lung for RSV (e.g., A2 strain) and HRV (e.g., A16 or B1 strains) do not increase from the initial amount of virus administered to the animal, even in mice that are immunocompromised due to age, genetic background, or targeted modifications to delete anti-viral host defense genes or overexpress viral receptors (17–25). Moreover, these immune alterations might have effects on acute infection that may or may not coincide with effects on the development of chronic lung disease, e.g., interferon (IFN) signaling separately influences viral replication and type 2 immune response (26, 27).
To improve a post-viral lung disease model using a human pathogen, we aimed to explore preliminary associations between influenza A virus (IAV) infection and subsequent wheezing illness in infants as well as an established connection of influenza A virus with exacerbations of asthma and COPD in children and adults (28–31). In addition, there were reports of persistent pod-like and cystic structures at 21–200 days after IAV (PR8 strain) infection in mice (32–34). Although these investigators connected these structures to lung regeneration and fibrotic disease, there were also reports of mucous-like cells after IAV infection (with PR8 and HKx31 strains) in mice (35, 36). These initial studies did not examine long-term time points or define mechanism. Our findings show that IAV replicates in mouse lung to levels that are a 1×106-fold higher than initial dosing and causes infection that progresses to chronic lung disease for at least 6 months. The disease is linked to initial viral load and severity of acute infection as well as subsequent IL-13 and mucin (MUC5AC) but not IL-17a induction at sites of viral-RNA remnants. The findings thereby establish the capacity of a virus with high-level replication in humans and mice to cause a severe acute illness and switch the susceptible host to chronic lung disease. This disease is linked at least in part to a localized, long-term type 2 immune response with excess inflammatory mucus production but also to an IL-13/IL-17-independent process for mucus formation as well as fibrosis and emphysema.
Materials and Methods
Mice
Wild-type C56BL/6J mice (000664), Stat6–/– mice (005977) (37), and Il17a–/– mice (016879) (38) mice were obtained from The Jackson Laboratory. Breeding pairs of Il13–/– mice were obtained from T. Wynn (39). Breeding was used to generate Il13–/––Stat6–/– and Il13–/––Il17a–/– mice. All mice were maintained on a C57BL/6J background and were co-housed in a barrier facility using cages fitted with micro-isolator lids. Animal husbandry and experimental procedures were approved by the Animal Studies Committees of Washington University School of Medicine in accordance with the guidelines from the National Institutes of Health.
Virus preparation and infection
IAV strain A/WS/33 was obtained from ATCC (VR-1520) and propagated in chicken embryo allantoic cavities as described previously (40). IAV strain A/PR/8/34 was obtained from Jacco Boon (Washington University). Aliquots of stock virus were stored at −70 °C and were titered using a plaque-forming assay with MDCK cells (ATCC CCL-34), 1 μg/ml of acetylated trypsin (Sigma T-6763), and agarose overlay as described previously (40). Mice were inoculated with IAV-WS/33 (0.04–5.0 pfu) or IAV-PR8 (10 pfu) in 30 μl of PBS given intranasally or with an equivalent amount of UV-inactivated virus or PBS at 5–6 weeks of age under ketamine/xylazine anesthesia. Results from male and female mice were pooled since no significant differences were found between sexes. Mouse lungs were frozen at −70 °C for homogenization in PBS with a cell disrupter (Mini-Beadbeater-96, Biospec Products) followed by viral plaque-forming assay to track pfu level normalized to gm of lung tissue (recognizing that one mouse lung weighs approximately 0.1 gm) (41). For viral titer and other assays in involved versus uninvolved lung tissue, the regions were determined by visual inspection and then separated by gross dissection.
RNA and protein analysis
Total RNA was isolated from lung. tracheobronchial lymph node, spleen, and thymus tissue using the Qiagen RNeasy kit and converted to cDNA using the High-Capacity cDNA Archive kit and random hexamer primers (Applied Biosystems). Levels of viral RNA were monitored using a real-time qPCR assay for the IAV segment 3 containing the polymerase acid (PA) gene or IAV segment 7 containing the matrix (M) gene with forward and reverse primers and MGB probes described in Supplemental Table I. To quantify the level of viral RNA, segments of the PA gene (nt 259–944) or M gene (nt 25–124) were cloned using the TA cloning kit (ThermoFisher Scientific) and used as standards. Lung levels of Muc5ac mRNA were determined using a real-time quantitative PCR (qPCR) assay as described previously (10). Lung levels of Clca1, IL13, IL17a and IL17f were determined using qPCR assay probes described in Supplemental Table II. All target mRNA and viral RNA levels were normalized to Gapdh or Mrlp19 mRNA level using the TaqMan Rodent GAPDH Control Kit or a Mrlp19-specific qPCR assay described in Supplemental Table II. All mRNA values were expressed as copy number based on comparison to the corresponding plasmid cDNA standard. Total lung protein was prepared using bead homogenization in T-per buffer with protease inhibitor (ThermoFisher Scientific). Homogenized samples were cleared with centrifugation, and the supernatant was analyzed using a quantitative ELISA for IL-13 (R&D DuoSet) according to the manufacturer’s instructions.
Strand-specific real-time qPCR assay for IAV RNA encoding the PA and NP genes was performed using tagged PCR primers and high-temperature reverse transcription based on an approach described previously (42) with primers and probes described in Supplemental Table I. Reverse transcription with the tagged primers was performed using SuperScript™ III reverse transcriptase (Invitrogen) and trehalose (Sigma) at 60 °C as described previously (43). For the PA gene, assay specificity was validated using positive and negative RNA strands generated from a Bluescript SK(+) plasmid encoding the PA gene segment (nt 259–944) under separate gene promoters with opposite orientations. For the NP gene, assay conditions were the same as those described previously for this method (43). In both cases, the DNA template was eliminated with TRIzol (Invitrogen) followed by treatment with DNase and the RNeasy Mini kit (QIAGEN).
Tissue staining and microscopy
Tissues were fixed with 10% neutral-buffered formalin, embedded in paraffin, cut into 5-μm thick sections, dewaxed, rehydrated, and incubated with mouse anti-MUC5AC mAb (clone 45M1, ThermoFisher Scientific) or control mAb as described previously (10). Immunofluorescence microscopy for virus was performed using goat anti-IAV H1N1 antibody (20-IG23, Fitzgerald Industries International) and tyramide-based signal amplification with Alexa Fluor 594 fluorochrome (Invitrogen). Slides were counterstained with DAPI-containing mounting media (Vector Laboratories). Sections were also subjected to PAS-hematoxylin and Gomori trichrome staining with quantification using a NanoZoomer S60 slide scanner (Hamamatsu) and Visiomorph (Visiopharm) or ImageJ (44) software with settings specific for the purple-magenta-tone of mucins or light-blue tone of collagen staining. Mean linear intercept values for alveolar size were derived as described previously (45).
Airway reactivity
Airway reactivity to nebulized methacholine (Sigma, St. Louis, MO) was determined by measurements of respiratory system resistance (RRS) using the FinePointe Resistance and Compliance system (DSI Buxco Research Systems, Wilmington, NC) as described previously (10, 11). Mice were anesthetized, ventilated via tracheostomy, and sequentially challenged with aerosolized PBS (baseline) followed by doubling doses of methacholine ranging from 0.6125 to 20 mg/ml. Methacholine was delivered at 3-min intervals using an in-line nebulizer (Aerogen Laboratory; 2.4–4 μm particle size). Resistance values were recorded during a 3-min period following each challenge. Data were manually verified, and spurious epochs removed from analysis.
Statistical analysis
Unless stated otherwise, all data are presented as mean ± SEM and two-tailed unpaired Student’s t-test was used to assess statistical significance between means. In all cases, the threshold for statistical significance was set at a P value less than 0.05. Airway reactivity was assessed using either two-way repeated measures analysis of variance or restricted maximum likelihood linear mixed model with genotype, infection, and methacholine dose as fixed effects and subject as a random effect.
Results
IAV infection results in dose-dependent illness and leaves active viral-RNA remnants
Initial experiments showed that intranasal delivery of IAV (A/WS/33 strain) caused dose-dependent levels of acute illness as monitored by body weight loss (Fig. 1A) in concert with progressive increases in viral load based on plaque-forming assay for infectious virus (Fig. 1B) or qPCR assay for the viral polymerase acidic (PA) gene product (Fig. 1C). The levels of infectious IAV detected in lung tissue indicated marked viral replication, e.g., mice dosed with 2 pfu resulted in recovery of 2 × 107 pfu/gm of lung tissue (equivalent to 2 × 106 pfu/lung) at 3 days after infection (Fig. 1D). Despite clearance of infectious IAV by 12 days after infection based on plaque assay (Fig. 1D) and viral immunostaining (Fig. 1E), IAV-specific RNA was detectable in the lung even at 105 days after infection (Fig. 1F). IAV RNA also persisted in lung lymph node at 77 days, spleen at 49 days, and thymus at 3 days after infection (Fig. 1G). Since conventional qPCR assay can produce non-strand-specific products, we also applied an approach based on the use of tagged PCR primers (i.e., tag-PCR assay) designed for the IAV PA gene to distinguish between positive and negative strands of viral RNA (Fig. 1H). This approach provided for specific detection of positive versus negative-strand RNA from the PA gene (Fig. 1I) and demonstrated persistence of positive-strand viral RNA for 49 days after infection (Figure 1J). Similarly, we found persistence of IAV NP gene expression based on conventional PCR assay (Fig. 1K), and positive-strand IAV RNA (Fig. 1L) using a strand-specific assay described previously (43). Together, these findings indicated that IAV is capable of producing coding RNA for prolonged periods of time, consistent with reports of chronic antigen and T cell activation (46–49).
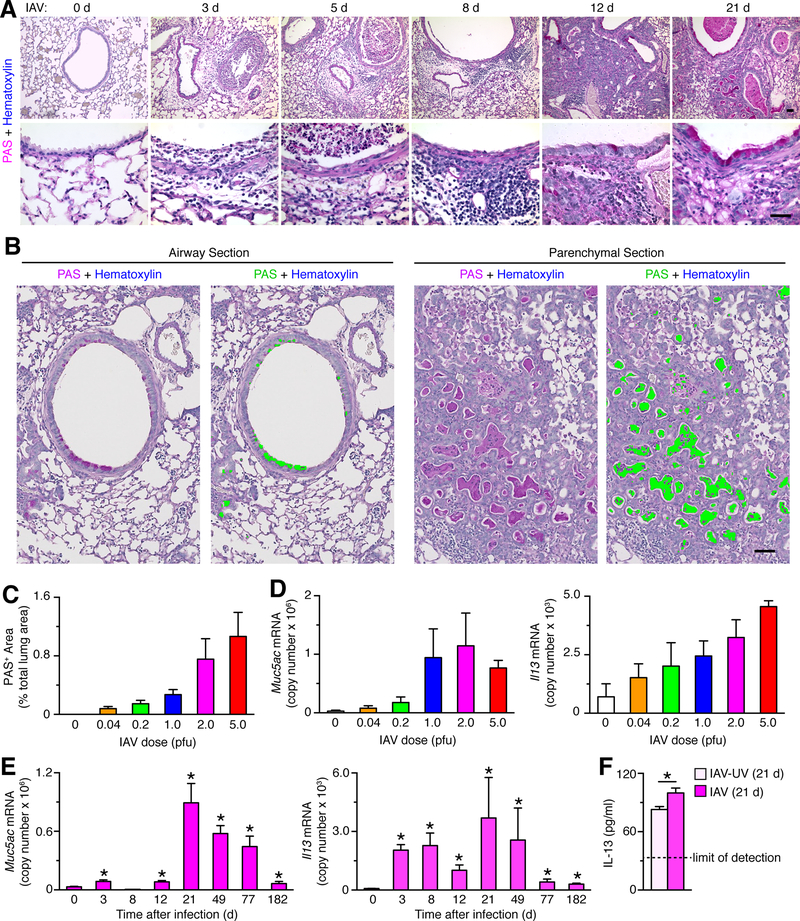
FIGURE 1.
Acute illness leaves viral remnants after IAV infection. (A) Time course of body weight change in wild-type C56BL/6J mice after infection with IAV (WS/33 strain, 0–5 pfu). Mice were euthanized at body weight <75% of initial value. (B) Corresponding lung levels of IAV monitored with plaque-forming assay for conditions in (A) at 3 d after infection. (C) Corresponding lung levels of IAV RNA encoding the PA gene monitored with PCR assay for conditions in (B). (D) Time course of lung levels of IAV monitored with plaque-forming assay after infection with IAV (2 pfu). (E) Time course for IAV immunostaining in lung sections after infection with IAV (2 pfu). Bar=400 μm. (F) Time course for lung levels of IAV RNA after infection with IAV (2 pfu). (G) Corresponding IAV-PA RNA levels in tracheobronchial lymph nodes, spleen, and thymus. For (H), * indicates p<0.05 versus no infection control. (H) Scheme for strand-specific qPCR assay in which the negative (–) RNA strand of viral RNA (vRNA) (labeled in magenta) or the positive (+) RNA strand of viral RNA (cRNA, mRNA) (labeled in blue) were reverse transcribed to corresponding cDNA using tagged primers that included a portion complementary to each strand (green) and another portion that was not related to viral sequence (orange). The resulting tagged cDNA was amplified using PCR in which one primer was specific to the tagged portion of cDNA (orange) and another primer (green) and probe (black) were specific to viral sequence. (I) Validation of strand-specific tag-PCR assay in which (+) and (–) strand RNA standards were transcribed from a plasmid encoding the IAV-PA gene and cDNA was synthesized using primers specific for the (–) or (+) strand of viral RNA. (J) Time course of lung levels of IAV-PA negative- and positive-strand RNA detected using tag-PCR assay. (K) Time course of lung levels of IAV RNA encoding the NP gene (IAV-NP) detected using PCR assay. (L) Time course of lung levels of IAV (+) strand RNA encoding IAV-NP detected using tag-PCR assay. For (A)-(G) and (I)-(L), values are representative of 3 separate experiments (n=8 mice per condition in each experiment). Values for 0 d were not significantly different than UV-inactivated IAV (IAV-UV) at 3–182 d (data not shown).
IAV infection also causes dose-dependent chronic lung disease
We next considered whether the persistence of IAV-RNA remnants might be associated with similarly long-lasting lung disease after viral infection. Indeed, we found that airway epithelial cell damage and immune cell accumulation was followed by the development of PAS-positive airway epithelial cells and bronchiolization consistent with the formation of mucous cells at 21 days after infection (Fig. 2A). Quantification of PAS-positive staining was performed using image analysis software (Fig. 2B) and showed increased staining in proportion to initial viral dose at 21 d after infection (Fig. 2C). Similarly, mucin Muc5ac and Il13 mRNA were also induced in proportion to viral dosing up to 2–5 pfu (Fig. 2D) and were maintained at increased levels with a maximum at 21 days and persistence to 182 days after infection (Fig. 2E). Consistent with induction of Il13 mRNA, we also detected a significant increase in IL-13 protein levels in lung tissue at 21 d after IAV infection (Fig. 2F), recognizing that this approach reflects IL-13 unbound to IL-13-receptor and the consequent need for additional experiments to establish pathophysiological significance of IL-13 expression in this model.
FIGURE 2.
Viral dose drives the development of chronic lung disease after IAV infection. (A) Time course for PAS-hematoxylin staining in lung sections after infection with IAV (WS/33 strain, 2 pfu). Bars=400 μm. (B) PAS-hematoxylin staining of lung sections for airway and parenchymal tissue at 21 d after IAV infection (2 pfu) with green colorization indicating computer-assigned PAS+ areas. Bar = 200 μm. (C) Quantitation of PAS+ area based on computer-assignment and quantification for conditions in (B). (D) Lung levels of Muc5ac and Il13 mRNA at 21 d after infection with IAV (0–5 pfu). (E) Time course for lung levels of Muc5ac and Il13 mRNA after infection with IAV (2 pfu). (F) Levels of IL-13 protein in lung tissue at 21 d after infection with IAV or IAV-UV. Dashed lines indicate the lower limit of detection for the assay. For (A)-(F), values are representative of 3 separate experiments (n≥8 mice per condition in each experiment). * indicates p<0.05 versus no infection or IAV-UV control (no difference was found for no infection versus IAV-UV).
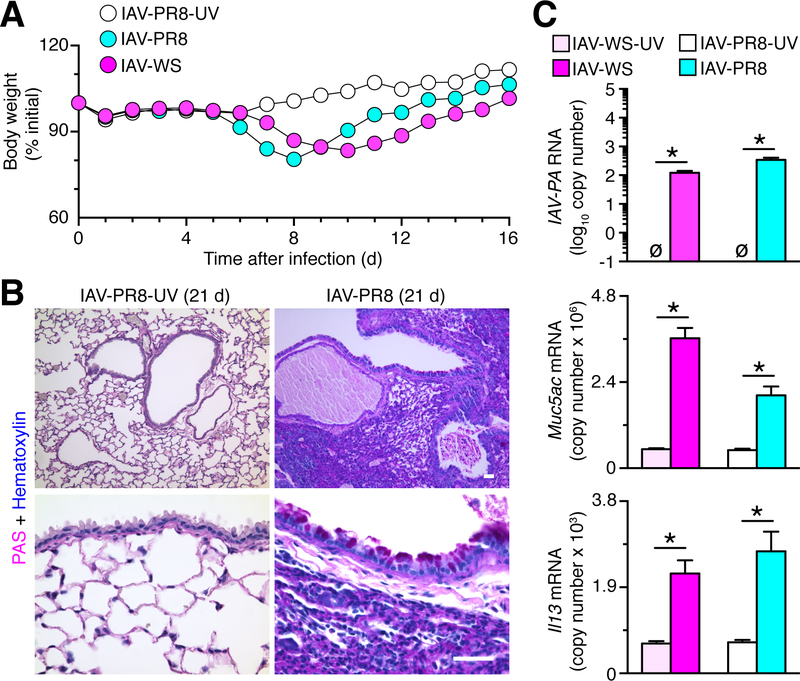
Meanwhile, however, a second IAV strain (A/PR/8/34) was dosed to produce a similar degree of weight loss as IAV-WS/33 (Fig. 3A) and was also found to produce similar lung disease (Fig. 3B) along with persistence of viral RNA and induction of Muc5ac and Il13 mRNA expression (Fig. 3C) at 21 d after infection, indicating that these post-viral effects were not specific to the WS/33 strain. Together, the results suggested a link between the severity of initial IAV infection and the subsequent development of the inflammatory mucus formation that is characteristic of chronic lung diseases.
FIGURE 3.
Severe acute illness leads to chronic lung disease after IAV-PR8 infection. (A) Time course of body weight after infection with IAV (PR8 strain, 10 pfu or WS/33 strain, 2 pfu) or corresponding UV-inactivated IAV-PR8 in wild-type C56BL/6J mice. (B) PAS and-hematoxylin staining of lung sections at 21 d after infection with IAV-PR8 (10 pfu) or IAV-PR8-UV. Bar=400 μm. (C) Corresponding lung levels of IAV-PA RNA and Muc5ac and Il13 mRNA for conditions in (B). For (A,C), values are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (C), * indicates p<0.05.
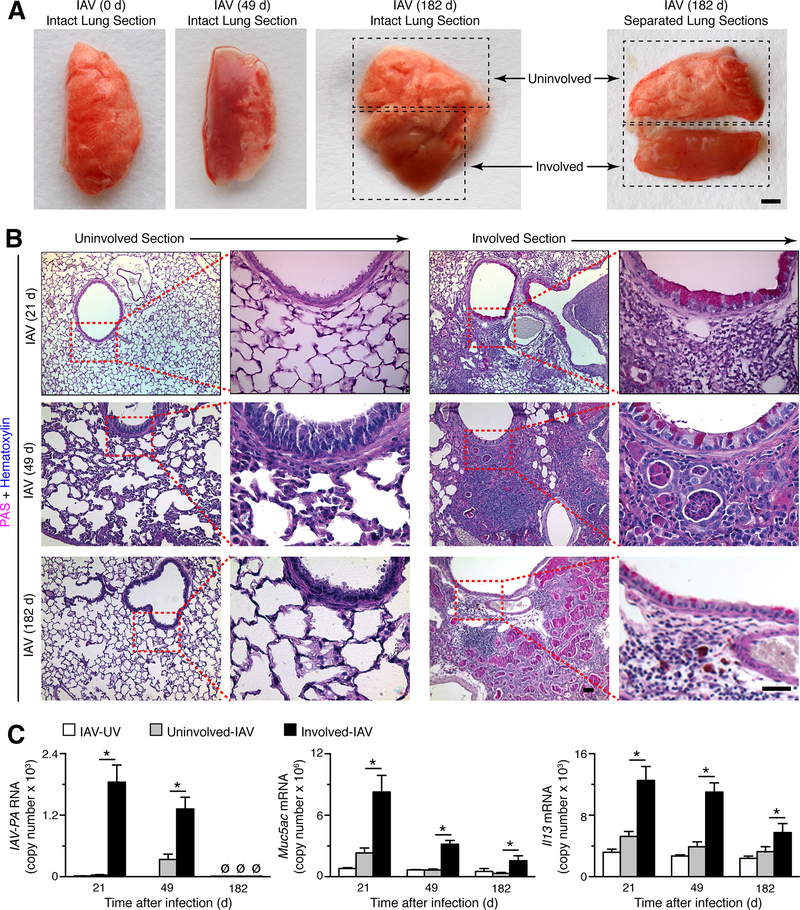
Detection of disease foci containing viral remnants and mucous cells
We recognized that chronic lung disease in humans is characterized by considerable heterogeneity across the lung tissue, so that some parts of the lung are markedly abnormal whereas other parts show little pathology even at advanced stages of disease (11, 50–52). We observed similar heterogeneity for post-viral lung disease in whole lung specimens of mice that were infected with IAV (Fig. 4A). We took advantage of this finding by dissecting involved from uninvolved sections of lung to assess levels of viral-RNA remnants and mucus production on a regional instead of whole lung level. PAS staining confirmed the gross pathology and showed evidence of mucous cell formation in the involved versus uninvolved sections at 21, 49, and 182 days after IAV infection (Figure 4B). In concert with the abnormalities in histopathology, we found corresponding increases in IAV-PA RNA in involved relative to uninvolved sections of lung at each of the same time points after IAV infection (Fig. 4C), although (as noted in Fig. 1F and Fig. 1K), IAV RNA was no longer detectable by 182 days after infection. We also found increased levels of Muc5ac and Il13 mRNA in involved versus uninvolved sections, with maximal values at 21 days declining to lower values at 182 days after IAV infection (Fig. 4C). The findings imply that the areas of the lung that were initially infected with virus and then leave viral-RNA remnants are most likely to manifest disease, but the continued presence of these remnants is not required for persistence of disease.
FIGURE 4.
Identification of focal areas of chronic lung disease after IAV infection. (A) Representative photographs of lung samples with grossly uninvolved or involved sections at 49 and 182 d (without and with separation by dissection) after infection with IAV (WS33 strain, 2 pfu). Bar=1 mm. (B) Representative PAS-hematoxylin staining of uninvolved or involved lung sections at 21, 49, and 182 d after infection with IAV (2 pfu). Bars=400 μm. (C) Levels of IAV-PA RNA and Muc5ac and Il13 mRNA in lung samples for conditions in (B) and control IAV-UV. For (A)-(C), values are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (C), * indicates p<0.05 versus uninvolved section.
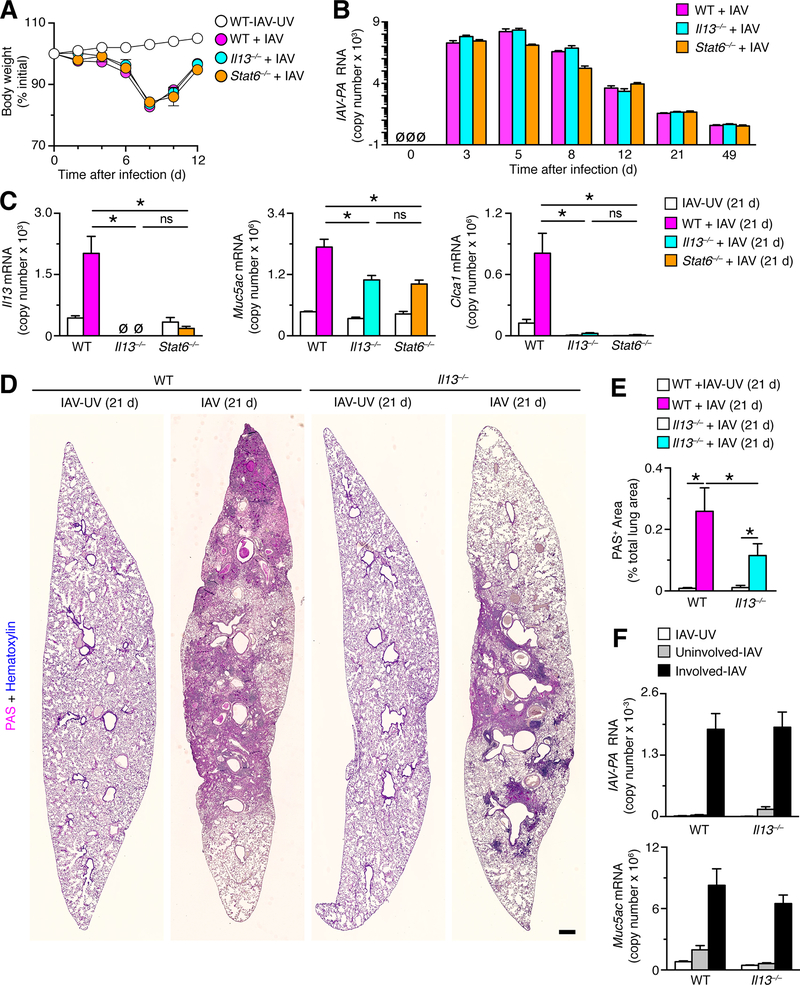
Post-IAV disease is linked in part to IL-13 induction
Levels of IL-13 production and signaling via the IL-13-receptor–STAT6 complex is a major determinant of airway mucus production after infection with SeV in mice (10, 11, 53). Accordingly, we next studied post-IAV disease in Il13–/– and Stat6–/– mice that exhibit body weight loss and viral levels in the lung that were no different than wild-type mice (Fig. 5A,B). In contrast to the similarities for acute illness among these mouse strains, the levels of Muc5ac mRNA induction were significantly attenuated in Il13–/– and Stat6–/– mice compared to wild-type control mice at 21 days after IAV infection (Fig. 5C). In addition, the usual induction of Clca1 mRNA was completely blocked in Il13–/– and Stat6–/– mice compared to wild-type control mice at 21 days after IAV infection (Fig. 5C). Expression of Clca1 mRNA is highly sensitive to IL-13 stimulation (14, 54), indicating that the IL-13-sensitive component of mucus production is effectively blocked in these mice and that there is an additional IL-13-independent component of inflammatory disease and/or mucus production after IAV infection. To address this issue, the level of PAS-hematoxylin staining (as an index of mucus-containing disease foci) was assessed using whole-lung scans of sections from Il13–/– and wild-type control mice at 21 d after infection with IAV or IAV-UV (Fig. 5D). With this approach, we found significant attenuation of PAS+-staining area in Il13–/– mice compared to wild-type mice (Fig. 5E). We also found that the persistent levels of IAV RNA and induction of Muc5ac mRNA in involved versus uninvolved lung sections from microdissection were no different in Il13–/– versus wild-type mice at 21 days after infection (Figure 5F). Together, these findings indicate that IAV drives focal lung disease with bronchiolization and mucus production that is at least partially but not completely dependent on IL-13 production and signaling. In addition, the results indicated that the levels but not the viral-RNA and mucus characteristics of disease foci were sensitive to IL-13 deficiency.
FIGURE 5.
IL-13 induction and STAT6 activation are linked to chronic lung disease after IAV infection. (A) Time course of body weight change in wild-type (WT), Il13–/–, and Stat6–/– mice on the indicated days after infection with IAV (2 pfu) or IAV-UV. (B) Time course for lung levels of IAV-PA RNA in the same mouse strains after infection with IAV (2 pfu). (C) Lung levels of Il13, Muc5ac, and Clca1 mRNA in the same mouse strains at 21 d after infection with IAV (2 pfu) or IA-UV. (D) PAS-hematoxylin staining of lung sections from WT and Il13–/–mice at 21 d after infection with IAV (2 pfu) or IAV-UV. Bar=1 mm. (E) Quantitation of PAS-positive areas in images in (D). (F) Quantitation of PAS+ area for conditions in (D). (F) Levels of IAV-PA RNA and Muc5ac mRNA in uninvolved or involved sections of lung from WT and Il13–/– mice at 21 d after infection with IAV or IAV-UV. For (A-C) and (E-F), values are representative of 3 separate experiments (n≥8 mice per condition in each experiment). * indicates p<0.05; ns=nonsignificant.
Post-IAV disease is not linked to reciprocal IL-17 production
Other investigators have reported that IL-17 might emerge as a candidate for driving airway inflammation and mucus production, particularly in the setting of IL-13 blockade that appears to enhance IL-17a expression level (55–57). In fact, we also observed an increase in Il17a mRNA in Il13–/– mice but not in wild-type mice at 21 d after IAV infection (Fig. 6A). We found no significant change in Il17f mRNA in Il13–/– or combined Il13–/––Il17a–/– mice. Accordingly, we next studied post-IAV lung disease Il17a–/– and combined Il13–/––Il17a–/– mice. Initial experiments showed that body weight loss and viral levels in the lung that were no different in these strains compared to Il13–/– or wild-type mice (Fig. 6B,6C). In addition, the levels of Muc5ac or Clca1 RNA induction were not significantly different in Il17a–/– mice compared to wild-type mice or in combined Il13–/––Il17a–/– mice compared to Il13–/–mice at 21 days after IAV infection (Fig. 6D), ruling against any effect of either Il17a or Il17f on Muc5ac expression. We also observed selective increases in neutrophil (but not eosinophil, macrophage, or lymphocyte) levels in BAL fluid in Il13–/– mice compared to wild-type mice, and this increase was blocked in both Il17a–/– and combined Il13–/––Il17a–/– mice (Fig. 6E), ruling against any effect of IL-17-dependent neutrophil influx on mucus production.
FIGURE 6.
Reciprocal IL-17a induction after IL-13 blockade is not linked to chronic lung disease after IAV infection. (A) Lung levels of Il17a and Il17f mRNA in WT, Il13–/–, Il17a–/–, and Il13–/––Il17a–/– mice at 21 d after infection with IAV (2 pfu) or IA-UV. (B) Time course of body weight change in the same mouse strains as (A) after infection with IAV (2 pfu) or IAV-UV. (C) Time course for lung levels of IAV-PA RNA for conditions in (B). (D) Lung levels of Il13, Muc5ac, and Clca1 mRNA in same mouse strains as (A) at 21 d after infection with IAV (2 pfu) or IAV-UV. (E) Levels of indicated immune cells in BAL fluid for conditions in (D). (F) Levels of airway reactivity using response of respiratory system resistance (RRS) to inhaled methacholine (MCh) and for baseline RRS for conditions in (D). For (A-F), values are representative of 3 separate experiments (n=5–8 mice per condition in each experiment). For (A-E), * indicates p<0.05; for (F), * indicates p<0.05 versus IAV-UV and ** indicates p<0.05 versus corresponding IAV-UV and IAV conditions.
In addition to excess mucus production, we also found significant increases in airway reactivity to inhaled methacholine in wild-type mice at 21 d after infection with IAV compared to IAV-UV (Fig. 6F). Similar to the pattern observed for regulation of Muc5ac expression, the development of airway hyper-reactivity was attenuated in Il13–/– mice compared to wild-type mice but were not significantly different in Il17a–/– mice compared to wild-type mice or in combined Il13–/––Il17a–/– mice compared to Il13–/–mice at 21 days after IAV infection (Fig. 6F). Comparable to our previous studies of SeV infection, we detected no significant increase in baseline respiratory system resistance (RRS) at 21 d after viral infection in wild-type or gene-knockout mice (Fig. 6F). Comparisons to pulmonary function testing in asthma and COPD are possible, and perhaps the combination of normal baseline but increased reactivity is more typical of asthma. However, it is prudent to recognize that the measurements in mice are made under general anesthesia that likely attenuates any increase in baseline or post-challenge respiratory resistance (58). Thus, IL-17a-dependent increases in neutrophil influx did not significantly influence key features of Muc5ac induction or airway hyper-reactivity that develop during chronic lung disease due to IAV infection.
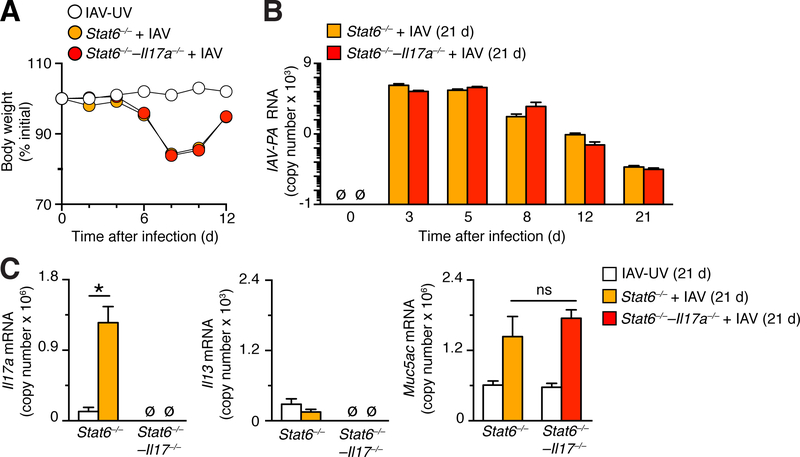
Based on the reported sensitivity of Stat6–/– mice to IL-17a stimulation of mucus production (56), we also studied combined Stat6–/––/––Il17a–/– mice to check for any effect of IL-17a on chronic lung disease after IAV infection. Initial experiments again demonstrated that body weight loss and viral levels in the lung that were no different in combined Stat6–/––/––Il17a–/– mice compared to Stat6–/–mice (Fig. 7A,7B). In addition, we found induction of Il17a mRNA and attenuated induction of Il13 mRNA in Stat6–/– mice that were similar to Il13–/– mice (Fig. 7C). Moreover, the levels of Muc5ac RNA induction were not significantly different in combined Stat6–/––Il17a–/– mice compared to Stat6–/–mice at 21 days after IAV infection (Fig. 7C). Thus, effects of IL-13 and IL-13R-signaling deficiencies were quite similar in the setting of IAV-induced lung disease.
FIGURE 7.
Reciprocal IL-17a induction due to STAT6-deficiency is not linked to chronic lung disease after IAV infection. (A) Time course of body weight change on the indicated days in Stat6–/– and Stat6–/––Il17a–/– mice after infection with IAV (2 pfu) or IAV-UV. (B) Time course for lung levels of IAV-PA RNA in same mouse strains as (A) after infection with IAV (2 pfu). (C) Lung levels of Il17a, Il13, and Muc5ac mRNA in same mouse strains as (A) at 21 d after infection with IAV (2 pfu) or IA-UV. For (A), (B), (C), values are representative of 3 separate experiments (n≥5 mice per condition in each experiment). * indicates p<0.05.
Stable characteristics of lung disease at later times after IAV infection
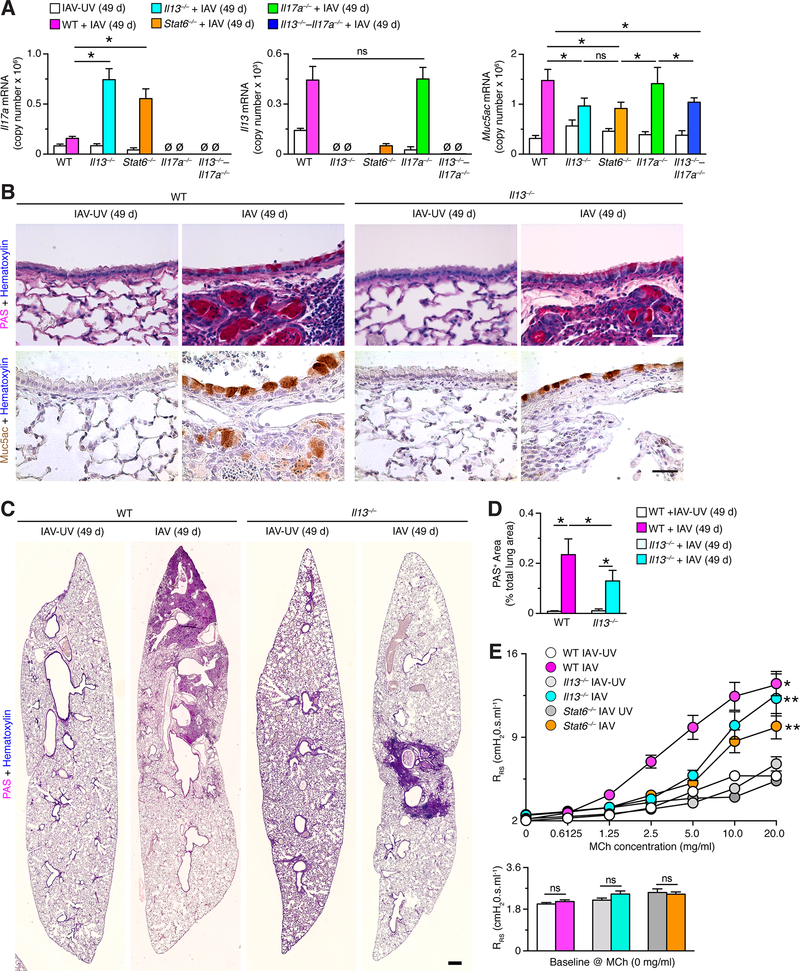
To determine whether longer-term post-viral disease was still due to a persistent type 2 immune mechanism, we also characterized chronic lung disease in mice at 49 days after IAV infection. We found that the pattern of Il17a, Il13, and Muc5ac mRNA expression in Il13–/–, Stat6–/–, Il17a–/–, and combined Il13–/––Il17a–/– mice were remarkably similar at 49 days compared to 21 days after IAV infection (Fig. 8A). Thus, Il13 and Muc5ac RNA induction were similarly attenuated in Il13–/–, Stat6–/– and combined Il13–/––Il17a–/– mice compared to wild-type mice at 49 days after IAV infection (Fig. 8A). In addition, PAS-hematoxylin and MUC5AC immunostaining of lung sections showed attenuation of mucus production in Il13–/– mice compared to wild-type mice at 49 days after IAV infection (Fig. 8B). Furthermore, PAS+-staining area was significantly attenuated in Il13–/– mice compared to wild-type mice at 49 days after IAV infection (Fig. 8C,D). The development of airway hyper-reactivity at 49 d was also similar to 21 d after IAV infection, exhibiting partial dependence on Il13 and Stat6 gene function (Fig. 8E). Together, these findings indicate that IAV drives chronic lung disease with excess mucus production that depends at least in part on a persistent IL-13-signaling mechanism that is similar at 21 and 49 d after infection.
FIGURE 8.
Persistence of IL-13–STAT6-linked chronic lung disease after IAV infection. (A) Lung levels of Il17a, Il13, and Muc5ac mRNA in WT, Il13–/–, Stat6–/–, Il17a–/–, and Il13–/––Il17a–/– mice at 49 d after infection with IAV (2 pfu) or IA-UV. (B) Representative PAS-hematoxylin staining and Muc5ac-hematoxylin immunostaining of lung sections from WT and Il13–/–mice at 49 d after infection with IAV (2 pfu) or IAV-UV. Bars=400 μm. (C) PAS-hematoxylin staining of lung sections from WT and Il13–/–mice at 21 d after infection with IAV (2 pfu) or IAV-UV. Bar=1 mm. (D) Quantitation of PAS-positive areas in images in (D). (E) Levels of airway reactivity using response RRS to inhaled MCh and for baseline RRS for conditions in (A). For (A)-(E), values are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (A), (C), and (D), * indicates p<0.05.
To further address the comparison between the post-IAV mouse model with chronic lung disease in humans, we also assessed the development of pulmonary fibrosis and emphysema during post-viral disease. We detected peribronchiolar and parenchymal fibrosis at 21 d after IAV infection based on Gomori trichrome staining for the blue-tone of collagen expression (Fig. 9A). The level of collagen staining was significantly increased in wild-type mice at 21 and 49 d after IAV infection, however, the same increase was also observed in Il13–/–, Il17a–/–, Il13–/––Il17a–/–, and Stat6–/– mice based on precision image-analysis of the blue-collagen signal (Fig. 9B-D). Significant increases in the same fibrotic signal were also detected at 21 d after IAV infection in wild-type and Il13–/– mice using the additional strain of IAV (A/PR/8/34) (Fig. 9E). In addition, we detected pulmonary emphysema in wild-type mice at 21 and 49 d after IAV infection based on mean linear intercept values for alveolar size (Fig. 9F,G). Similar to the data for fibrosis, we observed no difference in the degree of emphysema between wild-type and the four gene-knockout strains after IAV infection (Fig. 9F,G), and we confirmed similarly significant increases in alveolar size after IAV infection with a second strain of IAV (Fig. 9H). The values for alveolar enlargement were similar to those determined by hyperpolarized 3He MRI-based imaging in the post-SeV model (59). Together, the post-IAV mouse model exhibits key features of chronic lung disease (airway inflammation, mucus production, hyper-reactivity, fibrosis, and emphysema) as found in humans with asthma and COPD, but also provides additional insight into the variable dependence of these disease endpoints on IL-13-versus non-IL-13/IL-17-dependent signals.
FIGURE 9.
Persistent peribronchial and parenchymal fibrosis and pulmonary emphysema after IAV infection. (A) Gomori trichrome staining of lung sections from WT mice at 21 d after infection with IAV (2 pfu) or IAV-UV (WS/33 strain). Bar=1 mm. (B) Quantitation of trichrome-blue-positive areas for lung sections from the indicated mouse strains after infection with IAV or IAV-UV as described in (A). (C) Quantitation of trichrome-blue-positive areas for lung sections from the indicated mouse strains at 21 d after infection with IAV (10 pfu) or IAV-UV (PR8 strain). (D) Mean linear intercept values for indicated mouse strains after infection with IAV or IAV-UV (WS/33 strain) as described in (A). (E) Mean linear intercept values for indicated mouse strains after infection with IAV or IAV-UV (PR8 strain) as described in (C). For (B)-(E), values are representative of 3 separate experiments (n≥8 mice per condition in each experiment), and * indicates p<0.05.
Discussion
This study demonstrates previously unreported capacities of IAV to persist as active viral-RNA remnants in the lung and to cause a switch to chronic lung disease. The results provide for new host-pathogen principles as well as implications for the development of chronic inflammatory disease based on the following key findings: First, we show that IAV persists in a transcriptionally active form that is incapable of replication or generation of infectious virus but survives in the lung cell compartment for up to 3–6 months in wild-type mice. Second, in this mouse model, we demonstrate that IAV infection triggers long-term lung disease that continues after IAV-viral remnants are no longer detectable even in foci of inflammatory disease that are enriched for viral RNA, mucus production, and IL-13 expression. We further show that these disease foci of immune cell accumulation and mucus production are linked to induction of IL-13 expression and STAT6 activation but not reciprocal induction of IL-17a expression. Here we discuss the novelty and significance of these findings for the virus and the host.
In regards to the first issue related to IAV persistence, a combination of previous studies showed that viral antigen could be detected for prolonged periods. For example, viral antigen can be found for at least 70 days in draining lymph node based on activation of virus-specific CD4+ and CD8+ T cells (46–49, 60). These observations indicated the presence of a persistent depot of viral antigen long after clearance of infectious virus but left open the mechanism for the source of this antigen. Viral RNA was detected in the lymph node for up to 10 days and in the lung for up to 30 days after IAV infection in mice, leading to a proposal that respiratory dendritic cells serve to transport viral antigen from the lung to the draining lymph nodes as an explanation for long lasting stimulation of T cells at that site (49). However, the nature of viral RNA production remained uncertain. Here we show that viral RNA can be detected for at least 77 days in lung lymph nodes, suggesting this site as a possible source of viral antigen for the reported T cell stimulation. In support of this possibility, we also demonstrate that IAV remains transcriptionally active in this setting. We also find that IAV RNA can be present in the lung for at least 105 days after infection., and we localize the viral RNA to foci of disease. These findings provide a basis for even longer-term stimulation of the immune system that may have escaped detection in previous studies. The results support the possibility that the host may retain virus to keep the system armed for the next infection or that the virus may use the protected site in the host as an opportunity to improve infectious capability. For both strategies, it appears that virus and lung cells are able to co-exist without immune-mediated destruction for prolonged periods, in contrast to the cytopathic process found during acute infection. Further studies must determine the host cell for viral RNA remnants and the possible function of these cellular and molecular components in causing chronic inflammatory disease.
Co-persistence of viral RNA and post-viral lung disease is not specific to IAV infection. In particular, there is also persistent viral RNA in the lung for at least 49 d after infection after SeV infection in mice (10). In this case, we did not yet establish whether viral-RNA remnants are transcriptionally active or whether they become undetectable despite persistent lung disease. Similarly, we do not yet know whether SeV-RNA remnants are localized to a specific host tissue compartment or cell type that might be associated with lung disease in this model. This determination should also prove quite useful given the extensive definition for the immune mechanism of chronic lung disease after SeV infection (10–12, 53) and the possible overlap with at least the type 2 immune process after IAV infection that was found in the present study.
In regards to the second issue concerning post-viral lung disease, to our knowledge, our results are the first to fully model this type of disease for a potent human respiratory viral pathogen with highly efficient replication in a mouse model. In particular, IAV achieves peak viral titers that are increased 1 × 106-fold from the initial inoculum and maintains persistence of infectious virus for 10 days after inoculation. In contrast, RSV peak titer barely reaches the level of the initial inoculum, and virus is fully cleared by 8 days after infection (17–21). Even less impressive, HRV peak titer is generally 1×103-fold lower than initial inoculum and virus is cleared by 4 days after infection (22–25), thereby often requiring high-viral dosing or genetically-modified mice that still show relatively little viral replication (22, 24, 25). Moreover, the previous studies of IAV infection in mice suggest a degree of mucous cell metaplasia at 3 weeks after infection but did not study long-term outcome or viral persistence (35). Similarly, other studies suggest that IAV infection in mice might enhance or inhibit allergen-induced lung disease but do not assess the direct effects of viral infection on long-term lung disease (36, 61–63). None of these previous studies analyzed the critical effect of viral inoculum size and consequent viral titer and severity of acute illness on the subsequent development of chronic inflammatory disease as we show in the present study. This finding is consistent with the epidemiology of RSV-induced asthma, where children with the most severe cases of acute bronchiolitis are the most likely to develop chronic wheezing illnesses (2, 64). We would further expect that individuals with the most severe forms of acute influenza illness will also be at greatest risk to develop chronic lung disease, perhaps with the same chronic inflammatory foci as we observed in the mouse model of this process. The recent outbreak of H1N1 swine-origin influenza virus further suggests that the development of post-viral asthma could be compounded by an increased susceptibility to severe influenza in the asthmatic or asthma-prone population (65).
Given the possible relevance of our results to human disease, we also defined the immune basis for chronic lung disease after IAV infection. We established that the development of post-IAV disease, characterized by immune cell accumulation, bronchiolization, and excess mucus production depends at least in part on IL-13 expression and signaling via IL-13-receptor-associated STAT6 that is characteristic of the type 2 immune response. These findings are consistent with long-term IL-13-dependent disease after SeV infection in mice (10–12, 53). The combined results also define auto-amplification of IL-13 induction, given the decrease in IL-13 levels in Stat6–/– mice, consistent with IL-13 feed-forward and IL-13R induction in post-SeV disease in mice and COPD in humans (10–12). The present analysis also shows that the lung disease develops and persists as discrete foci within the lung at sites of active viral-RNA remnants that are likely also the locations of initial viral replication during acute infectious illness. In addition, we find that IL-13 drives an increase in foci of disease with no significant effect on the level of viral-RNA remnants or mucus production within these foci. The full significance of this finding still needs to be determined, but it already seems likely to be linked to the initial severity of infection and level of immune response, similar to the connection to viral dosing. In any case, these observations appear consistent with the localized abnormalities found with lung histology and imaging in humans with chronic lung disease due to asthma and COPD (66–68).
Our results also provide new insight into the relationship between the type 2 immune response and the possible role of IL-17 in the development of chronic lung disease. In that regard, previous work implicated increased IL-17A levels in the pathogenesis of this type of disease in humans and mouse models (69–75) under a mechanism that might be linked to neutrophil function (76–78) and might be increased with IL-13 or IL-13-signal blockade (55–57). However, we find that IL-17 does not contribute to foci of disease or excess mucus production after IAV infection, despite increases in IL-17- and IL-17A-dependent increases in neutrophils. Similarly, we found no effect of IL17a blockade on IL-13 expression, in contrast to other experimental models (57, 79) and no synergy for IL-17 and IL-13 despite reports of this effect in airway inflammatory disease (74). We also found no induction of IL-17F expression that might explain an independent effect on IL-17RA signaling despite reports of this mechanism in other models (80). Our findings are consistent with the lack of therapeutic effect in initial clinical trials of anti-IL-17RA mAb in asthma (81), although analysis of patient subsets and clinical trials with anti-IL-17A mAb (ClinicalTrials.gov NCT03299686) remain ongoing.
A key implication of our study is the extent of extrapolation from the post-viral mouse model to chronic lung disease in humans. In that regard, it is critical to recognize that respiratory viral infection and post-viral disease often involves both airway and alveolar sites. Indeed, the post-IAV mouse model shows elements of airway disease (i.e., airway inflammation, excess airway mucus production, airway hyper-reactivity, and peribronchiolar fibrosis) and alveolar/parenchymal disease (i.e., bronchiolization, emphysema, and fibrosis). Therefore, the model has been proposed to represent experimental counterparts of chronic airway disease (as found in chronic bronchitis/COPD and asthma) as well as pulmonary fibrosis (as found in interstitial lung diseases) and acute lung injury (as found in ARDS) (33, 82, 83). Each of these proposals offers support for corresponding mouse and human cell and molecular candidates for pathogenesis. The model will provide utility to the extent that these candidates prove useful as biomarkers and/or targets for therapeutic interventions in human disease. In the case of airway disease, the post-viral mouse models for IAV and SeV already provide a useful substrate to discover new small-molecule drugs for attenuating mucus production (14). In addition, the data from the post-viral mouse model bolsters ongoing development of biologics directed against IL-13. In that regard, a blocking mAb directed against the IL4Ralpha chain that is common to IL-13 and IL-4 receptors (84–86) but not against IL-13 alone (87–89) might be more effective in decreasing exacerbations of eosinophilic asthma. This difference might be due to the need to interrupt both IL-13- and IL-4-triggered signals in allergic asthma, but this possibility still needs to be tested directly, e.g., by direct comparison of mAb against IL-4/IL-13-receptor versus IL-13-receptor alone. This issue and the related issue of patient stratification will be equally challenging in COPD, where there also appears to be a type 2 immune response as reported by our group (10, 11, 13–15) and by others (90–93).
The present study establishes a new experimental model for developing precise therapeutic strategies for chronic lung disease based on viral-remnant RNA, IL-13-dependent, and non-IL-13–IL-17-dependent mechanisms for chronic lung disease in humans as well as insights into the upstream driver for IL-13 production. This will require definition of expression and function of epithelial cell-derived cytokines such as IL-33, IL-25, and TSLP in the post-IAV model as was done for SeV infection and was translated to humans as was done for COPD (11). This information will also serve to guide therapeutic strategies with mAbs against IL-33 and TSLP that are also being developed for use in humans with chronic lung disease due to asthma and COPD (94, 95). In fact, comparison of mechanism for post-viral lung disease after IAV versus SeV will likely prove useful for stratification of patients with viral exacerbations of chronic lung disease. In particular, the present findings expose disease endpoints that are independent of IL-13 and IL-17 expression and must therefore be controlled through new mechanisms that are not typically considered as a consequence of viral infection.
Supplementary Material
Acknowledgments
We thank Anand Patel, Suzanne Swanson, and Rose Tidwell for expert advice and assistance.
This work was supported by grants from the National Institutes of Health (NIAID, R01-AI111605 and R01-AI130591 and NHLBI R01-HL121791 and R01-HL120153).
Abbreviations used in this article:
- BAL
bronchoalveolar lavage
- Clca1
chloride channel accessory 1
- COPD
chronic obstructive pulmonary disease
- HRV
human rhinovirus
- IAV
influenza A virus
- MUC5AC
mucin 5AC
- NP
nucleoprotein
- pfu
plaque-forming unit
- RRS
respiratory system resistance
- RSV
respiratory syncytial virus
- SeV
Sendai virus
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Holtzman MJ 2012. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J. Clin. Invest 122: 2741–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigurs N, Gustafsson PM, Bjarnason R, Lundbeg F, Schmidt S, Sigurbergsson F, and Kjellman B. 2005. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med 171: 137–141. [DOI] [PubMed] [Google Scholar]
- 3.Castro M, Schweiger T, Yin-Declue H, Ramkumar T, Christie C, Zheng J, Cohen R, Schechtman K, Strunk R, and Bacharier L. 2008. Cytokine response after severe respiratory syncytial virus bronchiolitis in early life. J. Allergy Clin. Immunol 122: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R, Roberg KA, Anderson EL, Pappas TE, Gangnon R, Gern JE, and Lemanske RF Jr. 2011. Decreased lung function after preschool wheezing rhinovirus illness in children at risk to develop asthma. J. Allergy Clin. Immunol 128: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kan-o K, Ramirez R, MacDonald MI, Rolph M, Rudd PA, Spann KM, Mahalingam S, Bardin PG, and Thomas BJ. 2017. Human metapneumovirus infection in chronic obstructive pulmonary disease: impact of glucocorticosteroids and inteferon. J. Inf. Dis 215: 1536–1545. [DOI] [PubMed] [Google Scholar]
- 6.Footitt J, Malia P, Durham AL, Ho WE, Turjillo-Torralbo M-B, Telcian AG, Del Rosario A, Chang C, Peh H-Y, Kebadze T, Aniscenko J, Stanciu L, Essilfie-Quaye S, Ito K, Barnes PJ, Elkin SL, Kon OM, Wong F, Adcock IM, and Johnston SL. 2016. Oxidative and nitrosative stress and histone deacetylase-2 activity in exacerbations of COPD. Chest 149: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnelykke K, Coleman AT, Evans MD, Thorsen J, Waage J, Vissing HH, Carlsson CJ, Stokholm J, Chawes BL, Jessen LE, Fischer TK, Bochkov YA, Ober C, Lemanske RF Jr., Jackson DJ, Gern JE, and Bisgaard H. 2018. Cadherin-related family member 3 genetics and rhinovirus C respiratory illnesses. Am. J. Respir. Crit. Care Med 197: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnelykke K, Vissing NH, Sevelsted A, Johnston SL, and Bisgaard H. 2015. Association between respiratory infections in early life and later asthma is independent of virus type. J. Allergy Clin. Immunol 136: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter MJ, Morton JD, Kajiwara N, Agapov E, and Holtzman MJ. 2002. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J. Clin. Invest 110: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, Swanson S, Tidwell R, Tyner JW, Morton JD, Castro M, Polineni D, Patterson GA, Schwendener RA, Allard JD, Peltz G, and Holtzman MJ. 2008. Persistent activation of an innate immune response translates respiratory viral infection into chronic inflammatory lung disease. Nat. Med 14: 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy YG, Girard J-P, Stappenbeck TS, Patterson GA, Pierce RA, Brody SL, and Holtzman MJ. 2013. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Invest 123: 3967–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu K, Byers DE, Jin X, Agapov E, Alexander-Brett J, Patel AC, Cella M, Gilfilan S, Colonna M, Kober DL, Brett TJ, and Holtzman MJ. 2015. TREM-2 promotes macrophage survival and lung disease after respiratory viral infection. J. Exp. Med 212: 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agapov E, Battaile JT, Tidwell R, Hachem R, Patterson GA, Pierce RA, Atkinson JJ, and Holtzman MJ. 2009. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am. J. Respir. Cell Mol. Biol 41: 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alevy Y, Patel AC, Romero AG, Patel DA, Tucker J, Roswit WT, Miller CA, Heier RF, Byers DE, Brett TJ, and Holtzman MJ. 2012. IL-13–induced airway mucus production is attenuated by MAPK13 inhibition. J. Clin. Invest 122: 4555–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byers DE, Wu K, Dang-Vu G, Jin X, Agapov E, Zhang X, Battaile JT, Schechtman KB, Yusen R, Pierce RA, and Holtzman MJ. 2018. Triggering receptor expressed on myeloid cells-2 (TREM-2) expression tracks with M2-like macrophage activity and disease severity in COPD. Chest 153: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida N, and Homma M. 1978. Sendai virus. Adv. Virus Res 23: 349–381. [DOI] [PubMed] [Google Scholar]
- 17.Graham BS, Perkins MD, Wright PF, and Karzon DT. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26: 153–162. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto K, Durbin JE, Zhou W, Collins RD, Ho SB, Kolls JK, Dubin PJ, Sheller JR, Goleniewska K, O’Neal JF, Olson SJ, Mitchell D, Graham BS, and Peebles RSJ. 2005. Respiratory syncytial virus infection in the absence of STAT1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J. Allergy Clin. Immunol. 116: 550–557. [DOI] [PubMed] [Google Scholar]
- 19.Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS Jr., and Moore ML. 2011. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol 85: 5782–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Empey KM, Orend JG, Peebles RS, Egana L, Norris KA, Oury TD, and Kolls JK. 2012. Stimulation of immature lung macrophages with intranasal interferon gamma in a novel neonatal mouse model of resiratory syncytial virus infection. PloS ONE 7: e40499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, Quitalig M, Hotard AL, Moore ML, Hartert TV, Zhou B, McKenzie AN, and Peebles RSJ. 2016. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoic cells thorugh thymic stromal lymphopoietin. J. Allergy Clin. Immunol 138: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, Glanville N, Choy KJ, Jourdan P, Burnet J, Tuthill TJ, Pedrick MS, Hurle MJ, Plumpton C, Sharp NA, Bussell JN, Swallow DM, Schwarze J, Guy B, Almond JW, Jeffrey PK, Lloyd CM, Papi A, Killington RA, Rowlands DJ, Blain ED, Clarke NJ, and Johnston SL. 2008. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat. Med 14: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, Lukacs NW, Johnston SL, and Hershenson MB. 2008. Human rhinovirus 1B exposure induces phophatidylinositol 3-kinase-dependent airway inflammation in mice. Am. J. Respir. Crit. Care Med 177: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Miller DJ, Bowman ER, Nagarkar DR, Schneider D, Zhao Y, Linn MJ, Goldsmith AM, Bentley JK, Sajjan US, and Hershenson MB. 2011. MDA5 and TLR3 initiate pro-inflammatory signaling pathways leading to rhinovirus-induced airways inflammation and hyperresponsiveness. PLoS Pathog. 7: e1002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han M, Hong JY, Jaipalli S, Rajput C, Lei J, Hinde JL, Chen Q, Bentley JK, and Hershenson MB. 2016. IFN-g blocks development of an asthma phenotype in rhinovirus-infected baby mice by inhibiting type 2 innate lymphoid cells. Am. J. Respir. Cell Mol. Biol 56: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Mao D, Roswit WT, Jin X, Patel AC, Patel DA, Agapov E, Wang Z, Tidwell RM, Atkinson JJ, Huang G, McCarthy R, Yu J, Yun NE, Paessler SL, Lawson TG, Omattage NS, Brett TJ, and Holtzman MJ. 2015. PARP9-DTX3L ubiquitin ligase targets host histone H2BJ and viral 3C protease to enhance interferon signaling and control viral infection. Nat. Immunol 16: 1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber JP, Gonzales-van Horn SR, Roybal KT, Gill MA, and Farrar JD. 2014. IFN-a suppresses GATA3 transcription from a distal exon and promotes H3K27 trimethylaton of the CNS-1 enhancer in human Th2 cells. J. Immunol 192: 5687–5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksson M, Bennet R, and Nilsson A. 2002. Wheezing following lower respiratory tract infections with respiratory syncytial virus and influenza A in infancy. Ped. Allergy Immunol 11: 193–197. [DOI] [PubMed] [Google Scholar]
- 29.Walsh EE, Falsey A, R., and Hennessey PA. 1999. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am. J. Respir. Crit. Care Med 160: 791–795. [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, Bonini S, Bont L, Bossios A, Bousquet J, Braido F, Brusselle G, Canonica GW, Carlsen KH, Chanez P, Fokkens WJ, Garcia-Garcia M, Gjomarkaj M, Haahtela T, Holgate ST, Johnston SL, Konstantinou G, Kowalski M, Lewandowska-Polak A, and Lodrup-Carlsen K. 2011. Viruses and bacteria in acute asthma exacerbations–a GA2LEN-DARE systematic review. Allergy 66: 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandelswajg A, Moulin F, Menager C, Rozenberg F, Lebon P, and Gendrel D. 2010. Underestimation of influenza viral infection in childhood asthma exacerbations. J. Pediatr 157: 505–506. [DOI] [PubMed] [Google Scholar]
- 32.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang DY, Lim B, Chow VT, Crum CP, Xian W, and McKeon F. 2011. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147: 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo W, Zhang T, Zheng D, Wu A, Guan SP, Liew A-A, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, C. P. C, Xian W, and McKeon F. 2015. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature 517: 616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaughn AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, and Chapman HA. 2015. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517: 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchweitz JP, Harkema JR, and Kaminiski NE. 2007. Time-dependent airway epithelial and inflammatory cell responses induced by influenza virus A/PR/8/34 in C57BL/6 mice. Toxicol. Pathol 35: 424–435. [DOI] [PubMed] [Google Scholar]
- 36.Wohlleben G, Muller J, Tatsch U, Hambrecht C, Herz U, Renz H, Schmitt E, Moll H, and Erb KJ. 2003. Influenza A virus infection inhibits the efficient recruitment of Th2 cells into the airway and the development of airway eosinophilia. J. Immunol 170: 4601–4611. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan MH, Schindler U, Smiley ST, and Grusby MJ. 1996. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4: 313–319. [DOI] [PubMed] [Google Scholar]
- 38.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, and Iwakura Y. 2002. Antigen-specific T-cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17: 375–387. [DOI] [PubMed] [Google Scholar]
- 39.McKenzie GJ, Emson CL, Fallon P, Zurawski G, Murray R, Grencis R, and McKenzie ANJ. 1998. Impaired development of Th2 cells in IL-13-deficient mice. Immunity 9: 423–432. [DOI] [PubMed] [Google Scholar]
- 40.Szretter KJ, Balish AL, and Katz JM. 2006. Influenza: propagation, quantification, and storage. Curr. Protoc. Microbiol. Unit 15G.1: 1–22. [DOI] [PubMed] [Google Scholar]
- 41.Marino DJ 2011. Age-specific absolute and relative organ weight distributions for B6C3F1 mice. J. Toxicol. Environ. Health 75: 76–99. [DOI] [PubMed] [Google Scholar]
- 42.Lanford R, Sureau C, Jacob J, White R, and Fuerst T. 1994. Demonstration of in vitro infection of chimpanzee hepatocytes with hepatitis C virus using strand-specific RT/PCR. Virology 202: 606–614. [DOI] [PubMed] [Google Scholar]
- 43.Kawakami E, Watanabe T, Fujii K, Goto H, Watanabe S, Noda T, and Kawaoka Y. 2011. Strand-specific real-time RT-PCR for distinguishing influenza vRNA, cRNA, and mRNA. J. Virol. Methods 173: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, and Eliceiri KW. 2017. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods JC, Choong CK, Yablonskiy DA, Bentley J, Wong J, Pierce JA, Cooper JD, Macklem PT, Conradi MS, and Hogg JC. 2006. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn. Reson. Med 56: 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, and Swain SL. 2005. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med 202: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, and Cauley LS. 2006. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity 24: 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, and Swain SL. 2007. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J. Immunol 178: 7563–7570. [DOI] [PubMed] [Google Scholar]
- 49.Kim TS, Hufford MM, Sun J, Fu Y, and Braciale TJ. 2010. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J. Exp. Med 207: 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deslee G, Adair-Kirk TL, Betsuyaku T, Woods JC, Moore CH, Gierada DS, Conradi SH, Atkinson JJ, Toennies HM, Battaile JT, Kobayashi DK, Patterson GA, Holtzman MJ, and Pierce RA. 2010. Cigarette smoke induces nucleic acid oxidation in lung fibroblasts. Am. J. Respir. Cell Mol. Biol 43: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deslee G, Woods J, Moore C, Conradi S, Gierada D, Atkinson J, Battaile J, Liu L, Patterson A, Adair-Kirk T, Holtzman M, and Pierce R. 2009. Oxidative damage to nucleic acids in severe emphysema. Chest 135: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deslee G, Woods J, Moore C, Liu L, Conradi S, Milne M, Gierada D, Pierce J, Patterson G, Lewit R, Battaile J, Holtzman M, Hogg J, and Pierce R. 2009. Elastin expression in very severe human COPD. Eur. Respir. J 34: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, Spoor MS, You Y, Brody SL, and Holtzman MJ. 2006. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J. Clin. Invest 116: 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel AC, Morton JD, Kim EY, Alevy Y, Swanson S, Tucker J, Huang G, Agapov E, Phillips TE, Fuentes ME, Iglesias A, Aud D, Allard JD, Dabbagh K, Peltz G, and Holtzman MJ. 2006. Genetic segregation of airway disease traits despite redundancy of chloride channel calcium-activated (CLCA) family members. Physiol. Genomics 25: 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newcomb DC, Boswell MG, Huckabee MM, Goleniewska K, Dulek DE, Reiss S, Lukacs NW, Kolls JK, and Peebles RS Jr. 2012. IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner. J. Immunol. 188: 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newcomb DC, Boswell MG, Sherill TP, V. V. P, Boyd KL, Goleniewska K, Brody SL, Kolls JK, Adler KB, and Peebles RS Jr. 2013. IL17A induces signal transducers and activators of transcription-6-independent airway mucous cell metaplasia. Am. J. Respir. Cell Mol. Biol 48: 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, Butler CA, Hargadon B, Sciurba JC, Gieseck RL, Thompson RW, White S, Abbas AR, Jackman J, Wu LC, Egen JG, Heaney LG, Ramalingam TR, Arron JR, Wynn TA, and Bradding P. 2015. TH2 and TH17 inflammatory pathways are recriprocally regulated in asthma. Sci. Transl. Med 7: 301–129. [DOI] [PubMed] [Google Scholar]
- 58.Holtzman MJ, Hahn HL, Sasaki KA, Skoogh B-E, Graf PD, and Nadel JA. 1982. Selective effect of general anesthetics on reflex bronchoconstrictor responses in dogs. J. Appl. Physiol 53: 126–133. [DOI] [PubMed] [Google Scholar]
- 59.Wang W, Nguyen N, Agapov E, Holtzman MJ, and Woods JC. 2012. Monitoring in vivo changes in lung microstructure with 3He MRI in Sendai-virus infected mice. J. Appl. Physiol 112: 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, and Swain SL. 2009. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J. Immunol 182: 7353–7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, and Harmsen AG. 2001. Production of interferon-gamma by influenza hemaglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am. J. Pathol 158: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang Y, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, Savage PB, Endo S, Yamamura T, Maaskant J, Kitano N, Singh A, Bhatt A, Besra GS, van den Elzen P, Appelmelk B, Franck RW, Chen G, Dekruyff RH, Shimamura M, Illarionov P, and Umetsu DH. 2011. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J. Clin. Invest 121: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang Y, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, and Umetsu DT. 2011. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol 12: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, and Martinez FD. 1999. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 345: 541–545. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen-Van-Tam JS, Openshaw PJ, Hashim A, Gadd EM, Lim WS, Semple MG, Read RC, Taylor BL, Brett SJ, McMenamin J, Enstone JE, Armstrong C, and Nicholson AG. 2010. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-Sep 2009). Thorax 65: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, and Pare PD. 2004. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Eng. J. Med 350: 2645–2653. [DOI] [PubMed] [Google Scholar]
- 67.Kruger SJ, Nagle SK, Couch MJ, Ohno Y, Albert M, and Fain SB. 2016. Functional imaging of the lungs with gas agents. J. Magn Reson Imaging 43: 295–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, Raymond WW, Lachowicz-Scroggins ME, Di Maio S, Hoffman EA, Castro M, Fain SB, Jarjour NN, Israel E, Levy BD, Erzurum SC, Wenzel SE, Meyers DA, Bleecker ER, Phillips BR, Mauger DT, Gordon ED, Woodruff PG, Peters MC, and Fahy JV. 2018. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J. Clin. Invest 128: 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lajole S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, and Wills-Karp M. 2010. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat. Immunol 11: 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, and Hawrylowicz CM. Distinct endotypes of steroid-resistant asthma characterized by IL-17Ahigh and IFN-ghigh immunophenotypes: potential benefits of calcitriol. J. Allergy Clin. Immunol 136: 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willis CR, Siegel L, Leith A, Mohn D, Escobar S, Wamberg S, Misura K, Rickel E, Rottman JB, Comeau MR, Sullivan JK, Metz DP, Tocker J, and Budelsky AL. 2015. IL-17RA signaling drives airway inflammation and bronchial hyperreactivity in allergic asthma. Am. J. Respir. Cell Mol. Biol 53: 810–821. [DOI] [PubMed] [Google Scholar]
- 72.Ather JL, Ckless K, Martin R, Foley KL, Suratt BT, Boyson JE, Fitzgerald KA, Flavell RA, Eisenbarth SC, and Poynter ME. 2011. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J. Immunol 187: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roos AB, Sanden C, Mori M, Bjermer L, Stampfli MR, and Erjefalt JS. 2015. IL-17A is elevated in end-stage chronic obstructive pulmonary disease and contributes to cigarette smoke-induced lymphoid neogenesis. Am. J. Respir. Crit. Care Med 191: 1232–1241. [DOI] [PubMed] [Google Scholar]
- 74.Liu W, Liu S, Verma M, Zafar I, Good JT, Rollins D, Groshong S, Gorska MM, Martin RJ, and Alam R. 2017. Mechanism of TH2/TH27-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J. Allergy Clin. Immunol 139: 1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yanagisawa H, Hashimoto M, Minagawa S, Takasaka N, Ma R, Moermans C, Ito S, Araya J, Budelsky AL, Goodsell A, Baron JL, and Nishimura SL. 2017. Role of IL-17A in murine models of COPD airway disease. Am. J. Physiol. Lung Cell Mol. Physiol. 312: L122–L130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roos AB, Sethi S, Nikota J, Wrona CT, Dorrington MG, Sanden C, Bauer CMT, Shen P, Bowdish D, Stevenson CS, Erjefalt JS, and Stampfli MR. 2015. IL-17A and the promotion of neutrophilia in acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 192: 428–437. [DOI] [PubMed] [Google Scholar]
- 77.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, and Cook DN. 2009. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med 180: 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sichelstiel A, Yadava K, Trompette A, Salami O, Iwakura Y, Nicod LP, and Marsland BJ. 2014. Targeting IL-1b and IL-17A driven inflammation during influenza-induced exacerbations of chronic lung inflammation. PloS ONE 9: e98440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hall SL, Baker T, Lajoie S, Richgels PK, Yang Y, McAlees JW, van Lier A, Wills-Karp M, Sivaprasad U, Acciani TH, LeCras TD, Biagini Myers J, Butch Kovacic M, and Lewkowich IP. 2017. IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation. J. Allergy Clin. Immunol 139: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang Y-H, Schluns KS, Broaddus RR, Zhu Z, and Dong C. 2015. Regulation of inflammatory responses by IL-17F. J. Exp. Med 205: 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Busse WW, Holgate ST, Kerwin E, Chon Y, Feng J, Lin J, and Lin SL. 2013. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med 188: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 82.Xi Y, Kim T, Brumwell AN, Driver IH, Wei Y, Tan V, Jackson JR, Xu J, Lee DK, Gotts JE, Matthay MA, Shannon JM, Chapman HA, and Vaughan AE. 2017. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol 19: 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ray S, Chiba N, Yao C, Guan X, McConnell AM, Brockway B, Que L, McQualter JL, and Stripp BR. 2016. Rare SOX2+ airway progenitor cells generate KRT5+ cells that repopulate damaged alveolar parenchyma following influenza virus Infection. Stem Cell Reports 7: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkesseli S, Rocklin R, Bock B, Hamilton J, Ming JE, Radin A, Stahl N, Yancpoulos GD, Graham N, and Pirozzi G. 2013. Duplimab in persistent asthma with elevated eosinophil levels. N. Eng. J. Med 368: 2455–2466. [DOI] [PubMed] [Google Scholar]
- 85.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, Busse WW, Ford L, Sher L, FitzGerald JM, Katelaris C, Tohda Y, Zhang B, Staudinger H, Pirozzi G, Amin N, Ruddy M, Akinlade B, Khan A, Chao J, Martincova R, Graham NMH, Hamilton JD, Swanson BN, Stahl N, Yancopoulos GD, and Teper A. 2018. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med in press. [DOI] [PubMed] [Google Scholar]
- 86.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, Zhu H, Hamilton JD, Swanson BN, Khan A, Chao J, Staudinger H, Pirozzi G, Antoni C, Amin N, Ruddy M, Akinlade B, Graham NMH, Stahl N, Yancopoulos GD, and Teper A. 2018. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med in press. [DOI] [PubMed] [Google Scholar]
- 87.Corren J, Lemanske R, Hanania N, Korenblat P, Parsey M, Arron J, Harris J, Scheerens H, Wu L, Su Z, Mosesova S, Eisner M, Bohen S, and Matthews J. 2011. Lebrikizumab treatment in adults with asthma. New. Engl. J. Med 365: 1088–1098. [DOI] [PubMed] [Google Scholar]
- 88.Russell RJ, Chachi L, Fitzgerald JM, Backer V, Olivenstein R, Titlestad IL, Ulrik CS, Harrison T, Singh D, Chaudhuri R, Leaker B, McGarvey L, Siddiqui S, Wang MT, Braddock M, Nordenmark LH, Cohen D, Parikh H, Colice G, and Brightling CE. 2018. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir. Med 6: 499–510. [DOI] [PubMed] [Google Scholar]
- 89.Panettieri RA Jr, Sjobring U, Peterffy A, Wessman P, Bowen K, Piper E, Colice G, and Brightling CE. 2018. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir. Med 6: 511–525. [DOI] [PubMed] [Google Scholar]
- 90.Kitaguchi Y, Komatsu Y, Fujimoto K, Hanaoka M, and Kubo K. 2012. Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. Int. J. Chron. Obstruct. Pulmon. Dis 7: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, and Brightling CE. 2012. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am. J. Respir. Crit. Care Med 186: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, Lenburg ME, Spira A, and Woodruff PG. 2015. Asthma-COPD overlap: clinical relevance of genomic signatures of type 2 inflammation in COPD. Am. J. Respir. Crit. Care Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S, Lugogo NL, Martinot J-B, Sagar H, Albers FC, Bradford SS, Harris B, Mayer B, Rubin DB, Yancey SW, and Sciurba FC. 2017. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N. Engl. J. Med 377: 1613–1629. [DOI] [PubMed] [Google Scholar]
- 94.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, Fitzgerald JM, Boedigheimer M, Davis BE, Dias C, Gorski KS, Smith L, Bautista E, Comeau MR, Leigh R, and Parnes JR. 2014. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 370: 2102–2110. [DOI] [PubMed] [Google Scholar]
- 95.GlaxoSmithKline. 2017. Efficacy and Safety Study of GSK3772847 in Subjects With Moderately Severe Asthma (NCT03207243).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.