Abstract
Objective
To assess the effect of short message service (SMS) communication on facility delivery, exclusive breastfeeding (EBF) and contraceptive use.
Design
Mobile WACh was a 3-arm unblinded individually randomised controlled trial
Setting
A public sector maternal child health (MCH) clinic in Nairobi, Kenya.
Population
Three hundred women attending antenatal care were randomised, 100 to each arm, and followed for 24 weeks postpartum. Pregnant women 14 years old with access to a phone and able to read SMS were eligible for participation.
Methods
Women were randomised (1:1:1) to receive 1-way SMS versus 2-way SMS with a nurse versus control. Weekly SMS content was tailored for maternal characteristics and pregnancy or postpartum timing.
Main Outcome Measures
Facility delivery, EBF and contraceptive use were compared separately between each intervention arm and the control arm by Kaplan-Meier analysis and X2 tests using intent-to-treat analyses.
Results
The overall facility delivery rate was high (98%) and did not differ by arm. Compared to controls, probability of EBF was higher in 1-way SMS arm at 10 and 16 weeks, and in 2-way SMS arm at 10, 16, and 24 weeks (p<0·005 for all). Contraceptive use was significantly higher in both intervention arms by 16 weeks [1-way SMS: 72% and 2-way SMS: 73%; p=0·03 and 0·02 versus 57% control, respectively] however this difference was not significant when correcting for multiple comparisons.
Conclusion
One-way and 2-way SMS improved EBF practices and early contraceptive use. Two-way SMS had an added benefit on sustained EBF, providing evidence that SMS messaging influences uptake of interventions that improve maternal and neonatal health.
Keywords: mHealth, maternal child health, family planning, exclusive breastfeeding, facility delivery
Tweetable abstract
The Mobile WACh RCT demonstrates that SMS improved practice of exclusive breastfeeding and early postpartum contraception.
Introduction
Despite progress in improving maternal and infant health indicators, an unacceptable number of women and newborns continue to die from preventable complications and many countries face challenges in achieving the maternal, neonatal and child health (MNCH)-related Sustainable Development Goals (SDGs) indicators.(1–3) For infants, the peripartum period is particularly high risk, with over 40% of child deaths occurring in the neonatal period, the majority in sub-Saharan Africa.(3) Skilled delivery services, exclusive breastfeeding (EBF) and contraception have potential to decrease maternal and infant mortality rates; yet, uptake of these interventions is suboptimal in many low- and middle- income countries (LMIC).(4–8) Counselling and decision support during pregnancy and postpartum may increase uptake of effective practices.(9) Given high clinic volume and health care worker (HCW) shortages, counselling time may be limited and health concerns often occur between visits. Mobile health (mHealth) tools, those that utilize mobile phone and other wireless technologies to support health, provide an attractive strategy to augment clinic-based support.
Short message service (SMS) messaging, also known as text messaging or texting, could provide guidance to women between clinic visits. There is evidence that mHealth can be used to educate, provide reminders for visits and medications, improve communication between HCWs and patients, and improve self-efficacy, all potentially leading to better outcomes.(10–13) SMS programs for MNCH have been implemented in South Africa, Bangladesh, India, Nigeria and the United States.(14, 15) To date, program efficacy data are lacking.(16, 17) There is some evidence that multistep mHealth interventions improve antenatal care (ANC) attendance and skilled delivery uptake.(18–21) A quasi-experimental study of an SMS intervention demonstrated improved EBF.(22) One cluster randomised controlled trial (RCT) observed a significant decrease in perinatal mortality with a combined unidirectional SMS and voucher intervention.(23) Together, these studies suggest that mHealth may be a useful strategy to improve MNCH outcomes but more evidence is needed on their efficacy, mechanisms, and best implementation approach.
MNCH SMS programs are predominantly unidirectional (1-way). With this design, it is difficult to understand the real-time influence of the messages. In contrast, bidirectional (2-way) communication between a woman and a HCW not only enhances patient-provider communication, but also enables communication when women or infants are experiencing health issues. To our knowledge, no study has directly compared the efficacy of unidirectional versus bidirectional SMS approaches on MNCH intervention uptake or outcomes.
In this 3-arm RCT, we compared the effect of 1-way SMS versus 2-way interactive SMS text messaging versus control on facility delivery, EBF, contraceptive use, and MNCH outcomes.
Methods
Study design
The study was an unblinded 3-arm RCT implemented at the Mathare North Health Centre MCH clinic, a government health centre in Nairobi County offering antenatal, postpartum and maternity care and serving the surrounding settlements. Institutional Review Board approval for this study was obtained from the University of Washington and the Kenyatta National Hospital/University of Nairobi Ethics Review Committee.
Participants
Pregnant women seeking ANC at the MCH clinic were screened for participation between August 2013 and April 2014. Women were eligible if they were 14 years of age or older, pregnant and less than 36 weeks estimated gestational age (EGA), had access to a mobile phone (shared or personal) using the Safaricom Ltd. network, were able to communicate via SMS, planned to remain in the area for 6 months postpartum and were not part of another research study. Participants provided written informed consent and consent counselling was conducted in English or Kiswahili based on preference.
Randomization and masking
Participants were randomised to either: 1) 1-way SMS, 2) interactive 2-way SMS, or 3) control, using 1:1:1 allocation. An independent statistician generated a computer-generated randomisation list using random block sizes. The allocation codes were placed in sequentially numbered, opaque, sealed envelopes and distributed by research staff. Envelopes were sequentially provided to participants at randomization. Randomization allocation was unblinded to participants and study staff because the intervention required knowledge of group assignment. Those obtaining and analysing follow-up data (DM, KR, JS and JU) were masked to group assignment.
Procedures
ANC staff provided information about the study and referred interested women to the study nurse. Women who agreed to participate underwent screening and at randomisation, a tablet-based questionnaire designed in Open Data Kit (ODK) was administered to assess sociodemographic and medical history, and experience with mobile phones.
Women in the control arm received routine clinic-based counselling and care. Women in the 1-way and 2-way trial arms were registered into the Mobile WACh SMS delivery platform and indicated their preferences for message delivery including their name, language (English or Kiswahili), and day of the week and time for delivery.
Participants were classified into tracks (routine, adolescents, first time mothers, women with a previous caesarean section and those with multiple gestations) with messaging tailored to the specific track. Participants received routine messages unless they met criteria for another track. Adolescents were defined as 14-19 years of age. The automated system incorporated a personalized approach that provided gestational age-appropriate educational and counselling messaging. All messages included participant name, clinic and nurse name, an educational message and actionable advice targeting one of the main study outcomes. SMS topics included ANC, pregnancy complications, family planning, infant health, EBF, infant immunization and visit reminders. All messaging was free of charge to the participant using a reverse billed short code. Input from community-based focus group discussions among pregnant women and health care providers was used to design the SMS message bank.
Women randomised to the 1-way group received weekly “push” educational and motivational SMS. The 2-way group received the same weekly SMS; however, each SMS contained a question related to the content. During enrolment, the study nurse explained that replies to SMS questions were voluntary. Women were also encouraged to send SMS with concerns or questions. The study nurse was available to answer SMS daily on weekdays. A clinician (JU) reviewed messages twice monthly for quality assurance. All communication was conducted through our custom web application designed for three-way communication between the automated system, study staff, and participants. This system automated weekly messaging, facilitated message responses, and managed participant details. SMS were sent from enrolment until 12 weeks postpartum.
At study visits women completed questionnaires to assess primary and secondary outcomes including MNCH service utilization, breastfeeding practices and maternal and infant health status. Follow up visits were scheduled in conjunction with infant immunization visits at 2 (visit window: 1-<8 weeks), 10 (visit window: 8-<16 weeks) and 24 (visit window: 16-36 weeks) weeks postpartum. We compared study outcomes at 10, 16, and 24-week postpartum time-points.
Outcomes
Primary outcomes were facility delivery; EBF through 10, 16, and 24 weeks; and contraceptive use by 10, 16, and 24 weeks. Delivery information was ascertained by self-report at the 2-week visit. EBF was defined as no introduction of complementary feeding (including water) at the time of clinic visit. Contraceptive use was defined as current self-reported use of a modern contraceptive method [oral contraceptive pills (OCPs), injectables, implants, intrauterine devices (IUDs), condoms, or tubal ligation (TL)]. Secondary outcomes included clinic attendance (retention), maternal mortality, infant mortality, and maternal report of any other serious maternal or infant illness. Secondary contraceptive outcomes included use of long-acting, reversible contraception (LARC) defined as IUDs, implants or TL. Prior to the initiation of the trial, primary outcomes also included number of subsequent ANC visits and PMTCT uptake. Due to systems errors data on ANC visits was not collected. Only 6 women in the study reported being HIV+ and testing kits were not available for the majority of the study period, therefore we modified the protocol and clinicaltrials.gov profile during follow-up. The complete trial protocol is available as supplementary material.
Statistical analysis
We estimated that a sample size of 300 participants randomised at a 1:1:1 ratio would give 80% power to detect a 20% increase in both facility delivery and uptake of postpartum contraception between the control arm and each intervention arm, assuming α=0·05, 10% attrition, 65% facility deliveries in the control arm and 50% postpartum contraception uptake in the control arm. Sample size estimates for facility delivery and contraception rates utilized Nairobi informal settlement data from 2003, which was the most recent at the time of study design(24). The risk of facility delivery in each intervention arm relative to the control arm was compared by χ2 test. The Kaplan-Meier method was used to model the time to (1) contraceptive use and (2) introduction of complementary food and drink and estimate probabilities of contraceptive use and EBF at the pre-specified time points of 10, 16 and 24 weeks postpartum. Probabilities of these primary outcomes were compared by Wald test. As a secondary analysis, Cox regression was used to estimate hazard ratios comparing uptake of contraception and introduction of complementary foods between study arms throughout the follow-up period. Secondary outcomes were compared with the χ2 test. All analyses were intention to treat (ITT) and were completed in STATA (version 13). The Holm-Bonferroni method was used to correct for multiple comparisons in the primary outcomes.
For each of the primary outcomes of facility delivery, EBF and contraception use, two sensitivity analyses were conducted to assess the influence of missing data. For EBF and contraception use, the 22 participants who were missing follow-up visit data were assumed to have the outcome (cessation of EBF and uptake of contraception respectively) at either 1 week postpartum or at 25 weeks postpartum. Survival analysis and estimation of probabilities at 10, 16 and 24 weeks postpartum was conducted as described above. For facility delivery, the 23 participants who were missing delivery data were assumed to have delivered in a facility or not in a facility. Risk was compared by χ2 test as described above.
Funding was provided by the National Institutes of Health, the National Science and the University of Washington Global Center for Integrated Health of Women Adolescents and Children (Global WACh). The funders played no role in conducting the research or writing the paper.
Results
We recruited participants between August 20, 2013 and April 22, 2014. Of 312 women screened, 300 were eligible, enrolled and randomly assigned to one of three study groups (Figure 1). Eleven women were ineligible and 1 declined participation. Among enrolled participants, 100 were randomised to each trial arm but data were not entered into the SMS delivery platform for 2 women, 1 in the 1-way arm and 1 in the 2-way arm (Figure 1). Thus, 298 women participated in the study. Of these, 278 were included in the outcome analysis. Fourteen perinatal deaths, 3 withdrawals and 3 losses to follow-up occurred prior to the first follow up visit and these participants were not included in the primary outcome analysis. Retention in the study to the 24-week visit was 86 (87%), 82 (83%), 91 (91%) in the 1-way, 2-way, and control arms, respectively and did not differ significantly by arm (p=0.35 and p=0.09 comparing the control arm with one-way and two-way arms respectively). Overall, there were 16 (5%) stillbirths and neonatal deaths and 3 women withdrew from the study.
Figure 1.
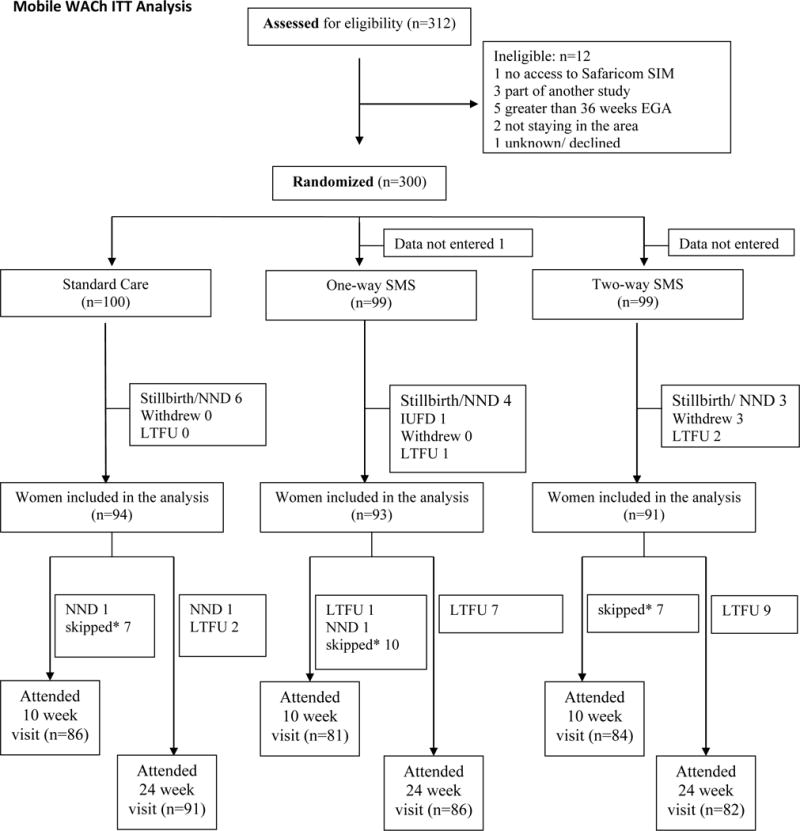
Trial profile. NND=neonatal demise. LTFU=lost to follow-up. *skipped = did not attend 10 week visit but attended 24 week visit.
Median age was 23 years (IQR 21-26) and median gestational age at enrolment was 26 weeks (IQR 21-30). Table 1 presents baseline characteristics. Most (93%) women were married, 55% completed primary school, and 46% had a prior pregnancy. The majority, 246 (83%), owned their own phone, but only 102 (34%) reported using SMS regularly, and less than 10% had ever used the internet.
Table 1.
Demographic and baseline characteristics of Mobile WACh study participants
| Control group (N= 100) |
1-way SMS group (N= 99a) |
2-way SMS group (N= 99a) |
|
|---|---|---|---|
| Age (years) | 23 (20-26) | 23 (21-26) | 24 (21-26) |
|
| |||
| Married monogamous | 82 (82%) | 89 (90%) | 87 (88%) |
|
| |||
| Currently has partner | 89 (90%) | 94 (95%) | 94 (95%) |
|
| |||
| Education completed | |||
| None | 0 (0%) | 0 (0%) | 0 (0%) |
| Lower primary 1-3 years | 2 (2%) | 1 (1%) | 0 (0%) |
| Upper primary 4-8 years | 44 (44%) | 48 (48%) | 37 (37%) |
| Secondary - not completed | 16 (16%) | 12 (12%) | 18 (18%) |
| Secondary - completed | 31 (31%) | 29 (29%) | 33 (33%) |
| Post-secondary | 7 (7%) | 9 (9%) | 11 (11%) |
|
| |||
| Estimated monthly income (KES) |
6000 (5000-7000) | 6000 (5000-8000) | 6000 (5000-9000) |
|
| |||
| Number of household residents | 3 (2-4) | 2 (2-3) | 3 (2-4) |
|
| |||
| Number of rooms in residence | 1 (1-1) | 1 (1-1) | 1 (1-1) |
|
| |||
| Shares phone | 15 (15%) | 9 (9%) | 22 (22%) |
|
| |||
| Using a mobile phone for <1 year | 43 (43%) | 36 (36%) | 41 (41%) |
|
| |||
| Uses SMS regularly | 28 (28%) | 40 (40%) | 34 (34%) |
|
| |||
| Ever used the internet | 3 (3%) | 10 (10%) | 7 (7%) |
|
| |||
| Gestational age weeks (LMP) | 27 (22-31) | 26 (20-30) | 25 (20-30) |
|
| |||
| Previously pregnant | 48 (48%) | 42 (42%) | 47 (47%) |
|
| |||
| Gravidity (including current) | 1 (1-2) | 1 (1-2) | 1 (1-2) |
|
| |||
| Parity (including current)b | 1 (1-2.5) | 1 (1-2) | 1 (1-2) |
|
| |||
| Number of ANC visits last pregnancyb | 3 (2-4) | 3 (2-4) | 3 (2-5) |
|
| |||
| Facility use for last deliveryb | 40 (83%) | 35 (83%) | 44 (94%) |
Data are n (%), or median (IQR).
Data missing for one participant in the 1-way group and one in the 2-way group.
Among those previously pregnant, N=48, 42, 47 for control, one-way and two-way. KES=Kenyan shillings. LMP=last menstrual period. ANC=antenatal care.
Facility delivery was very high in all 3 arms; the SMS interventions had no effect on uptake. Among 277 women providing delivery data, 273 (98.6%) reported delivering in a facility, with no difference between the 1-way and control arms [relative risk (RR) 1.00, 95% CI 0·97-1·03; p=0·99] or 2-way and control arms [RR 0·99, 95% CI 0·95-1·03; p=0·54]. Findings were similar in sensitivity analyses (Table S1).
Of 278 women who attended postpartum visits, all reported EBF for some duration. Women in both intervention arms were significantly more likely to EBF at 10 weeks and 16 weeks than women in the control arm (Table 2). The probability of EBF to 24 weeks postpartum was higher in both intervention groups than in the control, but only statistically significant in the 2-way messaging group [0·49 in 1-way, 0·62 in 2-way, and 0·41 in control, (p=0·30 and 0·005 for 1-way and 2-way vs. control, respectively)] (Table 2). Median time to introduction of food or water was 2 weeks longer (26 weeks) in the 2-way messaging group compared to the control group (24 weeks) (Figure 2). Findings were similar in sensitivity analyses assigning extreme timing of introduction of complementary feeding to participants with missing follow-up data (Table S2).
Table 2.
Effect of SMS interventions on primary outcomes among Mobile WACh participants
| Control group (n=94) |
1-way SMS group (n=93) |
p-value | 2-way SMS group (n=91) |
p-value | |
|---|---|---|---|---|---|
| Probability of eBF to 10 weeks | 0.79 (0.69-0.86) |
0.93 (0.86-0.97) |
0.003* | 0.96 (0.89-0.98) |
0.0004* |
| Probability of eBF to 16 weeks | 0.62 (0.52-0.71) |
0.82 (0.72-0.89) |
0.002* | 0.93 (0.85-0.97) |
<0.0001* |
| Probability of eBF to 24 weeks | 0.41 (0.31-0.51) |
0.49 (0.38-0.59) |
0.30 | 0.62 (0.51-0.72) |
0.005* |
| Probability of contraceptive use by 10 weeks postpartum | 0.33 (0.24-0.44) |
0.42 (0.32-0.53) |
0.24 | 0.31 (0.23-0.42) |
0.74 |
| Probability of contraceptive use by 16 weeks postpartum | 0.57 (0.47-0.67) |
0.72 (0.62-0.81) |
0.03 | 0.73 (0.63-0.82) |
0.02 |
| Probability of contraceptive use by 24 weeks postpartum | 0.77 (0.69-0.85) |
0.83 (0.74-0.90) |
0.39 | 0.83 (0.75-0.90) |
0.33 |
Data are probability (95% CI) based on survival analysis. P-values are for comparison of each intervention group with control by Wald test.
P-value statistically significant after correction for multiple comparisons. eBF=exclusive breastfeeding.
Figure 2.
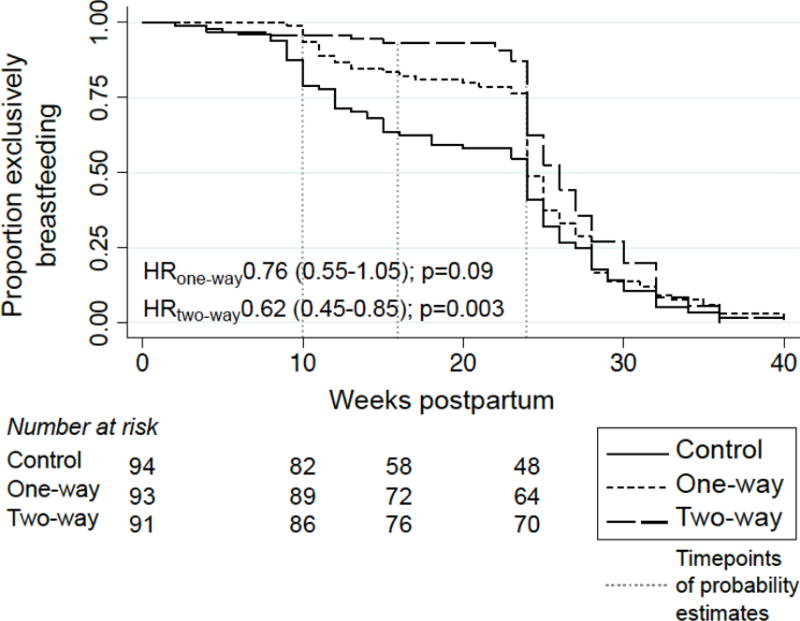
Probability of practice of exclusive breastfeeding over time by group assignment of women in the Mobile WACh study
Most of the 278 postpartum participants (89%) initiated contraception at some time during postpartum follow-up. In the control arm, probability of use was 0·33, 0·57, and 0·77 at 10, 16, and 24 weeks postpartum respectively (p<0·001 for change between 10 and 24 weeks). The probability of contraceptive use by 16 weeks postpartum was significantly higher in both intervention groups than in the control [0·72 in 1-way, 0·73 in 2-way, and 0·57 in control, (p=0·03 and 0·02 for 1-way and 2-way vs. control, respectively)], however this difference was not significant after correcting for multiple comparisons (Table 2). Contraceptive use remained higher in intervention arms at later time points but the difference was not statistically significant [0·83 in 1-way, 0·83 in 2-way and 0·77 in control at 24 weeks]. Median time to contraceptive initiation was shorter in intervention arms (1-way: 11 weeks, 2-way: 12 weeks) than the controls (13 weeks) but these differences were not significant (Figure 3). Findings were similar in sensitivity analyses assigning extreme timing of contraceptive uptake to participants with missing follow-up data (Table S3).
Figure 3.
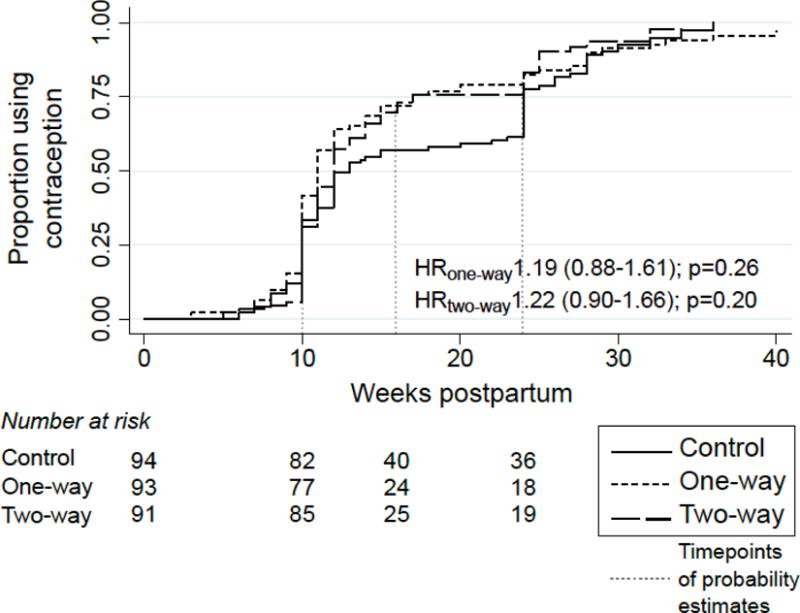
Probability of postpartum contraceptive initiation over time by group assignment of women in the Mobile WACh study
LARC use was similar across arms [1-way versus control (RR 1·16, 95% CI 0·44-3·03; p=0·77) and 2-way versus control (RR 1·41 95% CI 0·57-3·51; p=0·46)] with only 25 (11%) of all contraceptive users using LARC methods (IUCD and implants), the majority implants. Contraceptive continuation was high among women starting contraception at 10 weeks; however, 44 (30%) of contraceptive users across all arms switched methods between 10 and 24 weeks.
Overall, there were 16 stillbirths and neonatal deaths (5%); 14 occurred prior to the first postpartum follow-up visit. One miscarriage occurred before 20 weeks. There were fewer stillbirths and infant deaths in the 2-way group compared to the control group (3·1% versus 8%, p=0·21), however, this difference did not attain statistical significance (Table S4). There was one maternal death and her infant was among the neonatal deaths. No serious adverse events occurred as a result of the intervention.
The Mobile WACh platform delivered over 3000 messages to participants in the intervention arms and received over 1100 messages, with 83% engagement among 2-way users. Infant health and breastfeeding comprised 20% of all SMS received. Specific breastfeeding concerns included milk production and introduction of complementary feeds. Sixteen percent all messages received were about contraception, including concerns about myths and side effects.
Discussion
Main Findings
In this 3-armed RCT, a semi-automated yet tailored SMS intervention (Mobile WACh) significantly increased sustained EBF through six months and led to an increase in early uptake of postpartum contraception that did not maintain statistical significance after correcting for multiple comparisons. Women used 2-way SMS to address important concerns between clinic visits.
Strengths and Limitations
To our knowledge, this is the first individually randomised controlled trial assessing the influence of SMS on uptake of MNCH services and comparing 2-way SMS messaging to 1-way SMS. Unlike other studies, we did not include a package of interventions which allowed us to determine effects of SMS alone.
Our study did have some limitations. Major temporal changes occurred during the study period likely effecting uptake of services across all three arms. Free maternity care was rolled out in early 2013 and resulted in 98% facility delivery in all RCT arms. Additionally, the study nurse encouraged uptake of MNCH services at study visits, which likely caused increased uptake in the control arm. Women in the study were not blinded to their assignment which may have led to performance bias. Additionally, outcomes were obtained by self-report which could have introduced bias for social desirability, but this likely occurred across all arms.
The study was not powered to find a difference in maternal or infant mortality. Larger studies will be necessary to demonstrate these effects. In addition, mHealth interventions that promote engagement in care are partially dependent on high quality health systems to deliver good outcomes. Increasing uptake of proven MNCH services and behaviours does not guarantee impact on health outcomes and demand creation may encourage women to use services that do not meet their needs.
Interpretation
We found that both SMS intervention arms in our study demonstrated higher rates of EBF through 10 and 16 weeks, and women in the 2-way SMS arm were significantly more likely to adhere to recommendations to EBF through 24 weeks. Breastfeeding is a critical intervention for reducing under-5 mortality and provides benefits for both mother and infant.(7) There are few studies of SMS interventions to improve EBF.(25) A quasi-experimental study of 1-way SMS in China found longer duration of EBF in mothers receiving weekly SMS about infant feeding, compared to controls.(22) A cluster RCT of a multi-pronged intervention, including in-person meetings, text or voice messages and mini-dramas in Nigeria, also found higher rates of breastfeeding to 6 months in the intervention group.(26) In contrast, an RCT of twice-monthly voice-based lactation counselling did not find differences between EBF among women receiving the intervention.(27) In our study, messages were delivered throughout the critical time period for establishing and sustaining EBF. There is evidence that multi-visit counselling sessions during both the antenatal and postpartum period may increase EBF.(28, 29) Support tools that provide reassurance, information, and the opportunity to respond to questions may also increase EBF.(30) Our findings suggest that SMS augmented in-clinic counselling to sustain EBF. Two-way SMS was used to support EBF when challenges occurred and to discuss ways to overcome cultural pressures which can influence sustained EBF.(31)
In our study, we found that both 1-way and 2-way SMS approaches increased early uptake of contraception by 16 weeks postpartum, though this was not statistically significant when correcting for multiple comparisons. Early initiation of postpartum contraception has benefits including prevention of very short birth spacing and pregnancy prevention prior to the resumption of menses.(32) There have been conflicting findings regarding the effect of mHealth programs on contraceptive use. One RCT in the United States found that daily 1-way SMS resulted in higher OCP continuation at 6 months.(33) Another RCT in Cambodia observed higher rates of contraceptive use among women using an interactive voice recording (IVR) system and optional counsellor phone call. (34) In contrast, two SMS studies in Africa (m4RH in Kenya and 6001 system in Uganda) did not observe improved contraceptive uptake.(35, 36) There are important differences between our trial and these studies, both in terms of the intervention and the target population. The m4RH intervention targeted general consumers who signed up for 2-way informational family planning SMS. While messages improved knowledge, contraceptive use did not increase, and response rates were low (13.5-51.8%).(35) The 6001 system allowed phone users to text questions and responded with pre-prepared advice using an algorithm but found no increase in knowledge or change in attitudes.(36) In contrast, our RCT involved women attending care in a public MCH clinic and we initiated contact through push messaging rather than relying on participants to seek advice. Our finding of high engagement from women and high uptake of early contraception suggests that our trial population may have more motivation for FP than women in the general population. Increased early contraceptive use in the intervention arms suggests that larger studies should explore the use of this personalized approach to FP counselling.
Contrary to our hypothesis, 2-way SMS did not confer additional benefit for contraceptive use over 1-way. However, over 16% of all messages from 2-way participants were about contraception. Given high levels of contraception SMS engagement it is possible that 2-way SMS may demonstrate additional benefits in clinic settings with lower rates of postpartum contraception than in our trial.
SMS is low cost in comparison to voice calls and in-person visits, and the Mobile WACh platform allowed us to efficiently send and receive SMS. This type of efficiency has potentially major impacts on reducing human resource needs. One nurse responded to SMS from all women in the 2-way group while performing recruitment, follow-up and clinical duties. This messaging approach could potentially be integrated into routine clinical services but the 2-way SMS approach had added cost, both in terms of airtime and nurse effort. While it is difficult to ignore the enthusiasm of women in this trial for 2-way SMS for support, advice and triage of illness; further research on cost-effectiveness and potential for integration will be important to scale this type of intervention. Two-way communication may have averted unnecessary visits or expedited critical visits which could be cost-saving for both the family and health system. Mobile WACh messages were crafted to be personalized, actionable and outcome focused. However, it is not clear how much message content effects behaviour change. Other studies have used a simpler approach with significant effects on behaviour and health outcomes.(11)
Conclusion
In conclusion, our SMS intervention resulted in longer EBF and early contraceptive use during the postpartum period. These findings suggest that incorporating SMS messaging into MNCH care has the potential to engage patients and support the use of essential services and ultimately improve the health of women and infants. This trial suggests that rigorous evaluations of larger mHealth trials are warranted to guide the future of mHealth within MNCH and that caution should be taken in scaling SMS programs without a vision for determining impact on health outcomes.
Supplementary Material
Acknowledgments
This research received administrative support and mentorship from the Global Center for Integrated Health of Women, Adolescents and Children (Global WACh), which is jointly-supported by the Departments of Global Health, Pediatrics, and Obstetrics and Gynecology. We are grateful to the Kizazi group of the Global Center for Integrated Health of Women, Adolescents and Children (Global WACh), which provided feedback on study design.
Funding: Source of Funding: Funding was provided by the National Institutes of Health (K12HD001264 to JAU, R01HD080460, K24HD054314 and to GJS, K01AI116298 to ALD), the National Science Foundation (Graduate Research Fellowship to TP and BD), as well as the University of Washington Global Center for Integrated Health of Women Adolescents and Children (Global WACh).
Footnotes
Trial Registration: This trial was registered with ClinicalTrials.gov number NCT01894126. https://clinicaltrials.gov/ct2/show/NCT01894126?cond=SMS&cntry1=AF%3AKE&draw=1&rank=5
Disclosure of Interests
The authors have no conflicts of interests to disclose. Completed disclosure of interest forms are available to view online as supporting information.
Contribution to Authorship
JAU, TP, BD, ALD, JK and GJS made a substantial contribution to the design of the trial. JAU, TP, BD, DM, JK and GJS were responsible for the implementation of the study and acquisition of the data. JAU, JK and GJS were responsible for overall supervision. JAU, JS and KR performed the analysis and interpretation of the data. JAU, KR and GJS wrote the initial draft of the manuscript. All other authors provided significant feedback on the manuscript drafts. All authors agree to be accountable for the work.
Details of Ethics Approval: Institutional Review Board approval for this study was obtained from the University of Washington (42621) on 6/6/2013 and the Kenyatta National Hospital/University of Nairobi Ethics Review Committee (ERC) (P310/06/2012) on 9/21/2012.
References
- 1.Nations U. Transforming our world: the 2030 Agenda for Sustainable Development. Sustainable Development Knowledge Platform. 2015 [Google Scholar]
- 2.Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet. 2016;387(10017):462–74. doi: 10.1016/S0140-6736(15)00838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators GBDCM. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1725–74. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell OM, Graham WJ, Lancet Maternal Survival Series steering g Strategies for reducing maternal mortality: getting on with what works. Lancet. 2006;368(9543):1284–99. doi: 10.1016/S0140-6736(06)69381-1. [DOI] [PubMed] [Google Scholar]
- 5.Prata N, Sreenivas A, Vahidnia F, Potts M. Saving maternal lives in resource-poor settings: facing reality. Health Policy. 2009;89(2):131–48. doi: 10.1016/j.healthpol.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Tsui AO, McDonald-Mosley R, Burke AE. Family planning and the burden of unintended pregnancies. Epidemiol Rev. 2010;32:152–74. doi: 10.1093/epirev/mxq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–90. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 8.Yakoob MY, Ali MA, Ali MU, Imdad A, Lawn JE, Van Den Broek N, et al. The effect of providing skilled birth attendance and emergency obstetric care in preventing stillbirths. BMC Public Health. 2011;11(Suppl 3):S7. doi: 10.1186/1471-2458-11-S3-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleland J, Shah IH, Daniele M. Interventions to Improve Postpartum Family Planning in Low- and Middle-Income Countries: Program Implications and Research Priorities. Stud Fam Plann. 2015;46(4):423–41. doi: 10.1111/j.1728-4465.2015.00041.x. [DOI] [PubMed] [Google Scholar]
- 10.Boksmati N, Butler-Henderson K, Anderson K, Sahama T. The Effectiveness of SMS Reminders on Appointment Attendance: a Meta-Analysis. J Med Syst. 2016;40(4):90. doi: 10.1007/s10916-016-0452-2. [DOI] [PubMed] [Google Scholar]
- 11.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–45. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 12.Jareethum R, Titapant V, Chantra T, Sommai V, Chuenwattana P, Jirawan C. Satisfaction of healthy pregnant women receiving short message service via mobile phone for prenatal support: A randomized controlled trial. J Med Assoc Thai. 2008;91(4):458–63. [PubMed] [Google Scholar]
- 13.Ross R, Sawatphanit W, Suwansujarid T, Stidham AW, Drew BL, Creswell JW. The effect of telephone support on depressive symptoms among HIV-infected pregnant women in Thailand: an embedded mixed methods study. J Assoc Nurses AIDS Care. 2013;24(5):e13–24. doi: 10.1016/j.jana.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 14.(MAMA) MAMA. Research Agenda. :2015. [Available from: http://www.mobilemamaalliance.org.
- 15.Administration USDoHaHSHRaS. Promoting Maternal and Child Health Through Health Text Messaging An Evaluation of the Text4Baby Program- Final Report. Rockville, Maryland: 2015. [Google Scholar]
- 16.Evans WD, Wallace Bihm J, Szekely D, Nielsen P, Murray E, Abroms L, et al. Initial outcomes from a 4-week follow-up study of the Text4baby program in the military women’s population: randomized controlled trial. J Med Internet Res. 2014;16(5):e131. doi: 10.2196/jmir.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans WD, Wallace JL, Snider J. Pilot evaluation of the text4baby mobile health program. BMC Public Health. 2012;12:1031. doi: 10.1186/1471-2458-12-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund S, Hemed M, Nielsen BB, Said A, Said K, Makungu MH, et al. Mobile phones as a health communication tool to improve skilled attendance at delivery in Zanzibar: a cluster-randomised controlled trial. BJOG. 2012;119(10):1256–64. doi: 10.1111/j.1471-0528.2012.03413.x. [DOI] [PubMed] [Google Scholar]
- 19.Lund S, Nielsen BB, Hemed M, Boas IM, Said A, Said K, et al. Mobile phones improve antenatal care attendance in Zanzibar: a cluster randomized controlled trial. BMC Pregnancy Childbirth. 2014;14:29. doi: 10.1186/1471-2393-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaewkungwal J, Singhasivanon P, Khamsiriwatchara A, Sawang S, Meankaew P, Wechsart A. Application of smart phone in “Better Border Healthcare Program”: a module for mother and child care. BMC Med Inform Decis Mak. 2010;10:69. doi: 10.1186/1472-6947-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyeyemi SO, Wynn R. Giving cell phones to pregnant women and improving services may increase primary health facility utilization: a case-control study of a Nigerian project. Reprod Health. 2014;11(1):8. doi: 10.1186/1742-4755-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang H, Li M, Wen LM, Hu Q, Yang D, He G, et al. Effect of short message service on infant feeding practice: findings from a community-based study in Shanghai, China. JAMA Pediatr. 2014;168(5):471–8. doi: 10.1001/jamapediatrics.2014.58. [DOI] [PubMed] [Google Scholar]
- 23.Lund S, Rasch V, Hemed M, Boas IM, Said A, Said K, et al. Mobile phone intervention reduces perinatal mortality in zanzibar: secondary outcomes of a cluster randomized controlled trial. JMIR Mhealth Uhealth. 2014;2(1):e15. doi: 10.2196/mhealth.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macro KNBoSKaI. Kenya Demographic and Health Survey 2008-2009. Calverton, Maryland: KNBS and ICF Macro; 2010. [Google Scholar]
- 25.Sondaal SF, Browne JL, Amoakoh-Coleman M, Borgstein A, Miltenburg AS, Verwijs M, et al. Assessing the Effect of mHealth Interventions in Improving Maternal and Neonatal Care in Low- and Middle-Income Countries: A Systematic Review. PLoS One. 2016;11(5):e0154664. doi: 10.1371/journal.pone.0154664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flax VL, Negerie M, Ibrahim AU, Leatherman S, Daza EJ, Bentley ME. Integrating group counseling, cell phone messaging, and participant-generated songs and dramas into a microcredit program increases Nigerian women’s adherence to international breastfeeding recommendations. J Nutr. 2014;144(7):1120–4. doi: 10.3945/jn.113.190124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tahir NM, Al-Sadat N. Does telephone lactation counselling improve breastfeeding practices? A randomised controlled trial. Int J Nurs Stud. 2013;50(1):16–25. doi: 10.1016/j.ijnurstu.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Sinha B, Chowdhury R, Sankar MJ, Martines J, Taneja S, Mazumder S, et al. Interventions to improve breastfeeding outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):114–34. doi: 10.1111/apa.13127. [DOI] [PubMed] [Google Scholar]
- 29.Lumbiganon P, Martis R, Laopaiboon M, Festin MR, Ho JJ, Hakimi M. Antenatal breastfeeding education for increasing breastfeeding duration. Cochrane Database Syst Rev. 2016;(12):CD006425. doi: 10.1002/14651858.CD006425.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFadden A, Gavine A, Renfrew MJ, Wade A, Buchanan P, Taylor JL, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;(2):CD001141. doi: 10.1002/14651858.CD001141.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labbok MH. Transdisciplinary breastfeeding support: creating program and policy synergy across the reproductive continuum. Int Breastfeed J. 2008;3:16. doi: 10.1186/1746-4358-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiel de Bocanegra H, Chang R, Menz M, Howell M, Darney P. Postpartum contraception in publicly-funded programs and interpregnancy intervals. Obstet Gynecol. 2013;122(2 Pt 1):296–303. doi: 10.1097/AOG.0b013e3182991db6. [DOI] [PubMed] [Google Scholar]
- 33.Castano PM, Bynum JY, Andres R, Lara M, Westhoff C. Effect of daily text messages on oral contraceptive continuation: a randomized controlled trial. Obstet Gynecol. 2012;119(1):14–20. doi: 10.1097/AOG.0b013e31823d4167. [DOI] [PubMed] [Google Scholar]
- 34.Smith C, Ngo TD, Gold J, Edwards P, Vannak U, Sokhey L, et al. Effect of a mobile phone-based intervention on post-abortion contraception: a randomized controlled trial in Cambodia. Bull World Health Organ. 2015;93(12):842–50A. doi: 10.2471/BLT.15.160267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson D, Juras R, Riley P, Chatterji M, Sloane P, Choi SK, et al. A randomized controlled trial of the impact of a family planning mHealth service on knowledge and use of contraception. Contraception. 2017;95(1):90–7. doi: 10.1016/j.contraception.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Jamison JCKD, Raffler P. Mixed method evaluation of a passive mHealth sexual information texting service in Uganda. Cambridge, MA: 2013. (Contract No.: Working Paper 19107). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


