Abstract
Studies comparing endogenous and recombinant Serum Amyloid A (SAA) have generated conflicting data on the pro-inflammatory function of these proteins. In exploring this discrepancy, we found that in contrast to commercially sourced recombinant human SAA1 (hSAA1) proteins produced in E. coli, hSAA1 produced from eukaryotic cells did not promote pro-inflammatory cytokine production from human or mouse cells, induce Th17 differentiation, or stimulate TLR2. Proteomics analysis of E. coli derived hSAA1 revealed the presence of numerous bacterial proteins, with several being reported or probable lipoproteins. Treatment of hSAA1 with lipoprotein lipase, or addition of a lipopeptide to eukaryotic cell-derived hSAA1, inhibited or induced the production of TNFα from macrophages, respectively. Our results suggest that a function of SAA is in the binding of TLR2-stimulating bacterial proteins, including lipoproteins, and demand that future studies of SAA employ a recombinant protein derived from eukaryotic cells.
Introduction
The Serum Amyloid A (SAA) family of acute phase proteins have been studied for decades as robust biomarkers for a wide array of inflammatory and autoimmune disorders, as well as for their contribution to AA amyloidosis. Increasing ~1000 fold in the serum in response to infection and injury, SAA proteins are evolutionarily-conserved and are the major acute-phase proteins in vertebrates (1). Multiple isoforms of SAA are expressed in the liver (the largest source of acute-phase reactants) as well as in hematopoietic and non-hematopoietic cells throughout the body. SAA1 and SAA2 are highly homologous and predominantly produced by the liver, whereas SAA3 is an acutely-expressed isoform produced in non-primate mammals (2), and the constitutively-expressed SAA4 does not increase in response to infection or injury (3).
Given its rapid and robust increase under inflammatory and autoimmune conditions, it has long been speculated that SAA is a mediator of the inflammatory process. Specifically, we and others have reported that the pro-inflammatory effects of SAA are largely mediated through the activation of TLR2 (4–18). However, the E. coli-derived recombinant form of human SAA that is almost uniformly used by investigators (apoSAA, a lipid-binding apolipoprotein that is a constituent of plasma lipoprotein) elicits strong pro-inflammatory responses not shared with the endogenous form of SAA. Specifically, whereas recombinant apoSAA promotes neutrophil activation and pro-inflammatory cytokine production, human plasma containing highly elevated levels of SAA display neither of these effects (19, 20). Furthermore, transgenic overexpression of mouse (21) or human (20, 22) SAA1 in mice to levels that recapitulate those in the circulation of humans does not elicit a pro-inflammatory state. These results imply that further characterization of the differences in pro-inflammatory activities between recombinant and endogenous SAA is warranted.
SAA proteins associate with high-density lipoprotein (HDL), displacing ApoA1 as the predominant apolipoprotein during inflammation (1, 23). Interestingly, the capacity of SAA to stimulate cells via TLR2 has been reported to be only when it is not incorporated into HDL (21). SAA has been shown to prevent the entry of enveloped viruses into cells (24–26) and reported to opsonize Gram-negative bacteria (27, 28). The outer membrane protein A (OmpA) of E. coli has been reported to bind SAA1, enabling it to augment the ability of neutrophils to ingest invading bacteria (27). Furthermore, SAA functions as a circulating chaperone for the small lipophilic vitamin A derivative, retinoic acid (29), and can associate with phospholipids to form larger particles (30). As SAA clearly has the capacity to associate with lipid-rich particles and compounds, we hypothesized that other lipophilic molecules, such as bacterial lipopeptides, may also associate with SAA. Bacterial lipopeptides are potent activators of TLR2, whereby they induce the production of pro-inflammatory cytokines and other effects that have been attributed to recombinant forms of SAA produced in E. coli. The objective of our studies was to compare the effects of E. coli-derived and eukaryotic cell-derived SAA1 proteins on TNFα production from macrophages, the stimulation of TLR2, and the induction of Th17 responses, as well as to examine the contribution of bacterial lipoproteins to the capacity to induce the aforementioned pro-inflammatory effects.
Materials and Methods
Reagents
Chemicals were from Fisher Scientific (Hampton, NH) unless noted otherwise. Recombinant human apoSAA (apoSAA), SAA1 (hSAA1), and apoA1, all made in E. coli, were from Peprotech (Rocky Hill, NJ). Recombinant mouse SAA1 made in E. coli was from R&D Systems (Minneapolis, MN). Recombinant human SAA1 made in human embryonic kidney (HEK) cells was from Origene (Rockville, MD). Ultra-pure E. coli O111:B4 LPS and Pam3CSK4 were from Invivogen (San Diego, CA). Plasmid pcDNA3 was from Invitrogen (Carlsbad, CA). pcDNA3.1(+) encoding human SAA1 was from GenScript (Piscataway, NJ). Plasmid pCMV6-XL5 encoding human SAA1 was from Origene. E. coli transformed with pLX304 plasmids encoding human TLR1 and TLR2 were from the DNASU Plasmid Repository (Tempe AZ). Plasmids from these bacteria were purified using EndoFree Plasmid Kits from Qiagen (Germantown, MD). Polyethylenimine (PEI) was from Polysciences, Inc. (Warrington, PA). Mini-PROTEAN TGX precast 4–20% polyacrylamide gels were from Bio-Rad (Hercules, CA). Immobilon nitrocellulose transfer membranes and Amicon Ultra centrifugal filter units with a 3 kDa MW cutoff were from EMD Millipore (Billerica, MA). Lipoprotein lipase (LPL) from Pseudomonas sp. and ovalbumin (Ova) were from Sigma-Aldrich (St. Louis, MO). TOP10 chemically competent E. coli were from Invitrogen. anti-CD3 and anti-CD28 antibodies were from BD Biosciences (San Jose, CA).
Cells
Peripheral blood was collected from normal adult human donors under the approval of the University of Vermont Institutional Review Board (protocol #M10–171). For the preparation of peripheral blood monocuclear cells (PBMCs), blood collected into EDTA-coated tubes was diluted 1:1 in HBSS (without calcium or magnesium), layered over lymphocyte separation medium (LSM) (MP Biomedicals, Santa Ana, CA), and centrifuged at 500xg for 30 minutes at room temperature. The top layer of plasma was aspirated and discarded, leaving 2–3mm above the buffy coat, and the buffy coat and half the lower LSM layer was aspirated, mixed with HBSS, and washed twice. Neutrophils were enriched by resuspending the pellet obtained during the LSM preparation of PBMCs in 20 ml HBSS and adding 20 ml of 3% dextran in HBSS, which was subsequently mixed by inversion several times. Erythrocytes were allowed to settle for 20 minutes at room temperature, after which the neutrophil-rich supernatant was transferred to a new tube and washed with HBSS. Erythrocytes were lysed by two rounds of resuspending the pellet in 10 ml hypotinic lysis buffer for 30 seconds followed by the addition of an equal volume of reequilibration buffer and washing. Splenocytes and bone marrow were collected from C57BL/6J mice purchased from Jackson Laboratories (Bar Harbor, ME). Studies were approved by the University of Vermont’s Institutional Animal Care and Use Committee (protocol #12–018), in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and efforts were made to minimize suffering. Sodium pentobarbital was administered via intraperitoneal injection for euthanasia and cells were processed as described (4). Bone marrow-derived dendritic cells (BMDCs) were generated as previously described (31) and CD4+ T cells were isolated from splenocytes by negative selection (STEMCELL Technologies, Vancouver, BC, Canada) (32). RAW 264.7, J774A.1, and HEK293T cells from American Type Culture Collection (ATCC, Manassas, VA) were maintained in DMEM (Gibco, Grand Island, NY) supplemented with 10% FBS (Gibco), 1% L-Glutamine (Gibco), and 1x Primocin (Invivogen). For experiments in which cell supernatants were examined by ELISA, cells were plated at 2.5×105 cells/well in 250 μl of media in a 48-well plate and allowed to grow overnight. The following day, the cells were treated as indicated within the figure legends for each experiment. Cell supernatants were harvested at the end of each experiment, spun down at 3,300xg for 10 minutes to pellet cellular debris, transferred to new tubes, and frozen at −20˚C until analysis. For transfection, 5.6×104 HEK293T cells were seeded per cm2 in each well of 6- or 96-well plates (for expression of SAA or TLR1/2, respectively) and transfected one day later with 11.1 ¼l/cm2 DMEM containing 0.2 μg/cm2 plasmid and 0.9 μg/cm2 of PEI per well.
Cytokine and SAA quantitation
Cell supernatants were analyzed for mouse TNFα, IL-1β, or IL-4 using ELISA kits from BD Biosciences. Human IL-8, IL-6, TNFα, IL-1β, and SAA1, as well as mouse IL-5, IL-13, IL-17A, and IFNγ, were measured by ELISA using Duo-Set reagents from R&D Systems (Minneapolis, MN). All ELISAs were performed according to manufacturer’s instructions.
Fast protein liquid chromatography (FPLC)
250 µg of apoSAA was run on a Superdex75 size exclusion column (GE Healthcare Life Sciences, Pittsburgh, PA) in PBS, pH 7.2 running buffer, quantitated for total protein (OD280) as it eluted from the column (90 fractions of approximately 330 µl each were collected), and compared to the elution time of protein molecular weight standards to estimate its size. Briefly, a standard curve was generated by plotting the elution volume parameter (Kav) of several standards purchased from GE Healthcare (ribonuclease A, aprotinin, carbonic anhydrase, ovalbumin, conablbumin) versus their log MWs. The Kav of SAA was read from this curve to estimate its molecular weight (~76 kDa). Kav was calculated as follows: Blue dextran was used to calculate the void volume (Vo) of the column, and the total bed volume (Vt) was 24 ml. Kav = (Elution volume (Ve) – Vo)/(Vt-Vo).
Mass spectrometry analysis
For in-gel digestion and subsequent analyses, apoSAA (50 μg) in laemmli buffer with 5% 2-ME was separated on a 4–20% tris-glycine gel at 140 V for 1 hr 15 mins. The gel was then stained in 30% coomassie blue (40% methanol, 20% acetic acid, and 0.1% brilliant blue R (Sigma-Aldrich)) (diluted in destain) overnight, destained in 30% methanol, 20% acetic acid, imaged using a Canoscan 8800F scanner (Canon, Melville, NY), and a cut map was created to divide the lanes into 12 specific cuts. Proteins within the gel slices were reduced in 25 mM DTT for 30 mins, alkylated in-gel in 10 mM iodoacetamide for 45 mins, and subjected to two rounds of dehydration with acetonitrile and rehydration with water prior to a final dehydration in acetonitrile. To the dry gel slices, 25 ¼l of 12 ng/¼l sequence grade trypsin (Promega, Madison, WI) in 50 mM ammonium bicarbonate was added. The samples were placed on ice for 30 min and digested at 37ºC overnight. Tryptic peptides were extracted with 2.5% formic acid in 50% acetonitrile while spinning in a microcentrifuge at 13,000 rpm for 10 min. The supernatant was collected, and the gel slices were dehydrated by twice incubating with 100% acetonitrile and collecting all the extractions from a given gel slice in the same tube, and solvent was removed using a vacuum centrifuge at 37°C. The peptides were resuspended in 2.5% acetonitrile and 2.5% formic acid, loaded using a MicroAS autosampler (Thermo Scientific, Pittsburgh, PA), and separated in a microcapillary column packed with 12 cm of Magic C18, 200-Å, 5-μm material (Michrom Bioresources, Auburn, CA). The peptides were separated and eluted with a 5-to-35% acetonitrile (0.15% formic acid) gradient using a Surveyor pump plus HPLC instrument (Thermo Scientific) over 40 min, after a 15-min isocratic loading at 2.5% acetonitrile and 0.15% formic acid. Mass spectra were acquired in an LTQ-XL linear ion trap mass spectrometer (Thermo Scientific) using 10 tandem mass spectrometry scans following each survey scan over the entire run. The human and E. coli IPI forward and reverse concatenated databases were queried with SEQUEST software, requiring a 2-Da precursor mass tolerance, tryptic peptide matches, +57.02 Da on cysteine residues, and allowing +15.99 Da for oxidation of methionine residues. Using XCorr and ΔCn scores, peptide matches were filtered to a false discovery rate of less than 0.01% and when proteins were required to have at least three peptides identified there were no remaining hits matching to the reverse database. For in-solution digestion and subsequent analyses, recombinant proteins (6 μg) were concentrated using a Savant SpeedVac concentrator (Thermo Scientific) and then reconstituted in 50mM ammonium bicarbonate buffer containing 4% acetonitrile. Proteins were reduced in 100 mM DTT for 1 hr at 56°C, alkylated in 200 mM iodoacetamide for 45 min at room temp, and concentrated using a SpeedVac. Protein digestion was performed by reconstitution in digestion solution containing 100 mM ammonium bicarbonate, 4% acetonitrile, and 6 ng/¼l mass spec grade trypsin (Promega) and incubation at 37°C for 18 hr. Digestion was terminated with the addition of 10% formic acid, and samples were processed by ZipTip C18 P10 (Millipore). Liquid chromatography-mass spectrometry based protein identification was performed on a linear ion trap (LTQ)-Orbitrap Discovery mass spectrometer coupled to a Surveyor MS Pump Plus (Thermo Fisher Scientific). Tryptic peptides were loaded onto a 100 ¼m x 120 mm capillary column packed with MAGIC C18 (5 μm particle size, 20 nm pore size, Michrom Bioresources) at a flow rate of 500 nl/min. Separated peptides were introduced into the linear ion trap via a nanospray ionization source and a laser pulled ~3 μm orifice with a spray voltage of 1.8 kV. Standard “top-ten” data dependent acquisition was used in which an Orbitrap survey scan from m/z 360–1600 at 30,000 resolution was paralleled by 10 collision-induced dissociation MS/MS scans of the most abundant ions in the LTQ. Product ion spectra were searched in a target-decoy fashion using SEQUEST on Proteome Discoverer 1.4 (Thermo Fisher Scientific) against a curated E. coli database with apoSAA, human SAA1 and apoA1, and mouse SAA1 sequences incorporated. Search parameters were as follows: 1) full trypsin enzymatic activity; 2) two missed cleavages; 3) peptides between the MW of 350 – 5000 Da; 4) mass tolerance at 20 ppm for precursor ions and at 0.8 Da for fragment ions; and 5) dynamic modifications on methionine (+15.99 Da: oxidation) and static modification on cysteine (+57.02 Da: carbamidomethylation). Filters were applied to limit the false positive (FP) rates to less than 1% in the data sets. Assigned protein gene names and aliases were compared to the UniProtKB (33) and EcoProDB (34) databases, and the EcoTopic “LipoProteome” of EcoGene (35) was used to identify verified and probable E. coli lipoproteins. Data from the mass spectrometry analysis are included in Supplementary Table 1.
Statistics
Data were analyzed by one-way ANOVA and Dunnett’s or Tukey’s multiple comparisons tests, or by two-way ANOVA and Tukey’s multiple comparisons test using GraphPad Prism 7.04 for Windows (GraphPad Software, Inc., La Jolla, CA.). A post-test corrected p value smaller than 0.05 was considered statistically significant.
Results
E. coli-derived recombinant SAA proteins induce pro-inflammatory cytokine production, and Th17 polarization.
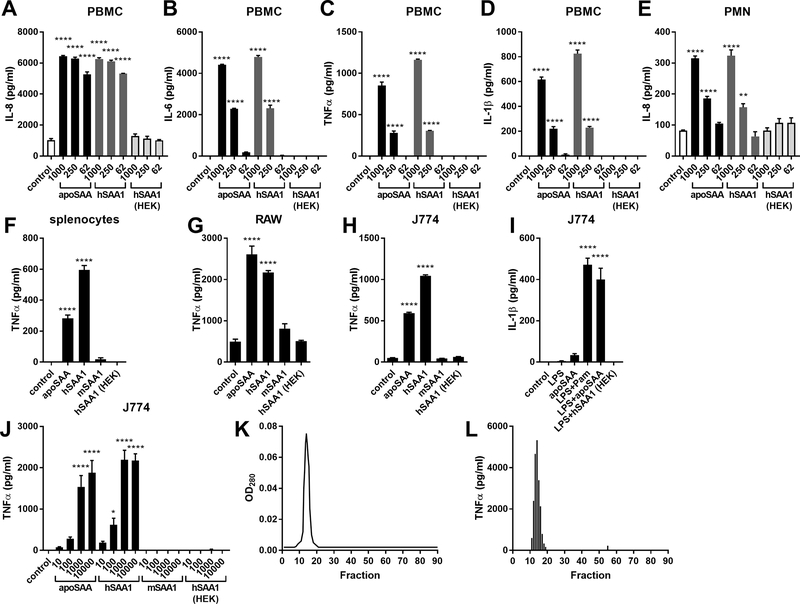
To compare the pro-inflammatory effects of SAA from different sources, we purchased recombinant SAA proteins from a number of vendors. apoSAA represents the most commonly used recombinant form of SAA, and was able to dose-dependently induce IL-8, IL-6, TNFα, and IL-1β production from human PBMCs (Fig 1A-D) and IL-8 from human neutrophils (Fig 1E). Since apoSAA is human SAA1 made in E. coli, but in which amino acids 60 and 71 are from SAA2, we also stimulated human PBMCs and neutrophils with human SAA1, also made from E. coli by the same vendor. Like apoSAA, hSAA1 induced robust pro-inflammatory cytokine production, whereas neither mouse SAA1 produced in E. coli (mSAA1) nor human SAA1 produced in human embryonic kidney (HEK) cells induced pro-inflammatory cytokine production from human PBMCs and neutrophils splenocytes (Fig 1A). Similar results, using TNFα as an indicator of pro-inflammatory cytokine production, were seen in primary mouse splenocytes (Fig 1F), as well as the RAW (Fig 1G) and J774 (Fig 1H) mouse macrophage cell lines. Consequently, subsequent studies were conducted using J774 cells. apoSAA has been reported by us (4, 36) and others (11, 17) to activate the Nlrp3 inflammasome to induce IL-1β secretion. Whereas the addition of apoSAA, or the synthetic bacterial lipopeptide Pam3CSK4, to LPS-primed J774 macrophages augmented IL-1β secretion, E. coli-derived mouse SAA1 and HEK-derived human SAA1 did not (Fig 1I). A dose-response study in J774 macrophages revealed that the E. coli-derived human SAA1 proteins elicited maximal TNFα production at 1 ¼g/ml, whereas the mouse SAA1 and HEK-derived human SAA1 did not induce TNFα production even at concentrations as high as 10 ¼g/ml (Fig 1J). apoSAA was subsequently separated into 90 fractions using size exclusion chromatography (FPLC). The fractions with the highest abundance of protein eluted at a time equivalent to an approximately 72 kDa standard (Fig 1K), suggesting that native SAA exists as a hexamer, as has been previously reported (37). We also found that only those fractions containing abundant quantities of SAA were capable of eliciting TNFα production (Fig. 1L). These results suggest that the stimulatory capacity of E. coli-derived SAA is associated with the native protein and cannot be physically separated by size-exclusion chromatography.
Figure 1.
Recombinant SAA produced in E. coli, but not produced from eukaryotic cells, stimulates pro-inflammatory cytokine production. Primary human PBMCs (A-D) and neutrophils (PMNs) (E) were unstimulated (control) or stimulated with 62, 250, or 1000 ng/ml of SAA from different sources for 24 (PBMC) or 3 hours (PMN), after which cytokine concentrations were measured by ELISA. Primary C57BL/6J mouse splenocytes (F), mouse RAW macrophage cells (G), and J774 mouse macrophages (H) were unstimulated (control) or stimulated with 1¼g/ml of SAA from different sources. Culture supernatants were collected 24 hours later and TNFα concentrations were measured by ELISA. J774 mouse macrophages were unstimulated (control) or stimulated for 24 hours with 100 ng/ml of LPS, 1 ¼g/ml of apoSAA, LPS + 100 ng/ml of the lipopeptide Pam3CSK4 (Pam), LPS + apoSAA, or LPS + 1 ¼g/ml of human SAA1 made in HEK cells, and IL-1β concentrations in culture supernatants were measured by ELISA (I). J774 mouse macrophages were unstimulated (control) or stimulated for 24 hours with 10, 100, or 1000 ng/ml of SAA from different sources and TNFα concentrations in culture supernatants were measured 24 hours later by ELISA (J). 250 ¼g of apoSAA was separated by FPLC using a size exclusion column and 90 fractions were collected (K). Fractions obtained from FPLC were diluted 1:10 in serum-free media and used to stimulate J774 cells for 24 hours after which TNFα concentrations were measured by ELISA (L). Data are mean ± SEM and are representative of three independent experiments with n = 4 per group. Statistics were analyzed using one-way ANOVA with Dunnett’s test for multiple comparisons. **** p < 0.0001, ** p < 0.01, * p < 0.05.
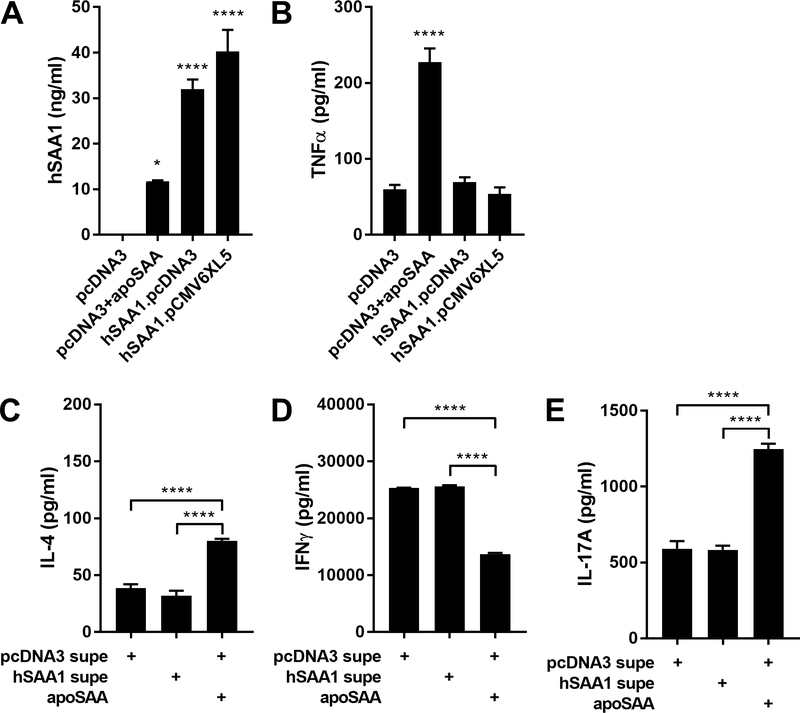
We next transfected HEK cells with empty vectors or plasmids encoding human SAA1, collected and concentrated the conditioned media, and measured production of SAA1 of approximately 30–40 ng/ml (Fig. 2A). However, when exposed to J774 cells, these same SAA1-rich culture supernatants were not able to stimulate TNFα production, in contrast to that elicited by the addition of 10 ng/ml apoSAA to the conditioned media from empty vector-transfected cells (Fig. 2B). In addition to its induction of pro-inflammatory cytokine production, we (4) and others (38) have reported that stimulation of dendritic cells (DCs) with apoSAA elicits the production of cytokines capable of promoting Th17 differentiation. However, exposure of DCs to conditioned media from empty vector- or human SAA1-transfected HEK cells, followed by subsequent polyclonal stimulation of naïve CD4+ T cells in the DC-derived media, was not able to decrease IFNγ production or promote IL-4 and IL-17A production, in contrast to apoSAA (Fig 2C-D). These results suggest that the pro-inflammatory and Th17-inducing effects of SAA may be limited to those proteins made in E. coli.
Figure 2.
apoSAA, but not SAA1 produced from eukaryotic cells, induces TNFα secretion from macrophages and promotes IL-17A production from CD4 T cells. HEK cells were transiently transfected with empty vector (pcDNA3) or plasmids encoding human SAA1 (hSAA1.pcDNA3 or hSAA1.pCMV6XL5). 48 hours later, culture supernatants were collected and concentrated using Amicon Ultra centrifugal concentrators, SAA1 concentrations were measured by ELISA (A), and the culture supernatants were used to stimulate J774 cells for 24 hours (in the absence or presence of 10 ng/ml apoSAA) after which TNFα production was measured by ELISA (B). Similarly-prepared HEK supernatants were added to BMDCs for 48 hours, their culture supernatants were collected and added to CD4+ T-cells that were stimulated with 5 ¼g/ml immobilized anti-CD3 and 2 ¼g/ml soluble anti-CD28 for 72 hours after which IL-4 (C), IFNγ (D), and IL-17A (E) were measured by ELISA. Data are mean ± SEM and are representative of three (A-B) or two (C-E) independent experiments with n = 4 per group. Statistics were analyzed using one-way ANOVA with Dunnett’s (A-B) or Tukey’s (C-E) test for multiple comparisons. * p < 0.05, ** p < 0.01, **** p < 0.0001.
Human SAA1 proteins made in E. coli stimulate TLR2.
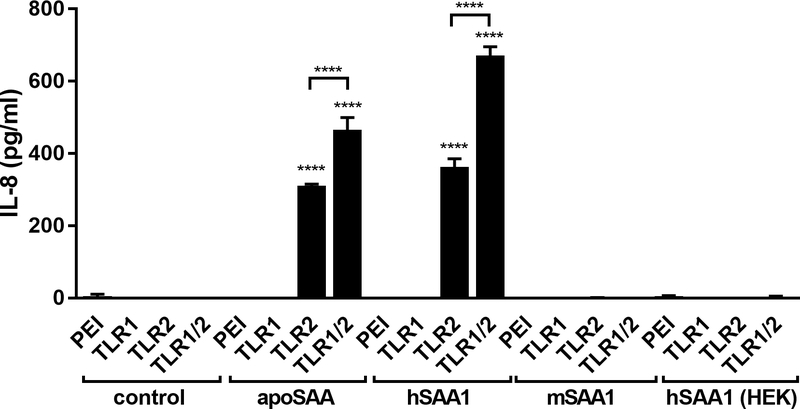
We (4) and others (5–18) have previously reported that the innate immune-stimulating capacity of apoSAA is not due to contaminating endotoxin (lipopolysaccharide) stimulating TLR4, but is instead mediated by protein in the preparation through stimulation of TLR2. Interestingly, the TLR2-stimulating capacity of human SAA1 has been demonstrated almost exclusively through the use of E. coli-derived recombinant human SAA1 proteins either generated by the investigators (6) or purchased from a reputable commercial vendor (5–17). A relatively common post-translational modification of bacterial proteins is through lipidation (acylation), which occurs at specific cysteine amino acids in the context of appropriate neighboring amino acids, a sequence defined as a lipobox (39). Heterodimers of TLR2 and TLR6 signal in response to diacylated lipoproteins, whereas heterodimers of TLR2 and TLR1 signal in response to triacylated lipoproteins. As apoSAA has been previously reported to signal through TLR1/2 heterodimers (7), we transfected HEK cells with TLR1, TLR2, or both TLR1 and TLR2, then stimulated the cells with commercially sourced recombinant SAA proteins. E. coli-derived human SAA1 proteins were able to stimulate TLR2- or TLR1/2-transfected cells and induce the production of IL-8, whereas mouse SAA1 or human SAA1 made in eukaryotic (HEK) cells were not (Fig. 3). Since the human SAA1 and apoSAA protein sequences do not contain a lipobox or even a cysteine that is required for acylation, these results implicate that bacterial lipoproteins may be present in the E. coli-derived SAA1 recombinant proteins and mediate the TLR2-dependent pro-inflammatory effects ascribed to SAA.
Figure 3.
apoSAA stimulates pro-inflammatory cytokine production via TLR2. HEK cells were exposed to transfection reagent (PEI) or were transiently transfected with plasmids encoding human TLR1, TLR2, or both TLR1 and TLR2 (TLR1/2). After 48 hours, the cells were then unexposed (control) or stimulated with 1¼g/ml of SAA from different sources for 24 hours, and IL-8 was measured by ELISA. Data are mean ± SEM and are representative of three independent experiments with n = 4 per group. Statistics were analyzed using one-way ANOVA with Tukey’s test for multiple comparisons. **** p < 0.0001.
Lipoproteins associated with human SAA1 elicit pro-inflammatory cytokine production.
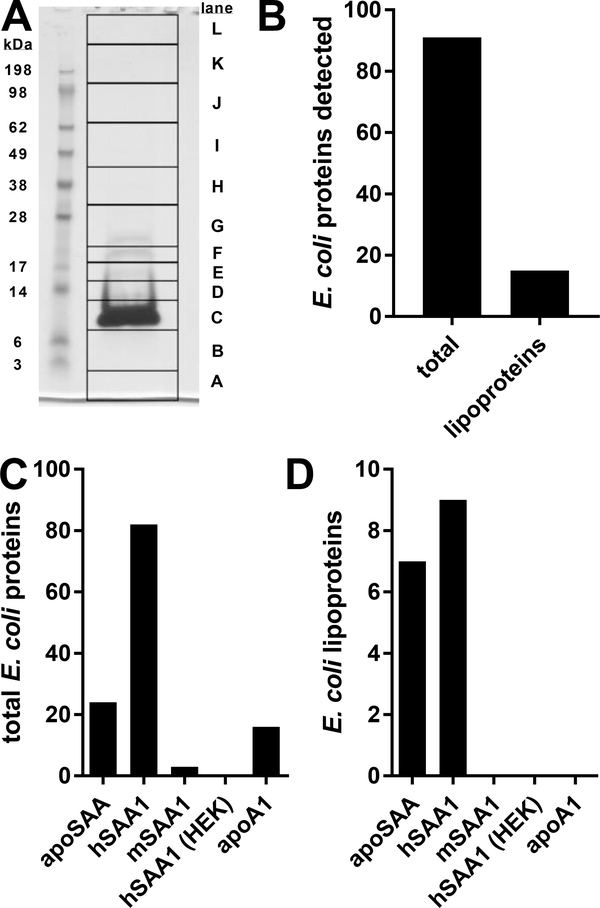
To determine whether bacterial proteins were present in apoSAA, we separated the preparation by reducing SDS-PAGE, extracted proteins from 12 gel slices, performed trypsin digestion, and analyzed tryptic peptides by mass spectrometry. We easily visualized on the stained gel a predominant 12 kDa band in the apoSAA prep, as was expected based on its molecular weight (Fig. 4A). An identical pattern was observed by the more sensitive technique of silver staining (not shown), indicating that there were not other proteins present besides SAA that were particularly abundant. Whereas the only human protein sequence present in our analysis was SAA1, the gel lane also contained fragments from 91 E. coli proteins, 15 of which were probable or predicted lipoproteins (Fig. 4B). Furthermore, mass spec analysis of tryptic peptides from additional commercial sources of SAA1 showed that only human SAA1 proteins derived from E. coli contained bacterial lipoproteins, whereas mouse SAA1 made from E. coli, human SAA1 made in HEK cells, and apoA1 made from E. coli by the same manufacturer as the E. coli-derived apoSAA and hSAA1 did not contain E. coli lipoproteins (Fig. 4C-D). These data implicate the selectivity of human SAA1 for interacting with specific bacterial proteins and lipoproteins, and the possibility that this activity is part of the protein’s pro-inflammatory function.
Figure 4.
apoSAA contains bacterial lipoproteins. 50 ¼g of apoSAA was reduced, denatured, and separated by PAGE (A). The 12 indicated regions were subjected to in-gel digestion with trypsin. The extracted tryptic peptides were analyzed by liquid chromatography-tandem mass spectrometry, and the resulting spectra were searched against a database for human and E. coli proteins, including predicted and probable E. coli liproteins (B). Six ¼g of SAA from different sources were digested in-solution and similarly analyzed by mass spectrometry. Total E. coli proteins identified (C) as well as predicted and probable E. coli lipoproteins identified (D) are indicated. Data are from two independent experiments.
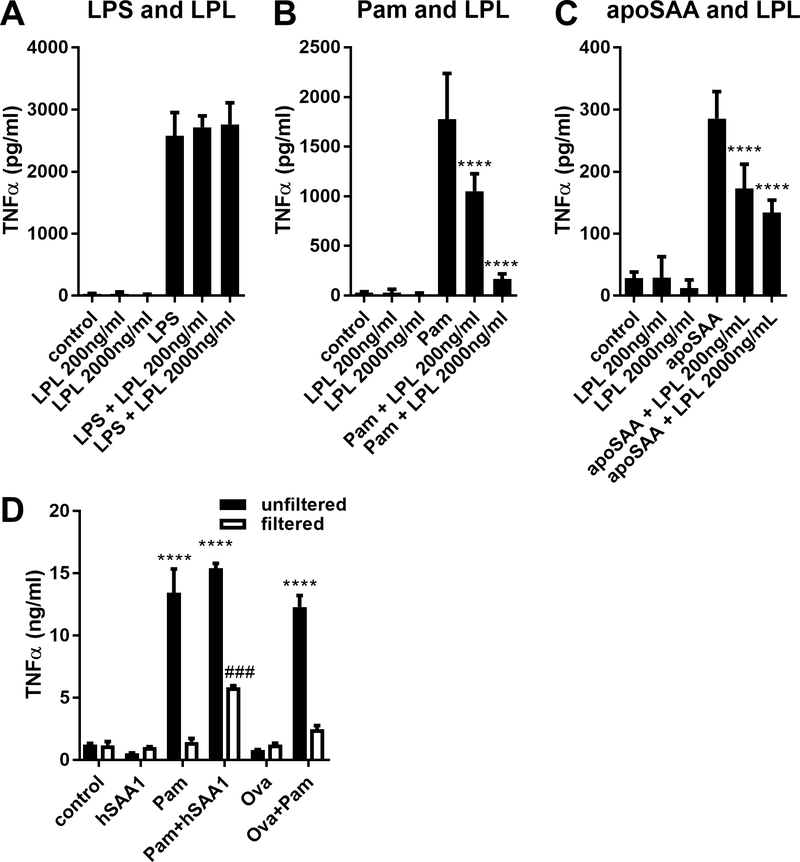
We next sought to determine whether lipoproteins were necessary and sufficient for the pro-inflammatory effects of human SAA1. Therefore, we treated LPS, the triacylated lipopeptide Pam3CSK4, or apoSAA with lipoprotein lipase (LPL), which deactivates the capacity of bacterial proteins to activate TLR2 (40). Whereas LPL had no impact on LPS (Fig. 5A), LPL dose-dependently inhibited the capacity of both Pam3CSK4 (Fig. 5B) and apoSAA (Fig. 5C) to stimulate TNFα production from J774 macrophages. We next exposed ovalbumin or human SAA1 produced in HEK cells to Pam3CSK4, subjected the preparations to centrifugation through 3 kDa cutoff filters to remove the 1.5 kDa Pam3CSK4, and stimulated J774 cells with the unfiltered preparation or the retained (filtered) fractions that were readjusted to the original volume. Whereas neither human SAA1 nor ovalbumin stimulated TNFα production, and the unfiltered preparations containing Pam3CSK4 all stimulated TNFα production, the filtered fractions from Pam3CSK4 incubated with human SAA1, but not from Pam3CSK4 incubated with ovalbumin, stimulated TNFα production (Fig. 5D). These results demonstrate that human SAA1 produced from eukaryotic cells can bind lipopeptides that stimulate pro-inflammatory cytokine production.
Figure 5.
Lipopeptides associated with SAA confer its ability to stimulate TNFα production. 100 ng/ml LPS (A), 100 ng/ml Pam3CSK4 (Pam, B), and 1¼g/ml apoSAA (C) were incubated with lipoprotein lipase (LPL), and then used to stimulate J774 cells. Culture supernatants were collected 24 hours later and TNFα concentrations were measured by ELISA. Human SAA1 from Origene produced in HEK cells (hSAA1) or ovalbumin (Ova) was unexposed or exposed to Pam3CSK4 (Pam) overnight (control), and some of the preparation was subjected to concentration of proteins >3 kDa using concentrated using Amicon Ultra centrifugal concentrators (filtered). Control and filtered preparations were used to stimulate J774 cells for 24 hours after which TNFα concentrations in culture media were measured by ELISA (D). Data are mean ± SEM and are representative of three (A-C) or two (D) independent experiments with n = 4 per group. Statistics were analyzed using one-way ANOVA with Dunnett’s test for multiple comparisons (A-C) or two-way ANOVA with Tukey’s test for multiple comparisons (D). **** p < 0.0001 compared to Pam (B), apoSAA (C), or control (D). ### p < 0.001 compared to unfiltered Pam+hSAA1 (D).
Discussion
Studies conducted using recombinant Serum Amyloid A made in E. coli have implicated this family of acute phase proteins as pro-inflammatory mediators (5–18), yet several other reports demonstrate no pro-inflammatory effect of the endogenous protein enriched from human serum (19, 20) or in transgenic mice producing high levels of circulating human (20, 22) or mouse (21) SAA1. Our own previous work using recombinant apoSAA demonstrated that the pro-inflammatory activity of human SAA1 is mediated by TLR2 and is inhibitable by proteinase K, and is present in TLR4-deficient cells and not inhibitable by polymyxin B (4). Consequently, it is widely appreciated that the pro-inflammatory activities of SAA are not a consequence of endotoxin contamination. Instead, our studies reported herein demonstrate that bacterial proteins, including lipoproteins, associate with recombinant human SAA1 produced in E. coli and mediate the activation of TLR2 and Nlrp3 to induce the production of pro-inflammatory and Th17-promoting cytokines. This association between hSAA1 and bacterial lipoproteins appears to be necessary and sufficient for the cytokine-inducing effects since even though recombinant apoA1 contained some bacterial proteins, they were not lipoproteins or known TLR2 agonists, and were not able to induce TNFα production from J774 macrophages. Whereas the ability of lipoprotein lipase to diminish apoSAA-induced TNFα production is dose-dependent, significant, and substantial, it is incomplete. While reasons for this are uncertain, possibilities include inadequate access of lipoproteins to the enzyme’s active site, in contrast to the effective inactivation of the lipopeptide Pam3CSK4. Additionally, non-lipopeptides, such as outer membrane protein A (OmpA), are present in the apoSAA preparation that remain capable of stimulating TLR2 and inducing TNFα production despite lipoprotein lipase. Whereas lipoprotein lipase has activity on both lipoteichoic acids and lipoproteins (40), Gram-negative E. coli do not contain lipotochoic acid, implicating that the pro-inflammatory activity of recombinant human SAA1 is indeed largely conferred by a lipoprotein. In addition to their ability to stimulate TLR2, lipoproteins such as palmitate-conjugated albumin can serve as a strong signal for activation of the intracellular pattern recognition receptor, Nlrp3 (41). Although SAA is not directly palmitoylated, perhaps contaminating bacterial (lipo)proteins are also responsible for the Nlrp3-stimulating capacity of apoSAA (4, 11, 17, 36), especially considering the capacity of Pam3CSK4 to augment IL-1β release.
The acylation of bacterial lipoproteins occurs at the cell membrane (39), whereas recombinant proteins accumulate in intracellular inclusion bodies prior to acylation (42). Consequently, it is likely that the association of hSAA1 and bacterial lipoproteins occurs during processing to extract the recombinant protein, not during their biosynthesis, implying that similar associations may also occur between circulating SAA and bacterial proteins in vivo during infection. Interestingly, the E. coli-derived recombinant apoSAA and hSAA1 preparations used in our studies contained outer membrane protein A (OmpA), a bacterial protein previously reported to activate macrophages (43) and DCs (44) via TLR2 and to interact with SAA (27). The E. coli-derived recombinant human SAA1 proteins also contained several bacterial lipoproteins, including periplasmic methionine binding lipoprotein (MetQ), peptidoglycan-associated lipoprotein (Pal), DUF3053 family lipoprotein (YiaF), and the lipoproteins DcrB and AcrA (Supplemental Table). However, neither apoSAA nor hSAA1 contained the triacylated Braun Lipoprotein that is abundant in the E. coli outer membrane and stimulates TLR2 (45). Furthermore, none of these bacterial proteins were present in the E. coli-derived recombinant mouse SAA1 used in our studies.
Our results support several earlier studies implicating that acute phase SAA lacks pro-inflammatory activity (19–21). Interestingly, these studies were conducted using SAA enriched from the serum/plasma of subjects with rheumatoid arthritis, a sterile inflammatory disease, or from cardiac surgery patients and associated with HDL. It will be important to examine whether during bacterial infection the highly-induced levels of SAA, some of which exist as soluble proteins independent of HDL but that can also form HDL-sized complexes with phospholipids (30), interact with bacterial lipoproteins, and gain pro-inflammatory activity.
The reasons for the distinct differences between human and mouse SAA1 proteins to associate with bacterial (lipo)proteins are uncertain. Despite their evolutionary relatedness based on genomic organization and regulation, the secreted form of human SAA1 has 75% amino acid identity with mouse SAA1 (by BLAST (46)), which may account for the aforementioned differences. However, there is 80% identity between the N-terminal sequences of human SAA1 (aa 11–58) that have been reported to confer SAA’s TLR2-dependent pro-inflammatory effects (18) and those in mouse SAA1. Whereas human SAA1 derived from HEK cells did not promote the Th17 differentiation induced by E. coli-derived human SAA1, it is very interesting that SAA1/2 double knockouts elicit diminished responses to the Th17 induction elicited by Segmented Filamentous Bacteria infection, and that high concentrations of recombinant mouse SAA1 were able to increase IL-17A and IL-17F production in a dendritic cell-independent manner (47). These results suggest additional activities of SAA1 in innate and adaptive immunity that are perhaps related to cell survival and immunometabolism (48). In fact, endogenous SAA1 isolated from human serum that lacked pro-inflammatory effects did enhance the survival of primary human neutrophils by promoting anti-apoptotic pathways (19). This ability of SAA1 to affect inflammatory cells may be due to differences between recombinant and endogenous forms of SAA1, differences between the responses of primary cells in the setting of acute inflammation in vivo, differential SAA isoform expression at sites of inflammation, and the stimulation of cells through receptors besides TLR2, TLR4, and NLRP3 (e.g. FPR2/FPRL1, SB-R1, RAGE) that have been implicated to elicit the effects of SAA.
Our results call for a reevaluation of the pro-inflammatory effects ascribed to SAA through the use of E. coli-derived recombinant proteins. For researchers requiring a recombinant form of human SAA for experimentation, it is reassuring that a commercial source made in eukaryotic cells and containing no bacterial proteins is available. It is highly recommended that such a eukaryotic cell-derived source of SAA be used for all future studies in which its potential participation in inflammation is evaluated.
Supplementary Material
Acknowledgments
This paper is in honor of Dr. Edward Burgess, a promising doctoral candidate whose life was cut short by malignant mesothelioma and who was posthumously conferred the PhD degree. We thank Marion Weir for helping with some of the mass spectrometry analyses. We thank Renee Stapleton, MD, PhD and Sara Ardren for assistance in procuring human blood samples.
This work was funded by National Institutes of Health grants R01 HL107291 (MEP), R01 HL133920 (MEP), R01 AI103003 (MJW), T32 HL076122 (EJB and MJR), P30 GM103532, P20 GM103496, and P20GM103449. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103449. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Abbreviations used in this article:
- apoA1
human apolipoprotein A1
- apoSAA
the apolipoprotein form of human SAA1
- BMDC
bone marrow-derived dendritic cell
- DC
dendritic cell
- HEK
human embryonic kidney
- FPLC
fast performance liquid chromatography
- Nlrp3
NACHT, LRR and PYD domains-containing protein
- Pam3CSK4
synthetic triacylated lipoprotein (N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-[R]-cysteinyl-[S]-seryl-[S]-lysyl-[S]-lysyl-[S]-lysyl-[S]-lysine)
- PEI
polyethylenimine
- SAA
Serum Amyloid A
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Uhlar CM, and Whitehead AS 1999. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 265: 501–523. [DOI] [PubMed] [Google Scholar]
- 2.Ramadori G, Sipe JD, and Colten HR 1985. Expression and regulation of the murine serum amyloid A (SAA) gene in extrahepatic sites. J Immunol 135: 3645–3647. [PubMed] [Google Scholar]
- 3.Steel DM, Sellar GC, Uhlar CM, Simon S, DeBeer FC, and Whitehead AS 1993. A constitutively expressed serum amyloid A protein gene (SAA4) is closely linked to, and shares structural similarities with, an acute-phase serum amyloid A protein gene (SAA2). Genomics 16: 447–454. [DOI] [PubMed] [Google Scholar]
- 4.Ather JL, Ckless K, Martin R, Foley KL, Suratt BT, Boyson JE, Fitzgerald KA, Flavell RA, Eisenbarth SC, and Poynter ME 2011. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol 187: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chami B, Barrie N, Cai X, Wang X, Paul M, Morton-Chandra R, Sharland A, Dennis JM, Freedman SB, and Witting PK 2015. Serum amyloid A receptor blockade and incorporation into high-density lipoprotein modulates its pro-inflammatory and pro-thrombotic activities on vascular endothelial cells. Int. J. Mol. Sci 16: 11101–11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Zhou H, Cheng N, Qian F, and Ye RD 2014. Serum amyloid A1 isoforms display different efficacy at Toll-like receptor 2 and formyl peptide receptor 2. Immunobiology 219: 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng N, He R, Tian J, Ye PP, and Ye RD 2008. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol 181: 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly M, Rooney PR, McGarry T, Maratha AX, McCormick J, Miggin SM, Veale DJ, and Fearon U 2016. Acute serum amyloid A is an endogenous TLR2 ligand that mediates inflammatory and angiogenic mechanisms. Ann. Rheum. Dis 75: 1392–1398. [DOI] [PubMed] [Google Scholar]
- 9.He RL, Zhou J, Hanson CZ, Chen J, Cheng N, and Ye RD 2009. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood 113: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakota K, Mrak-Poljsak K, Bozic B, Tomsic M, and Sodin-Semrl S 2013. Serum amyloid A activation of human coronary artery endothelial cells exhibits a neutrophil promoting molecular profile. Microvasc. Res 90: 55–63. [DOI] [PubMed] [Google Scholar]
- 11.Niemi K, Teirila L, Lappalainen J, Rajamaki K, Baumann MH, Oorni K, Wolff H, Kovanen PT, Matikainen S, and Eklund KK 2011. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol 186: 6119–6128. [DOI] [PubMed] [Google Scholar]
- 12.Nishida E, Aino M, Kobayashi SI, Okada K, Ohno T, Kikuchi T, Hayashi JI, Yamamoto G, Hasegawa Y, and Mitani A 2016. Serum Amyloid A Promotes E-Selectin Expression via Toll-Like Receptor 2 in Human Aortic Endothelial Cells. Mediators Inflamm. 2016: 7150509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Reilly S, Cant R, Ciechomska M, Finnigan J, Oakley F, Hambleton S, and van Laar JM 2014. Serum amyloid A induces interleukin-6 in dermal fibroblasts via Toll-like receptor 2, interleukin-1 receptor-associated kinase 4 and nuclear factor-kappaB. Immunology 143: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passey SL, Bozinovski S, Vlahos R, Anderson GP, and Hansen MJ 2016. Serum Amyloid A Induces Toll-Like Receptor 2-Dependent Inflammatory Cytokine Expression and Atrophy in C2C12 Skeletal Muscle Myotubes. PLoS One 11: e0146882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidl SE, Pessolano LG Jr., Bishop CA, Best M, Rich CB, Stone PJ, and Schreiber BM 2017. Toll-like receptor 2 activation and serum amyloid A regulate smooth muscle cell extracellular matrix. PLoS One 12: e0171711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Zhu Z, Cheng N, Yan Q, and Ye RD 2014. Serum amyloid A induces interleukin-33 expression through an IRF7-dependent pathway. Eur J Immunol 44: 2153–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu N, Liu S, Yi X, Zhang S, and Ding Y 2015. Serum amyloid A induces interleukin-1beta secretion from keratinocytes via the NACHT, LRR and PYD domains-containing protein 3 inflammasome. Clin. Exp. Immunol. 179: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Chen M, Zhang G, and Ye RD 2017. Suppression of Lipopolysaccharide-Induced Inflammatory Response by Fragments from Serum Amyloid A. J Immunol 199: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 19.Bjorkman L, Raynes JG, Shah C, Karlsson A, Dahlgren C, and Bylund J 2010. The proinflammatory activity of recombinant serum amyloid A is not shared by the endogenous protein in the circulation. Arthritis Rheum 62: 1660–1665. [DOI] [PubMed] [Google Scholar]
- 20.Christenson K, Bjorkman L, Ahlin S, Olsson M, Sjoholm K, Karlsson A, and Bylund J 2013. Endogenous Acute Phase Serum Amyloid A Lacks Pro-Inflammatory Activity, Contrasting the Two Recombinant Variants That Activate Human Neutrophils through Different Receptors. Front Immunol 4: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MH, de Beer MC, Wroblewski JM, Webb NR, and de Beer FC 2013. SAA does not induce cytokine production in physiological conditions. Cytokine 61: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahlin S, Olsson M, Olsson B, Svensson PA, and Sjoholm K 2013. No evidence for a role of adipose tissue-derived serum amyloid a in the development of insulin resistance or obesity-related inflammation in hSAA1(+/−) transgenic mice. PLoS One 8: e72204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Buck M, Gouwy M, Wang JM, Van Snick J, Opdenakker G, Struyf S, and Van Damme J 2016. Structure and Expression of Different Serum Amyloid A (SAA) Variants and their Concentration-Dependent Functions During Host Insults. Curr. Med. Chem 23: 1725–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Z, Cai L, Jiang J, Chang KS, van der Westhuyzen DR, and Luo G 2007. Human serum amyloid A protein inhibits hepatitis C virus entry into cells. J Virol 81: 6128–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavie M, Voisset C, Vu-Dac N, Zurawski V, Duverlie G, Wychowski C, and Dubuisson J 2006. Serum amyloid A has antiviral activity against hepatitis C virus by inhibiting virus entry in a cell culture system. Hepatology 44: 1626–1634. [DOI] [PubMed] [Google Scholar]
- 26.Misse D, Yssel H, Trabattoni D, Oblet C, Lo Caputo S, Mazzotta F, Pene J, Gonzalez JP, Clerici M, and Veas F 2007. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J Immunol 178: 407–415. [DOI] [PubMed] [Google Scholar]
- 27.Shah C, Hari-Dass R, and Raynes JG 2006. Serum amyloid A is an innate immune opsonin for Gram-negative bacteria. Blood 108: 1751–1757. [DOI] [PubMed] [Google Scholar]
- 28.Hari-Dass R, Shah C, Meyer DJ, and Raynes JG 2005. Serum amyloid A protein binds to outer membrane protein A of gram-negative bacteria. J Biol Chem 280: 18562–18567. [DOI] [PubMed] [Google Scholar]
- 29.Derebe MG, Zlatkov CM, Gattu S, Ruhn KA, Vaishnava S, Diehl GE, MacMillan JB, Williams NS, and Hooper LV 2014. Serum amyloid A is a retinol binding protein that transports retinol during bacterial infection. Elife 3: e03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frame NM, Jayaraman S, Gantz DL, and Gursky O 2017. Serum amyloid A self-assembles with phospholipids to form stable protein-rich nanoparticles with a distinct structure: A hypothetical function of SAA as a “molecular mop” in immune response. J. Struct. Biol. 200: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ather JL, and Poynter ME 2018. Serum amyloid A3 is required for normal weight and immunometabolic function in mice. PLoS One 13: e0192352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ather JL, Burgess EJ, Hoyt LR, Randall MJ, Mandal MK, Matthews DE, Boyson JE, and Poynter ME 2016. Uricase Inhibits Nitrogen Dioxide-Promoted Allergic Sensitization to Inhaled Ovalbumin Independent of Uric Acid Catabolism. J Immunol 197: 1720–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.UniProt Consortium T 2018. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 46: 2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yun H, Lee JW, Jeong J, Chung J, Park JM, Myoung HN, and Lee SY 2007. EcoProDB: the Escherichia coli protein database. Bioinformatics 23: 2501–2503. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, and Rudd KE 2013. EcoGene 3.0. Nucleic Acids Res. 41: D613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poynter ME 2012. Airway epithelial regulation of allergic sensitization in asthma. Pulm. Pharmacol. Ther 25: 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu J, Yu Y, Zhu I, Cheng Y, and Sun PD 2014. Structural mechanism of serum amyloid A-mediated inflammatory amyloidosis. Proc Natl Acad Sci U S A 111: 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, and Littman DR 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babu MM, Priya ML, Selvan AT, Madera M, Gough J, Aravind L, and Sankaran K 2006. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J. Bacteriol 188: 2761–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seo HS, and Nahm MH 2009. Lipoprotein lipase and hydrofluoric acid deactivate both bacterial lipoproteins and lipoteichoic acids, but platelet-activating factor-acetylhydrolase degrades only lipoteichoic acids. Clin. Vaccine Immunol. 16: 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, and Ting JP 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12: 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kane JF, and Hartley DL 1991. Properties of recombinant protein-containing inclusion bodies in Escherichia coli. Bioprocess Technol. 12: 121–145. [PubMed] [Google Scholar]
- 43.Soulas C, Baussant T, Aubry JP, Delneste Y, Barillat N, Caron G, Renno T, Bonnefoy JY, and Jeannin P 2000. Outer membrane protein A (OmpA) binds to and activates human macrophages. J Immunol 165: 2335–2340. [DOI] [PubMed] [Google Scholar]
- 44.Jeannin P, Renno T, Goetsch L, Miconnet I, Aubry JP, Delneste Y, Herbault N, Baussant T, Magistrelli G, Soulas C, Romero P, Cerottini JC, and Bonnefoy JY 2000. OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat Immunol 1: 502–509. [DOI] [PubMed] [Google Scholar]
- 45.Neilsen PO, Zimmerman GA, and McIntyre TM 2001. Escherichia coli Braun lipoprotein induces a lipopolysaccharide-like endotoxic response from primary human endothelial cells. J Immunol 167: 5231–5239. [DOI] [PubMed] [Google Scholar]
- 46.Boratyn GM, Schaffer AA, Agarwala R, Altschul SF, Lipman DJ, and Madden TL 2012. Domain enhanced lookup time accelerated BLAST. Biol. Direct 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee JY, Ziel JW, Miraldi ER, Domingos AI, Bonneau R, and Littman DR 2015. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 163: 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ather JL, Fortner KA, Budd RC, Anathy V, and Poynter ME 2013. Serum amyloid A inhibits dendritic cell apoptosis to induce glucocorticoid resistance in CD4(+) T cells. Cell Death Dis. 4: e786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.