Abstract
Diabetes is a metabolic disease afflicting millions of people worldwide. A substantial fraction of world’s total healthcare expenditure is spent on treating diabetes. Hypoglycemia is a serious consequence of anti-diabetic drug therapy, because it induces metabolic alterations in brain. Metabolic alterations are one of the central mechanisms mediating hypoglycemia-related functional changes in brain. Acute, chronic, and/or recurrent hypoglycemia modulates multiple metabolic pathways, and exposure to hypoglycemia increases consumption of alternate respiratory substrates such as ketone bodies, glycogen and monocarboxylates in brain. The aim of this review is to discuss hypoglycemia-induced metabolic alterations in brain in glucose counterregulation, uptake, utilization and metabolism, cellular respiration, amino acid and lipid metabolism, and the significance of other sources of energy. The present review summarizes information on hypoglycemia-induced metabolic changes in brain of diabetic and non-diabetic subjects, and the manner in which they may affect brain function.
Keywords: Neuron, glucose, metabolomics, glucose transporters, lipid metabolism, amino acid metabolism
Introduction
Diabetes is a metabolic disease afflicting 425 million people worldwide. About $727 billion (12 percent of the world’s total healthcare expenditure) are spent on diabetes [1]. Multiple effective therapeutic approaches are available to treat diabetes and its related complications by attenuating hyperglycemia. However, these approaches also elicit hypoglycemia as a side effect [2–4]. Therefore, drug therapy-related hypoglycemia is an important problem in the management of both type 1 (T1D) and type 2 diabetes (T2D) [5]. The American Diabetes Association defines hypoglycemia as plasma glucose level of ≤70 mg/dL (≤3.9 mmol/L) [6]. Based on epidemiological and clinical studies, it appears that episodes of hypoglycemia are commonly observed in both T1D and T2D patients [3,7,8]. Episodes of hypoglycemia are frequently observed in either patients suffering from severe insulin-dependent T1D or longstanding T2D [3,9].
Severe hypoglycemia impairs cognition and is associated with dementia in diabetic subjects [10–13]. Long-term cerebral hypoperfusion in T1D patients is associated with repeated episodes of hypoglycemia [14] and is linked with increased risk of cognitive impairment and dementia [15]. Hypoglycemia-associated cognitive impairment is associated with vascular changes in brain [11]. Oxidative stress is concomitant with hypoglycemia-associated cognitive deficit [16]. Thus, the literature suggests that exposure to hypoglycemia causes brain-related dysfunction in diabetics. A detailed understanding of the effect of hypoglycemia on brain metabolites may help us design approaches to lower hypoglycemia-induced brain damage.
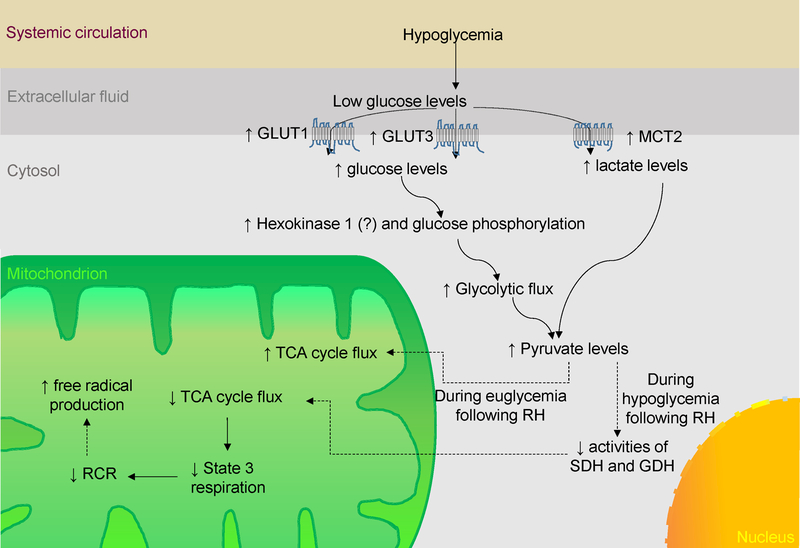
In this review article, we provide an overview of brain metabolism and how it is altered by exposure to acute, chronic and/or recurrent hypoglycemia (RH) (Fig. 1). We also summarized information about hypoglycemia-induced increase in consumption of sources of energy other than glucose.
Figure 1:
Schematic representation of the interplay of signaling pathways modulated by hypoglycemia. GLUT, Glucose transporter; GDH, Glutamate dehydrogenase; MCT2, Monocarboxylate transporter 2; RH, Recurrent hypoglycemia; RCR, Respiratory control ratio; SDH, Succinate dehydrogenase
Glucose counterregulatory mechanisms
Typically, during hypoglycemia, patients experience palpitations, anxiety, hunger, sweating, confusion, loss of consciousness and seizures, and if the hypoglycemia is sustained and severe it may result in coma and ultimately death [17]. An episode of hypoglycemia activates a number of adaptive mechanisms, which correct the fall in blood glucose levels. These mechanisms include drop in pancreatic insulin and epinephrine secretion, increase in glucagon secretion, and modulation of the autonomic nervous system [18–20]. Reduction in insulin secretion decreases metabolic dissipation of glucose from circulation and an increase in glucagon secretion stimulates hepatic glucose production by activating glycogenolysis and gluconeogenesis, ultimately leading to increased blood glucose levels [21,22]. Epinephrine activates adrenergic pathways that increase blood glucose levels by modulating the secretion of insulin and glucagon, and the rates of glucose uptake and utilization [21,22]. Sustained hypoglycemia causes the release of growth hormone and cortisol, which corrects hypoglycemia [23–25]. A prior episode of acute hypoglycemia causes decreased glucose counterregulation and autonomic responses to hypoglycemia leading to hypoglycemia unawareness, hypoglycemia-associated autonomic failure (HAAF) and problems with hypoglycemia management [26–28]. HAAF increases the risk of severe hypoglycemia many fold when the patient is under intensive anti-diabetic therapy [29,30]. Prior exposure to hypoglycemia decreases hypoglycemia-linked changes in glucagon and epinephrine levels [26,31]. Therefore, the literature cited above shows that intrinsic adaptive mechanisms of glucose counterregulation are blunted in T1D patients previously exposed to hypoglycemia, resulting in increased severity of pronounced hypoglycemia.
Alterations of metabolism in brain during hypoglycemia
Brain glucose levels during systemic hypoglycemia
Glucose is a critical energy substrate required for brain functioning [32]. During euglycemic conditions as well as during mild to moderate hypoglycemia, normal human brain depends primarily on glucose as the principal metabolic substrate obtained from the systemic circulation [33,34]. A study observed a linear relationship between plasma glucose levels and brain glucose concentration when plasma glucose values were within the physiological range [35]. It is predicted that during hypoglycemia when blood glucose concentration is 2.1 mM, the brain glucose level goes down to zero owing to glucose consumption [36]. Therefore, the glucose content in brain is regulated by blood glucose levels, and systemic hypoglycemia is expected to reduce glucose availability to the brain.
Glucose uptake
Glucose transporters (GLUTs) belong to the Major Facilitator Superfamily group of proteins [37] that mediates facilitative passive diffusion of glucose across membranes. GLUTs are approximately 500 amino acid-long proteins containing 12 transmembrane-spanning α helices and N-linked oligosaccharide moieties [38–41]. GLUTs are subdivided into three classes based on amino acid sequence homology. Class I is comprised of GLUT1 to GLUT4 (differentiated by varied tissue distributions and hormonal regulation). Class II contains GLUT5 (a fructose-specific transporter) and three related transporter proteins - GLUT7, GLUT9, and GLUT11 [42]. Class III of GLUTs are characterized by an absence of a glycosylation site on the first extracellular linker domain and the presence of glycosylation sites in the ninth transmembrane domain [43]. GLUT1, GLUT3, and GLUT5 are expressed in brain [44–52]. Glucose transporters expressed on microglia are GLUT5 [51]. Glucose is transported from the systemic circulation into the brain across the blood brain barrier via GLUT1 present on microvascular endothelial cells [53,54]. GLUT1 is also expressed on astrocytes [54]. Glucose transport occurs in neurons via GLUT3 (an isoform of GLUT abundantly expressed in the neurons) [55]. Hypoglycemia causes upregulation of GLUT1 mRNA as well as protein levels in the blood brain barrier and brain tissue [53,56–58]. Further, chronic hypoglycemia increases GLUT1 levels in brain capillaries [58]. Repeated insulin administration-induced chronic hypoglycemia for 4–8 days increases the levels of GLUT3 in rat brain [59–61]. Hypoglycemia causes upregulation of monocarboxylic acid transporters (MCT-2) and GLUT3 [62–64]. Continued hypoglycemia enhances glucose transport in brain by increasing GLUT levels, extent of glucose uptake, and related brain functions [58,65–67]. An in vitro study showed that hypoglycemia along with serum deprivation also enhances glucose uptake [68]. Chronic hypoglycemia causes increased transportation and utilization of glucose in brain [66,67]. A normal rate of brain glucose uptake is maintained during hypoglycemia [65,69]. After antecedent RH, brain glucose metabolism during euglycemic conditions increases in terms of higher neuronal glucose oxidation [70]. The cerebral metabolic rate of glucose correlates with hypoglycemia-induced changes in GLUT levels [71], whereas hypoglycemia in animals previously exposed to RH causes a decrease in total tricarboxylic acid (TCA) cycle flux [70]. Moreover, the extent of glucose metabolism in brain increases after RH along with a concomitant increase in lactate uptake [72].
Cerebral glucose utilization
Normal cerebral glucose utilization varies in different parts of brain, e.g., cerebral glucose consumption in gray matter is more heterogeneous and relatively high as compared to the white matter [73,74]. An acute episode of hypoglycemia decreases cerebral glucose consumption [67,74–77]. The extent of hypoglycemia-induced decrease in glucose utilization is more prominent in areas of brain where normal cerebral glucose utilization is higher [74,76]. Segal et al have reported that glucose uptake and consumption rate were not significantly different in human subjects after 24 h of hypoglycemia [78]. A single episode of hypoglycemia does not produce any significant effect on metabolic fluxes affecting mechanisms of glucose catabolism in the brains of healthy subjects [79]. Continued hypoglycemia for a period of one week decreases cerebral glucose utilization [71]. However, subjects suffering from uncontrolled T1D display an impaired stimulation of hyperinsulinemic hypoglycemia-induced glucose oxidation [80]. Cerebral glucose utilization in animals previously exposed to RH later increases when they are euglycemic, but decreases when they are hypoglycemic [70]. During consequent euglycemia, RH-exposed animals perform better on spatial memory tests but they did worse when exposed to hypoglycemia, likely owing to limited availability of glucose [81].
Glycolysis
Once glucose enters the cells, it serves as the principal substrate of glycolysis [82]. A study by Marín-Hernández et al on HeLa cells exposed to hypoglycemia for 24 hours showed that acute hypoglycemia causes increase in the protein levels of GLUT1, GLUT3 and hexokinase I, as well as glycolytic flux [83]. Prior exposure to recurrent hypoglycemia increases glucose phosphorylation in rat hypothalamus [84]. These observations implicate that hypoglycemia affects delivery of glucose as well as glycolysis. A 48 hour period of fasting decreases glucose metabolism and production of malonyl-coenzyme A in the hypothalamus and cortex [85]. This study implies that fasting, to compensate the limited availability of glucose, causes reprogramming of substrate utilization away from glycolysis and more toward lipid oxidation. A study in cerebellar neuron cultures showed that aglycemia is associated with decreased consumption of glucose, lactate production rate, and lactate to glucose ratio resulting in an increased extent of glucose oxidation by approximately 35% [86]. They also demonstrated that hypoglycemia increased the ratio of TCA cycle to glycolytic fluxes, indicative of enhanced oxidative metabolism when compared to glycolysis. An acute episode of hypoglycemia inhibits glycolysis and glycogenolysis [87]. Severe hypoglycemia leads to a decrease in the levels of glucose 1,6-bisphosphate, fructose 2,6-bisphosphate, and fructose 1,6-bisphosphate, allosteric activators of phosphofructokinase (the rate-limiting enzyme in glycolysis) [88]. Glucose consumption increases during euglycemia in brain previously exposed to RH
TCA cycle
After glycolysis, which takes place in the cytosol, mitochondria play a critical role in mediating ATP generation via the TCA cycle and electron transport chain [82]. TCA cycle flux in healthy subjects at glucose levels of approximately 3 mmol/l was not significantly different from that observed at euglycemic levels [79]. However, hypoglycemia in type 1 diabetic subjects caused increase in TCA cycle flux in comparison to heathy subjects, possibly due to cerebral adaptations to RH [89]. During the substantial decline in brain energy production associated with severe hypoglycemia, a modified form of TCA cycle continues to work which involves aspartate aminotransferase-mediated formation of α-ketoglutarate from oxaloacetate and supports neurons during hypoglycemia-associated energy deprivation [90]. Using a radio-labeled lactate microdialysis study followed by nuclear magnetic resonance analysis of microdialysate, Gallagher et al. showed that lactate may be directly used as a TCA cycle substrate [91]. In previously RH-exposed animals, euglycemia increases glucose utilization and TCA cycle flux, but hypoglycemia causes a decrease in the same [70]. An acute episode of hypoglycemia decreases activities of succinate dehydrogenase and glutamate dehydrogenase [87]. Overall, these studies indicate differential effects of hypoglycemia on TCA cycle during euglycemia and hypoglycemia.
Mitochondrial respiration
Mitochondrial respiration is the principal source of energy in neurons. Electron donors like NADH and FADH2 produced in the TCA cycle supply electrons to the electron transport chain in mitochondria [92]. This produces a proton gradient across mitochondrial membrane, which drives production of ATP via complex V [92,93]. Insulin-induced acute hypoglycemia decreases the respiratory control ratio (RCR: ratio of state 3 to state 4 respiration) when compared with control groups, indicating hypoglycemia-induced impairment of electron transport chain function in mitochondria [94]. Exposure to chronic moderate hypoglycemia in normal as well as streptozotocin-diabetic rats for a period of one week causes lower state 3 respiration and RCR [95]. Chronic hypoglycemia exacerbates mitochondrial respiratory chain impairments in the hippocampus of an animal model of T1D as assessed in terms of RCR and ADP/O index [96]. Our laboratory has previously shown that RH changes the ratio of mitochondrial respiratory chain complex I subunits, indicating that hippocampal mitochondria are very sensitive to variations in glucose levels in diabetics [97]. Hypoglycemia-induced mitochondrial substrate limitation causes increased mitochondrial free radical production [98]. Therefore, hypoglycemia causes impairment of mitochondrial respiration and results in an increase in free radical production, a pathway having a significant potential to cause brain damage.
Amino acid metabolism
Physiological hyperinsulinemia produces hypoglycemia by causing increased tissue glucose uptake and inhibiting release of newly synthesized glucose into the circulation [99,100]. Leucine oxidation data has implied that insulin inhibits release of glucose from protein stores [101,102]. Hypoglycemia causes an adaptive release of regulatory hormones such as glucagon, epinephrine and cortisol to counter hypoglycemia as described in a previous section of this article [18–20,23–25]. Besides increasing glucose production, glucagon also enhances proteolysis, uptake of glutamine, and oxidation of amino acids [103–108]. Epinephrine and cortisol increases amino acid uptake and proteolysis [109–114]. Hyperinsulinemic hypoglycemia induces proteolysis as assessed in terms of leucine kinetics and oxidation [115]. Acute hypoglycemia among human subjects increases uptake of glutamine, a gluconeogenic amino acid, without affecting protein and leucine kinetics [116]. Sustained moderate hypoglycemia causes a decrease in amino acid levels in blood [117]. During the 12 hr period of moderate progressive hypoglycemia, the level of plasma branched-chain amino acids decreases in the first 6 hours but increases in the later 6-hour period. In contrast, the levels of essential non-branched-chain amino acids continued to decrease at a slower rate [117]. Amino acid supplementation suppresses glucose oxidation in fasted human subjects [118]. An earlier study observed that an episode of acute hypoglycemia caused in streptozotocin (Stz)-diabetic rats increases plasma levels of aspartate and GABA when compared to Stz-diabetic rats that were not subjected to hypoglycemia [119]. The same study also observed increased synaptosomal levels of glutamate and GABA in Stz-diabetic rats exposed to hypoglycemia when compared to Stz-diabetic that were not exposed to hypoglycemia. Evaluation of extracellular (microdialysate) amino acid levels in striatum of hypoglycemia-exposed perinatal rats (P7) observed that the levels of glutamate, aspartate and taurine increased, while levels of glutamine decreased over time during hypoglycemia [120]. Exposure of synaptosomes to hypoglycemia led to lower levels of ATP and increased levels of ADP, and subsequent depletion in synaptosomal membrane potential with increased release of aspartate [121]. This depleted energy status may be responsible for increased cytosolic free Ca2+ levels, which in turn, may contribute to brain damage during severe hypoglycemia [122]. Thus, the existing literature shows a differential effect of hypoglycemia on amino acid metabolism in brain. However, future research is required to determine the potential role of amino acid metabolism in hypoglycemia-induced brain dysfunction.
Lipid metabolism
Hypoglycemia causes activation of counterregulatory mechanisms for the attainment of euglycemia [18–20,23–25]. Lipolysis causes generation of glycerol and free fatty acids as a source of energy and substrates for the gluconeogenic processes. Fasting enhances fatty acid and ketone levels in blood [123]. Moderate hypoglycemia for a short duration (23 min) does not stimulate lipolysis [124]. However, moderate hypoglycemia for 4 hours inhibits the effect of insulin-associated decrease in free fatty acid levels [125]. Attenuation of insulin-induced hypoglycemia is associated not only with alterations in glucose kinetics but also with a rebound increase in lipolysis-mediated production of glucose in liver [125,126]. Free fatty acids mediate glucose counter-regulation by modulating hepatic glucose production [126]. Lipolysis participates in catecholamine-mediated acute phase of glucose counter-regulation elicited by hypoglycemia [127]. Lipolysis also mediates delayed glucose counter-regulatory pathways stimulated by growth hormone and cortisol [24,25]. Free fatty acids in the blood mediates the development of post-hypoglycemic insulin resistance [128]. Non-esterified fatty acids production increases with hyperinsulinemic hypoglycemia indicating the potential role of the lipids as an alternate source of energy [129]. Hypoglycemia inhibits uptake of arachidonate into glycerophospholipids of brain membranes [130]. It is possible that this decreased fatty acid uptake may lead to altered membrane function as well as synaptic processes during and after hypoglycemia. However, the extent of the role of lipid metabolism in mediating hypoglycemia-induced metabolic adaptations in brain is unknown and requires critical assessment.
Effect of hypoglycemia on cellular metabolism
Severe hypoglycemia causes a significant decrease in the number of metabolites in brain in terms of the phosphocreatine, ATP, ADP, and AMP concentrations [131]. Starvation for a period of 3–4 days causes decrease in the levels of alanine, glutamate and glutamine as well as increases in the levels of glycine in brain [132]. Insulin-induced moderate to severe hypoglycemia in pregnant mothers increases glutamate and glutamine levels in the fetal brain, and decreases alanine and GABA levels in young adults. Moreover, an NMR study indicates that hypoglycemia increases glucose flux in young adults via the pyruvate carboxylase and pyruvate dehydrogenase pathways [133]. MRS-based metabolomic analysis shows that insulin-induced hypoglycemia causes a decrease in the levels of alanine, β-hydroxybutyrate, lactate, threonine, and valine, and increase in the phenylalanine and Ƭ-methyl histidine concentrations in brain [134]. Repeated and severe hypoglycemia in neonates increases the levels of creatine, glutamate, glutamine, γ-aminobutyric acid, aspartate, succinate, taurine, and myoinositol in the occipital cortex, and levels of N-acetyl aspartate and choline were increased in hippocampus of hypoglycemia-exposed neonates [135]. In addition to the metabolite increases in neonates, levels of lactate, N-acetyl aspartate, alanine, choline, glycine, acetate, and ascorbate are also observed to be higher in the occipital cortex of adolescent animals that were exposed to hypoglycemia during the neonatal period. This study observed brain area-specific effects as patterns of metabolic alteration were different in hippocampus of adolescent animals that were exposed to hypoglycemia during the neonatal period [135]. A recent study investigated the effect of RH on reduced glutathione levels (GSH) in parietal cortex, striatum, and hippocampus [136]. They observed that levels of GSH decreased when measured at 12 and 24 h after last hypoglycemia exposure in all three brain regions studied. Their results suggest overall oxidative alteration of proteins. It is possible that oxidized proteins may affect overall cellular metabolism owing to altered functioning capacities of these proteins. These studies demonstrate a profound effect of hypoglycemia on the metabolome. However, detailed studies identifying the effect of recurrent hypoglycemia on cellular metabolism are thus warranted.
Importance of alternate sources of energy for brain during RH
Although the brain depends on glucose as its main source of energy, it possesses an ability to use other sources of energy such as lactate and ketones; viz., β-hydroxybutyrate and acetoacetate. After being converted into pyruvate or acetyl coenzyme A, these substrates can enter the TCA cycle and contribute toward energy production. Such alternate sources of energy facilitate brain physiology during hypoglycemia. For example, increased lactate is taken up into the brain for respiration but without a concomitant increase in lactate oxidation particularly during hypoglycemia [137,138]. Although glucose is the only metabolic substrate that can rescue brain from hypoglycemia [139], supplementation of alternate fuels like lactate and β-hydroxybutyrate during hypoglycemia can enhance oxidative metabolism, decrease autonomic and neuroglycopenic symptoms and enhance glucose counterregulatory mechanisms [72,140–143]. RH increases neuronal uptake of lactate, which serves as a metabolic regulator that preserves metabolism of glucose in brain during hypoglycemia [72]. Brain acetate concentrations, metabolism and monocarboxylic acid transport increases two-fold in T1D patients in comparison to non-diabetic control subjects [144]. Developing rats (age equivalent to full-term newborn human infants) were able to maintain phosphocreatine/creatine ratio in the physiological range for almost 2.5 hrs during experimentally-induced neuroglycopenia [145]. This study also observed that lactate levels decreased during initial phase of hypoglycemia suggesting that lactate may be utilized for energy production during this phase. Results also indicated that glutamate and glutamine were major energy substrates in the subsequent phase of hypoglycemia and once those energy substrates were depleted, aspartate was used as the final energy source. Their results indicate that brain relies on various sources of energy during different phases of hypoglycemia. The use of glutamate and glutamine as a source of energy substrates is further implicated by an earlier study [146]. Using immunogold staining in hippocampus and striatum, they observed that levels of both glutamate and glutamine decreased in most tissue compartments following hypoglycemia. In vitro study observed that isolated synaptosomes were able to maintain high ATP/ADP ratios when exposed to hypoglycemia (glucose-free media) further supporting the role of alternate fuels in maintaining energy status during hypoglycemia [147]. An earlier study observed reduced cerebral arteriovenous difference for glucose during the period of hypoglycemia, further supporting a view that brain relies on alternate energy substrates during hypoglycemia [148].
Ketone bodies
Ketone bodies like acetoacetate, 3-β-hydroxybutyrate and acetone are products of fatty acid metabolism, which serve as a source of energy during conditions of nutrient deprivation as reviewed previously [149,150]. Prolonged fasting increases the level of ketone bodies in blood [151]. Ketone metabolism in brain is dependent on its blood levels and blood-brain barrier permeability [152–154]. Ketone bodies are metabolized into acetyl-CoA which feeds the TCA cycle to meet the increased metabolic demand in the brain [155,156]. β-hydroxybutyric acid contributes toward the neuronal synthesis of glutamate and glutamine [157]. Although β-hydroxybutyric acid facilitates ATP synthesis in brain, it cannot replace glucose as a respiratory substrate [142,158–160]. Lipid administration normalizes hypoglycemia-induced changes in cognitive function of brain and glucose counterregulation [142,161,162]. Ketone bodies decrease oxidative stress, energy deficit, excitotoxicity and neuronal death associated with glycolysis inhibition and hypoglycemia [163–167]. Moreover, a ketogenic diet ameliorates hypoglycemia-induced neuronal death in rats [168]. Therefore, current data implicates that ketone bodies may serve as an alternate metabolic fuel during hypoglycemia. In comparison to other metabolites, understanding the time course and extent of hypoglycemia-induced increase in ketone bodies and their metabolism can provide valuable insights.
Glycogen
Glycogen is a reserve respiratory substrate for brain during metabolic stress conditions like hypoglycemia. Although the basal level of glycogen in brain is modest, its exceptionally slow respiratory consumption [169,170] causes its increased metabolism during brain activation [171–174]. Glycogen is stored in cytosol of astrocytes in the form of electron-dense isodiametric (10–30 nm) β-particles adjacent to the enzymes needed for its synthesis and degradation, namely glycogen synthase and glycogen phosphorylase, respectively [175–177]. During hypoglycemia, stored glycogen is mobilized and acted upon by glycogen phosphorylase in astrocytes and is converted into glucose-1-phosphate and glucose-6-phosphate to eventually feed glycolysis [178,179]. Metabolism of astrocytic glycogen into lactate fuels brain cells during glucose deprivation and increased metabolic demand [171,180–182]. There is a correlation between glucose deprivation-induced decrease in glycogen levels and delay in the loss of action potential of neurons [181,182]. Inhibition of glycogen phosphorylase-induced increase in astrocytic glycogen levels preserves normal neuronal activity during hypoglycemia and decreases associated neuronal death [183]. Acute moderate hypoglycemia enhances glycogen utilization to produce glucose [178]. During restoration of euglycemia after hypoglycemia, glycogen levels in brain go well above the pre-hypoglycemia levels within few hours indicating the presence of ‘glycogen supercompensation’ [178]. Duarte and colleagues evaluated the effect of post-hypoglycemia glucose levels on hypoglycemia-induced glycogen supercompensation [184]. They observed that brain glycogen concentrations remained elevated in studied brain areas (cortex, hippocampus, and striatum) when measured at 24 h post-hypoglycemia. They also observed that post-hypoglycemia, glucose (normoglycemia vs hyperglycemia) levels did not affect the extent of post-hypoglycemia glycogen supercompensation. During RH, glycogen supercompensation is blunted and does not mediate hypoglycemia unawareness and HAAF [185,186]. Further studies are required to understand the role of glycogen as an alternative fuel for brain during hypoglycemia.
Other alternate sources of energy
During hypoglycemia, pyruvate treatment produces neuroprotection in brain [187,188]. Pyruvate treatment of hippocampal slices before hypoglycemia improves neuronal function [189]. Pyruvate administration attenuates RH-induced brain damage in diabetic rats by circumventing sustained impairment of glycolysis induced via activation of PARP-1 [190]. Hypoglycemia increases monocarboxylate transport and acetate uptake in diabetic brains [144]. Lactate may be directly used as a TCA cycle substrate [91]. While RH decreases glucose metabolism during an eventual episode of hypoglycemia, acetate metabolism remains unchanged [70]. During hypoglycemia, an increase in aspartate, and decrease in the glutamine and glutamate levels is associated with a low energy status of brain [90,191]. Glutamine [86] serves as an alternate source of energy for neurons during recovery from hypoglycemia. The pyruvate recycling pathway is involved in maintaining respiration during hypoglycemia [86]. Typically, pyruvate recycling involves glutamate or glutamine oxidation in the TCA cycle by facilitating the outward movement of a four-carbon unit from the malate or oxaloacetate steps and reentry at the pyruvate step of the cycle [192,193]. Glutamate/glutamine oxidation via pyruvate recycling maintains energy supply for homeostasis during sustained hypoglycemia in developing rat hippocampus [145], while a 12-hour exposure of cultured cerebellar neurons to aglycemia decreases glucose uptake and glycolysis and increases glutamine uptake [86]. Hypoglycemia enhances fluxes involved in the pyruvate recycling pathway; viz., pyruvate-Acetyl-CoA flux, α-ketoglutarate-succinyl-CoA flux, glutamine-glutamate flux and malate pyruvate flux. However, the sites of these biochemical changes are debated between neurons and astrocytes and are yet to be determined [192–198]. Glutamate oxaloacetate transaminase mediated metabolism of experimentally increased extracellular glutamate levels takes place through the truncated TCA cycle under hypoglycemic conditions [199].
Research implicating the therapeutic potential of modulating metabolic changes in brain during RH
Considering the literature cited above, it is evident that RH induces multiple changes in brain metabolism. Modulation of some of these changes may have a potential beneficial effect on the hypoglycemic brain. An intraperitoneal dose of sodium L-lactate prevents hypoglycemia-induced neuronal death by serving as an alternate source of energy in the brain during hypoglycemia. This effect is due to lactate-induced correction of PARP-1 activation-linked decrease in NAD(+) levels, causing direct activation of the TCA cycle and maintenance of cellular ATP levels [200]. Pyruvate treatment along with glucose administration post-hypoglycemia protects neurons from oxidative injury, microglial activation and loss of endogenous antioxidant system in the cerebral cortex after RH [190]. Administration of both a ketogenic diet and β-hydroxybutyrate produces a beneficial effect on neuronal death associated with hypoglycemia [164,165,168]. Moreover, medium chain fatty acid supplementation improves cognitive deficit in treated T1D patients undergoing hypoglycemia [142]. Therefore, it may be deduced that modulation of metabolic changes in hypoglycemic brain possesses therapeutic potential, provided it is augmented by future in-depth mechanistic studies.
Summary and Future Directions
Therefore, as discussed above, RH exposure induces adaptive changes in glucose counter-regulatory mechanisms, uptake, utilization, cellular respiration, amino acid metabolism, and lipid metabolism. Further, we summarized literature on RH-induced adaptive changes in brain involving utilization of alternate sources of energy. Additional information about changes in activity of key respiratory enzymes and determination of the extent of dependence of hypoglycemic brain on alternate sources of energy during diabetes can contribute towards attainment of the clinical potential of modulating such changes in brain. Therefore, studies on the effect produced by mild, moderate and severe hypoglycemia on the individual metabolic pathways, their influence on overall brain energetics and functioning will help us understand the metabolomic changes in brain. This may help in tailoring new clinical approaches to treat RH-induced dysfunction of brain seen during diabetes. Further, studying the crosstalk between different metabolic pathways and their overall influence on temporal changes in brain function may help to identify key pathways which are involved in the pathophysiology of diabetes-induced brain dysfunction. Studying the time course of changes in brain metabolism with the progression of diabetes may also help us identify the therapeutic windows of potential treatment strategies. Diabetic patients are suffering from other comorbid conditions like aging, hyperlipidemia and hypertension [201–204]. These comorbidities produce their independent effects on brain metabolism as well [205–207]. Therefore, it is worthwhile to study the extent and time course of the cumulative metabolic effect of the concomitant presence of hypoglycemia during diabetes with these comorbid conditions. Moreover, prior to studying the beneficial effect of modulating the metabolic changes in brain induced by RH, it is also important to test the effect of such interventions on brain functioning per se, hypoglycemia unawareness, and the pathogenesis of RH. Overall, such analysis may help us continue to study the clinical potential of modulating the metabolic changes induced by hypoglycemia in brain.
Conclusions
Considering the profound effect of hypoglycemia on brain in diabetes and that not much information is available on its mechanism, studying hypoglycemia-induced adaptive changes in brain metabolism promises to have important pathological ramifications. Even in the presence of literature produced by exhaustive research work carried out by several research groups world-wide, some basic information on the effect of hypoglycemia on metabolic pathways is still missing. Given the epidemiological significance of hypoglycemia in diabetics, continued studies on metabolomic changes in brain elicited by RH are expected to identify novel therapeutic principles for hypoglycemia-induced detrimental effects on brains of diabetic subjects. Additionally, research integrating the qualitative and quantitative differences between metabolic effects produced by hypoglycemia on brain might help us further appreciate the overall influence of diabetes on brain function.
Acknowledgements
This study was supported by National Institutes of Health grant NS073779. We would like to thank Dr. Brant Watson for critical reading of this manuscript.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.International Diabetes Federation (2017). http://www.diabetesatlas.org/key-messages.html. Accessed 1.3.2018 [DOI] [PubMed]
- 2.Diabetes Control Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329 (14):977–986. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 3.U. K. Hypoglycaemia Study Group (2007) Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 50 (6):1140–1147. 10.1007/s00125-007-0599-y [DOI] [PubMed] [Google Scholar]
- 4.Zammitt NN, Frier BM (2005) Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care 28 (12):2948–2961 [DOI] [PubMed] [Google Scholar]
- 5.Cryer PE (2002) Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 45 (7):937–948. 10.1007/s00125-002-0822-9 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association Workgroup on Hypoglycemia (2005) Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 28 (5):1245–1249 [DOI] [PubMed] [Google Scholar]
- 7.Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, Band MM, Reekie G, Leese GP, Collaboration DM (2005) Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med 22 (6):749–755. 10.1111/j.1464-5491.2005.01501.x [DOI] [PubMed] [Google Scholar]
- 8.Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH (2015) Hypoglycemia in Type 2 Diabetes--More Common Than You Think: A Continuous Glucose Monitoring Study. J Diabetes Sci Technol 9 (5):999–1005. 10.1177/1932296815581052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holstein A, Hammer C, Plaschke A, Ptak M, Kuhn J, Diekmann J, Kleesiek K, Egberts EH (2004) Hormonal counterregulation during severe hypoglycaemia under everyday conditions in patients with type 1 and insulin-treated type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 112 (8):429–434. 10.1055/s-2004-821188 [DOI] [PubMed] [Google Scholar]
- 10.Hansen TI, Olsen SE, Haferstrom ECD, Sand T, Frier BM, Haberg AK, Bjorgaas MR (2017) Cognitive deficits associated with impaired awareness of hypoglycaemia in type 1 diabetes. Diabetologia 60 (6):971–979. 10.1007/s00125-017-4233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan CM, Klein BEK, Lee KE, Cruickshanks KJ, Klein R (2016) Associations between recent severe hypoglycemia, retinal vessel diameters, and cognition in adults with type 1 diabetes. J Diabetes Complications 30 (8):1513–1518. 10.1016/j.jdiacomp.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr., Selby JV (2009) Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 301 (15):1565–1572. 10.1001/jama.2009.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Languren G, Montiel T, Julio-Amilpas A, Massieu L (2013) Neuronal damage and cognitive impairment associated with hypoglycemia: An integrated view. Neurochem Int 63 (4):331–343. 10.1016/j.neuint.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 14.Keymeulen B, Jacobs A, de Metz K, de Sadeleer C, Bossuyt A, Somers G (1995) Regional cerebral hypoperfusion in long-term type 1 (insulin-dependent) diabetic patients: relation to hypoglycaemic events. Nucl Med Commun 16 (1):10–16 [DOI] [PubMed] [Google Scholar]
- 15.Sarti C, Pantoni L, Bartolini L, Inzitari D (2002) Cognitive impairment and chronic cerebral hypoperfusion: what can be learned from experimental models. J Neurol Sci 203–204:263–266 [DOI] [PubMed] [Google Scholar]
- 16.Won SJ, Yoo BH, Kauppinen TM, Choi BY, Kim JH, Jang BG, Lee MW, Sohn M, Liu J, Swanson RA, Suh SW (2012) Recurrent/moderate hypoglycemia induces hippocampal dendritic injury, microglial activation, and cognitive impairment in diabetic rats. J Neuroinflammation 9:182 10.1186/1742-2094-9-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Towler DA, Havlin CE, Craft S, Cryer P (1993) Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 42 (12):1791–1798 [DOI] [PubMed] [Google Scholar]
- 18.Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Ciofetta M, Modarelli F, Di Vincenzo A, Annibale B, Lepore M, Lalli C, et al. (1994) Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia 37 (8):797–807 [DOI] [PubMed] [Google Scholar]
- 19.Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J (1991) Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 260 (1 Pt 1):E67–74 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz NS, Clutter WE, Shah SD, Cryer PE (1987) Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest 79 (3):777–781. 10.1172/JCI112884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cryer PE (1994) Banting Lecture. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes 43 (11):1378–1389 [DOI] [PubMed] [Google Scholar]
- 22.Rizza RA, Cryer PE, Gerich JE (1979) Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest 64 (1):62–71.doi: 10.1172/JCI109464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle PJ, Cryer PE (1991) Growth hormone, cortisol, or both are involved in defense against, but are not critical to recovery from, hypoglycemia. Am J Physiol 260 (3 Pt 1):E395–402 [DOI] [PubMed] [Google Scholar]
- 24.De Feo P, Perriello G, Torlone E, Ventura MM, Fanelli C, Santeusanio F, Brunetti P, Gerich JE, Bolli GB (1989) Contribution of cortisol to glucose counterregulation in humans. Am J Physiol 257 (1 Pt 1):E35–42 [DOI] [PubMed] [Google Scholar]
- 25.De Feo P, Perriello G, Torlone E, Ventura MM, Santeusanio F, Brunetti P, Gerich JE, Bolli GB (1989) Demonstration of a role for growth hormone in glucose counterregulation. Am J Physiol 256 (6 Pt 1):E835–843 [DOI] [PubMed] [Google Scholar]
- 26.Dagogo-Jack SE, Craft S, Cryer PE (1993) Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 91 (3):819–828. 10.1172/JCI116302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis SN, Shavers C, Mosqueda-Garcia R, Costa F (1997) Effects of differing antecedent hypoglycemia on subsequent counterregulation in normal humans. Diabetes 46 (8):1328–1335 [DOI] [PubMed] [Google Scholar]
- 28.Heller SR, Cryer PE (1991) Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 40 (2):223–226 [DOI] [PubMed] [Google Scholar]
- 29.Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Benedetti MM, Santeusanio F, Gerich JE, Brunetti P (1984) A reliable and reproducible test for adequate glucose counterregulation in type I diabetes mellitus. Diabetes 33 (8):732–737 [DOI] [PubMed] [Google Scholar]
- 30.White NH, Skor DA, Cryer PE, Levandoski LA, Bier DM, Santiago JV (1983) Identification of type I diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med 308 (9):485–491. 10.1056/NEJM198303033080903 [DOI] [PubMed] [Google Scholar]
- 31.Segel SA, Paramore DS, Cryer PE (2002) Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes 51 (3):724–733 [DOI] [PubMed] [Google Scholar]
- 32.Harvard Mahoney Neuroscience Institute (2017). http://neuro.hms.harvard.edu/harvard-mahoney-neuroscience-institute/brain-newsletter/and-brain-series/sugar-and-brain. Accessed 01.03.2018
- 33.Lubow JM, Pinon IG, Avogaro A, Cobelli C, Treeson DM, Mandeville KA, Toffolo G, Boyle PJ (2006) Brain oxygen utilization is unchanged by hypoglycemia in normal humans: lactate, alanine, and leucine uptake are not sufficient to offset energy deficit. Am J Physiol Endocrinol Metab 290 (1):E149–E153. 10.1152/ajpendo.00049.2005 [DOI] [PubMed] [Google Scholar]
- 34.Wahren J, Ekberg K, Fernqvist-Forbes E, Nair S (1999) Brain substrate utilisation during acute hypoglycaemia. Diabetologia 42 (7):812–818 [DOI] [PubMed] [Google Scholar]
- 35.Gruetter R, Ugurbil K, Seaquist ER (1998) Steady-state cerebral glucose concentrations and transport in the human brain. J Neurochem 70 (1):397–408 [DOI] [PubMed] [Google Scholar]
- 36.Choi IY, Lee SP, Kim SG, Gruetter R (2001) In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab 21 (6):653–663. 10.1097/00004647-200106000-00003 [DOI] [PubMed] [Google Scholar]
- 37.Pao SS, Paulsen IT, Saier MH Jr. (1998) Major facilitator superfamily. Microbiol Mol Biol Rev 62 (1):1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arbuckle MI, Kane S, Porter LM, Seatter MJ, Gould GW (1996) Structure-function analysis of liver-type (GLUT2) and brain-type (GLUT3) glucose transporters: expression of chimeric transporters in Xenopus oocytes suggests an important role for putative transmembrane helix 7 in determining substrate selectivity. Biochemistry 35 (51):16519–16527. 10.1021/bi962210n [DOI] [PubMed] [Google Scholar]
- 39.Fukumoto H, Kayano T, Buse JB, Edwards Y, Pilch PF, Bell GI, Seino S (1989) Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem 264 (14):7776–7779 [PubMed] [Google Scholar]
- 40.Fukumoto H, Seino S, Imura H, Seino Y, Eddy RL, Fukushima Y, Byers MG, Shows TB, Bell GI (1988) Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A 85 (15):5434–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF (1985) Sequence and structure of a human glucose transporter. Science 229 (4717):941–945 [DOI] [PubMed] [Google Scholar]
- 42.Doege H, Bocianski A, Scheepers A, Axer H, Eckel J, Joost HG, Schurmann A (2001) Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. Biochem J 359 (Pt 2):443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schurmann A, Seino S, Thorens B (2002) Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 282 (4):E974–976. 10.1152/ajpendo.00407.2001 [DOI] [PubMed] [Google Scholar]
- 44.Birnbaum MJ, Haspel HC, Rosen OM (1986) Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A 83 (16):5784–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brant AM, Jess TJ, Milligan G, Brown CM, Gould GW (1993) Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem Biophys Res Commun 192 (3):1297–1302. 10.1006/bbrc.1993.1557 [DOI] [PubMed] [Google Scholar]
- 46.Kasanicki MA, Cairns MT, Davies A, Gardiner RM, Baldwin SA (1987) Identification and characterization of the glucose-transport protein of the bovine blood/brain barrier. Biochem J 247 (1):101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maher F, Vannucci SJ, Simpson IA (1994) Glucose transporter proteins in brain. FASEB J 8 (13):1003–1011 [DOI] [PubMed] [Google Scholar]
- 48.Mantych GJ, James DE, Chung HD, Devaskar SU (1992) Cellular localization and characterization of Glut 3 glucose transporter isoform in human brain. Endocrinology 131 (3):1270–1278. 10.1210/endo.131.3.1505464 [DOI] [PubMed] [Google Scholar]
- 49.Mantych GJ, James DE, Devaskar SU (1993) Jejunal/kidney glucose transporter isoform (Glut-5) is expressed in the human blood-brain barrier. Endocrinology 132 (1):35–40. 10.1210/endo.132.1.8419132 [DOI] [PubMed] [Google Scholar]
- 50.Nagamatsu S, Sawa H, Kamada K, Nakamichi Y, Yoshimoto K, Hoshino T (1993) Neuron-specific glucose transporter (NSGT): CNS distribution of GLUT3 rat glucose transporter (RGT3) in rat central neurons. FEBS Lett 334 (3):289–295 [DOI] [PubMed] [Google Scholar]
- 51.Payne J, Maher F, Simpson I, Mattice L, Davies P (1997) Glucose transporter Glut 5 expression in microglial cells. Glia 21 (3):327–331 [DOI] [PubMed] [Google Scholar]
- 52.Shepherd PR, Gibbs EM, Wesslau C, Gould GW, Kahn BB (1992) Human small intestine facilitative fructose/glucose transporter (GLUT5) is also present in insulin-responsive tissues and brain. Investigation of biochemical characteristics and translocation. Diabetes 41 (10):1360–1365 [DOI] [PubMed] [Google Scholar]
- 53.Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR (1999) Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem 72 (1):238–247 [DOI] [PubMed] [Google Scholar]
- 54.Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27 (11):1766–1791. 10.1038/sj.jcbfm.9600521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maher F, Davies-Hill TM, Simpson IA (1996) Substrate specificity and kinetic parameters of GLUT3 in rat cerebellar granule neurons. Biochem J 315 (Pt 3):827–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boado RJ, Pardridge WM (1993) Glucose deprivation causes posttranscriptional enhancement of brain capillary endothelial glucose transporter gene expression via GLUT1 mRNA stabilization. J Neurochem 60 (6):2290–2296 [DOI] [PubMed] [Google Scholar]
- 57.Koranyi L, Bourey RE, James D, Mueckler M, Fiedorek FT Jr., Permutt MA (1991) Glucose transporter gene expression in rat brain: Pretranslational changes associated with chronic insulin-induced hypoglycemia, fasting, and diabetes. Mol Cell Neurosci 2 (3):244–252 [DOI] [PubMed] [Google Scholar]
- 58.Kumagai AK, Kang YS, Boado RJ, Pardridge WM (1995) Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes 44 (12):1399–1404 [DOI] [PubMed] [Google Scholar]
- 59.Antony S, Peeyush Kumar T, Mathew J, Anju TR, Paulose CS (2010) Hypoglycemia induced changes in cholinergic receptor expression in the cerebellum of diabetic rats. J Biomed Sci 17:7 10.1186/1423-0127-17-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee DH, Chung MY, Lee JU, Kang DG, Paek YW (2000) Changes of glucose transporters in the cerebral adaptation to hypoglycemia. Diabetes Res Clin Pract 47 (1):15–23 [DOI] [PubMed] [Google Scholar]
- 61.Uehara Y, Nipper V, McCall AL (1997) Chronic insulin hypoglycemia induces GLUT-3 protein in rat brain neurons. Am J Physiol 272 (4 Pt 1):E716–719 [DOI] [PubMed] [Google Scholar]
- 62.Vavaiya KV, Briski KP (2008) Effects of caudal fourth ventricular lactate infusion on hypoglycemia-associated MCT2, GLUT3, GLUT4, GCK, and sulfonylurea receptor-1 gene expression in the ovariectomized female rat LHA and VMH: impact of estradiol. J Mol Neurosci 34 (2):121–129. 10.1007/s12031-007-9020-z [DOI] [PubMed] [Google Scholar]
- 63.Vavaiya KV, Briski KP (2008) Effects of caudal hindbrain lactate infusion on insulin-induced hypoglycemia and neuronal substrate transporter glucokinase and sulfonylurea receptor-1 gene expression in the ovariectomized female rat dorsal vagal complex: Impact of estradiol. J Neurosci Res 86 (3):694–701. 10.1002/jnr.21530 [DOI] [PubMed] [Google Scholar]
- 64.Vavaiya KV, Paranjape SA, Patil GD, Briski KP (2006) Vagal complex monocarboxylate transporter-2 expression during hypoglycemia. Neuroreport 17 (10):1023–1026. 10.1097/01.wnr.0000224766.07702.51 [DOI] [PubMed] [Google Scholar]
- 65.Boyle PJ, Nagy RJ, O’Connor AM, Kempers SF, Yeo RA, Qualls C (1994) Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci U S A 91 (20):9352–9356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCall AL, Fixman LB, Fleming N, Tornheim K, Chick W, Ruderman NB (1986) Chronic hypoglycemia increases brain glucose transport. Am J Physiol 251 (4 Pt 1):E442–447 [DOI] [PubMed] [Google Scholar]
- 67.Pelligrino DA, Segil LJ, Albrecht RF (1990) Brain glucose utilization and transport and cortical function in chronic vs. acute hypoglycemia. Am J Physiol 259 (5 Pt 1):E729–735 [DOI] [PubMed] [Google Scholar]
- 68.Russo VC, Kobayashi K, Najdovska S, Baker NL, Werther GA (2004) Neuronal protection from glucose deprivation via modulation of glucose transport and inhibition of apoptosis: a role for the insulin-like growth factor system. Brain Res 1009 (1–2):40–53. 10.1016/j.brainres.2004.02.042 [DOI] [PubMed] [Google Scholar]
- 69.Boyle PJ, Kempers SF, O’Connor AM, Nagy RJ (1995) Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med 333 (26):1726–1731. 10.1056/NEJM199512283332602 [DOI] [PubMed] [Google Scholar]
- 70.Jiang L, Herzog RI, Mason GF, de Graaf RA, Rothman DL, Sherwin RS, Behar KL (2009) Recurrent antecedent hypoglycemia alters neuronal oxidative metabolism in vivo. Diabetes 58 (6):1266–1274. 10.2337/db08-1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duelli R, Staudt R, Duembgen L, Kuschinsky W (1999) Increase in glucose transporter densities of Glut3 and decrease of glucose utilization in rat brain after one week of hypoglycemia. Brain Res 831 (1–2):254–262 [DOI] [PubMed] [Google Scholar]
- 72.Herzog RI, Jiang L, Herman P, Zhao C, Sanganahalli BG, Mason GF, Hyder F, Rothman DL, Sherwin RS, Behar KL (2013) Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. J Clin Invest 123 (5):1988–1998. 10.1172/JCI65105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28 (5):897–916 [DOI] [PubMed] [Google Scholar]
- 74.Suda S, Shinohara M, Miyaoka M, Lucignani G, Kennedy C, Sokoloff L (1990) The lumped constant of the deoxyglucose method in hypoglycemia: effects of moderate hypoglycemia on local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab 10 (4):499–509. 10.1038/jcbfm.1990.92 [DOI] [PubMed] [Google Scholar]
- 75.Abdul-Rahman A, Siesjo BK (1980) Local cerebral glucose consumption during insulin-induced hypoglycemia, and in the recovery period following glucose administration. Acta Physiol Scand 110 (2):149–159. 10.1111/j.1748-1716.1980.tb06645.x [DOI] [PubMed] [Google Scholar]
- 76.Bryan RM Jr., Keefer KA, MacNeill C(1986) Regional cerebral glucose utilization during insulin-induced hypoglycemia in unanesthetized rats. J Neurochem 46 (6):1904–1911 [DOI] [PubMed] [Google Scholar]
- 77.Ghajar JB, Plum F, Duffy TE (1982) Cerebral oxidative metabolism and blood flow during acute hypoglycemia and recovery in unanesthetized rats. J Neurochem 38 (2):397–409 [DOI] [PubMed] [Google Scholar]
- 78.Segel SA, Fanelli CG, Dence CS, Markham J, Videen TO, Paramore DS, Powers WJ, Cryer PE (2001) Blood-to-brain glucose transport, cerebral glucose metabolism, and cerebral blood flow are not increased after hypoglycemia. Diabetes 50 (8):1911–1917 [DOI] [PubMed] [Google Scholar]
- 79.van de Ven KC, de Galan BE, van der Graaf M, Shestov AA, Henry PG, Tack CJ, Heerschap A (2011) Effect of acute hypoglycemia on human cerebral glucose metabolism measured by (1)(3)C magnetic resonance spectroscopy. Diabetes 60 (5):1467–1473. 10.2337/db10-1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caprio S, Amiel S, Tamborlane WV, Gelfand RA, Sherwin RS (1990) Defective free-fatty acid and oxidative glucose metabolism in IDDM during hypoglycemia. Influence of glycemic control. Diabetes 39 (2):134–141 [DOI] [PubMed] [Google Scholar]
- 81.McNay EC, Sherwin RS (2004) Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes 53 (2):418–425 [DOI] [PubMed] [Google Scholar]
- 82.Meisenberg G, Simmons WH (2012) Tricarboxylic Acid Cycle, and Oxidative Phosphorylation. In: Principles of Medical Biochemistry. Third edn Saunders, an imprint of Elsevier, Inc, pp 347–373 [Google Scholar]
- 83.Marin-Hernandez A, Lopez-Ramirez SY, Del Mazo-Monsalvo I, Gallardo-Perez JC, Rodriguez-Enriquez S, Moreno-Sanchez R, Saavedra E (2014) Modeling cancer glycolysis under hypoglycemia, and the role played by the differential expression of glycolytic isoforms. FEBS J 281 (15):3325–3345. 10.1111/febs.12864 [DOI] [PubMed] [Google Scholar]
- 84.Osundiji MA, Hurst P, Moore SP, Markkula SP, Yueh CY, Swamy A, Hoashi S, Shaw JS, Riches CH, Heisler LK, Evans ML (2011) Recurrent hypoglycemia increases hypothalamic glucose phosphorylation activity in rats. Metabolism 60 (4):550–556. 10.1016/j.metabol.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poplawski MM, Mastaitis JW, Yang XJ, Mobbs CV (2010) Hypothalamic responses to fasting indicate metabolic reprogramming away from glycolysis toward lipid oxidation. Endocrinology 151 (11):5206–5217. 10.1210/en.2010-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amaral AI, Teixeira AP, Sonnewald U, Alves PM (2011) Estimation of intracellular fluxes in cerebellar neurons after hypoglycemia: importance of the pyruvate recycling pathway and glutamine oxidation. J Neurosci Res 89 (5):700–710. 10.1002/jnr.22571 [DOI] [PubMed] [Google Scholar]
- 87.Telushkin PK, Nozdrachev AD, Potapov PP, Medvedeva NB, Stel’makh AY (2005) Glycolysis and oxidtion enzyme activity in rat brain during insulin-induced hypoglycemia against the background of alloxan-induced diabetes mellitus. Bull Exp Biol Med 140 (6):695–697 [DOI] [PubMed] [Google Scholar]
- 88.Magen A, Koren-Schwartzer N, Chen-Zion M, Beitner R (1995) Effect of insulin-induced hypoglycemia on cytoskeleton-bound and cytosolic phosphofructokinase and the levels of glucose 1,6-bisphosphate in rat brain. Biochem Mol Med 56 (2):94–98 [DOI] [PubMed] [Google Scholar]
- 89.van de Ven KC, Tack CJ, Heerschap A, van der Graaf M, de Galan BE (2013) Patients with type 1 diabetes exhibit altered cerebral metabolism during hypoglycemia. J Clin Invest 123 (2):623–629. 10.1172/JCI62742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sutherland GR, Tyson RL, Auer RN (2008) Truncation of the krebs cycle during hypoglycemic coma. Med Chem 4 (4):379–385 [DOI] [PubMed] [Google Scholar]
- 91.Gallagher CN, Carpenter KL, Grice P, Howe DJ, Mason A, Timofeev I, Menon DK, Kirkpatrick PJ, Pickard JD, Sutherland GR, Hutchinson PJ (2009) The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 132 (Pt 10):2839–2849.doi: 10.1093/brain/awp202 [DOI] [PubMed] [Google Scholar]
- 92.Nelson DL, Cox MM (2004) Oxidative phosphorylation and photophosphorylation. In: Nelson DL, Cox MM (ed) Lehninger’s principles of biochemistry. 4th edn W. H. Freeman, New York, pp 691–750 [Google Scholar]
- 93.Schultz BE, Chan SI (2001) Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu Rev Biophys Biomol Struct 30:23–65. 10.1146/annurev.biophys.30.1.23 [DOI] [PubMed] [Google Scholar]
- 94.Cardoso S, Santos MS, Seica R, Moreira PI (2010) Cortical and hippocampal mitochondria bioenergetics and oxidative status during hyperglycemia and/or insulin-induced hypoglycemia. Biochim Biophys Acta 1802 (11):942–951. 10.1016/j.bbadis.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 95.Pelligrino DA, Becker GL, Miletich DJ, Albrecht RF (1989) Cerebral mitochondrial respiration in diabetic and chronically hypoglycemic rats. Brain Res 479 (2):241–246 [DOI] [PubMed] [Google Scholar]
- 96.Cardoso S, Santos RX, Correia SC, Carvalho C, Santos MS, Baldeiras I, Oliveira CR, Moreira PI (2013) Insulin-induced recurrent hypoglycemia exacerbates diabetic brain mitochondrial dysfunction and oxidative imbalance. Neurobiol Dis 49:1–12. 10.1016/j.nbd.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 97.Dave KR, Tamariz J, Desai KM, Brand FJ, Liu A, Saul I, Bhattacharya SK, Pileggi A (2011) Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin-induced diabetic rats. Stroke 42 (5):1404–1411. 10.1161/STROKEAHA.110.594937 [DOI] [PubMed] [Google Scholar]
- 98.McGowan JE, Chen L, Gao D, Trush M, Wei C (2006) Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia. Neurosci Lett 399 (1–2):111–114. 10.1016/j.neulet.2006.01.034 [DOI] [PubMed] [Google Scholar]
- 99.Caprio S, Sacca L, Tamborlane WV, Sherwin RS (1988) Relationship between changes in glucose production and gluconeogenesis during mild hypoglycemia in humans. Metabolism 37 (8):707–710 [DOI] [PubMed] [Google Scholar]
- 100.Lecavalier L, Bolli G, Cryer P, Gerich J (1989) Contributions of gluconeogenesis and glycogenolysis during glucose counterregulation in normal humans. Am J Physiol 256 (6 Pt 1):E844–851 [DOI] [PubMed] [Google Scholar]
- 101.Castellino P, Luzi L, Simonson DC, Haymond M, DeFronzo RA (1987) Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J Clin Invest 80 (6):1784–1793. 10.1172/JCI113272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fukagawa NK, Minaker KL, Rowe JW, Goodman MN, Matthews DE, Bier DM, Young VR (1985) Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest 76 (6):2306–2311. 10.1172/JCI112240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Battezzati A, Simonson DC, Luzi L, Matthews DE (1998) Glucagon increases glutamine uptake without affecting glutamine release in humans. Metabolism 47 (6):713–723 [DOI] [PubMed] [Google Scholar]
- 104.Cherrington AD, Williams PE, Shulman GI, Lacy WW (1981) Differential time course of glucagon’s effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes 30 (3):180–187 [DOI] [PubMed] [Google Scholar]
- 105.Couet C, Fukagawa NK, Matthews DE, Bier DM, Young VR (1990) Plasma amino acid kinetics during acute states of glucagon deficiency and excess in healthy adults. Am J Physiol 258 (1 Pt 1):E78–85 [DOI] [PubMed] [Google Scholar]
- 106.Magnusson I, Rothman DL, Gerard DP, Katz LD, Shulman GI (1995) Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes 44 (2):185–189 [DOI] [PubMed] [Google Scholar]
- 107.Pacy PJ, Cheng KN, Ford GC, Halliday D (1990) Influence of glucagon on protein and leucine metabolism: a study in fasting man with induced insulin resistance. Br J Surg 77 (7):791–794 [DOI] [PubMed] [Google Scholar]
- 108.Tessari P, Inchiostro S, Barazzoni R, Zanetti M, Vettore M, Biolo G, Iori E, Kiwanuka E, Tiengo A (1996) Hyperglucagonemia stimulates phenylalanine oxidation in humans. Diabetes 45 (4):463–470 [DOI] [PubMed] [Google Scholar]
- 109.Darmaun D, Matthews DE, Bier DM (1988) Physiological hypercortisolemia increases proteolysis, glutamine, and alanine production. Am J Physiol 255 (3 Pt 1):E366–373 [DOI] [PubMed] [Google Scholar]
- 110.Fong YM, Albert JD, Tracey K, Hesse DG, Calvano S, Matthews DE, Lowry SF (1991) The influence of substrate background on the acute metabolic response to epinephrine and cortisol. J Trauma 31 (11):1467–1476 [DOI] [PubMed] [Google Scholar]
- 111.Gelfand RA, Matthews DE, Bier DM, Sherwin RS (1984) Role of counterregulatory hormones in the catabolic response to stress. J Clin Invest 74 (6):2238–2248. 10.1172/JCI111650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matthews DE, Pesola G, Campbell RG (1990) Effect of epinephrine on amino acid and energy metabolism in humans. Am J Physiol 258 (6 Pt 1):E948–956 [DOI] [PubMed] [Google Scholar]
- 113.Shamoon H, Jacob R, Sherwin RS (1980) Epinephrine-induced hypoaminoacidemia in normal and diabetic human subjects: effect of beta blockade. Diabetes 29 (11):875–881 [DOI] [PubMed] [Google Scholar]
- 114.Simmons PS, Miles JM, Gerich JE, Haymond MW (1984) Increased proteolysis. An effect of increases in plasma cortisol within the physiologic range. J Clin Invest 73 (2):412–420. 10.1172/JCI111227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hourani H, Williams P, Morris JA, May ME, Abumrad NN (1990) Effect of insulin-induced hypoglycemia on protein metabolism in vivo. Am J Physiol 259 (3 Pt 1):E342–350 [DOI] [PubMed] [Google Scholar]
- 116.Battezzati A, Benedini S, Fattorini A, Piceni Sereni L, Luzi L (2000) Effect of hypoglycemia on amino acid and protein metabolism in healthy humans. Diabetes 49 (9):1543–1551 [DOI] [PubMed] [Google Scholar]
- 117.De Feo P, Perriello G, Santeusanio F, Brunetti P, Bolli G, Haymond MW (1992) Differential effects of insulin-induced hypoglycaemia on the plasma branched-chain and non-branched-chain amino acid concentrations in humans. Diabete Metab 18 (4):277–282 [PubMed] [Google Scholar]
- 118.Flakoll PJ, Wentzel LS, Rice DE, Hill JO, Abumrad NN (1992) Short-term regulation of insulin-mediated glucose utilization in four-day fasted human volunteers: role of amino acid availability. Diabetologia 35 (4):357–366 [DOI] [PubMed] [Google Scholar]
- 119.Cardoso S, Carvalho C, Santos R, Correia S, Santos MS, Seica R, Oliveira CR, Moreira PI (2011) Impact of STZ-induced hyperglycemia and insulin-induced hypoglycemia in plasma amino acids and cortical synaptosomal neurotransmitters. Synapse 65 (6):457–466. 10.1002/syn.20863 [DOI] [PubMed] [Google Scholar]
- 120.Silverstein FS, Simpson J, Gordon KE (1990) Hypoglycemia alters striatal amino acid efflux in perinatal rats: an in vivo microdialysis study. Ann Neurol 28 (4):516–521. 10.1002/ana.410280408 [DOI] [PubMed] [Google Scholar]
- 121.Santos MS, Moreno AJ, Carvalho AP (1996) Relationships between ATP depletion, membrane potential, and the release of neurotransmitters in rat nerve terminals. An in vitro study under conditions that mimic anoxia, hypoglycemia, and ischemia. Stroke 27 (5):941–950 [DOI] [PubMed] [Google Scholar]
- 122.Uematsu D, Greenberg JH, Reivich M, Karp A (1989) Cytosolic free calcium, NAD/NADH redox state and hemodynamic changes in the cat cortex during severe hypoglycemia. J Cereb Blood Flow Metab 9 (2):149–155. 10.1038/jcbfm.1989.22 [DOI] [PubMed] [Google Scholar]
- 123.Syamsunarno MR, Iso T, Hanaoka H, Yamaguchi A, Obokata M, Koitabashi N, Goto K, Hishiki T, Nagahata Y, Matsui H, Sano M, Kobayashi M, Kikuchi O, Sasaki T, Maeda K, Murakami M, Kitamura T, Suematsu M, Tsushima Y, Endo K, Hotamisligil GS, Kurabayashi M (2013) A critical role of fatty acid binding protein 4 and 5 (FABP4/5) in the systemic response to fasting. PLoS One 8 (11):e79386 10.1371/journal.pone.0079386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Godfried MH, Romijn JA, Endert E, Sauerwein HP (1994) Metabolic effects of hypoglycemic counterregulation during sustained mild hyperinsulinemia and constant glucose availability in healthy men. Nutrition 10 (1):5–10 [PubMed] [Google Scholar]
- 125.Caprio S, Gelfand RA, Tamborlane WV, Sherwin RS (1989) Oxidative fuel metabolism during mild hypoglycemia: critical role of free fatty acids. Am J Physiol 256 (3 Pt 1):E413–419 [DOI] [PubMed] [Google Scholar]
- 126.Fanelli C, Calderone S, Epifano L, De Vincenzo A, Modarelli F, Pampanelli S, Perriello G, De Feo P, Brunetti P, Gerich JE, et al. (1993) Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest 92 (4):1617–1622. 10.1172/JCI116746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fanelli CG, De Feo P, Porcellati F, Perriello G, Torlone E, Santeusanio F, Brunetti P, Bolli GB (1992) Adrenergic mechanisms contribute to the late phase of hypoglycemic glucose counterregulation in humans by stimulating lipolysis. J Clin Invest 89 (6):2005–2013. 10.1172/JCI115809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lucidi P, Rossetti P, Porcellati F, Pampanelli S, Candeloro P, Andreoli AM, Perriello G, Bolli GB, Fanelli CG (2010) Mechanisms of insulin resistance after insulin-induced hypoglycemia in humans: the role of lipolysis. Diabetes 59 (6):1349–1357. 10.2337/db09-0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Voss TS, Vendelbo MH, Kampmann U, Pedersen SB, Nielsen TS, Johannsen M, Svart MV, Jessen N, Moller N (2017) Effects of insulin-induced hypoglycaemia on lipolysis rate, lipid oxidation and adipose tissue signalling in human volunteers: a randomised clinical study. Diabetologia 60 (1):143–152. 10.1007/s00125-016-4126-x [DOI] [PubMed] [Google Scholar]
- 130.Strosznajder J (1984) Effect of hypoglycemia on the brain free fatty acid level and the uptake of fatty acids by phospholipids. Neurochem Res 9 (4):465–476 [DOI] [PubMed] [Google Scholar]
- 131.Agardh CD, Kalimo H, Olsson Y, Siesjo BK (1981) Hypoglycemic brain injury: metabolic and structural findings in rat cerebellar cortex during profound insulin-induced hypoglycemia and in the recovery period following glucose administration. J Cereb Blood Flow Metab 1 (1):71–84. 10.1038/jcbfm.1981.8 [DOI] [PubMed] [Google Scholar]
- 132.Haber S, Lapidot A (2001) Energy fuel utilization by fetal versus young rabbit brain: a 13C MRS isotopomer analysis of [U-(13)C]glucose metabolites. Brain Res 896 (1–2):102–117 [DOI] [PubMed] [Google Scholar]
- 133.Lapidot A, Haber S (2000) Effect of acute insulin-induced hypoglycemia on fetal versus adult brain fuel utilization, assessed by (13)C MRS isotopomer analysis of [U-(13)C]glucose metabolites. Dev Neurosci 22 (5–6):444–455. 10.1159/000017474 [DOI] [PubMed] [Google Scholar]
- 134.Ennis K, Lusczek E, Rao R (2017) Characterization of the concurrent metabolic changes in brain and plasma during insulin-induced moderate hypoglycemia using (1)H NMR spectroscopy in juvenile rats. Neurosci Lett 653:370–375. 10.1016/j.neulet.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 135.Liu K, Ye XJ, Hu WY, Zhang GY, Bai GH, Zhao LC, He JW, Zhu H, Shao JB, Yan ZH, Gao HC (2013) Neurochemical changes in the rat occipital cortex and hippocampus after repetitive and profound hypoglycemia during the neonatal period: an ex vivo (1)H magnetic resonance spectroscopy study. Mol Neurobiol 48 (3):729–736. 10.1007/s12035-013-8446-2 [DOI] [PubMed] [Google Scholar]
- 136.Languren G, Montiel T, Ramirez-Lugo L, Balderas I, Sanchez-Chavez G, Sotres-Bayon F, Bermudez-Rattoni F, Massieu L (2017) Recurrent moderate hypoglycemia exacerbates oxidative damage and neuronal death leading to cognitive dysfunction after the hypoglycemic coma. J Cereb Blood Flow Metab:271678X17733640. 10.1177/0271678X17733640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Feyter HM, Mason GF, Shulman GI, Rothman DL, Petersen KF (2013) Increased brain lactate concentrations without increased lactate oxidation during hypoglycemia in type 1 diabetic individuals. Diabetes 62 (9):3075–3080. 10.2337/db13-0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB (2009) Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29 (6):1121–1129. 10.1038/jcbfm.2009.35 [DOI] [PubMed] [Google Scholar]
- 139.Clarke DD, Sokoloff L (1994) Circulation and energy metabolism of brain. In: Siegel G, Agranoff B, Albers RW, Molinoff P (ed) Basic Neurochemistry : Molecular, Cellular and Medical Aspects. 5th edn Raven Press, New York, pp 645–680 [Google Scholar]
- 140.Avogaro A, Nosadini R, Doria A, Tremolada C, Baccaglini U, Ambrosio F, Merkel C, Nosadini A, Trevisan R, Fioretto P (1990) Substrate availability other than glucose in the brain during euglycemia and insulin-induced hypoglycemia in dogs. Metabolism 39 (1):46–50 [DOI] [PubMed] [Google Scholar]
- 141.Maran A, Crepaldi C, Trupiani S, Lucca T, Jori E, Macdonald IA, Tiengo A, Avogaro A, Del Prato S (2000) Brain function rescue effect of lactate following hypoglycaemia is not an adaptation process in both normal and type I diabetic subjects. Diabetologia 43 (6):733–741. 10.1007/s001250051371 [DOI] [PubMed] [Google Scholar]
- 142.Page KA, Williamson A, Yu N, McNay EC, Dzuira J, McCrimmon RJ, Sherwin RS (2009) Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes 58 (5):1237–1244. 10.2337/db08-1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J (1994) Effect of hyperketonemia and hyperlacticacidemia on symptoms, cognitive dysfunction, and counterregulatory hormone responses during hypoglycemia in normal humans. Diabetes 43 (11):1311–1317 [DOI] [PubMed] [Google Scholar]
- 144.Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI (2006) Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes 55 (4):929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rao R, Ennis K, Long JD, Ugurbil K, Gruetter R, Tkac I (2010) Neurochemical changes in the developing rat hippocampus during prolonged hypoglycemia. J Neurochem 114 (3):728–738. 10.1111/j.1471-4159.2010.06797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gundersen V, Fonnum F, Ottersen OP, Storm-Mathisen J (2001) Redistribution of neuroactive amino acids in hippocampus and striatum during hypoglycemia: a quantitative immunogold study. J Cereb Blood Flow Metab 21 (1):41–51. 10.1097/00004647-200101000-00006 [DOI] [PubMed] [Google Scholar]
- 147.Kauppinen RA, Nicholls DG (1986) Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem 158 (1):159–165 [DOI] [PubMed] [Google Scholar]
- 148.Belik J, Wagerle LC, Stanley CA, Sacks LM, Herbert DW, Delivoria-Papadopoulos M (1989) Cerebral metabolic response and mitochondrial activity following insulin-induced hypoglycemia in newborn lambs. Biol Neonate 55 (4–5):281–289. 10.1159/000242930 [DOI] [PubMed] [Google Scholar]
- 149.Laffel L (1999) Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 15 (6):412–426 [DOI] [PubMed] [Google Scholar]
- 150.Nehlig A (2004) Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fatty Acids 70 (3):265–275. 10.1016/j.plefa.2003.07.006 [DOI] [PubMed] [Google Scholar]
- 151.Cahill GF Jr., Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA Jr., Kipnis DM (1966) Hormone-fuel interrelationships during fasting. J Clin Invest 45 (11):1751–1769. 10.1172/JCI105481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gjedde A, Crone C (1975) Induction processes in blood-brain transfer of ketone bodies during starvation. Am J Physiol 229 (5):1165–1169 [DOI] [PubMed] [Google Scholar]
- 153.Hasselbalch SG, Knudsen GM, Jakobsen J, Hageman LP, Holm S, Paulson OB (1995) Blood-brain barrier permeability of glucose and ketone bodies during short-term starvation in humans. Am J Physiol 268 (6 Pt 1):E1161–1166 [DOI] [PubMed] [Google Scholar]
- 154.Hawkins RA, Williamson DH, Krebs HA (1971) Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J 122 (1):13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Williamson DH, Bates MW, Krebs HA (1968) Activity and intracellular distribution of enzymes of ketone-body metabolism in rat liver. Biochem J 108 (3):353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Williamson DH, Bates MW, Page MA, Krebs HA (1971) Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J 121 (1):41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pan JW, de Graaf RA, Petersen KF, Shulman GI, Hetherington HP, Rothman DL (2002) [2,4–13 C2]-beta-Hydroxybutyrate metabolism in human brain. J Cereb Blood Flow Metab 22 (7):890–898. 10.1097/00004647-200207000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arakawa T, Goto T, Okada Y (1991) Effect of ketone body (D-3-hydroxybutyrate) on neural activity and energy metabolism in hippocampal slices of the adult guinea pig. Neurosci Lett 130 (1):53–56 [DOI] [PubMed] [Google Scholar]
- 159.Brooks KJ, Clark JB, Bates TE (1998) 3-Hydroxybutyrate aids the recovery of the energy state from aglycaemic hypoxia of adult but not neonatal rat brain slices. J Neurochem 70 (5):1986–1990 [DOI] [PubMed] [Google Scholar]
- 160.Wada H, Okada Y, Nabetani M, Nakamura H (1997) The effects of lactate and beta-hydroxybutyrate on the energy metabolism and neural activity of hippocampal slices from adult and immature rat. Brain Res Dev Brain Res 101 (1–2):1–7 [DOI] [PubMed] [Google Scholar]
- 161.Evans ML, Matyka K, Lomas J, Pernet A, Cranston IC, Macdonald I, Amiel SA (1998) Reduced counterregulation during hypoglycemia with raised circulating nonglucose lipid substrates: evidence for regional differences in metabolic capacity in the human brain? J Clin Endocrinol Metab 83 (8):2952–2959.doi: 10.1210/jcem.83.8.4937 [DOI] [PubMed] [Google Scholar]
- 162.Haywood SC, Bree AJ, Puente EC, Daphna-Iken D, Fisher SJ (2009) Central but not systemic lipid infusion augments the counterregulatory response to hypoglycemia. Am J Physiol Endocrinol Metab 297 (1):E50–56. 10.1152/ajpendo.90673.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Camberos-Luna L, Geronimo-Olvera C, Montiel T, Rincon-Heredia R, Massieu L (2016) The Ketone Body, beta-Hydroxybutyrate Stimulates the Autophagic Flux and Prevents Neuronal Death Induced by Glucose Deprivation in Cortical Cultured Neurons. Neurochem Res 41 (3):600–609. 10.1007/s11064-015-1700-4 [DOI] [PubMed] [Google Scholar]
- 164.Haces ML, Hernandez-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L (2008) Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol 211 (1):85–96. 10.1016/j.expneurol.2007.12.029 [DOI] [PubMed] [Google Scholar]
- 165.Julio-Amilpas A, Montiel T, Soto-Tinoco E, Geronimo-Olvera C, Massieu L (2015) Protection of hypoglycemia-induced neuronal death by beta-hydroxybutyrate involves the preservation of energy levels and decreased production of reactive oxygen species. J Cereb Blood Flow Metab 35 (5):851–860. 10.1038/jcbfm.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Massieu L, Haces ML, Montiel T, Hernandez-Fonseca K (2003) Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience 120 (2):365–378 [DOI] [PubMed] [Google Scholar]
- 167.Mejia-Toiber J, Montiel T, Massieu L (2006) D-beta-hydroxybutyrate prevents glutamate-mediated lipoperoxidation and neuronal damage elicited during glycolysis inhibition in vivo. Neurochem Res 31 (12):1399–1408. 10.1007/s11064-006-9189-5 [DOI] [PubMed] [Google Scholar]
- 168.Yamada KA, Rensing N, Thio LL (2005) Ketogenic diet reduces hypoglycemia-induced neuronal death in young rats. Neurosci Lett 385 (3):210–214. 10.1016/j.neulet.2005.05.038 [DOI] [PubMed] [Google Scholar]
- 169.Oz G, Henry PG, Seaquist ER, Gruetter R (2003) Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochem Int 43 (4–5):323–329 [DOI] [PubMed] [Google Scholar]
- 170.Oz G, Seaquist ER, Kumar A, Criego AB, Benedict LE, Rao JP, Henry PG, Van De Moortele PF, Gruetter R (2007) Human brain glycogen content and metabolism: implications on its role in brain energy metabolism. Am J Physiol Endocrinol Metab 292 (3):E946–951. 10.1152/ajpendo.00424.2006 [DOI] [PubMed] [Google Scholar]
- 171.Brown AM, Baltan Tekkok S, Ransom BR (2004) Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem Int 45 (4):529–536. 10.1016/j.neuint.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 172.Cruz NF, Dienel GA (2002) High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab 22 (12):1476–1489. 10.1097/01.WCB.0000034362.37277.C0 [DOI] [PubMed] [Google Scholar]
- 173.Dienel GA, Ball KK, Cruz NF (2007) A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J Neurochem 102 (2):466–478. 10.1111/j.1471-4159.2007.04595.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morgenthaler FD, van Heeswijk RB, Xin L, Laus S, Frenkel H, Lei H, Gruetter R (2008) Non-invasive quantification of brain glycogen absolute concentration. J Neurochem 107 (5):1414–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cataldo AM, Broadwell RD (1986) Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol 15 (4):511–524 [DOI] [PubMed] [Google Scholar]
- 176.Ibrahim MZ (1975) Glycogen and its related enzymes of metabolism in the central nervous system. Adv Anat Embryol Cell Biol 52 (1):3–89 [DOI] [PubMed] [Google Scholar]
- 177.Pellegri G, Rossier C, Magistretti PJ, Martin JL (1996) Cloning, localization and induction of mouse brain glycogen synthase. Brain Res Mol Brain Res 38 (2):191–199 [DOI] [PubMed] [Google Scholar]
- 178.Choi IY, Seaquist ER, Gruetter R (2003) Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res 72 (1):25–32. 10.1002/jnr.10574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Oz G, Kumar A, Rao JP, Kodl CT, Chow L, Eberly LE, Seaquist ER (2009) Human brain glycogen metabolism during and after hypoglycemia. Diabetes 58 (9):1978–1985. 10.2337/db09-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623 (2):208–214 [DOI] [PubMed] [Google Scholar]
- 181.Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR (2005) Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res 81 (5):644–652. 10.1002/jnr.20573 [DOI] [PubMed] [Google Scholar]
- 182.Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR (2000) Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci 20 (18):6804–6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K, Swanson RA (2007) Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmethyl)pro pyl]-1H-indole-2-carboxamide). J Pharmacol Exp Ther 321 (1):45–50. 10.1124/jpet.106.115550 [DOI] [PubMed] [Google Scholar]
- 184.Duarte JMN, Morgenthaler FD, Gruetter R (2017) Glycogen Supercompensation in the Rat Brain After Acute Hypoglycemia is Independent of Glucose Levels During Recovery. Neurochem Res 42 (6):1629–1635. 10.1007/s11064-017-2178-z [DOI] [PubMed] [Google Scholar]
- 185.Herzog RI, Chan O, Yu S, Dziura J, McNay EC, Sherwin RS (2008) Effect of acute and recurrent hypoglycemia on changes in brain glycogen concentration. Endocrinology 149 (4):1499–1504. 10.1210/en.2007-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oz G, DiNuzzo M, Kumar A, Moheet A, Khowaja A, Kubisiak K, Eberly LE, Seaquist ER (2017) Cerebral glycogen in humans following acute and recurrent hypoglycemia: Implications on a role in hypoglycemia unawareness. J Cereb Blood Flow Metab 37 (8):2883–2893. 10.1177/0271678X16678240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA (2005) Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes 54 (5):1452–1458 [DOI] [PubMed] [Google Scholar]
- 188.Zhou D, Qian J, Chang H, Xi B, Sun RP (2012) Pyruvate administered to newborn rats with insulin-induced hypoglycemic brain injury reduces neuronal death and cognitive impairment. Eur J Pediatr 171 (1):103–109. 10.1007/s00431-011-1489-3 [DOI] [PubMed] [Google Scholar]
- 189.Shetty PK, Sadgrove MP, Galeffi F, Turner DA (2012) Pyruvate incubation enhances glycogen stores and sustains neuronal function during subsequent glucose deprivation. Neurobiol Dis 45 (1):177–187. 10.1016/j.nbd.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Choi BY, Kim JH, Kim HJ, Yoo JH, Song HK, Sohn M, Won SJ, Suh SW (2013) Pyruvate administration reduces recurrent/moderate hypoglycemia-induced cortical neuron death in diabetic rats. PLoS One 8 (11):e81523.doi: 10.1371/journal.pone.0081523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Behar KL, den Hollander JA, Petroff OA, Hetherington HP, Prichard JW, Shulman RG (1985) Effect of hypoglycemic encephalopathy upon amino acids, high-energy phosphates, and pHi in the rat brain in vivo: detection by sequential 1H and 31P NMR spectroscopy. J Neurochem 44 (4):1045–1055 [DOI] [PubMed] [Google Scholar]
- 192.Cerdan S, Kunnecke B, Seelig J (1990) Cerebral metabolism of [1,2–13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem 265 (22):12916–12926 [PubMed] [Google Scholar]
- 193.Cruz F, Scott SR, Barroso I, Santisteban P, Cerdan S (1998) Ontogeny and cellular localization of the pyruvate recycling system in rat brain. J Neurochem 70 (6):2613–2619 [DOI] [PubMed] [Google Scholar]
- 194.Bakken IJ, White LR, Aasly J, Unsgard G, Sonnewald U (1997) Lactate formation from [U-13C]aspartate in cultured astrocytes: compartmentation of pyruvate metabolism. Neurosci Lett 237 (2–3):117–120 [DOI] [PubMed] [Google Scholar]
- 195.Chapa F, Cruz F, Garcia-Martin ML, Garcia-Espinosa MA, Cerdan S (2000) Metabolism of (1-(13)C) glucose and (2-(13)C, 2-(2)H(3)) acetate in the neuronal and glial compartments of the adult rat brain as detected by [(13)C, (2)H] NMR spectroscopy. Neurochem Int 37 (2–3):217–228 [DOI] [PubMed] [Google Scholar]
- 196.Hassel B, Sonnewald U (1995) Glial formation of pyruvate and lactate from TCA cycle intermediates: implications for the inactivation of transmitter amino acids? J Neurochem 65 (5):2227–2234 [DOI] [PubMed] [Google Scholar]
- 197.Olstad E, Olsen GM, Qu H, Sonnewald U (2007) Pyruvate recycling in cultured neurons from cerebellum. J Neurosci Res 85 (15):3318–3325. 10.1002/jnr.21208 [DOI] [PubMed] [Google Scholar]
- 198.Sonnewald U, Westergaard N, Jones P, Taylor A, Bachelard HS, Schousboe A (1996) Metabolism of [U-13C5] glutamine in cultured astrocytes studied by NMR spectroscopy: first evidence of astrocytic pyruvate recycling. J Neurochem 67 (6):2566–2572 [DOI] [PubMed] [Google Scholar]
- 199.Rink C, Gnyawali S, Stewart R, Teplitsky S, Harris H, Roy S, Sen CK, Khanna S (2017) Glutamate oxaloacetate transaminase enables anaplerotic refilling of TCA cycle intermediates in stroke-affected brain. FASEB J 31 (4):1709–1718. 10.1096/fj.201601033R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Won SJ, Jang BG, Yoo BH, Sohn M, Lee MW, Choi BY, Kim JH, Song HK, Suh SW (2012) Prevention of acute/severe hypoglycemia-induced neuron death by lactate administration. J Cereb Blood Flow Metab 32 (6):1086–1096. 10.1038/jcbfm.2012.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hypertension in Diabetes Study (HDS): II. Increased risk of cardiovascular complications in hypertensive type 2 diabetic patients (1993). J Hypertens 11 (3):319–325 [DOI] [PubMed] [Google Scholar]
- 202.Abbatecola AM, Paolisso G, Sinclair AJ (2015) Treating diabetes mellitus in older and oldest old patients. Curr Pharm Des 21 (13):1665–1671 [DOI] [PubMed] [Google Scholar]
- 203.Alonso-Moran E, Orueta JF, Esteban JI, Axpe JM, Gonzalez ML, Polanco NT, Loiola PE, Gaztambide S, Nuno-Solinis R (2015) Multimorbidity in people with type 2 diabetes in the Basque Country (Spain): Prevalence, comorbidity clusters and comparison with other chronic patients. Eur J Intern Med 26 (3):197–202.doi: 10.1016/j.ejim.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 204.Du Y, Heidemann C, Gosswald A, Schmich P, Scheidt-Nave C (2013) Prevalence and comorbidity of diabetes mellitus among non-institutionalized older adults in Germany - results of the national telephone health interview survey ‘German Health Update (GEDA)’ 2009. BMC Public Health 13:166 10.1186/1471-2458-13-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cao Z, Ye BD, Shen ZW, Cheng XF, Yang ZX, Liu YY, Wu RH, Geng K, Xiao YY (2015) 2D-1H proton magnetic resonance spectroscopic imaging study on brain metabolite alterations in patients with diabetic hypertension. Mol Med Rep 11 (6):4232–4238. 10.3892/mmr.2015.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Duarte JM, Do KQ, Gruetter R (2014) Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy. Neurobiol Aging 35 (7):1660–1668. 10.1016/j.neurobiolaging.2014.01.135 [DOI] [PubMed] [Google Scholar]
- 207.Ivanisevic J, Stauch KL, Petrascheck M, Benton HP, Epstein AA, Fang M, Gorantla S, Tran M, Hoang L, Kurczy ME, Boska MD, Gendelman HE, Fox HS, Siuzdak G (2016) Metabolic drift in the aging brain. Aging (Albany NY) 8 (5):1000–1020.doi: 10.18632/aging.100961 [DOI] [PMC free article] [PubMed] [Google Scholar]