Abstract
New diagnoses of HIV-1 infection among people who inject drugs (PWID) rocketed in Athens, Greece between 2011 and 2014 (HIV-1 outbreak). Our aim was to identify, during that period, potential cross-group transmissions between the within-Greece PWID and other risk or national groups using molecular methods.
Sequences from 33 PWID were outside the PWID-outbreak networks in Greece (PWID-imported transmissions). Phylogenetic analyses on 28 of these sequences (subtypes A and B) showed that 11 subtype B infections originated from Greece, whereas 8 and 7 subtype A strains were from former Soviet Union countries (AFSU) and Greece, respectively. The putative source in half of the PWID-imported transmissions with Greek origin was an individual who acquired HIV via sexual contact.
During four years of an HIV-1 outbreak among PWID in Athens, Greece, 33 individuals in this group (4.6% of all diagnoses with phylogenetic analyses) are likely to represent infections, sexually or injection-acquired, outside the within-Greece-PWID-outbreak networks. Combined molecular and traditional HIV surveillance to monitor introductions of new strains, and interventions that aim at reducing the rate of both injection and sexual risky practices are needed during drug injection-related HIV outbreaks.
Keywords: HIV-1, PWID, cross-group transmissions
1. Introduction
Global numbers show patterns of decreasing HIV incidence but outbreaks occur often in groups at risk for infection in Europe and worldwide. Greece experienced a large HIV-1 outbreak among people who inject drugs (PWID) between 2011–2014 (Bonovas and Nikolopoulos, 2012; Paraskevis et al., 2011; Pharris et al., 2011). The number of HIV-1 diagnoses among PWID in Greece exceeded 500 in 2012, while, before 2011, the HIV-1 epidemic was confined mainly to men who have sex with men (MSM) with sporadic cases among PWID (HCDCP, 2018). The epidemic receded after 2014 but figures still remain higher than the pre-outbreak period (HCDCP, 2014; Nikolopoulos et al., 2015b). Another European Union (EU) state, Romania, also saw large numbers of HIV infection among PWID in its capital city, Bucharest, after 2011 (Niculescu et al., 2015; Nikolopoulos et al., 2015a; Pharris et al., 2011). In recent years, increased numbers of HIV-1 diagnoses among PWID have also been reported in Dublin, Glasgow, and Luxembourg (EMCDDA, 2016).
The exponential rise of HIV-1 rates in PWID in Athens, Greece coincided with the serious economic and social crisis that Greece has been facing over the last years. There is evidence that the socio-economic turbulence hit first marginalized groups including drug injectors, led parts of the population to unemployment and homelessness, increased the size of risk networks in downtown Athens, the capital city of Greece, and allowed the interaction of distant ethnic groups (Nikolopoulos et al., 2015a, 2015b, Paraskevis et al., 2017, 2013; Sypsa et al., 2015). In a setting of low coverage of harm reduction services, newly circulating HIV strains were able to spread rapidly. Despite difficulties, the epidemic was substantially controlled (Nikolopoulos et al., 2017; Paraskevis et al., 2015; Sypsa et al., 2017) due to the coordinated response of health authorities, universities, and non-governmental organizations (NGOs). ARISTOTLE, a large seek, test, treat and retain intervention, the scale-up of needle/syringes programs (NSPs), and the increased access to substitution and antiretroviral treatment contributed to the successful containment of the outbreak (Hatzakis et al., 2015; HCDCP, 2014; Nikolopoulos et al., 2015b; Sypsa et al., 2017).
In molecular terms, the HIV epidemic has been extensively studied in Greece. Subtype B was the predominant clade in all transmission groups including PWID but subtype A has taken over in new diagnoses after 2004 (Nikolopoulos et al., 2008; Paraskevis et al., 2007). The HIV-1 outbreak among PWID was investigated using also molecular methodology (Paraskevis et al., 2015, 2013, 2011). This allowed us to observe the circulating new strains and to date their introduction into the injecting population, to identify four major phylogenetic clusters, and to investigate transmission dynamics and patterns (Kostaki et al., 2017; Paraskevis et al., 2013, 2011). In particular, analyses showed that 630 (88.6%) of 711 HIV-1 sequences of people who had been diagnosed between Jan 1st, 2011 and August 5th, 2014 (61% of all HIV-1 diagnoses among PWID in Greece over that period) fell within four PWID-outbreak transmission networks that have been described in Paraskevis et al. (2015, 2013): CRF14_BG (50.1%), CRF35_AD (17.3%), subtype B (14.9%), and A (6.3%). Moreover, 48 (6.8%) sequences were recombinants that consisted of partial regions originating from the four PWID-outbreak clades. Given the molecular evidence, we became confident about the recent start of the outbreak and its association with drugs injection. Therefore, we carefully designed and implemented interventions targeted at a drug injection-related epidemic (Hatzakis et al., 2015; Nikolopoulos et al., 2015b). This type of response was proven to be successful in other settings as well (Des Jarlais et al., 2011).
In an HIV outbreak, the majority of viral transmissions occur between individuals who are jointly engaged in risky practices (e.g. unprotected anal sex for MSM or sharing of injecting equipment for PWID). These transmissions can be identified as monophyletic clades of viral sequences and are named “outbreak lineages”. Transmissions, however, even during outbreaks, may also occur between individuals who belong to different risk groups, such as between drug injectors and MSM or heterosexuals, or to different national groups. Infections in non-PWID that originate from the primary outbreak group (e.g. PWID) are named “PWID-exported transmissions”, while transmissions in PWID that occur outside the major PWID-related outbreak phylogenetic clusters are named “PWID-imported transmissions”. The latter category probably includes many sexual transmissions of HIV. Therefore, during an ongoing drug injection-related epidemic, new isolates, beyond strains that have already established themselves in the PWID population, are likely to be introduced in drug injectors. Being aware of these events could be useful in terms of taking appropriate public health measures to prevent long-term transmission chains.
The aim of this analysis was thus to detect the presence of PWID-imported transmissions among HIV-1 infected drug injectors in Athens, Greece during the outbreak years, and to identify their geographic origin.
2. Materials and Methods
Samples of 711 HIV-1 infected PWID were collected between Jan 1st, 2011 and August 5th, 2014 in the context of regular genotypic drug resistance testing at HIV diagnosis, from the ARISTOTLE program, and from the Transmission Reduction Intervention Project (TRIP) (Friedman et al., 2014; Hatzakis et al., 2015). Surveillance data were available from the Hellenic Center for Disease Control and Prevention (HCDCP). ARISTOTLE was approved by the Institutional Review Board (IRB) of the National and Kapodistrian University of Athens, Greece, while TRIP was approved by the IRBs of the National Development and Research Institutes in New York City and of the Hellenic Scientific Society for the Study of AIDS and Sexually Transmitted Diseases (Athens, Greece).
HIV-1 subtypes were determined by the COMET HIV-1 subtyping tool and by further confirmatory phylogenetic analysis (Struck et al., 2014) as described previously (Paraskevis et al., 2015). Phylogenetic trees were inferred using, as references, sequences from pure subtypes and circulating recombinant forms (CRFs) available on the HIV Los Alamos sequence database (HIV Databases, 2018), and sequences from the four local transmission groups identified in PWID during the outbreak (2011–2014) (Paraskevis et al., 2015, 2013).
We use the term “PWID-imported transmissions” to refer to infections among PWID diagnosed during the outbreak years (2011–2014) whose sequences clustered outside the four outbreak transmission networks (Paraskevis et al., 2015, 2013) or were not recombinants of the PWID-outbreak sequences [i.e. unique recombinant forms (URFs) that contained partial genomic regions originating from outbreak lineages]. Further phylogenetic analyses were conducted on the sequences of the PWID-imported transmissions. Specifically, phylogenetic trees were inferred for subtype A PWID-imported transmissions using as references 488 and 1,463 sequences sampled from Greece during 1998–2013 and from a global dataset, respectively. For subtype B PWID-imported transmissions, we used 1,656 sequences from Greece and 3,984 sequences that had been sampled globally. Both global datasets were downloaded after a random selection from the HIV Los Alamos sequence database (HIV Databases, 2018). The sequences used as references in phylogenetic analyses and their country of origin are shown in Supplementary Table 1. For subtypes A and B, we also included the five most closely related sequences to each one of the PWID-imported transmissions as resulted after a BLAST search using the HIV BLAST tool (HIV Databases, 2018). For subtypes F1 and G PWID-imported transmissions, we used as references all sequences (n1=785 and n2=1452, respectively) available on the HIV sequence database (HIV Databases, 2018). The length of the alignment was 765, 663, 924, and 918 nucleotides for subtypes A, B, F1, and G, respectively. Additional phylogenetic analysis for the URF and the two unclassified sequences could not be done as no reference sequences were available. The putative origin of infection for these sequences could not be identified by BLAST search.
The analysis was conducted on protease (PR) and partial reverse transcriptase (RT) sequences. Phylogenetic trees were inferred by the approximate maximum likelihood method using the GTR model with the discrete gamma model and with 20 rate categories as implemented in FastTree 2 program (Price et al., 2010).
The origin of infection for the study subjects was estimated based on the branching order of their sequences. Specifically, for subtype A, we considered that all sequences from PWID falling within the large monophyletic groups of Greek sequences (Paraskevis et al., 2007) had their origin in Greece. Similarly, for subtypes B and F1 sequences that belonged to clusters of Greek or Romanian lineages at proportion ≥ 80% and receiving Shimodaira-Hasegawa (SH) support higher than 0.85, the origin was also believed to be in Greece and Romania, respectively. The sister clade to the PWID sequences of individuals with Greek origin was considered the most putative source of their infection.
Statistical analyses were performed using STATA 14. Statistical significant results were those with p-value less than 0.05.
3. Results
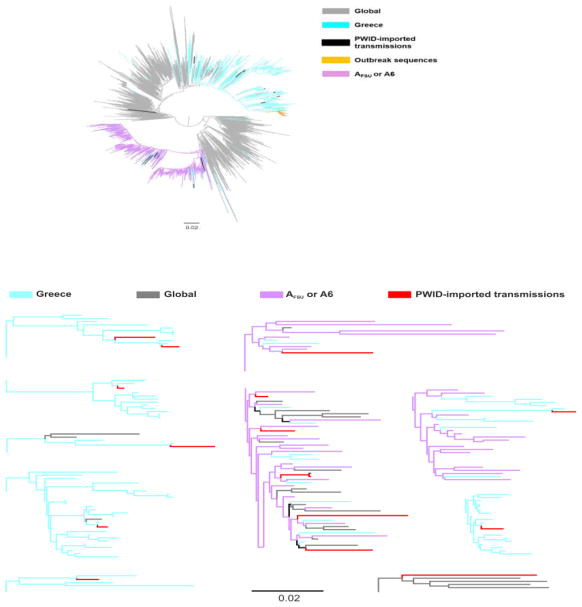
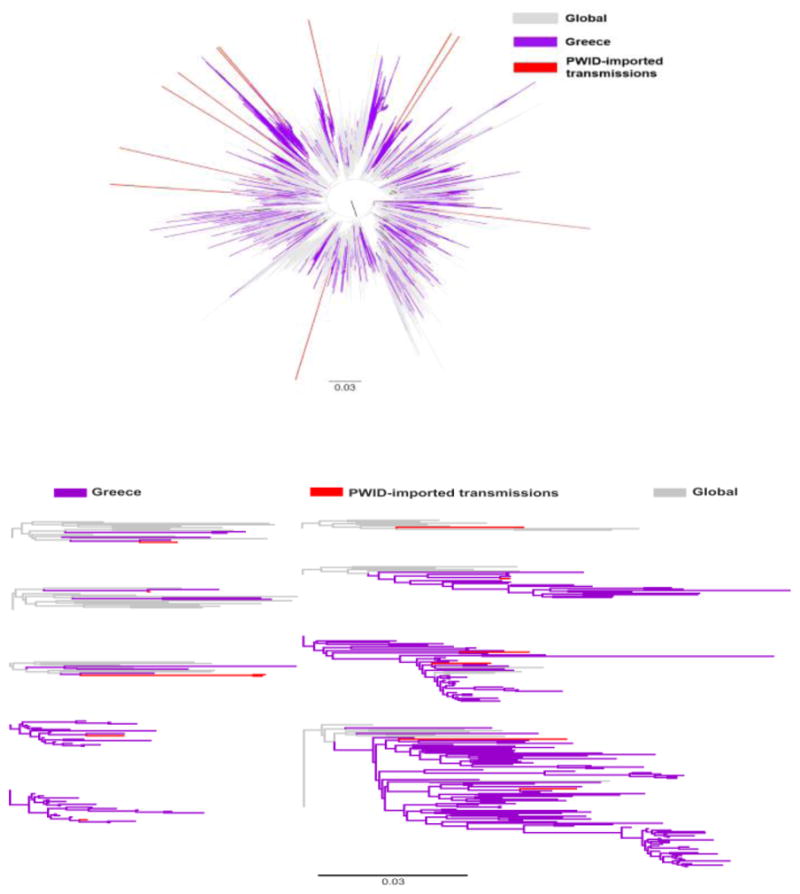
Sequences from 33 PWID (4.6% of the 711 PWID who were diagnosed between Jan 1st, 2011 and August 5th, 2014 and underwent phylogenetic analysis) did not belong either to the PWID-outbreak transmission networks or to their recombinants. The proportion of these PWID-imported transmissions among the annual number of diagnoses fluctuated over time reaching the highest value in 2014 (2011, 7.4%; 2012, 1.4%; 2013, 6.0%; 2014, 8.5% - p=0.01). The PWID-imported transmissions included subtype A1 (n=16, 48.5%), B (n=12, 36.5%), F1 (n=1, 3.0%), G (n=1, 3.0%), and URF (n=1, 3.0%), while 2 sequences (6.1%) remained unclassified. Further phylogenetic analysis for 28 subtypes (A1 and B) of the PWID-imported transmissions revealed that most of these infections originated from non-PWID-transmission networks in Greece and from PWID transmission networks in former Soviet Union countries (A6, formerly named AFSU) (Bobkov et al., 2001). In particular, 11 (91.7%) of the PWID-imported transmissions-subtype B lineages originated from Greece (Figure 1), whereas 8 (50.0%) and 7 (43.8%) of the PWID-imported transmissions-subtype A strains were of A6 and of Greek origin, respectively (Figure 2). Phylogenetic trees revealed that the origin of the subtype F1 infection was from Romania (Niculescu et al., 2015), while the exact origin of the PWID-imported subtype G transmission could not be identified (data not shown).
Figure 1.
A. Unrooted phylogenetic tree of HIV-1 subtype B sequences from Greece (marked in purple) and a global reference dataset (marked in grey). Sequences isolated from HIV-1 people who inject drugs (PWID) that were not clustered are marked in red (PWID-imported transmissions). Red highlight from non-clustered sequences does not correspond to branch lengths. B. Detail of the phylogenetic tree showing the clustering patterns of sequences from PWID-imported transmissions. The scale bar indicates the number of nucleotide substitutions per site.
Figure 2.
A. Unrooted phylogenetic tree of HIV-1 subtype A sequences from Greece (marked in light blue), AFSU* or A6 (marked in light purple), and a global reference dataset (marked in grey). Outbreak-PWID** sequences are marked in blue and PWID-imported transmissions are indicated with red. (*FSU: Former Soviet Union countries; **PWID: people who inject drugs).B. Detail of the phylogenetic tree showing the clustering patterns of sequences from PWID-imported transmissions. The scale bar indicates the number of nucleotide substitutions per site.
Additional analyses were conducted for the closest sister clades (putative source) of the sequences that were in the Greek clusters. The closest relative was identified for 6 out of 7 PWID-imported transmissions-subtype A1 sequences: for 3 sequences (50%), the source was in MSM or in heterosexuals, for 2 sequences (33.3%) the risk group of the source was unknown, and for 1 sequence (16.7%), the source of infection was a PWID. For the 11 PWID-imported transmissions-subtype B sequences, the closest relative was estimated for 10 of them: for 5 sequences (50%), the putative source was either a MSM or a heterosexual individual, for 3 sequences (30%) a PWID, while for 2 sequences (20%), the risk group of the source was unknown.
4. Discussion
Approximately 5% of the HIV-1 sequences in PWID in Greece during the outbreak years (2011–2014) were not part of the outbreak-associated phylogenetic groups. The proportion of these PWID-imported transmissions among the annual number of HIV-1 diagnoses peaked in 2014 when the drug injection-related outbreak showed clear signs of recession. Nearly half of the non-outbreak sequences were classified as subtype A1 and one third as subtype B.
The majority (91.7%) of the PWID-imported-subtype B transmissions in PWID were phylogenetically related with non-outbreak sequences circulating in Greece. As a matter of fact, before the HIV-1 outbreak in PWID, subtype B was prevalent among MSM (Paraskevis et al., 2007). The identification of these new single introductions of subtype B into the drug-injecting population implies, probably, sexually-acquired infections. This was further confirmed phylogenetically since the putative source of these non-PWID-outbreak infections was either a MSM or a heterosexual in at least 50% of cases. Of note, the PWID-imported-subtype B sequences that originated from non-outbreak-related infections in PWID (30%) were in small clusters of two sequences suggesting limited transmission networking and lower infection rate in these groups. The low number of clustered, PWID-imported transmissions is in great contrast with the large clusters (approximately 60–450 individuals) observed among the outbreak sequences.
Although the total number of sequences outside the major PWID clusters is relatively small and these introductions have not resulted insofar in long transmission chains, they should be carefully monitored. The history of the New York City (NYC) HIV epidemic has shown that sexual contacts can fuel transmissions following an outbreak in drug injectors. Nowadays in NYC, the prevalence of HIV is higher in non-injecting drug users than in PWID (Des Jarlais et al., 2014, 2011). Given the continuous introduction in PWID in Greece of strains from other risk or national groups, interventions such as increasing HIV testing, scaling-up NSPs, and improving access to opioid substitution and antiretroviral treatment have to be sustainable. In addition, the existence of sexual transmissions in PWID requires the promotion of safe sexual practices. Given the heightened viral load of people with recent HIV-1 infection and the documented contribution of early infections to HIV-1 spread (Hamlyn et al., 2010), contact or network tracing of PWID with molecular evidence of sexually or injection-acquired HIV-1 infection, combined with laboratory and/or epidemiologic evidence that the infection occurred recently, could be another safe and feasible approach to stopping transmission chains (Friedman et al., 2015).
Eight PWID during the outbreak years were infected with a non-outbreak-related subtype A1 and were phylogenetically grouped with A6 sequences. It seems that, during the outbreak, new strains were introduced in PWID in Greece representing either drug injectors living in Greece who became infected abroad or imported infections from other countries into the Greek drug scene. Previous HIV-1 subtype analyses in Europe have showed the predominance of subtype B and suggested compartmentalized epidemics and clustering between individuals within the same country (Abecasis et al., 2013; Frentz et al., 2013). However, travelling patterns of HIV infection have also been observed (Niculescu et al., 2015; Paraskevis et al., 2009). For example, the largest PWID-outbreak transmission network in Athens (CRF14_BG) originated from Romania, while the second in size (CRF35_AD) from Afghanistan/Iran (Paraskevis et al., 2015). In the Romanian epidemic, PWID who had been infected by CRF14_BG reported recent travel to Greece and Spain (Niculescu et al., 2015). While the drug injection-related epidemic is levelling off in Greece, the molecular evidence of introductions of new isolates through drug injection raises issues of concern. Of course, single introductions of viral strains do not necessarily generate large transmission networks (Paraskevis et al., 2013). This requires the coexistence of factors that facilitate rapid and easy transmission including socio-economic (homelessness) and other parameters (low coverage of prevention and harm reduction programs) as those that had been present in Greece before the crisis began and early on into the crisis years (Nikolopoulos et al., 2015b; Paraskevis et al., 2013; Sypsa et al., 2017). However, given the continuous fall of the Gross Domestic Product and the increasing income inequalities in Greece, new chains of infection are likely to be observed in unsaturated for HIV groups of drug injectors in downtown Athens or in other drug settings in the Athenian metropolitan area, especially in remote suburbs that were less or perhaps not affected at all by the outbreak (Nikolopoulos et al., 2015a). The sustainability of interventions such as ARISTOTLE (Hatzakis et al., 2015) and TRIP (Friedman et al., 2015, 2014; Nikolopoulos et al., 2016) might substantially decrease the likelihood of new HIV transmissions.
Cross-border infections have also implications at the European level. Distant outbreaks are likely to occur very fast following an epidemic in an EU region. Greece and a couple of other European countries including Portugal, Serbia, and Spain, for example, were found to be exporting subtype B infections to other parts of the European continent (Paraskevis et al., 2009). These underscore the need for coordinated responses at European level, which should be informed by timely and accurate molecular evidence that can reduce the lead time to outbreak detection (Paraskevis et al., 2015).
The strengths of our analyses include the high sampling coverage of PWID (61% of all HIV-1 diagnoses in PWID over the certain period) and the use of a large database of previously diagnosed HIV-1 individuals in Greece for the conduct of phylogenetic analyses. A potential weakness is that the putative origin of an infection can be traced accurately only if the source is sampled and the alignment contains adequate phylogenetic signal to reconstruct the evolutionary history of the study group.
Molecular biology and epidemiology come together to give insights into public health problems and emergencies. Based on molecular and surveillance information, we were able to identify and comprehend the transmission pattern of HIV, and take the necessary steps to reduce the infection rate in PWID (Paraskevis et al., 2015, 2013, 2011). The updated phylogenetic analyses presented here show that new isolates, unrelated to the sequences that caused the outbreak and comprised the primary transmission networks, are continuously introduced in drug injectors as the result of unprotected sexual contact or of injecting drug use. Interventions that have shown to work should be in place to limit the development of new, long chains of HIV transmission.
Supplementary Material
Supplementary Table 1. Sequences used as references in phylogenetic analyses and their country of origin.
Highlights.
Greece experienced an outbreak of HIV-1 among people who inject drugs-PWID in 2011–2014.
HIV-1 infections in PWID were mainly grouped in four phylogenetic clusters.
About 5% of the HIV-1 infections in PWID were outside these outbreak clusters.
Many of these PWID-imported infections may have been acquired sexually.
Acknowledgments
Funding
Support for ARISTOTLE and several authors was provided by EU NSRF 2007–2013 that was co-funded by the European Social Fund and Greek national resources. Additional financial support was provided by the Hellenic Scientific Society for the study of AIDS and Sexually Transmitted Diseases. We also acknowledge support from the National Institute on Drug Abuse (NIDA) (grant DP1 DA034989 - Preventing HIV Transmission by Recently-Infected Drug Users). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding agencies.
Footnotes
Declarations of interest
none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abecasis AB, Wensing AMJ, Paraskevis D, Vercauteren J, Theys K, Van de Vijver DAMC, Albert J, Asjö B, Balotta C, Beshkov D, Camacho RJ, Clotet B, De Gascun C, Griskevicius A, Grossman Z, Hamouda O, Horban A, Kolupajeva T, Korn K, Kostrikis LG, Kücherer C, Liitsola K, Linka M, Nielsen C, Otelea D, Paredes R, Poljak M, Puchhammer-Stöckl E, Schmit JC, Sönnerborg A, Stanekova D, Stanojevic M, Struck D, Boucher CAB, Vandamme AM. HIV-1 subtype distribution and its demographic determinants in newly diagnosed patients in Europe suggest highly compartmentalized epidemics. Retrovirology. 2013;10:7. doi: 10.1186/1742-4690-10-7. https://doi.org/10.1186/1742-4690-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobkov A, Kazennova E, Khanina T, Bobkova M, Selimova L, Kravchenko A, Pokrovsky V, Weber J. An HIV Type 1 Subtype A Strain of Low Genetic Diversity Continues to Spread among Injecting Drug Users in Russia: Study of the New Local Outbreaks in Moscow and Irkutsk. AIDS Res Hum Retroviruses. 2001;17:257–261. doi: 10.1089/088922201750063188. https://doi.org/10.1089/088922201750063188 [DOI] [PubMed] [Google Scholar]
- Bonovas S, Nikolopoulos G. High-burden epidemics in Greece in the era of economic crisis. Early signs of a public health tragedy. J Prev Med Hyg. 2012;53:169–71. [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, Friedman SR. HIV among drug users at Beth Israel Medical Center, New York City, the first 25 years. Subst Use Misuse. 2011;46:131–9. doi: 10.3109/10826084.2011.521456. https://doi.org/10.3109/10826084.2011.521456 [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, McKnight C, Arasteh K, Feelemyer J, Perlman DC, Hagan H, Dauria EF, Cooper HLF. A perfect storm: crack cocaine, HSV-2, and HIV among non-injecting drug users in New York City. Subst Use Misuse. 2014;49:783–92. doi: 10.3109/10826084.2014.880176. https://doi.org/10.3109/10826084.2014.880176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA-European Monitoring Center for Drugs and Drug Addiction. Drug-related infectious diseases in Europe. Lisbon: 2016. https://doi.org/10.2810/139972 [Google Scholar]
- Frentz D, Wensing AMJ, Albert J, Paraskevis D, Abecasis AB, Hamouda O, Jørgensen LB, Kücherer C, Struck D, Schmit JC, Åsjö B, Balotta C, Beshkov D, Camacho RJ, Clotet B, Coughlan S, De Wit S, Griskevicius A, Grossman Z, Horban A, Kolupajeva T, Korn K, Kostrikis LG, Liitsola K, Linka M, Nielsen C, Otelea D, Paredes R, Poljak M, Puchhammer-Stöckl E, Sönnerborg A, Stanekova D, Stanojevic M, Vandamme AM, Boucher CAB, Van de Vijver DAMC. Limited cross-border infections in patients newly diagnosed with HIV in Europe. Retrovirology. 2013;10:36. doi: 10.1186/1742-4690-10-36. https://doi.org/10.1186/1742-4690-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, Downing MJ, Smyrnov P, Nikolopoulos G, Schneider JA, Livak B, Magiorkinis G, Slobodianyk L, Vasylyeva TI, Paraskevis D, Psichogiou M, Sypsa V, Malliori MM, Hatzakis A. Socially-Integrated Transdisciplinary HIV Prevention. AIDS Behav. 2014;18:1821–1834. doi: 10.1007/s10461-013-0643-5. https://doi.org/10.1007/s10461-013-0643-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, Williams L, Muth S, Hatzakis A, Paraskevis D, Psichogiou M, Al E. Finding the recently-infected to enhance medical care and prevention of transmission: A network approach and study in Athens, Greece. 143rd Meeting of the American Public Health Association.2015. [Google Scholar]
- Hamlyn E, Jones V, Porter K, Fidler S. Antiretroviral treatment of primary HIV infection to reduce onward transmission. Curr Opin HIV AIDS. 2010;5:283–90. doi: 10.1097/COH.0b013e32833a6b11. https://doi.org/10.1097/COH.0b013e32833a6b11 [DOI] [PubMed] [Google Scholar]
- Hatzakis A, Sypsa V, Paraskevis D, Nikolopoulos G, Tsiara C, Micha K, Panopoulos A, Malliori M, Psichogiou M, Pharris A, Wiessing L, van de Laar M, Donoghoe M, Heckathorn DD, Friedman SR, Des Jarlais DC. Design and baseline findings of a large-scale rapid response to an HIV outbreak in people who inject drugs in Athens, Greece: the ARISTOTLE programme. Addiction. 2015;110:1453–1467. doi: 10.1111/add.12999. https://doi.org/10.1111/add.12999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HCDCP. [accessed 2.13.13];Hellenic Center for Disease Control and Prevention [WWW Document] 2018 URL http://www.keelpno.gr.
- Hellenic Center for Disease Control and Prevention. HIV/AIDS Surveillance Report in Greece. 2014. [Google Scholar]
- HIV Databases. [accessed 2.13.18];HIV Databases. 2018 URL https://www.hiv.lanl.gov.
- Kostaki E, Magiorkinis G, Psichogiou M, Flampouris A, Iliopoulos P, Papachristou E, Daikos GL, Bonovas S, Otelea D, Friedman SR, Hatzakis A, Paraskevis D. Detailed molecular surveillance of the HIV-1 outbreak among people who inject drugs (PWID) in Athens during a period of four years. Curr HIV Res. 2017:15. doi: 10.2174/1570162X15666171120104048. https://doi.org/10.2174/1570162X15666171120104048 [DOI] [PubMed]
- Niculescu I, Paraschiv S, Paraskevis D, Abagiu A, Batan I, Banica L, Otelea D. Recent HIV-1 Outbreak Among Intravenous Drug Users in Romania: Evidence for Cocirculation of CRF14_BG and Subtype F1 Strains. AIDS Res Hum Retroviruses. 2015;31:488–95. doi: 10.1089/aid.2014.0189. https://doi.org/10.1089/aid.2014.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos G, Paraskevis D, Hatzakis A. HIV epidemiology in Greece. Future Microbiol. 2008;3:507–516. doi: 10.2217/17460913.3.5.507. https://doi.org/10.2217/17460913.3.5.507 [DOI] [PubMed] [Google Scholar]
- Nikolopoulos G, Pavlitina E, Muth S, Schneider J, Psichogiou M, Williams L, Paraskevis D, Sypsa V, Magiorkinis G, Smyrnov P, Korobchuk A, Vasylyeva T, Skaathun B, Malliori M, Kafetzopoulos E, Hatzakis A, Friedman S. A network intervention that locates and intervenes with recently HIV-infected persons: The Transmission Reduction Intervention Project (TRIP) Sci Rep. 2016;6:38100. doi: 10.1038/srep38100. https://doi.org/10.1038/srep38100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos GK, Fotiou A, Kanavou E, Richardson C, Detsis M, Pharris A, Suk JE, Semenza JC, Costa-Storti C, Paraskevis D, Sypsa V, Malliori MM, Friedman SR, Hatzakis A. National Income Inequality and Declining GDP Growth Rates Are Associated with Increases in HIV Diagnoses among People Who Inject Drugs in Europe: A Panel Data Analysis. PLoS One. 2015a;10:e0122367. doi: 10.1371/journal.pone.0122367. https://doi.org/10.1371/journal.pone.0122367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos GK, Katsoulidou A, Kantzanou M, Rokka C, Tsiara C, Sypsa V, Paraskevis D, Psichogiou M, Friedman S, Hatzakis A. Evaluation of the limiting antigen avidity EIA (LAg) in people who inject drugs in Greece. Epidemiol Infect. 2017;145:401–412. doi: 10.1017/S0950268816002417. https://doi.org/10.1017/S0950268816002417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos GK, Sypsa V, Bonovas S, Paraskevis D, Malliori-Minerva M, Hatzakis A, Friedman SR. Big Events in Greece and HIV Infection Among People Who Inject Drugs. Subst Use Misuse. 2015b;50:825–38. doi: 10.3109/10826084.2015.978659. https://doi.org/10.3109/10826084.2015.978659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevis D, Kostaki E, Nikolopoulos GK, Sypsa V, Psichogiou M, Del Amo J, Hodges-Mameletzis I, Paraskeva D, Skoutelis A, Malliori M, Williams L, Friedman SR, Daikos GL, Hatzakis A. Molecular Tracing of the Geographical Origin of Human Immunodeficiency Virus Type 1 Infection and Patterns of Epidemic Spread Among Migrants Who Inject Drugs in Athens. Clin Infect Dis. 2017;65:2078–2084. doi: 10.1093/cid/cix717. https://doi.org/10.1093/cid/cix717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevis D, Magiorkinis E, Magiorkinis G, Sypsa V, Paparizos V, Lazanas M, Gargalianos P, Antoniadou A, Panos G, Chrysos G, Sambatakou H, Karafoulidou A, Skoutelis A, Kordossis T, Koratzanis G, Theodoridou M, Daikos GL, Nikolopoulos G, Pybus OG, Hatzakis A. Increasing prevalence of HIV-1 subtype A in Greece: estimating epidemic history and origin. J Infect Dis. 2007;196:1167–76. doi: 10.1086/521677. https://doi.org/10.1086/521677 [DOI] [PubMed] [Google Scholar]
- Paraskevis D, Nikolopoulos G, Fotiou A, Tsiara C, Paraskeva D, Sypsa V, Lazanas M, Gargalianos P, Psichogiou M, Skoutelis A, Wiessing L, Friedman SR, Jarlais DCDES, Terzidou M, Kremastinou J, Malliori M, Hatzakis A. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: a longitudinal study. PLoS One. 2013;8:e78941. doi: 10.1371/journal.pone.0078941. https://doi.org/10.1371/journal.pone.0078941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevis D, Nikolopoulos G, Tsiara C, Paraskeva D, Antoniadou A, Lazanas M, Gargalianos P, Psychogiou M, Malliori M, Kremastinou J, Hatzakis A. HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. Euro Surveill. 2011:16. doi: 10.2807/ese.16.36.19962-en. [DOI] [PubMed] [Google Scholar]
- Paraskevis D, Paraschiv S, Sypsa V, Nikolopoulos G, Tsiara C, Magiorkinis G, Psichogiou M, Flampouris A, Mardarescu M, Niculescu I, Batan I, Malliori M, Otelea D, Hatzakis A. Enhanced HIV-1 surveillance using molecular epidemiology to study and monitor HIV-1 outbreaks among intravenous drug users (IDUs) in Athens and Bucharest. Infect Genet Evol. 2015;35:109–21. doi: 10.1016/j.meegid.2015.08.004. https://doi.org/10.1016/j.meegid.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Paraskevis D, Pybus O, Magiorkinis G, Hatzakis A, Wensing AM, van de Vijver DA, Albert J, Angarano G, Asjo B, Balotta C, Boeri E, Camacho R, Chaix ML, Coughlan S, Costagliola D, De Luca A, de Mendoza C, Derdelinckx I, Grossman Z, Hamouda O, Hoepelman IM, Horban A, Korn K, Kuecherer C, Leitner T, Loveday C, Macrae E, Maljkovic I, Meyer L, Nielsen C, Op de Coul EL, Ormaasen V, Perrin L, Puchhammer-Stockl E, Ruiz L, Salminen M, Schmit JC, Schuurman R, Soriano V, Stanczak J, Stanojevic M, Struck D, Van Laethem K, Violin M, Yerly S, Zazzi M, Boucher CA, Vandamme AM, Programme S. Tracing the HIV-1 subtype B mobility in Europe: a phylogeographic approach. Retrovirology. 2009;6:49. doi: 10.1186/1742-4690-6-49. https://doi.org/10.1186/1742-4690-6-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharris A, Wiessing L, Sfetcu O, Hedrich D, Botescu A, Fotiou A, Nikolopoulos GK, Malliori M, Salminen M, Suk JE, Griffiths P, van de Laar MJ. Human immunodeficiency virus in injecting drug users in Europe following a reported increase of cases in Greece and Romania, 2011. Euro Surveill. 2011:16. [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. https://doi.org/10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D, Lawyer G, Ternes A-M, Schmit J-C, Bercoff DP. COMET: adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res. 2014;42:e144. doi: 10.1093/nar/gku739. https://doi.org/10.1093/nar/gku739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypsa V, Paraskevis D, Malliori M, Nikolopoulos GK, Panopoulos A, Kantzanou M, Katsoulidou A, Psichogiou M, Fotiou A, Pharris A, Van De Laar M, Wiessing L, Des Jarlais D, Friedman SR, Hatzakis A. Homelessness and Other Risk Factors for HIV Infection in the Current Outbreak Among Injection Drug Users in Athens, Greece. Am J Public Health. 2015;105:196–204. doi: 10.2105/AJPH.2013.301656. https://doi.org/10.2105/AJPH.2013.301656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypsa V, Psichogiou M, Paraskevis D, Nikolopoulos G, Tsiara C, Paraskeva D, Micha K, Malliori M, Pharris A, Wiessing L, Donoghoe M, Friedman S, Des Jarlais D, Daikos G, Hatzakis A. Rapid decline of HIV incidence among people who inject drugs during a fast-track combination prevention programme following an HIV outbreak in Athens. J Infect Dis. 2017;215:1496–1505. doi: 10.1093/infdis/jix100. https://doi.org/10.1093/infdis/jix100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Sequences used as references in phylogenetic analyses and their country of origin.