Abstract
Objective
To determine whether patient-specific differences in motor control quantified using muscle synergy analysis were associated with changes in gait after treatment in cerebral palsy across two clinical centers with different treatments and clinical protocols.
Design
Retrospective Cohort Study.
Setting
Clinical Medical Center.
Participants
Center 1: 473 children with cerebral palsy and 84 typically-developing children. Center 2: 163 children with cerebral palsy and 12 typically-developing children.
Interventions
Standard clinical care at each center.
Outcome Measures
The dynamic motor control index during walking (walk-DMC) was computed from electromyography data during gait using muscle synergy analysis. Regression analysis was used to evaluate whether pre-treatment walking speed or kinematics, muscle synergies, treatment group, prior treatment, or age were associated with post-treatment changes in gait at both clinical centers.
Results
Walk-DMC was significantly associated with changes in speed and kinematics after treatment with similar regression models at both centers. Children with less impaired motor control were more likely to have improvements in walking speed and gait kinematics after treatment, independent of treatment group.
Conclusions
Dynamic motor control evaluated with synergy analysis was associated with changes in gait after treatment at both centers, despite differences in treatments and clinical protocols. This study further supports the finding that walk-DMC provides additional information, not captured in traditional gait analysis, that may be useful for treatment planning.
Keywords: CP (Cerebral Palsy), Gait, Motor Disorders, Walking Speed, Electromyography
Poor motor control often arises after neurologic injury, such as in cerebral palsy (CP). Reduced motor control hinders movement and has been suggested as an important factor influencing treatment outcomes (1). Recent research has suggested that measures derived from muscle synergy analysis, such as the Dynamic Motor Control Index during Walking (walk-DMC), may provide a clinical tool to quantify poor motor control and inform treatment planning (2,3). These studies also suggested that individuals with less impaired motor control, or higher walk-DMC scores, have greater improvements in walking ability after treatment than individuals with poor motor control. These results support the common clinical belief that poor motor control can contribute to worse treatment outcomes. Despite these initial promising results, the relationship between walk-DMC and treatment outcomes has only been evaluated at one center. Reproducibility is critical to understand the generalizability and potential impact of new measures in the clinic, especially when considering that many published biomedical findings fail to be reproduced (4).
Whether walk-DMC’s association with treatment outcomes extends across clinical centers remains an open question, especially considering differences in clinical protocols and treatments between centers. For example, between centers, the dosage and number of muscles treated with botulinum toxin type A injection (5), amount of transection in selective dorsal rhizotomy (6), and choice of procedures in single-event multi-level orthopedic surgeries for children with CP (7) can a vary. In clinical gait analyses, the number of muscles monitored with electromyography (EMG) varies between centers which may impact calculations of synergies and walk-DMC. Thus, it is important to determine whether differences between centers in procedures, outcomes, or collected data, refute or alter conclusions about whether walk-DMC is a useful measure to inform treatment planning.
Walk-DMC leverages the theory of muscle synergies to create an objective measure of motor control that quantifies an individual’s complexity of neuromuscular control during walking (2,8). Synergy analysis uses EMG data and matrix factorization algorithms to identify a small set of weighted muscles groups (synergies) that are recruited together during functional tasks (9–11). After neurologic injury (e.g. stroke and CP), even fewer synergies are required to describe the EMG data, suggesting a simplified control strategy that may contribute to impaired movement (12–16). Since EMG data are collected as part of standard care in clinical gait analysis laboratories, synergy methods are attractive for quantifying patient-specific deficits in neuromuscular control. Walk-DMC represents a summary measure of an individual’s synergy complexity.
The aim of this research was to investigate whether walk-DMC is associated with treatment outcomes across clinical centers. A secondary goal was to determine whether differences in clinical treatment or data collection protocols (e.g. the number of muscles included in synergy analysis) impact these results. Determining if walk-DMC is associated with changes in kinematics and walking speed following treatment will help determine the clinical utility and generalizability of walk-DMC as a measure of motor control for treatment planning in CP.
Methods
The data used in this study received ethical approval by the [Center 2 ethics board] and from the [Center 1 ethics board].
Participants
Children with CP from two clinical centers with pre-treatment electromyography (EMG) and kinematic data, collected as standard clinical care, were retrospectively analyzed. For Center 1, 473 children (Table 1) from [Center 1] were analyzed across the following treatment groups: selective dorsal rhizotomy (SDR), single-event multi-level orthopedic surgery (SEMLS), singlelevel orthopedic surgery, or conservative treatment (physical therapy, excluding botulinum toxin type A injection, BTA). Full details on the collection methodology from Center 1 can be found in (3).
Table 1.
Subject Demographics, average (one standard deviation).
| Treatment | N | GMFCS | Age | Gender | Speed | Speed | GDI | GDI | Follow-up Time |
|---|---|---|---|---|---|---|---|---|---|
| I II/III | y+mo | F: | M | Pre | Post | Pre | Post y+mo | ||
| Center 1 | |||||||||
| CONS | 76 | 22/28/26 | 6+8 (2+7) | 35:41 | 0.33 (0.11) | 0.36 (0.12) | 71.3 (11.4) | 73.7 (12.2) | 1+7 (0+7) |
| ORTHO-1 | 39 | 16/15/8 | 6+11 (3+4) | 16:23 | 0.34 (0.11) | 0.36 (0.12) | 71.5 (9.7) | 75.7 (10.7) | 1+6 (0+4) |
| SEMLS | 176 | 25/85/66 | 10+0 (3+5) | 77:99 | 0.31 (0.11) | 0.29 (0.11) | 67.4 (9.7) | 76.3 (10.2) | 1+5 (0+5) |
| SDR | 182 | 35/76/71 | 5+7 (2+0) | 82:100 | 0.32 (0.12) | 0.35 (0.11) | 69.5 (9.4) | 75.1 (8.5) | 1+6 (0+4) |
| Center 2 | |||||||||
| BTA | 60 | 20/20/20 | 6+9 (2+11) | 20:40 | 0.31 (0.14) | 0.29 (.015) | 73.3 (12.1) | 74.1 (10.8) | 0+2 (0+2) |
| SEMLS | 59 | 20/19/20 | 12+1 (3+1) | 24:35 | 0.28 (0.11) | 0.25 (.013) | 66.1 (11.7) | 76.7 (12.0) | 1+1 (0+2) |
| SDR | 44 | 12/28/4 | 9+1 (2+0) | 23:21 | 0.33 (0.11) | 0.31 (.010) | 72.1 (10.5) | 74.0 (13.9) | 1+1 (0+5) |
BTA: Botulinum Toxin Type A Injection, CONS: Conservative Treatment, F: Female, GDI: Gait Deviation Index, N: number of participants, M: Male, ORTHO-1: Single-Level Orthopaedic Surgery, Post: Post Treatment, Pre: Pre Treatment, SDR: Selective Dorsal Rhizotomy, SEMLS: Single-Event Multi-Level Orthopedic Surgery, Speed: Non-Dimensional Walking Speed, y+mo: Years + Months.
For Center 2, we analyzed motion analysis data (Vicon)a from 163 children with CP (Table 1), collected at [Center 2], distributed between three treatment groups: BTA, SDR, and SEMLS. We selected a roughly even distribution between Gross Motor Function Classification System (GMFCS) Levels I–III for BTA and SEMLS groups. The SDR group was selected based upon data availability (fewer individuals, mostly GMFCS Level II). Participants walked barefoot at their self-selected speed. Data from 12 typically-developing (TD) children were included for comparison to the CP groups.
Electromyography
Surface EMG data (Wave Wireless EMG)b were collected at either 1000 or 1500 Hz from four muscles (rectus femoris, medial hamstrings, gastrocnemius, and tibialis anterior). Four additional muscles (gluteus medius, vastus lateralis, lateral hamstrings, and soleus) were recorded from 147 children with CP and the TD children at Center 2. Since we were using retrospective clinical data, some walking trials contained one or more poor or missing EMG channels. In some cases, clinicians switched electrodes between walking trials such that each muscle was recorded in at least one trial. We analyzed each child’s more impaired side, when indicated by clinician assessment, or otherwise selected a random side. EMG data from the middle 80% of each trial were used to avoid transient accelerations and decelerations near the beginning and end of each trial, and maximize data for synergy analysis (11).
Raw EMG data were band-pass filtered between 20 and 500 Hz upon collection, then digitally pre-processed with a 20 Hz high-pass filter, rectified, and low-pass filtered at 10 Hz (17). Each EMG channel’s amplitude was scaled to its maximum across all trials. EMG data were then down-sampled to 100 Hz and concatenated from all available trials for each individual to maximize the number of steps for analysis.
Synergy Analysis
We used walk-DMC to evaluate whether synergies were associated with treatment outcomes. Walk-DMC converts total variance accounted for in the one-synergy solution (tVAF1) to a z-score using the average and standard deviation of tVAF1 from the TD group (tVAFAVG and tVAFSD). The average walk-DMC of the TD group is 100, and each 10 point change in walk-DMC represents one standard deviation from the TD group (eq. 1). A higher tVAF1 results in a lower walk-DMC score, suggesting a simplified neuromuscular control strategy. Walk-DMC was created in a manner similar to other common clinical scales that enable easy comparison with TD controls, such as the Gait Deviation Index (GDI) (18).
| (1) |
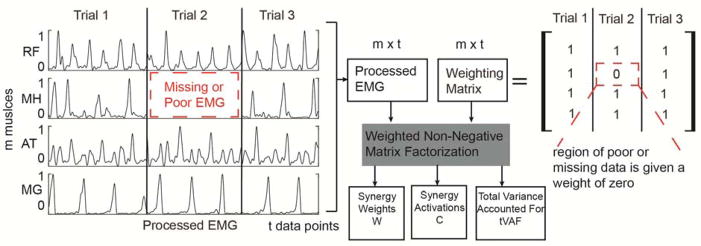
The one synergy solution was calculated for each child from EMG data using weighted non-negative matrix factorization (WNMF) (19,20). Non-negative matrix factorization (NMF) (3,21) finds a set of synergy weights (Wmxn) and activations (Cnxt) such that EMG=W × C + error, where n is the number of synergies (1 in this study), m is the number of muscles (4, 8, or 16 in this study), and t is the number of EMG data points. WNMF extends NMF by assigning each data point a weight, allowing us to assign a weight of zero to all missing or corrupted EMG channels (Figure 1). We calculated synergies in Matlabc using the following parameters: 50 replicates, 1000 maximum iterations, a 1×10−4 minimum convergence threshold, and a 1×10−6 completion threshold.
Figure 1.
Data from multiple walking trials are concatenated together. In some trials there is poor or missing data for some channels. When weighted non-negative matrix factorization is used, the regions of poor or missing data are given zero weight and not incorporated into the update rules for non-negative matrix factorization. This approach allowed us to maximize the amount of data for analysis As with traditional non-negative matrix factorization, synergy weights, activations and total variance accounted for by a given number of synergies are the outputs.
Outcome Measures
Changes in walking ability were assessed after treatment using two measures: GDI (18) and non-dimensional walking speed (22). GDI measures an individual’s deviation in kinematics compared to an unimpaired dataset using nine joint angles. GDI is scaled such that the average ± standard deviation of the control group is 100 ±10 points (each child was analyzed with respect to their center’s control kinematic database). Dimensionless walking speed was calculated as to account for differences in speed due to leg length (22).
To determine whether walk-DMC was associated with treatment outcomes, stepwise linear regression models were created for each outcome measure (post-treatment GDI and walking speed), starting with a constant model to identify the fewest explaining factors. The four-muscle set was used to replicate the methods from Center 1 (3). The initial potential regressors supplied to this model were the pre-treatment outcome measure (pre-treatment GDI or walking speed), walk-DMC, treatment group, age, and whether the limb had undergone prior surgery (yes/no). Regressors were added into the model such that the sum of squared errors was minimized using an F statistic at an alpha of 0.05 and critical p<0.05. To minimize the effect of outliers, robust fitting using a bi-square weighting algorithm was applied to the model output from the stepwise regression. Additionally, we applied the linear regression models originally identified from Center 1 (3) to compare the regression coefficients between the two centers.
To examine sensitivity of the prior results to clinical protocols, we analyzed the impact of number of muscles on the regression results by including four-or eight-muscles in the synergy analysis. These represent two of the most common muscle sets used in clinical gait analyses. Since inter-limb effects may be important (23,24), we also calculated bilateral walk-DMC using either the four- or eight-muscle sets from both legs. The same methods for the linear regression models were applied to each set of synergy outputs. Of the 163 individuals from Center 2 with EMG data for four muscles unilaterally, 152 had data bilaterally, and 147/136 had data for eight muscles unilaterally/bilaterally. To examine the robustness of the resulting models (across muscle sets for GDI and walking speed) a 10-fold cross-validation (25) was performed by replicating regressions on a random 90% of the data and testing the resulting models on the remaining 10% of observations. Model robustness was assessed by examining the cross-validated errors in relation to the original model errors.
Results
Gait Deviation Index
At Center 2, the average pre/post-treatment GDI scores were 73/74, 72/74, and 66/77 for the BTA, SDR, and SEMLS treatment groups, respectively. Pre-treatment GDI was significantly lower in the SEMLS group compared to the BTA or SDR groups (one-way ANOVA, Tukey Kramer post-hoc p<.001). For each treatment group, GDI increased more than 5 pts (minimum clinically significant difference) (26) in 28% (BTA), 27% (SDR), and 68% (SEMLS) of individuals. The percentage of individuals whose GDI decreased more than 5 pts was 25% (BTA), 16% (SDR), and 5% (SEMLS).
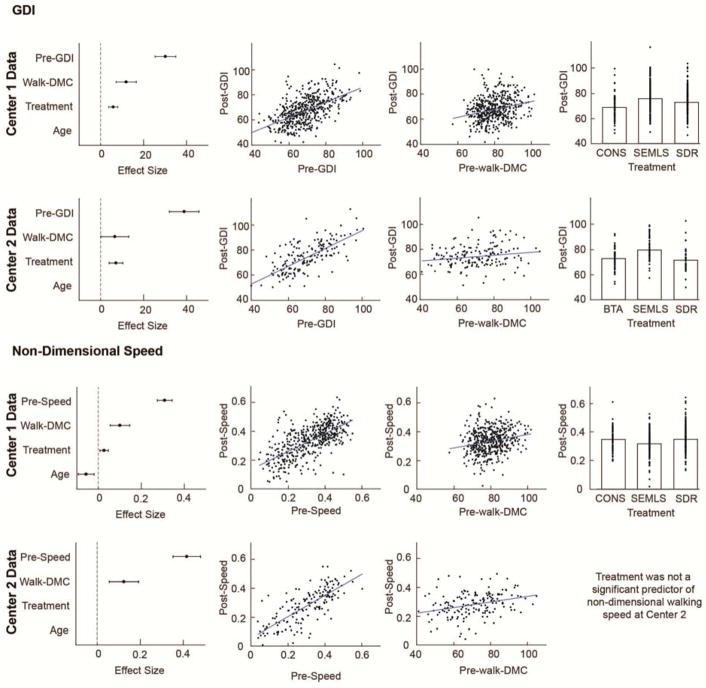
Pre-treatment GDI, walk-DMC, and treatment group were significantly associated with post-treatment GDI, with an interaction between pre-treatment GDI and treatment group (Table 2). This exactly matches the form of the model identified at Center 1 (3). The effect sizes (95% CI) were 43.2 (±7.7) for pre-treatment GDI, 7.4 (±7.2) for walk-DMC, and 8.0 (±3.6) for treatment group (Figure 2). Both centers models indicated a positive coefficient for walk-DMC, (higher walk-DMC scores corresponded to higher post-treatment GDI), but the slope magnitude was smaller at Center 2 (0.11 vs 0.26 at Center 1).
Table 2.
Regression models of post-treatment GDI and walking speed at two centers.
| Regression Model* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| GDI Post = | GDI Pre × Treatment + | Treatment Intercept + | walk-DMC | |||||||||
|
|
||||||||||||
| treatment group | estimate | p | estimate | p | estimate | p | ||||||
| GDI | Center 1 ** | R^2 | 0.42 | Cons | 0.74 | <.01 | −0.68 | 0.91 | 0.26 | <.01 | ||
| RMSE | 7.8 | SEMLS | 0.51 | <.01 | 20.87 | <.01 | ||||||
| SDR | 0.38 | <.01 | 27.49 | <.01 | ||||||||
| Ortho-1 | 0.61 | <.01 | 8.94 | 0.37 | ||||||||
| Center 2 | R^2 | 0.52 | BTA | 0.57 | <.01 | 24.44 | <.01 | 0.11 | <.05 | |||
| RMSE | 8.7 | SEMLS | 0.65 | <.01 | 25.57 | <.01 | ||||||
| SDR | 0.99 | <.01 | −6.60 | 0.49 | ||||||||
| Center 1 Regression Model | ||||||||||||
|
|
||||||||||||
| Speed Post = | Speed Pre + | Treatment Intercept + | walk-DMC + | Age | ||||||||
|
|
||||||||||||
| treatment group | estimate | p | estimate | p | estimate | p | estimate | p | ||||
| Non-Dimensional Speed | Center 1 ** | R^2 | 0.53 | Cons | 0.62 | <.01 | −0.010 | 0.81 | 0.0023 | <.01 | −0.0044 | <.05 |
| RMSE | 0.081 | SEMLS | −0.037 | 0.36 | ||||||||
| SDR | −0.009 | 0.82 | ||||||||||
| Ortho-1 | −0.011 | 0.8 | ||||||||||
| Center 2 | R^2 | 0.66 | BTA | 0.72 | <.01 | −0.073 | <.05 | 0.0021 | <.01 | −0.0018 | 0.42 | |
| RMSE | 0.078 | SEMLS | −0.075 | 0.07 | ||||||||
| SDR | −0.0606 | 0.12 | ||||||||||
| Center 2 Regression Model | ||||||||||||
|
| ||||||||||||
| Speed Post = | Speed Pre + | Intercept + | walk-DMC | |||||||||
|
| ||||||||||||
| treatment group | estimate | p | estimate | p | estimate | p | ||||||
| Center 2 | R^2 | 0.67 | ||||||||||
| RMSE | 0.077 | All | 0.74 | <.01 | −0.08 | <.05 | 0.0019 | <.01 | ||||
BTA: Botulinum Toxin Type A Injection, CONS: Conservative Treatment, GDI: Gait Deviation Index, ORTHO-1: Single-Level Orthopaedic Surgery, Post: Post Treatment, Pre: Pre Treatment, SDR: Selective Dorsal Rhizotomy, SEMLS: Single-Event Multi-Level Orthopedic Surgery, Speed: Non-Dimensional Walking Speed, Walk-DMC: Dynamic Motor Control Index during Walking
For GDI, the stepwise regression identified the same model for Center 1 and Center 2 data
Data reproduced from Schwartz et al. 2016 (3)
Figure 2.
Adjusted response plots and effect sizes are shown for statistically significant regressors for GDI and non-dimensional walking speed models. Models are identified by the stepwise regression after applying robust fitting from the original paper (Schwartz 2016) and the data from Center 2 (four muscles). The estimated effect sizes and 95% confidence interval show which regressors are present in the Center 1 and Center 2 models. Adjusted response plots show the relation between the outcome (post-treatment GDI or non-dimensional walking speed) and each predictor after removing the effect of the other predictors. For GDI, walk-DMC, treatment group, and pre-treatment GDI were significant regressors in the Center 1 data (top row, reproduced from the prior paper) and Center 2 data (second row). For Non-Dimensional walking speed, treatment and age (not shown) were significant regressors in the Center 1 data (third row, reproduced from the prior paper), but not in the Center 2 data (bottom row) Walk-DMC and pre-treatment walking speed were present in both models.
Walking Speed
The average pre/post-treatment dimensionless walking speeds at Center 2 were 0.31/0.29, 0.28/0.25, and 0.33/0.31 for the BTA, SDR, and SEMLS treatment groups, respectively. Pre-treatment dimensionless speeds were not significantly different between treatment groups (one-way ANOVA, p = 0.18). After treatment, 17% (BTA), 27% (SDR), and 27% (SEMLS) increased their walking speed by more than 10%, while 50% (BTA), 36% (SDR), and 51% (SEMLS) decreased walking speed by more than 10%.
Stepwise linear regression indicated that pre-treatment dimensionless walking speed and walk-DMC were significantly associated with post-treatment walking speed at Center 2 (Table 2). The effect sizes were 0.42 (±0.06) for pre-treatment walking speed and 0.12 (±0.07) for walk-DMC (Figure 2). The coefficient for walk-DMC was similar between centers (0.0019 vs 0.0023 at Center 1), with the positive slopes indicating higher walk-DMC scores were associated with faster post-treatment walking speeds. The model at Center 1 also found treatment group and age to be significant predictors. Applying Center 1’s model to Center 2’s data described 66% of the variance in post-treatment walking speed (vs 67% with the Center 2 model), with effect sizes of 0.41 (±0.07) for pre-treatment walking speed, 0.13 (±0.07) for walk-DMC, −0.03 (±0.07) for age, and 0.01 (±0.03) for treatment group (Figure 2).
Muscle Sets
Children with CP had lower walk-DMC than TD children across four- or eight-muscle sets, and unilateral or bilateral muscle sets (one-way Anova, Tukey Kramer Post-hoc p<0.001). Walk-DMC was significantly associated with post-treatment GDI and walking speed for all muscle sets, although after applying robust fitting, the coefficient between post-treatment GDI and walk-DMC was no longer significant for the unilateral eight-muscle set (p=0.12). The effect sizes for post-treatment GDI were similar across all muscle sets for walk-DMC (range: 5.4–8.3), pre-treatment GDI (41.0–42.5), and treatment (6.8–7.4). The effect sizes for post-treatment dimensionless walking speed were similar across muscle sets (0.42–0.43 pre-treatment speed and 0.12–0.13 walk-DMC).
Cross-Validation
Cross-Validation errors (Table 3) were similar to the original errors for all models at both centers. On average 10-fold validation errors between the modeled and measured outcomes were smaller than the model calculated from the full dataset at Center 2.
Table 3.
Cross-Validation Results
|
|
||||
|---|---|---|---|---|
| Root Mean Square Errors | ||||
|
| ||||
| GDI | Non-Dimensional Walking Speed | |||
|
| ||||
| Original Model | Cross Validation | Original Model | Cross Validation | |
|
|
||||
| Four Muscle model | ||||
|
|
||||
| Center 1 | 7.8 | 7.7 | 0.08 | 0.08 |
| Center 2 | 8.7 | 8.7 | 0.08 | 0.08 |
|
|
||||
| Alternate Muscle Sets | ||||
|
|
||||
| Center 2 (8 muscles) | 8.9 | 8.4 | 0.08 | 0.07 |
| Center 2 (4 muscles bilateral) | 9.0 | 8.6 | 0.08 | 0.07 |
| Center 2 (8 muscles bilateral) | 9.2 | 8.3 | 0.08 | 0.07 |
GDI: Gait Deviation Index
Discussion
Walk-DMC was associated with changes in GDI and walking speed after treatment at a second clinical center, and showed results strikingly similar to those found at the first clinical center. Walk-DMC was positively associated with post-treatment walking speed, such that individuals with less impaired motor control pre-treatment (higher walk-DMC) had greater increases in walking speed after treatment. A change in walking speed of roughly 10% represents a clinically important difference (27), which corresponds to a preoperative difference of 16 pts walk-DMC at Center 1 or 15 pts walk-DMC at Center 2. Walk-DMC was also positively associated with changes in walking kinematics, as measured with GDI, but was weaker at Center 2. A change of 5 points GDI represents a clinically important difference (26) which corresponds to a preoperative difference of 19 points in walk-DMC at Center 1 and 45 points in walk-DMC at Center 2. These differences may be due to differences in clinical outcomes and treatment groups between centers, as discussed below.
For both walking speed and GDI, children with lower walk-DMC scores generally had worse outcomes after treatment compared to children with higher walk-DMC scores. These findings contrast with other pre-treatment variables. For example, kinematics or spatio-temporal measures have previously been used to predict treatment outcomes, and have indicated that greater impairments were generally associated with better treatment outcomes (28,29). Similarly, in this study, lower pre-treatment walking speed and GDI were associated with greater improvements after treatment. The positive slope of walk-DMC with treatment outcomes suggests that walk-DMC can provide unique information about a child’s walking pattern that is not reflected by other measures.
Children with less impaired motor control (higher walk-DMC) but greater impairments in walking (low GDI or speed) had the best outcomes across treatments at both clinical centers. Synergies have been theorized to reflect simplified control strategies, such as from central-pattern generators or other lower-levels of control in the central nervous system (23). While these hypotheses are challenging to verify in humans, the results of this research suggest that walk-DMC may serve as a warning sign to identify children who are more dependent on lower-level control and may have smaller improvements in walking after treatment.
There were several differences between the analysis of walk-DMC and outcomes at the two centers. In comparison to Center1, we grouped all orthopedic surgeries together (versus separating single or multiple orthopedic surgeries) and there was no conservative treatment group. Instead, a BTA group was included. Center 2 included a higher percentage of GMFCS Level I children (32% vs 21% at Center 1). Importantly, we also noted smaller changes in GDI after SDR at Center 2 (2 pts) compared to Center 1 (5 pts). These differences in outcomes following SDR from the two centers highlights the need to evaluate differences in protocols between centers. A separate examination by Huenaerts (30) of patients at Center 2 who received SDR showed that small changes in the gait profile score (similar to GDI) were due to improved knee kinematics combined with worsening hip flexion and anterior pelvic tilt. The differences in SDR outcomes impacted our regression models at Center 2 by increasing the effect sizes of treatment and pre-treatment GDI, while decreasing the effect size of walk-DMC. Re-computing the linear models without the SDR treatment group caused all effect sizes to more closely match Center 1.
The muscles measured with EMG were dictated by the current standard of care at both centers. This study demonstrated that walk-DMC was significantly associated with treatment outcomes regardless of choice of muscles (i.e., unilateral/bilateral and four/eight-muscle sets). The similarity of unilateral and bilateral walk-DMC also suggests that these methods provide a more global, rather than limb-specific, measure of motor control. A further methodological difference was the application of WNMF to the Center 2 data (versus NMF at Center 1), increasing the amount of EMG data available for analysis by including trials with missing or corrupted channels (11% of trials for four muscles). Tests on data sets with no missing EMG data showed no differences in synergy outputs between the two algorithms.
Study Limitations
The results of this study were limited by the use of retrospective data, which affects our ability to prospectively evaluate the use of walk-DMC or other measures of motor control in treatment planning. The other variables included in the model were selected to match the prior study, which focused on variables previously suggested to be associated with treatment outcomes in CP. Other variables (e.g., metabolic costs, physical exam measures, or neurologic injury) may also be important predictors of treatment outcomes in CP. In this study, we used GDI as a global measure of improvements in gait. However, improving GDI is not the only goal targeted by interventions in CP. For the BTA treatment group, a series of repeated treatments shifts the focus from altering kinematics to maintaining improvements (31) and most subjects included in this study had received prior BTA treatments (68%). Additional clinical measures, such as spasticity, (which is targeted by BTA and SDR treatments) were not included in this analysis. Specific details of the treatments including which muscles were targeted with BTA, or what specific surgical procedures were administered during SEMLS were also not included. The heterogeneity in treatments in CP remains a challenge in predicting patient-specific improvements in walking ability after treatment.
Conclusions
This study demonstrated that walk-DMC was associated with treatment outcomes at two separate centers across a variety of treatments. These associations were not sensitive to which muscles were included in the analysis, demonstrating robust patterns that can help inform the use of motor control to determine which children are more likely to benefit from treatments to improve gait in CP.
Highlights.
Synergies were associated with post-treatment gait outcomes
This association was independent of treatment group at both centers
Less impaired motor control was associated with faster walking post-treatment
Less impaired motor control was associated with greater improvements in kinematics
Associations were similar across two commonly used clinical muscle sets
Acknowledgments
Acknowledgment of presentation: Data from the first clinical center have been previously published in Developmental Medicine and Child Neurology under Schwartz MH, Rozumalski A, Steele KM. Dynamic motor control is associated with treatment outcomes for children with cerebral palsy. Dev Med Child Neurol. 2016;58(11):1139–45. Portions of this work was prented as an abstract at the 41st American Society of Biomechanics meeting in Boulder, Co, August 8–11, 2017.
Financial Support: Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under award number R01NS091056, as well as graduate student funding from the Washington Research Foundation Funds for Innovation in Neuroengineering.
Abbreviations
- ANOVA
analysis of variance
- BTA
botulinum toxin type A injection
- CP
cerebral palsy
- EMG
electromyography
- GDI
gait deviation index
- GMFCS
Gross Motor Function Classification System
- NMF
non-negative matrix factorization
- SDR
selective dorsal rhizotomy
- SEMLS
single-event multilevel orthopedic surgery
- TD
typically-developing
- tVAF
total variance accounted for
- walk-DMC
the Dynamic Motor Control Index during Walking
- WNMF
weighted non-negative matrix factorization
Footnotes
Vicon 10 or 15 cameras and Nexus software, 14 Minns Business Park West Way, oxford, OX2 0JB, United Kingdom
Wave Wireless EMG, Cometa, via G. Falcone, 43 - 20010 Bareggio (MI) Italy
MathWorks, Inc., 1 Apple Hill Drive, Natick, Massachusetts 017602098, United States
Conflict of interest: The authors declare no conflict of interest.
Clinical Trial Registration Number: N/A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cahill-rowley K, Rose J. Etiology of impaired selective motor control : emerging evidence and its implications for research and treatment in cerebral palsy. Dev Med Child Neurol. 2014;56(6):522–8. doi: 10.1111/dmcn.12355. [DOI] [PubMed] [Google Scholar]
- 2.Steele KM, Rozumalski A, Schwartz MH. Muscle synergies and complexity of neuromuscular control during gait are altered in individuals with cerebral palsy. Dev Med Child Neurol. 2015;57(45):1176–82. doi: 10.1111/dmcn.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz MH, Rozumalski A, Steele KM. Dynamic motor control is associated with treatment outcomes for children with cerebral palsy. Dev Med Child Neurol. 2016;58(11):1139–45. doi: 10.1111/dmcn.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begley CG, Ioannidis JPA. Reproducibility in Science Improving the Standard for Basic and Preclinical Research. Circ Res. 2015;116:116–26. doi: 10.1161/CIRCRESAHA.114.303819. [DOI] [PubMed] [Google Scholar]
- 5.Gormley ME, Gaebler-spira D, Delgado MR. Use of Botulinum Toxin Type A in Pediatric Patients With Cerebral Palsy: A Three-Center Retrospective Chart Review. J Child Neurol. 2001;16(2):113–8. doi: 10.1177/088307380101600209. [DOI] [PubMed] [Google Scholar]
- 6.Mclaughlin J, Bjornson K, Temkin N, Steinbok P, Wright V, Reiner A, et al. Selective dorsal rhizotomy: meta-analysis of three randomized controlled trials. Dev Med Child Neurol. 2002;44:17–25. doi: 10.1017/s0012162201001608. [DOI] [PubMed] [Google Scholar]
- 7.Lamberts RP, Burger M, Toit J, Langerak NG. A Systematic Review of the Effects of Single-Event Multilevel Surgery on Gait Parameters in Children with Spastic Cerebral Palsy. PLoS One. 2016;11(10):1–22. doi: 10.1371/journal.pone.0164686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ting LH, Chiel HJ, Trumbower RD, Allen JL, Mckay JL, Hackney ME, et al. Neuron [Internet] 1. Vol. 86. Elsevier Inc; 2015. Perspective Neuromechanical Principles Underlying Movement Modularity and Their Implications for Rehabilitation; pp. 38–54. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol. 2009;19(6):601. doi: 10.1016/j.conb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neptune RR, Clark DJ, Kautz Sa. Modular control of human walking: a simulation study. J Biomech [Internet] 2009 Jun 19;42(9):1282–7. doi: 10.1016/j.jbiomech.2009.03.009. [cited 2013 Sep 23] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2696580&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira AS, Gizzi L, Farina D, Kersting UG. Motor modules of human locomotion: influence of EMG averaging, concatenation, and number of step cycles. Front Hum Neurosci [Internet] 2014;8(May):335. doi: 10.3389/fnhum.2014.00335. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4033063&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz Sa. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103:844–57. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Routson RL, Clark DJ, Bowden MG, Kautz Sa, Neptune RR. Gait Posture [Internet] 3. Vol. 38. Elsevier B.V; 2013. Jul, The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance; pp. 511–7. [cited 2014 Sep 27] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3687005&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang L, Li F, Cao S, Zhang X, Wu D, Chen X. Muscle synergy analysis in children with cerebral palsy. J Neural Eng. 2015;12:046017. doi: 10.1088/1741-2560/12/4/046017. [DOI] [PubMed] [Google Scholar]
- 15.Cappellini G, Ivanenko YP, Martino G, Maclellan MJ, Sacco A, Morelli D, et al. Immature Spinal Locomotor Output in Children with Cerebral Palsy. Front Physiol. 2016;7(October):478. doi: 10.3389/fphys.2016.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuman B, Goudriaan M, Bar-On L, Schwartz MH, Desloovere K, Steele KM. Repeatability of muscle synergies within and between days for typically developing children and children with cerebral palsy. Gait Posture. 2016;45:127–32. doi: 10.1016/j.gaitpost.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Shuman BR, Schwartz MH, Steele KM. Electromyography Data Processing Impacts Muscle Synergies during Gait for Unimpaired Children and Children with Cerebral Palsy. Front Comput Neurosci. 2017;11(June):1–9. doi: 10.3389/fncom.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz MH, Rozumalski A. The gait deviation index: A new comprehensive index of gait pathology. Gait Posture. 2008;28:351–7. doi: 10.1016/j.gaitpost.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Ngom A. The non-negative matrix factorization toolbox for biological data mining. Source Code Biol Med. 2013;8(10):1–26. doi: 10.1186/1751-0473-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Park H. Gene expression Sparse non-negative matrix factorizations via alternating non-negativity-constrained least squares for microarray data analysis. Bioinformatics. 2007;23(12):1495–502. doi: 10.1093/bioinformatics/btm134. [DOI] [PubMed] [Google Scholar]
- 21.Tresch MC, Cheung VCK, d’Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol. 2006 Jan;95(2006):2199–212. doi: 10.1152/jn.00222.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hof AL. Scaling gait data to body size. Gait Posture. 1996;4:222–3. doi: 10.1016/s0966-6362(01)00097-2. [DOI] [PubMed] [Google Scholar]
- 23.Dominici N, Dominici N, Ivanenko YP, Cappellini G, Avella A, Mondì V, et al. Locomotor Primitives in Newborn Babies and Their Development. Science (80-) 2011;334:997–9. doi: 10.1126/science.1210617. [DOI] [PubMed] [Google Scholar]
- 24.Maclellan MJ, Ivanenko YP, Massaad F, Bruijn SM, Duysens J, Lacquaniti F. Muscle activation patterns are bilaterally linked during split-belt treadmill walking in humans. J Neurophysiol. 2014;111:1541–52. doi: 10.1152/jn.00437.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauerbrei W. The use of resampling methods to simplify regression models in medical statistics. Appl Stat. 1999;48:313–29. [Google Scholar]
- 26.Baker R, Mcginley JL, Schwartz M, Thomason P, Rodda J, Graham HK. Gait Posture [Internet] 4. Vol. 35. Elsevier B.V; 2012. Gait & Posture The minimal clinically important difference for the Gait Profile Score; pp. 612–5. Available from: [DOI] [PubMed] [Google Scholar]
- 27.Oeffinger D, Bagley A, Rogers S, Gorton G, Kryscio R, Abel M, et al. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Dev Med Child Neurol. 2010;50(12):918–25. doi: 10.1111/j.1469-8749.2008.03150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutz E, Donath S, Tirosh O, Graham HK, Baker R. Gait Posture [Internet] 3. Vol. 38. Elsevier B.V; 2013. Gait & Posture Explaining the variability improvements in gait quality as a result of single event multi-level surgery in cerebral palsy; pp. 455–60. Available from: [DOI] [PubMed] [Google Scholar]
- 29.Hicks JL, Delp SL, Schwartz MH. Can biomechanical variables predict improvement in crouch gait? Gait Posture. 2012;34(2):197–201. doi: 10.1016/j.gaitpost.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huenaerts C, Molenaers G, Nieuwenhuys A, Pauwels P, Monari D, Desloovere K. Gait Posture [Internet] Vol. 42. Elsevier B.V; 2015. Session OS12 Multilevel Problems in Cerebral Palsy; pp. S54–5. Available from: [DOI] [Google Scholar]
- 31.Molenaers G, Campenhout A, Van Fagard K, De Cat J, Desloovere K. The use of botulinum toxin A in children with cerebral palsy, with a focus on the lower limb. J Child Orthop. 2010;4:183–95. doi: 10.1007/s11832-010-0246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]