Abstract
Background
This study aimed to review the long-term clinical outcomes and graft patency of coronary artery bypass grafting (CABG) using arterial grafts in patients with Kawasaki disease (KD) affecting the coronary artery.
Methods
Twenty patients with KD who underwent CABG from January 2002 to June 2014 were enrolled. There were 4 male (20%) and 16 female (80%) patients with ages at operation ranging from 2 to 42 years (median, 17.5 years). Our routine operative strategy was off-pump CABG with arterial grafts. The mean follow-up duration was 59.5 ± 48.5 months (range, 1–159 months). Coronary angiogram or computed tomography angiogram was used to evaluate graft patency in 16 patients (80%).
Results
All patients survived CABG without late mortality. Left internal thoracic arteries were used in 19 patients, while right internal thoracic arteries were used in 10 patients. Right gastroepiploic arteries were used in 3 patients, and a saphenous vein graft (SVG) was used in 1 patient. Among the 20 patients, 2 patients underwent coronary reintervention with balloon angioplasty because of graft failure. Two patients underwent coronary reintervention because of new obstructive lesions that were not significant at the time of the initial operation. Patency rates at 5 and 10 years were 94% and 87%, respectively. The rate of freedom from coronary reintervention at 10 years was 82%.
Conclusion
Off-pump CABG with mainly arterial graft revascularization may be considered a good surgical option for coronary lesions caused by KD.
Keywords: Coronary Artery Bypass Grafting, Kawasaki Disease, Graft
Graphical Abstract
Introduction
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome, is an acute febrile vasculitis that affects medium-sized arteries. In 2011, the average annual incidence of KD in Korea was 134.4 per 100,000 children under the age of 5 years, which is the second highest incidence of KD worldwide, with the highest incidence occurring in Japan.1 Serious complications of this disease include coronary artery aneurysms and subsequent stenotic lesions, which can lead to life-threatening myocardial infarction (MI) or sudden death.2 Over the past 15 years, clinical experience with interventional catheter treatment in KD patients has gradually increased. However, the experiences remain limited because unlike atherosclerotic coronary artery stenosis, KD coronary artery stenosis commonly involves severe calcification.3,4,5,6 Therefore, coronary artery bypass grafting (CABG) is currently the preferred treatment. The first pediatric CABG was performed in 1976 using a saphenous vein graft (SVG); since then, CABG using internal thoracic arterial grafts with or without SVGs has been successfully reported in KD patients.7,8,9,10,11,12 However, long-term coronary graft patency rates are not satisfactory, especially with the use of venous grafts and in patients under the age of 8 years. Thus, since 2002, arterial conduits, including the internal thoracic artery, have been used as grafts. The aim of this study was to review the long-term clinical outcomes and graft patency of CABG using arterial conduits in Kawasaki patients with coronary artery involvement.
Methods
Patients
We reviewed the medical records of 20 KD patients who underwent CABG at Samsung Medical Center by a single surgeon from January 2002 to June 2014. All patients had coronary artery lesions caused by KD, including giant aneurysms or stenosis. During the acute phase, treatment consisted of aspirin plus gamma-globulin. All patients were administered aspirin (100 mg) from the time the coronary disease was first detected. Patient demographic data are summarized in Table 1.
Table 1. Baseline patient characteristics.
| Variables | All patients (n = 20)a | |
|---|---|---|
| Age at KD onset, yr, median (range) | 3 (0–7) | |
| Age at operation, yr, median (range) | 17.5 (2–42) | |
| Sex, female, No. (%) | 4 (20) | |
| Previous MI, No. (%) | 8 (40) | |
| Preoperative LVEF, %, mean | 55.84 ± 14.05 | |
| Low preoperative EF (< 0.40), No. (%) | 3 (15) | |
| Involved coronary lesions, including stenosis and aneurysms, No. (%) | ||
| Left main coronary artery | 4 (20) | |
| 1 vessel disease | 1 (5) | |
| 2 vessel disease | 10 (50) | |
| 3 vessel disease | 5 (25) | |
KD = Kawasaki disease, MI = myocardial infarction, LVEF = left ventricle ejection fraction, EF = ejection fraction.
aData acquired between January 2002 and June 2014.
Operative techniques
In patients with KD, the main strategy for coronary revascularization was off-pump CABG with arterial grafts. Off-pump CABG was performed in 16 patients (80%), with 4 patients (20%) undergoing conventional CABG using cardiopulmonary bypass owing to hemodynamic instability during off-pump CABG. All operations were performed through standard median sternotomy, and a skeletonized internal thoracic artery was harvested by sharp dissection, clipping, and branch ligation. The saphenous vein was harvested from the patient's upper or lower leg with split incisions via a no-touch technique. Heparinized saline was used to dilate the diameter of the SVG. The right gastroepiploic artery (GEA) was prepared with a pedicled technique and grafted in situ. After the pericardium was opened, the right internal thoracic artery (RITA) was anastomosed to the left side of the left internal thoracic artery (LITA) to construct a Y composite graft. Then, the distal end of the internal thoracic arteries was clipped for pressure dilatation. In most cases, the LITA was anastomosed to the left anterior descending artery (LAD), sequentially anastomosing its branches, after which the right internal thoracic arterial graft was anastomosed to the branches of the circumflex artery, specifically the obtuse marginal arteries. The right coronary artery (RCA) was revascularized last. If proximal RCA stenosis was greater than 80%, the RITA was typically used. If the length of the harvested RITA was not sufficient to reach the RCA anastomosis due to left ventricle dilatation or other causes, the right GEA was used. If proximal RCA stenosis was less than 80%, aortocoronary bypass using the SVG was chosen. The quality of our anastomosis technique was evaluated by transit time flow rate with a Transonic Flowmeter (Transonic Systems, Ithaca, NY, USA). Protamine sulfate (0.5 mg/kg) was used to reverse the effect of heparin.
Follow-up
The mean follow-up duration was 59.5 ± 48.5 months (range, 1–159 months). Coronary angiography or computed tomography angiography was used to evaluate graft patency in 16 patients (80%). The median interval from initial operation to latest graft patency evaluation was 23.5 months (range, 3 days–150 months). In addition, a treadmill test was conducted to screen for inducible ischemia. Postoperative outcomes and events were acquired by reviewing medical records, directly interviewing patients or their families via telephone, and from cross-referencing the National Registry of Births and Deaths. Data were expressed either as the mean ± standard deviation or as frequency and proportion. Graft patency rates were analyzed by the Kaplan-Meier method. Statistical analyses were performed using SPSS, version 22.0 (SPSS, Chicago, IL, USA).
Ethics statement
The present study protocol was reviewed and approved by the Samsung Medical Center Institutional Review Board (2017-03-042), which waived the requirement for individual consent from patients or relatives.
Results
The absolute indications of CABG in our series were ischemic symptoms with definite coronary artery lesions. However, CABG was performed in 4 patients (patients 1, 3, 8, and 16) who had aneurysmal change without definite flow decrease on coronary angiography. In patient 1, left ventricular infarction (apicoanterior wall) was observed on thallium scan irrespective of the findings of coronary angiography. Patient 3 visited the pediatrician prior to his scheduled appointment due to sudden chest pain. A large, thrombosed coronary aneurysm in the LAD was observed, and a perfusion defect in the anterior wall was evident on thallium scan. Patient 8 was hospitalized due to sudden dyspnea on exertion during follow-up. On coronary angiography, coronary artery dilatation including thrombosis and heavy calcification was observed in the LAD. A small sized, mild, persistent perfusion defect in the mid and basal anteroseptum was observed. In patient 16, abrupt decreased wall motion was found during the regular echocardiographic follow-up. Coronary angiography showed 50% stenosis of the dilated and thrombosed LAD and total occlusion (TO) of the left circumflex artery (LCX). The RCA was also dilated and narrowed up to 50%. Although the target vessel lesions were not severe in these patients, CABG was performed considering the rapid exacerbation of coronary stenosis.
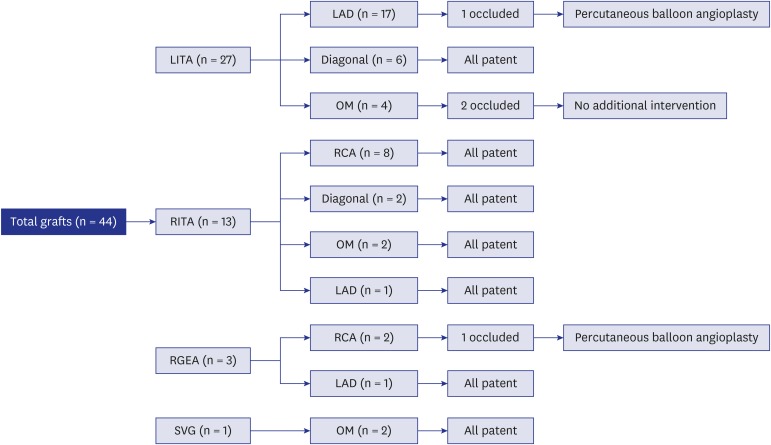
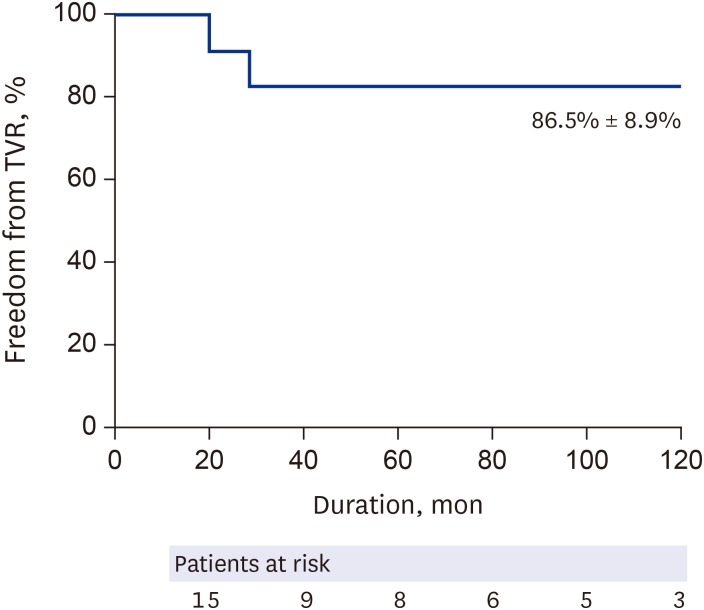
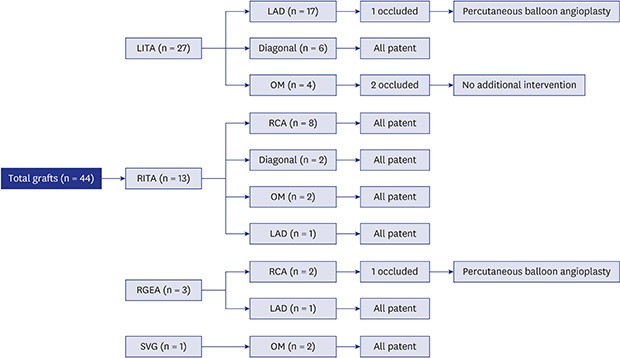
All patients survived CABG without late mortality. The LITA was used in 19 patients, while the RITA was grafted in 10 patients. The right GEA was used in 3 patients, and a SVG was used in 1 patient. Target vessels for each graft are summarized in Table 2. Among the 27 left internal thoracic arterial grafts, 3 (11%) were occluded, and among the 13 right internal thoracic arterial grafts, no graft occlusions were observed (Fig. 1). Patency rates at 5 and 10 years were 94% and 82%, respectively. Freedom from target coronary revascularization at 10 years was 86.7% ± 8.9% (Fig. 2). At the final follow-up, the clinical condition and functional health of all patients were good without any subjective symptoms.
Table 2. Summarized data of the grafts used and the target vessels.
| Variables | Value | |
|---|---|---|
| No. of grafts per patient, mean (range) | 1.7 ± 0.6 (1–3) | |
| No. of grafted target vessels per patient, mean (range) | 2.2 ± 1.1 (1–4) | |
| No. of total grafted target vessels | 44 | |
| No. of grafted LITA | 27 | |
| LAD | 17 | |
| D | 6 | |
| OM | 3 | |
| RI | 1 | |
| No. of grafted RITA | 13 | |
| RCA | 6 | |
| PDA | 2 | |
| OM | 2 | |
| D | 2 | |
| LAD | 1 | |
| No. of grafted GEA | 3 | |
| RCA | 2 | |
| LAD | 1 | |
| No. of grafted SVG | 1 | |
| OM | 1 | |
LITA = left internal thoracic artery, LAD = left anterior descending artery, D = diagonal branch, OM = obtuse marginal branch, RI = ramus intermedius branch, RITA = right internal thoracic artery, RCA = right coronary artery, PDA = posterior descending artery, GEA = gastroepiploic artery, SVG = saphenous vein graft.
Fig. 1. Flow diagram of the outcomes of each graft.
LITA = left internal thoracic artery, RITA = right internal thoracic artery, RGEA = right gastroepiploic artery, SVG = saphenous vein graft, LAD = left anterior descending artery, OM = obtuse marginal branch, RCA = right coronary artery, PBA = percutaneous balloon angioplasty.
Fig. 2. Freedom from coronary reintervention: online only.
TVR = target vessel revascularization.
The patients' pertinent clinical features are summarized in Table 3. In patient 6, the atherosclerotic change of the LAD was extensive and severe, and angioplasty using a SVG was inevitable. After long angioplasty, the length of the LITA was not sufficient to reach the distal LAD; hence, the right GEA was used for revascularization of the LAD territory.
Table 3. Clinical outcomes of CABG.
| Case number | Sex | Age, yr | Preoperative lesions | Surgical procedure | Off-pump | Graft patency | Patency follow-up, mon | Coronary reintervention |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 2 | LAD-A | LITA-LAD | X | P | 150 | PTRA on new LCX lesion |
| RCA-A | ||||||||
| 2 | M | 20 | LM (STO)-A | LITA-LAD | O | Not done | - | (-) |
| LAD (TO) | RITA(Y)-D1-D2 | |||||||
| 3 | M | 6 | LAD-A | LITA-LAD | X | P | 63 | (-) |
| RCA-A | ||||||||
| 4 | M | 16 | LAD (TO)-A | LITA-LAD | O | P | 47 | (-) |
| RCA-A | ||||||||
| 5 | M | 30 | LAD (TO)-A | LITA-D1 | O | P | 90 | (-) |
| LCX (95%) | RITA (Y)-LAD | |||||||
| SVG-OM | ||||||||
| 6 | M | 42 | LAD (95%) | LITA-OM | X | P | 56 | (-) |
| LCX (95%) | Small LITA (Y)-D | |||||||
| RI (TO) | GEA-distal LAD | |||||||
| RCA (95%) | LAD endarterectomy and angioplasty with SVG (6 cm) | |||||||
| 7 | M | 10 | LAD-A | GEA-RCA | O | Not done | - | (-) |
| RCA (TO)-A | ||||||||
| 8 | F | 14 | LAD-A | LITA-LAD | O | O: string-sign | 19 | PBA on LITA |
| RCA-A | ||||||||
| 9 | M | 5 | LAD (STO)-A | LITA-LAD | O | P | 83 | (-) |
| RCA-A | ||||||||
| 10 | F | 17 | LAD (TO) | LITA-D-LAD | O | Not done | - | (-) |
| LCX (SS)-A | RITA(Y)-OM-PDA | |||||||
| RCA (TO) | ||||||||
| 11 | M | 16 | LM-A | LITA-LAD | O | LITA:P | 27 | PBA on GEA |
| LAD (90%) | Small LITA (Y)-RI | Small LITA: O | ||||||
| LCX (90%) | GEA-RCA | GEA: 75% | ||||||
| RCA (90%)-A | ||||||||
| 12 | F | 30 | LAD (TO)-A | LITA-LAD | O | P | 16 | Redo CABG on new LCX lesion with RITA |
| LCX-A | ||||||||
| 13 | M | 23 | LAD (TO) | LITA-LAD | O | P | 35 | (-) |
| RCA (TO)-A | RITA-RCA | |||||||
| 14 | M | 28 | LAD (TO)-A | LITA-D2-LAD | O | P | 12 | (-) |
| Small LITA(Y)-D1 | ||||||||
| 15 | M | 18 | LAD (TO)-A | LITA-LAD | O | P | 7 | (-) |
| RCA (TO)-A | RITA-RCA | |||||||
| 16 | M | 9 | LM-A | LITA-LAD | X | LAD: P | 7 | (-) |
| LAD-A | Small LITA(Y)-OM | OM: O | ||||||
| LCX (TO) | RITA-RCA | RCA: P | ||||||
| RCA-A | ||||||||
| 17 | M | 12 | LAD (90%)-A | LITA-LAD | X | Not done | - | (-) |
| LCX (90%) | Small LITA(Y)-OM | |||||||
| RCA (80%)-A | RITA-RCA | |||||||
| 18 | M | 28 | LM-A | LITA-LAD | O | P | 20 | (-) |
| LAD (STO)-A | RITA-RCA | |||||||
| LCX-A | ||||||||
| RCA (TO) | ||||||||
| 19 | M | 38 | LM (STO) | LITA-D-LAD | O | P | 0 | (-) |
| LAD (TO)-A | RITA(Y)-OM-PDA | |||||||
| LCX (MO)-A | ||||||||
| RCA (TO)-A | ||||||||
| 20 | M | 39 | LAD (TO)-A | LITA-LAD | O | P | 0 | (-) |
| RI (60%) | RITA-RCA | |||||||
| LCX-A | ||||||||
| RCA (TO)-A |
M = male, F = female, LM = left main lesion, LAD = left anterior descending artery, D = diagonal branch, LCX = left circumflex artery, OM = obtuse marginal branch, RI = ramus intermedius branch, RCA = right coronary artery, PDA = posterior descending artery, STO = subtotal occlusion, TO = total occlusion, O = occluded, P = patent, PTRA = percutaneous transluminal rotablation, LITA = left internal thoracic artery, RITA = right internal thoracic artery, Y = y configuration anastomosis, SVG = saphenous vein graft, GEA = gastroepiploic artery, CABG = coronary artery bypass grafting, PBA = percutaneous balloon angioplasty.
Four patients underwent coronary reintervention. Coronary angiography of patient 1, a 2-year-old girl who underwent CABG using the LITA, was performed 12 years after the operation and demonstrated graft patency. However, in the LCX territory, multiple new stenotic lesions were observed, and percutaneous transluminal rotational ablation was performed. Patient 8, a 14-year-old girl who underwent off-pump CABG using the LITA, presented graft failure after 19 months. Percutaneous balloon angioplasty (PBA) was successfully performed on the graft. Patient 11, a 16-year-old male, underwent off-pump CABG using the LITA and the right GEA. After 27 months, a coronary angiography was performed and revealed occlusion of the gastroepiploic arterial graft to the RCA. A PBA was conducted on the right gastroepiploic arterial graft. Coronary angiography of patient 12, a 30-year-old female who underwent off-pump CABG using the LITA, was performed 16 months later and showed graft patency; however, new lesions on the LCX territory were detected and required repeat CABG.
Discussion
In KD patients who developed a coronary aneurysm, our strategy of off-pump CABG using arterial grafts produced favorable long-term outcomes and graft patency. At 10 years post-operation, freedom from repeat coronary revascularization was 86.7% ± 8.9%, and all patients demonstrated satisfactory functional health at the final follow-up.
Although catheter intervention has been used to recanalize coronary arteries with severe calcification, the frequent presence of calcified aneurysms, with clots inside, makes the procedure less than satisfactory.2 Since 1976, when Kitamura et al.7,13 reported the first successful CABG in a KD patient, CABG has been preferred over percutaneous coronary intervention (PCI) for the treatment of coronary lesions in KD patients. However, Yoshikawa et al.8 reported that the long-term patency of SVGs was not satisfactory. According to that study, the low patency of SVGs may be attributed to the frequent and rapid degeneration of vein grafts in small children. The investigators suggested that the use of SVGs should be avoided unless the internal thoracic artery is unavailable. Therefore, since 2002, our center has used the internal thoracic artery as the first choice graft for CABG in KD patients. In addition, numerous studies have reported that for KD patients, internal thoracic artery grafts are superior to SVGs in terms of long-term patency.14,15,16 Furthermore, while the internal thoracic artery demonstrates favorable long-term graft patency, the conduit is also known to have somatic growth potential.17 The present study confirmed that using the internal thoracic artery is optimal for CABG in KD patients.
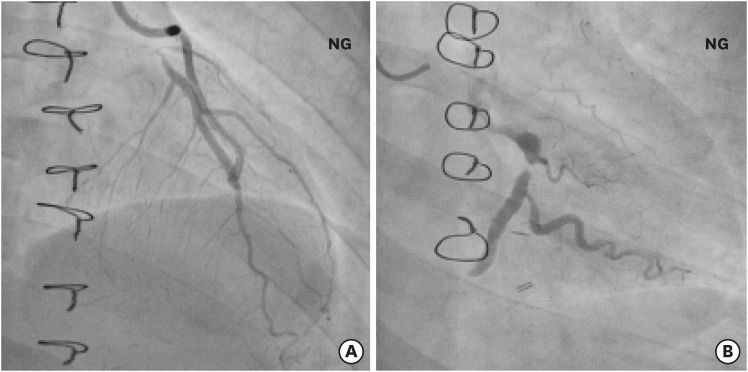
According to Suzuki et al.,18 coronary aneurysms induced by KD usually involve bifurcation of the left coronary artery system, thus making it difficult to accurately assess the LCX. In addition, the investigators suggested that underestimating stenotic lesions may lead to incomplete revascularization and early reintervention after CABG. Tsuda et al.19 reported that unlike adult-type atherosclerotic coronary lesions, coronary disease in KD patients can either progress or regress owing to its childhood-onset, inflammatory nature, and the effect its bidirectional remodeling can have on CABG outcomes. In our series, 2 patients (10%) required PCI because of newly developed lesions in the LCX branches after CABG. The first patient (a 2-year-old female) required percutaneous transluminal rotational ablation at the 12-year postoperative interval because of newly developed multiple stenotic lesions in the LCX branches. The second patient (a 30-year-old female) required repeat CABG due to obtuse marginal arteries (Fig. 3). Thus, in this clinical setting, meticulous preoperative evaluation of the LCX and postoperative anti-inflammatory management is critical.
Fig. 3. Coronary angiography in a patient requiring repeat CABG. (A) Patent LITA graft to the LAD. (B) Newly detected occlusion of the left circumflex artery: online only.
CABG = coronary artery bypass grafting, LITA = left internal thoracic artery, LAD = left anterior descending artery, NG = nitroglycerin injection.
An additional complication for KD patients undergoing CABG is early graft malfunction because of competitive blood flow between the native coronary artery and grafts. Competitive blood flow is known to be a major risk factor for graft failure in adult-type atherosclerotic lesions.20 In the present study, this finding was observed in 2 patients. In the first patient (patient 8), echocardiography detected a 4.5-mm-diameter aneurysm in the left main coronary artery, and no definitive stenosis was confirmed. Diffuse narrowing of the LITA graft by string-sign was observed after 19 months. The second patient (patient 11) underwent off-pump CABG because of proximal left circumflex stenosis (90%), which was too small to be revascularized. Instead, isolated bypass of the LITA to the ramus intermedius was performed. However, the ramus intermedius was not significantly narrowed, and complete degeneration of the LITA graft was observed after 27 months. Several authors reported similar experiences of flow competition after CABG in KD patients.8,19 These findings suggest that CABG in a coronary aneurysm without significant localized stenosis should be avoided. Moreover, the status of the collaterals associated with TO of coronary arteries is critical when deciding on a particular treatment. Interestingly, new onset RCA TO was detected after CABG to the LAD which was occluded and supplied with collaterals from the patent RCA preoperatively in patient 9. Based on this experience, we previously suggested that the status of collaterals associated with TO of coronary arteries in KD should be evaluated regularly for the prevention of further MI.21
For KD patients, studies have indicated an adverse correlation between younger ages at operation and long-term graft patency.22,23 Recently, Legendre et al.24 reported good patency of CABG in young children; however, the procedure was technically challenging and required careful follow-up. In the present study, one graft was occluded because of blood flow competition (14%; 1/7) in a patient under the age of 12 years, and this was not different in patients over the age of 12 years (9%; 2/23; P = 0.594). Further, 2 out of the 3 grafts that were occluded were successfully resolved by PBA. This result is consistent with the findings of Tsuda et al.11 which demonstrated long-term graft patency via the proper application of catheter intervention after CABG in KD patients. Therefore, pediatric CABG is a potentially better and safer alternative to catheter intervention in KD patients with coronary artery lesions.
This study has several limitations. First, this was a retrospective and descriptive study of a small number of patients. However, KD involving the coronary artery and requiring CABG is rare in Korea; therefore, a retrospective review was the only feasible study design. Second, the extent of coronary angiographic follow-up was not sufficient. Longer and more frequent angiographic follow-up is necessary in future studies.
In conclusion, off-pump CABG with predominantly arterial graft revascularization may be a better and safer surgical option for coronary lesions caused by KD. Arterial conduits, somatic growth potential, and flow competition should be considered in pediatric CABG.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Lee YT. Data curation: Song J, Kang I, Han W. Formal analysis: Jeong DS, Han W. Methodology: Jeong DS. Validation: Lee YT, Park PW. Investigation: Song J, Kang I. Writing - original draft: Jeong DS. Writing - review & editing: Jeong DS, Kim WS.
References
- 1.Ha S, Seo GH, Kim KY, Kim DS. Epidemiologic study on Kawasaki disease in Korea, 2007–2014: based on health insurance review & assessment service claims. J Korean Med Sci. 2016;31(9):1445–1449. doi: 10.3346/jkms.2016.31.9.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94(6):1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 3.Akagi T, Ogawa S, Ino T, Iwasa M, Echigo S, Kishida K, et al. Catheter interventional treatment in Kawasaki disease: a report from the Japanese pediatric interventional cardiology investigation group. J Pediatr. 2000;137(2):181–186. doi: 10.1067/mpd.2000.107164. [DOI] [PubMed] [Google Scholar]
- 4.Ino T, Akimoto K, Ohkubo M, Nishimoto K, Yabuta K, Takaya J, et al. Application of percutaneous transluminal coronary angioplasty to coronary arterial stenosis in Kawasaki disease. Circulation. 1996;93(9):1709–1715. doi: 10.1161/01.cir.93.9.1709. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa S, Fukazawa R, Ohkubo T, Zhang J, Takechi N, Kuramochi Y, et al. Silent myocardial ischemia in Kawasaki disease: evaluation of percutaneous transluminal coronary angioplasty by dobutamine stress testing. Circulation. 1997;96(10):3384–3389. doi: 10.1161/01.cir.96.10.3384. [DOI] [PubMed] [Google Scholar]
- 6.Hijazi ZM, Smith JJ, Fulton DR. Stent implantation for coronary artery stenosis after Kawasaki disease. J Invasive Cardiol. 1997;9(8):534–536. [PubMed] [Google Scholar]
- 7.Kitamura S, Kawashima Y, Fujita T, Mori T, Oyama C. Aortocoronary bypass grafting in a child with coronary artery obstruction due to mucocutaneous lymphnode syndrome: report of a case. Circulation. 1976;53(6):1035–1040. doi: 10.1161/01.cir.53.6.1035. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa Y, Yagihara T, Kameda Y, Taniguchi S, Tsuda E, Kawahira Y, et al. Result of surgical treatments in patients with coronary-arterial obstructive disease after Kawasaki disease. Eur J Cardiothorac Surg. 2000;17(5):515–519. doi: 10.1016/s1010-7940(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Otaki M, Oku H, Fukuda T, Shinohara T. Follow-up study of coronary artery bypass grafting in patients with Kawasaki disease. Am Heart J. 2001;142(4):740–744. doi: 10.1067/mhj.2001.117316. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Narisawa T, Hirano J, Suzuki T, Asano F, Ebato B, et al. Coronary artery bypass grafting for coronary aneurysms due to Kawasaki disease. Ann Thorac Cardiovasc Surg. 2001;7(5):307–310. [PubMed] [Google Scholar]
- 11.Tsuda E, Kitamura S, Kimura K, Kobayashi J, Miyazaki S, Echigo S, et al. Long-term patency of internal thoracic artery grafts for coronary artery stenosis due to Kawasaki disease: comparison of early with recent results in small children. Am Heart J. 2007;153(6):995–1000. doi: 10.1016/j.ahj.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Wakisaka Y, Tsuda E, Yamada O, Yagihara T, Kitamura S. Long-term results of saphenous vein graft for coronary stenosis caused by Kawasaki disease. Circ J. 2009;73(1):73–77. doi: 10.1253/circj.cj-08-0225. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura S, Kameda Y, Seki T, Kawachi K, Endo M, Takeuchi Y, et al. Long-term outcome of myocardial revascularization in patients with Kawasaki coronary artery disease. A multicenter cooperative study. J Thorac Cardiovasc Surg. 1994;107(3):663–673. [PubMed] [Google Scholar]
- 14.Muta H, Ishii M. Percutaneous coronary intervention versus coronary artery bypass grafting for stenotic lesions after Kawasaki disease. J Pediatr. 2010;157(1):120–126. doi: 10.1016/j.jpeds.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda E, Kitamura S Cooperative Study Group of Japan. National survey of coronary artery bypass grafting for coronary stenosis caused by Kawasaki disease in Japan. Circulation. 2004;110(11) Suppl 1:II61–II66. doi: 10.1161/01.CIR.0000138194.61225.10. [DOI] [PubMed] [Google Scholar]
- 16.Tsuda E, Hamaoka K, Suzuki H, Sakazaki H, Murakami Y, Nakagawa M, et al. A survey of the 3-decade outcome for patients with giant aneurysms caused by Kawasaki disease. Am Heart J. 2014;167(2):249–258. doi: 10.1016/j.ahj.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura S, Seki T, Kawachi K, Morita R, Kawata T, Mizuguchi K, et al. Excellent patency and growth potential of internal mammary artery grafts in pediatric coronary artery bypass surgery. New evidence for a “live” conduit. Circulation. 1988;78(3 Pt 2):I129–39. [PubMed] [Google Scholar]
- 18.Suzuki A, Kamiya T, Kuwahara N, Ono Y, Kohata T, Takahashi O, et al. Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases. Pediatr Cardiol. 1986;7(1):3–9. doi: 10.1007/BF02315475. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda E, Fujita H, Yagihara T, Yamada O, Echigo S, Kitamura S. Competition between native flow and graft flow after coronary artery bypass grafting. Impact on indications for coronary artery bypass grafting for localized stenosis with giant aneurysms due to Kawasaki disease. Pediatr Cardiol. 2008;29(2):266–270. doi: 10.1007/s00246-007-9114-y. [DOI] [PubMed] [Google Scholar]
- 20.Berger A, MacCarthy PA, Siebert U, Carlier S, Wijns W, Heyndrickx G, et al. Long-term patency of internal mammary artery bypass grafts: relationship with preoperative severity of the native coronary artery stenosis. Circulation. 2004;110(11) Suppl 1:II36–40. doi: 10.1161/01.CIR.0000141256.05740.69. [DOI] [PubMed] [Google Scholar]
- 21.Kwak JH, Song J, Kang IS, Huh J, Lee HJ. Changes in coronary perfusion after occlusion of coronary arteries in Kawasaki disease. Yonsei Med J. 2014;55(2):353–359. doi: 10.3349/ymj.2014.55.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavroudis C, Backer CL, Duffy CE, Pahl E, Wax DF. Pediatric coronary artery bypass for Kawasaki congenital, post arterial switch, and iatrogenic lesions. Ann Thorac Surg. 1999;68(2):506–512. doi: 10.1016/s0003-4975(99)00588-3. [DOI] [PubMed] [Google Scholar]
- 23.Brackenbury E, Gardiner H, Chan K, Hickey M. Internal mammary artery to coronary artery bypass in paediatric cardiac surgery. Eur J Cardiothorac Surg. 1998;14(6):639–642. doi: 10.1016/s1010-7940(98)00231-0. [DOI] [PubMed] [Google Scholar]
- 24.Legendre A, Chantepie A, Belli E, Vouhé PR, Neville P, Dulac Y, et al. Outcome of coronary artery bypass grafting performed in young children. J Thorac Cardiovasc Surg. 2010;139(2):349–353. doi: 10.1016/j.jtcvs.2009.07.061. [DOI] [PubMed] [Google Scholar]