Abstract
As violence against self and others is an important outcome in the treatment of patients with psychosis-spectrum disorders and hostility is an important indicator for violence, we set out to evaluate the effects of different types of antipsychotic agents in reducing hostility. We performed a systematic literature search, which provided 18 suitable randomized studies comparing typical to atypical antipsychotics for at least 4 weeks in patients with psychotic disorders. Results showed a small (0.26) but significant effect for atypical as compared to typical antipsychotics, with high heterogeneity, even though the mean dose of typical antipsychotics was higher. This effect size remained similar when separately analyzing sponsored and non-sponsored studies. When differentiating between high and low-dose studies, the high-dose group showed a significant difference between typical and atypical antipsychotics whereas the low-dose group did not. An analysis comparing clozapine to typical antipsychotics showed a moderate effect size (0.415), with low heterogeneity. These results are important for clinicians to help their shared decision making with patients when choosing maintenance treatment, as next to efficacy for psychosis and tolerability, safety for the patient and their environment is an important outcome.
Introduction
Violence is an important outcome measure in patients who suffer from psychosis-spectrum disorders. A large meta-analysis including 45,533 patients with psychosis-spectrum disorders or bipolar disorder found that 18.5% had committed a verbal aggressive or physical violent act [1]. An earlier meta-analysis including 18,432 patients with psychosis-spectrum disorders found that 9.9% of the patients committed at least one act of interpersonal violence or violent criminality including threats [2]. This meta-analysis also found an increased risk for homicide in individuals with schizophrenia of 0.3% vs. 0.02% in the general population [2]. A third meta-analysis including only patients with a first-episode psychosis found that 34.5% had shown physical violence with almost half of these (16.6%) having committed serious violence (resulting in injury/using a weapon/sexual assault) [3]. Factors such as the severity of positive symptoms, comorbid substance abuse, economic deprivation, childhood conduct disorder, and poor treatment adherence are well known prospective predictors, while negative symptoms predict lower rates of violence [1, 4, 5].
Violence has several negative consequences for patients themselves and for the general public. Although 95–99% of violent incidents in society is not caused by individuals with psychosis-spectrum disorders, a certain public fear still exists [6]. Violence of patients with psychosis-spectrum disorders contributes to the stigmatization for those suffering from psychotic illness, affecting also the large majority without any history of violence. The consequences of violence for individual patients include legal involvement, prolonged hospitalization, individual stigmatization but also victimization [7].
Previous reports suggest that in the acute phase of psychosis, antipsychotic medication prevents or reduces aggressive behavior [3, 4]. There is the suggestion for superiority of clozapine in reducing violence, specifically in treatment-resistant psychotic disorders [8–10]. However, most of these studies are in retrospect. It has also been suggested that atypical antipsychotics, which on average are more potent 5-HT antagonists, could have a better anti-aggressive effect than more specific D2 blockading drugs [11, 12]. Yet, there is no consistent evidence to make a rational decision as to which type of antipsychotic may be most effective in reducing aggression, and even the superiority of clozapine is not well documented. The aim of the current review study is to provide evidence to support a rational decision on which type of antipsychotic medication most effectively reduces hostility, and thereby possibly affecting violence in patients with psychosis-spectrum disorders.
Violence and aggression can be assessed in many different ways. Unfortunately, very few studies have investigated the influence of different antipsychotic drugs on actual rates of violent incidence or added specific questionnaires inquiring about such incidents. However, many randomized controlled trials provide data on the hostility item of the Positive and Negative Symptom Scale (PANSS; [13]) or the Brief Psychiatric Rating Scale (BPRS; [14]). This item is often used to give an indication of the aggressiveness of a patient. There is a close association between the hostility item and aggression since some of the descriptions of the hostility item include verbal aggression and physical violence [5]. Indeed, a significant correlation (r = 0.77, p < 0.01) between the hostility item of the PANSS and the Modified Overt Aggression Scale (MOAS) has been found [15] as well as a significant association (OR = 1.5, 95% CI 1.0–2.1) between PANSS hostility scores and verbal aggressive or physical violent incidents [1].
Therefore, we assessed the efficacy of various antipsychotic drugs on this PANSS/BPRS item, focusing on the comparison between typical and atypical antipsychotics, in studies of at least 4 weeks in duration.
Materials and methods
Search
A meta-analysis was conducted according to the PRISMA statement [16]. An electronic search was conducted using PubMed, Cochrane, Embase, and PsychInfo. The following Mesh search terms were used both alone and in combinations: “violence,” “aggression,” “hostility,” “schizophrenia,” “psychotic disorders,” “antipsychotic agents” and the generic names of 25 commonly used antipsychotics. There was no limitation on year or language. The reference lists of the obtained studies were checked for cross references.
Inclusion
The following inclusion criteria were used:
Method: randomized studies, blinded or open label.
Population: patients diagnosed with a psychosis-spectrum disorder (schizophrenia, schizoaffective disorder or psychotic disorder not otherwise specified) according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-III, DSM-III-R, DSM-IV, DSM-IV-TR or DSM-V) or International Classification of Diseases (ICD 9 or 10). Both in and out patients were included, but not patients under current treatment in forensic facilities.
Medication: studies comparing at least one typical to one atypical antipsychotic.
Treatment duration: studies testing the antipsychotic for 4 weeks or more.
Outcome measure: studies reporting the hostility item of the PANSS or BPRS, the PANSS hostility/excitement factor score, the BPRS hostile/suspiciousness cluster or the Behavioural Agitation Score (BAS) derived from the BPRS.
Data: studies reporting sufficient information to compute common effect size statistics (means and SDs, exact p, t, or z values) or corresponding authors could supply these data upon request.
We excluded case studies, cross sectional studies, and studies using a mixed sample of psychotic disorders and other diagnosis. The search included studies aimed specifically on hostility (i.e., studies that reported on hostility or violence in title, abstract or keywords). After the initial search, we used cross references from the studies we found and other reviews.
Outcome measures
On the PANSS, the hostility item is defined as “verbal and nonverbal expressions of anger and resentment, including sarcasm, passive-aggressive behavior, verbal abuse and assaultiveness”. The score is based on interpersonal behavior during the interview and reports by primary care workers or family. It is, like the other PANSS items, scored from 1–7. A score of 1 indicates absent hostility, whereas a score of 7 indicates marked anger, resulting in physical assault towards others. The hostility item of the BPRS is similarly scored on a scale 1–7 and is defined as “Animosity, contempt, belligerence, threats, arguments, tantrums, property destruction, fights, and any other expression of hostile attitudes or actions. Do not infer hostility from neurotic defenses, anxiety or somatic complaints. Do not include incidents of appropriate anger or obvious self-defense”. Although both instruments use a scale of 1–7 and assess the same construct, there are differences. Where the PANSS instructions include anchors for each score of 1 through 7, the original BPRS does not include anchors for individual scores but only a description of the hostility item.
For both the PANSS and the BPRS, clusters have been made to assess and differentiate domains in psychopathology more accurately. The PANSS five factor model is developed by Lindenmayer et al. [17] and sometimes called “uncontrolled hostility/excitement factor”. This cluster consists of four items: P4 excitement, G14 poor impulse control, P7 hostility, and G4 tension. The BPRS factor is named “hostility/ suspiciousness” and consists of the items hostility, suspiciousness, and uncooperativeness [14]. For the BPRS, a second cluster is sometimes used: the BAS, also called the BPRS agitation score. The BAS contains the items: hostility, anxiety, tension, uncooperativeness, and excitement [18]. The primary outcome measure was the mean change in the hostility item of the PANSS or BPRS, the PANSS hostility/excitement factor score, the BPRS hostile/suspiciousness cluster or BAS for typical versus atypical antipsychotics. If both the hostility item and the factor or cluster score were reported, the hostility item was preferred.
Patient data of last observation carried forward were used for analysis. If studies did not provide the (exact) data, the authors were contacted requesting the data.
Comparisons
Primary analysis is to compare atypical to typical antipsychotics.
To check for a possible dose-effect, the studies were divided into two groups: one with the typical and atypical antipsychotics dosed below 500 mg chlorpromazine equivalent, and one with dosages above 500 mg chlorpromazine equivalent. Moreover, since clozapine is suggested to be particularly effective in reducing hostility [9, 10], a separate analysis was performed including only this agent in comparison to typical antipsychotic medication. Also, the effect sizes of the sponsored versus non-sponsored studies as well as the open label versus double-blind studies were calculated.
Analysis
Two reviewers independently extracted data. Disagreements were resolved by consensus. Hedges’ g was used to quantify effect sizes for the mean difference (typical vs atypical) of the change score (end of treatment vs baseline). These change scores were preferred instead of pre-treatment and post-treatment scores in order to avoid overestimation of the true effect because of the pre-post treatment correlation [19]. If no change score was reported, pre-treatment and post-treatment means and standard deviations (SDs) or exact F, t, or p values were used. The pre-post correlation was posited as 0.5 in terms of Pearson correlations. If a comparison was reported to be significant, but no exact p value was reported, the reported p value threshold was used. For example, if only p < 0.01 was given; p = 0.01 was used. The effect was considered positive when the atypical antipsychotic had a better effect on hostility than the typical antipsychotic.
When studies had more than one arm of atypical antipsychotics (e.g., two or more atypical antipsychotics or different dose of the same antipsychotic), the data of the typical antipsychotics arm was used multiple times as a control. Since this could cause an underestimation or overestimation of the effect of the typical antipsychotic in terms of significance, the N of the typical group was divided by the number of atypical arms in the study.
A random effects model was used because of the differences between studies in participants, treatment duration and size [20]. To measure the heterogeneity the I2 was calculated. Heterogeneity of <25% is considered low, >50% is moderate, and a value of >75% indicates a high variance [21]. High heterogeneity poses a limitation to a reliable interpretation of the results. However, it had been discussed that I2 does not indicate how much the effect size varies [21]. A sensitivity analysis was undertaken by repeating the analysis excluding outlier studies. All calculations were executed using Comprehensive Meta-Analysis Version 2.0, Biostat [22].
The chlorpromazine equivalent levels were calculated according to the study of Gardner et al. [23]. The reported fixed or mean dose of the antipsychotics in the included studies were used. These equivalents were used to divide the studies into groups using high (>500 mg chlorpromazine equivalent) and low (<500 mg chlorpromazine equivalent) dose antipsychotics. When studies reported the fixed or mean dose at baseline as well as during the study and at endpoint, the dose at endpoint was used. When studies did not report a mean or fixed dosed but a range, the minimum dose of the range should be >500 mg for the arm to be included in the high-dose group.
Results
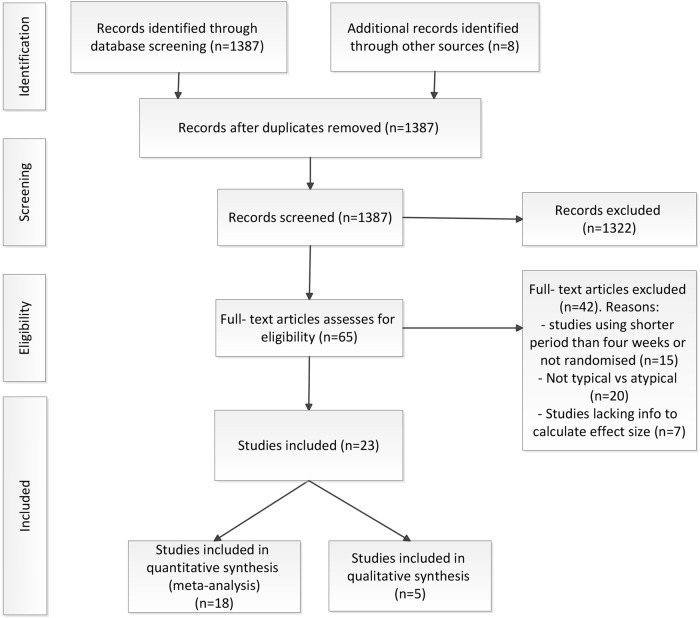
The literature search resulted in 1395 studies, which were screened for inclusion (Fig. 1). After this first screen, 65 articles were assed for eligibility. A total of 42 studies were excluded for specific reasons, for example studies comparing two atypical antipsychotic drugs, non-randomized studies or studies using a treatment period shorter than 4 weeks. A total of 18 randomized controlled trials, providing specific information on hostility comparing an atypical antipsychotic to a typical drug for at least 4 weeks could be included in the meta-analysis (Table 1). Data from 6799 patients were used. From these patients, 4969 were assigned to an atypical antipsychotic and 1830 to a typical antipsychotic. Of these 18 studies, 15 studies used haloperidol as a typical comparator, two used chlorpromazine, and one study used perphenazine. Two studies used four different atypical antipsychotics and one study used three. Also, three studies differentiated the groups according to different atypical antipsychotic dosages. One study used two dose groups, one study four, and one study used five different dose groups. This resulted in a total of 34 subgroups used for the analysis.
Fig. 1.
PRISMA Flow diagram of the literature search
Table 1.
Description of included studies in meta-analysis
| Study | Country | Total no. of patients | Study design | Duration in weeks | Mean age (SD) | % male | Duration of illness in years (SD) | Previous violence or hostility | Outcome measure | No. of pts per arm | Dose (fixed, range or mean) | Chlorpromazine equivalent | Sponsored | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [40] | US | 39 | RAN, DB | 10 | CLO 34 (6) HAL 35 (8) |
CLO 68.42 HAL 75 |
CLO 14 (6) HAL 14 (6) |
No | BPRS hostility item | CLO 19 HAL 20 |
Mean (SD) CLO 410.5 (45.8) HAL 24.8 (5.5) | CLO 616 HAL 1488 |
No | CLO superior to HAL |
| [18] | US | 217a | RAN, DB, PLA | 6 | QUE 37.4 (9.4) HAL 37.3 (10.9) | QUE 73.1 HAL 78.6 | UN | No | BAS | QUE 175 HAL 42 | Fixed: QUE 150, 300, 600 or 750 HAL 12 | QUE 120, 240, 480 or 600 HAL 720 |
Yes | QUE therapeutic effects on hostility and behavioral agitation |
| [41] | US | 157 | RAN, DB | 14 | 40.8 (9.2) | 85 | 19.5 (8.4) | No | PANSS hostility item | OLA 39 CLO 40 RIS 41 HAL 37 |
Mean (SD) CLO 526.6 (140.3) OLA 30.4 (6.6) RIS 11.6 (3.2) HAL 25.7 (5.7) |
CLO 789.9 OLA 912 RIS 1160 HAL 1542 |
Yes | CLO advantage to HAL and RIS but not to OLA |
| [26] | 20 countries | 567 | RAN, SB | 6 | ZIP 34 (10.5) HAL 34.6 (10.5) | ZIP 66.7 HAL 65.9 |
UN | No | BPRS hostility item | ZIP 429 HAL 138 |
Mean (SD) ZIP 116 (30.4) HAL11.5 (3.6) |
ZIP 435 HAL 690 |
Yes | ZIP superior to HAL |
| [42] | US | 107 | RAN, DB | 8 | 30 (median) | 60.92 | UN | No | BPRS hostile suspiciousness cluster | CLO 52 CHL 55 |
Mean: CLO 390 CHL 698 | CLO 585 CHL 698 |
No | CLO superior to CHL |
| [43] | US | 109a | RAN, DB, PLA | 9 | UN | UN | UN | PANSS hostility ≥3 | PANSS hostility item | RIS 85 HAL 24 | Fixed: RIS 6, 10, or 16 HAL 20 | RIS 600, 1000, or 1600 HAL 1200 | Yes | RIS greater selective effect on hostility than HAL |
| [44] | GER | 37 | RAN, DB | 6 | AMI 43.3 (9.1) HAL 40.1 (10.2) | AMI 81 HAL 80 | AMI 17.3 HAL 14.3 | No | BPRS hostility item | AMI 20 HAL 17 | Range: AMI 490–1000 HAL 5-40 | AMI 421–860 HAL 300–2400 |
No | Positive but not significant trend in favor of AMI |
| [45] | GER | 151 | RAN, DB | 52 | RIS 30.9 (9.6) HAL 32.3 (10.5) | RIS 68.8 HAL 47.3 |
UN | No | PANSS hostility/ excitement | RIS 77 HAL 74 |
Mean (SD) RIS 3.9 (2.0) HAL 3.6 (1.8) |
RIS 390 HAL 216 |
Yes | HAL superior to RIS |
| [46] | US | 268 | RAN, DB | 6 | UN | UN | UN | No | BPRS hostility | CLO 126 CHL 142 |
Mean: CLO 450 CHL 900 |
CLO 675 CHL 900 |
Yes | CLO superior to CHL |
| [47] | US | 71 | RAN, DB | 29 | CLO 41 (10) HAL 40 (8) | CLO 70 HAL 71 | UN | No | BPRS hostile suspiciousness cluster | CLO 37 HAL 34 | Mean (SD) CLO 523 (171) HAL 18.9 (7.0) |
CLO 784.5 HAL 1134 | Yes | CLO superior to HAL |
| [48] | US and EUR | 1996 | RAN, DB | 6 | OLA 38.7 (11.6) HAL 38.3 (11.1) |
OLA 65 HAL 64.7 |
OLA 14.5 (10.5) HAL 14.9 (10.1) |
No | BPRS hostility | OLA 1336 HAL 660 |
Mean (SD) OLA 10.47 (3.54) HAL 9.38 (3.48) |
OLA 314 HAL 562.8 |
Yes | OLA greater improvement than HAL |
| [49] | US CAN | 397 | RAN, DB | 8 | 37 (10.3) | 83 | UN | No | PANSS hostility/excitement | RIS2 87 Ris 6–16 225 HAL 85 |
Fixed: RIS 2 or 6–16 HAL 20 | RIS 200 or 600–1600 HAL 1200 |
Yes | RIS superior to HAL |
| [50] | US | 63 | RAN, DB | 104 | RIS 43.7 (9.2) HAL 43.3 (8.4) |
RIS 87.9 HAL 96.7 |
UN | No | BPRS hostile suspiciousness cluster | RIS 33 HAL 30 |
Mean (SD) RIS 5.7 (2.5) HAL 4.5 (2.4) | RIS 570 HAL 270 | Yes | No significant difference |
| [24] | KOR | 35 | RAN, DB | 8 | 34.1 | 48.7 | UN | No | BPRS hostility item | RIS 16 HAL 19 |
Mean: RIS 7.5 HAL 8.95 |
RIS 750 HAL 537 |
Yes | No significant difference |
| [51] | 5 countries | 307 | RAN, DB | 4 | 36 (11.3) | 62 | UN | No | BPRS hostile suspiciousness cluster | AMI 100 58 AMI 400 62 AMI 800 63 AMI1200 64 HAL 60 |
Fixed: AMI100, 400, 800, or 1200 HAL 16 | AMI 86, 344, 688 or 1032 HAL 960 | Yes | AMI superior to HAL |
| [25] | 15 countries | 1362 | RAN, DB | 8 | RIS 1 38.4 RIS 4 38.1 RIS 8 37.6 RIS12 37.9 RIS 16 38.5 HAL 38.1 |
RIS 1 72.48 RIS4 66.96 RIS 8 62.61 RIS 12 62.83 RIS 16 62.50 HAL 66.3 |
UN | No | BPRS hostility cluster scores | RIS 1 229 RIS 4 227 RIS 8 230 RIS 12 226 RIS 16 224 HAL 226 |
Fixed: RIS 1, 4, 8, 12 or 16 HAL 10 | RIS 100, 400, 800, 1200 or 1600 HAL 600 | Yes | |
| [27] (Eufest) | 14 countries | 302 | RAN, OL | 52 | AMI 25.4 (5.1) OLA 26.3 (6.1) QUE 26.3 (5.7) ZIP 26.3 (5.5) HAL 24.5 (5.4) |
AMI 58 OLA 57 QUE 64 ZIP 46 HAL 61 |
UN | PANSS hostility ≥1 | PANSS hostility item | AMI 62 OLA 67 QUE 67 ZIP 52 HAL 54 |
Mean (SD): AMI 448.4 (221.2) OLA 11.5 (6.0) QUE 509.7 (310.7) ZIP 96.0 (52.1) HAL 3.3 (2.3) |
AMI 385.96 OLA 345 QUE 407.76 ZIP 360 HAL 198 |
Yes | OLA superior to HAL, QUE and Ami in the first 3 months but not significant in the last 6 months |
| [52] (Catie) | US | 614 | RAN, DB | 78 | OLA 40.3 (10.4) QUE 40.4 (11.6) RIS 39.9 (11.8) ZIP 39.8 (11.1) PER 38.7 (11.1) |
OLA 70 QUE 72 RIS 75 ZIP 66 PER 71 |
UN | PANSS hostility ≥ 2 | PANSS hostility item | OLA 136 QUE 147 RIS 144 ZIP 74 PER 113 |
Range: OLA 7.5–30 QUE 200–800 RIS 1.5–6.0 ZIP 40–160 PER 8–32 |
OLA 225–900 QUE 160–640 RIS 150–600 ZIP 150–600 PER 160–640 |
No | OLA superior to other |
HAL haloperidol, AMI amisulpride, RIS risperidon, OLA olanzapine, CLO clozapine, QUE quetiapine, ZIP ziprasidone, PER perphenazine, CHL chlorpromazine, RAN randomized, DB double blind, PLA placebo controlled, UN unknown, BPRS Brief Psychiatric Rating Scale, PANSS Positive and Negative Symptom Scale, BAS Behavioral Agitation Score (from BPRS agitation item, combined anxiety, tension, hostility, uncooperativeness and excitement)
aThe placebo arms are not included in this meta-analysis and the total number of patients is reduced by the number of patients in the placebo arm
As for the atypical antipsychotic drugs, seven studies investigated risperidone, five studies clozapine, four studies olanzapine and quetiapine, ziprasidone and amisulpride were all used in three studies. Two studies were placebo-controlled but the placebo arms are not included in this meta-analysis. With the exception of two studies, all studies were double blind. A large proportion of the studies (14 studies; 5676 (83%) patients) were sponsored by pharmaceutical companies.
Primary outcome measure: hostility scores
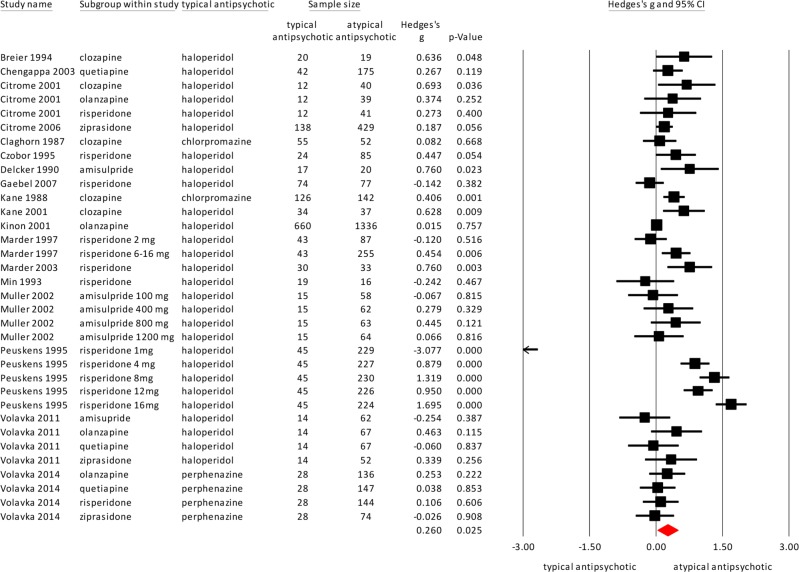
Atypical antipsychotics were superior in reducing hostility compared to the typical antipsychotics with a small effect size, as shown in Fig. 2 (18 studies, N = 6799, Hedges’ g = 0.260, p = 0.025). Heterogeneity, however, was high (I2 = 92.65). The study of Min et al. [24] was considered an outlier (see Fig. 2). Excluding this study, the effect size remained similar and heterogeneity remained high (17 studies, Hedges’ g = 0.273, p = 0.020, I2 = 92.84). The study of Peuskens [25] included a large group, and for a sensitivity analysis, we repeated the meta-analysis without this study. The effect size remained similar, with increased significance and decreased heterogeneity (17 studies, Hedges’ g = .212, p = 0.000, I2 = 44.36).
Fig. 2.
Meta-analysis of the effect of typical versus atypical antipsychotics on hostility
Differences between high and low dosage on effect size
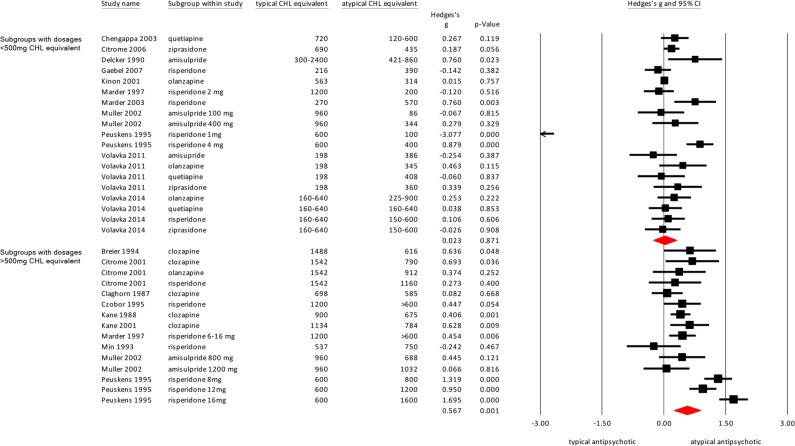
The threshold for the high and low dosed groups was set at 500 mg chlorpromazine equivalent. A total of 19 of the subgroups had a dose below 500 mg and 15 subgroups had a dose above the 500 mg threshold.
The high-dose subgroups showed a significantly superior effect of the atypical antipsychotics in reducing hostility compared with typical antipsychotics (Fig. 3; Hedges’ g = .567, p = 0.001), but this was not found in the analysis of the low-dose groups (Hedges’ g = .023, p = 0.871). The heterogeneity for the high dosed arms is somewhat lower (I2 = 82.79) than the low-dose groups (I2 = 93.39).
Fig. 3.
Meta-analysis of the effect of typical versus atypical antipsychotics on hostility. Effect sizes are grouped by dose: studies with doses < 500 mg CHL equivalent and studies > 500 mg CHL equivalent
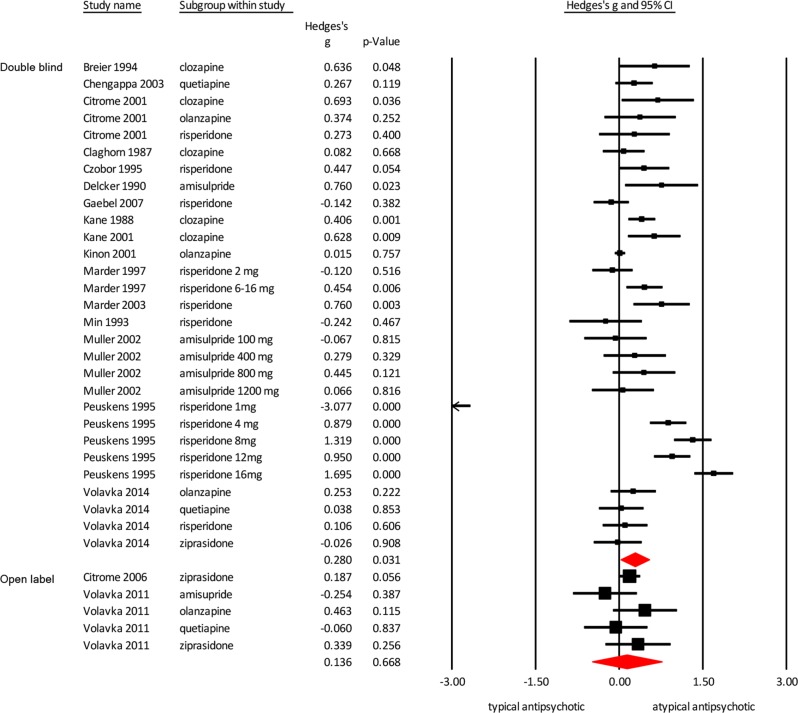
Open label vs double-blind studies
Two studies were open label [26, 27]. These open-label studies had a lower effect size compared with the double-blind studies (Fig. 4; open-label studies Hedges’ g = 0.136 p = 0.668; double-blind studies Hedges’ g = 0.280, p = 0.031).
Fig. 4.
Meta-analysis of the effect of typical versus atypical antipsychotics on hostility. Effect sizes are grouped by the type of study: double blind and open label
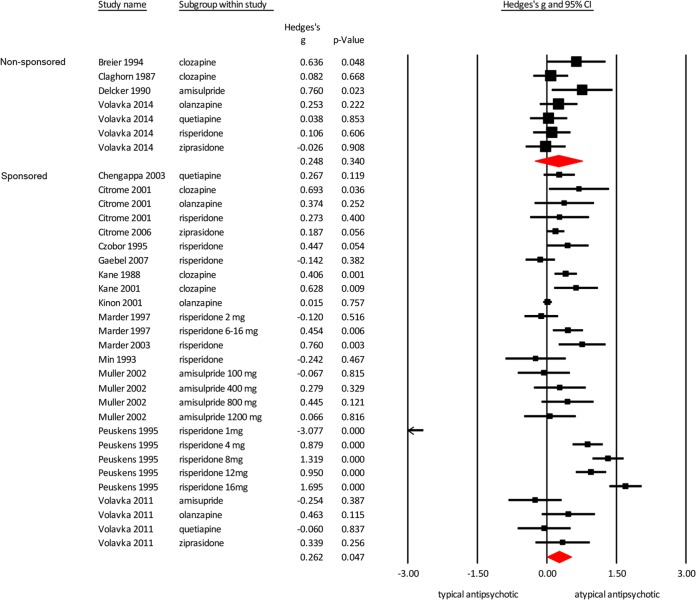
Sponsored vs non-sponsored studies
For both the non-sponsored as well as the sponsored studies, the atypical antipsychotics were superior in reducing hostility compared to typical antipsychotics (Fig. 5; non-sponsored studies Hedges’ g = 0.248 p = 0.340; sponsored studies Hedges’ g = 0.262 p = 0.047). Significance was lost for the non-sponsored studies because of reduced statistical power. While mean weighted effect sizes of sponsored and non-sponsored studies were similar, heterogeneity was much higher in the sponsored group (I2 = 94.12) compared to the non-sponsored group (I2 = 12.65).
Fig. 5.
Meta-analysis of the effect of typical versus atypical antipsychotics on hostility. Effect sizes are grouped by sponsoring
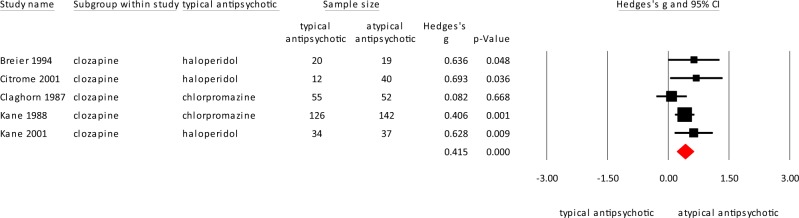
Clozapine
Five studies compared clozapine to typical antipsychotics, of which three used haloperidol and two studies used chlorpromazine as a comparator. Clozapine was significantly superior in reducing hostility compared to typical antipsychotics with a moderate effect size (Fig. 6; clozapine N = 290; typical antipsychotic (haloperidol or chlorpromazine) N = 247; Hedges’ g = 0.415, p = 0.0000). Heterogeneity was low (I2 = 19.16).
Fig. 6.
Meta-analysis of the effect of clozapine vs typical antipsychotics on hostility
Discussion
This study found that atypical antipsychotics are marginally more effective in reducing hostility compared to typical antipsychotics, with a small effect size. Heterogeneity, however, was high and the majority of studies were sponsored. Nonetheless, the non-sponsored studies also found atypical antipsychotics to be superior in reducing hostility with a similar effect size. Only two open-label studies were included, which had a lower effect size than the double-blind studies. Clozapine was significantly better in treating hostility than typical antipsychotic drugs, with a moderate effect size and low heterogeneity. In general, typical drugs were dosed higher than atypical drugs. However, with the studies divided into high and low-dose groups, the high dosed atypical antipsychotics show an even greater and significant effect size. It should be noted that the superiority of the high dose atypical antipsychotics could be influenced by the clozapine studies, since these studies are all included in the high-dose group.
The results of this meta-analysis largely confirm previous studies [10, 28–31] and reviews [9, 11, 32] suggesting superiority of atypical antipsychotics and particularly clozapine in treating violence in patients with psychosis-spectrum disorders. However, previous studies did not perform a meta-analysis of different atypical antipsychotics, and therefore could not quantify these differences. All previous reviews and expert opinions pointed to clozapine as being most effective. This quantitative systematic review confirms these statements using all currently available evidence from randomized controlled trials.
Some atypical antipsychotic drugs, especially olanzapine, quetiapine, and clozapine are more sedative than haloperidol [33], which could explain their effect on hostility. However, most clinical studies control for the effect of sedation and activation as well as psychosis, which could indicate that the anti-hostility effect was at least partially independent from the sedating, activating, and antipsychotic effects of the drug. Moreover, augmentation with benzodiazepine, which does not reduce hostility, shows that sedation alone is not responsible for this effect [34]. Indeed, animal work using knockout mouse models that are insensitive to sedative effects of clozapine, still show efficacy of clozapine in reducing aggression in these animals [35], suggesting the superiority of clozapine and to a lesser degree of other atypicals, may be independent of sedation. Another explanation for the superiority of atypical antipsychotics is their effects on 5-HT receptors. Aggression and hostility have been linked to serotonergic abnormalities both in human and animal studies [36]. In patients with psychosis-spectrum disorders, polymorphism of the 5HT2a promoter region is associated with social cognition and anger management [37]. SSRIs have been suggested to be effective in treating hostility in autism, but for schizophrenia this evidence is lacking [36].
Of course, we need to keep in mind that hostility and violent behavior in psychosis-spectrum disorders have heterogeneous etiology, and may include fear, delusional beliefs, command hallucinations, lack of impulse control or comorbid antisocial personality, or drug addiction. We should therefore not expect that any given pharmacological treatment will be equally effective in reducing violent behavior caused by psychosis, impaired impulse control, craving, or personality disorder [38].
Strengths and weaknesses of the study
This study has several limitations. First, the search initially included studies that focused on violence or hostility and reported as such in the title or abstract. Studies without any of our keywords in title or abstract may have been missed. However, the cross reference search was performed thoroughly and included other meta analyses and reviews. Second, the PANSS and the BPRS are instruments with different scoring instructions. While both have a similar outcome measure, the scores are not interchangeable. We, however, could not analyze them separately because of the loss in power. Third, the large number of sponsored studies could cause bias. However, the separate analysis of the non-sponsored studies also showed very similar effects. The heterogeneity of the sponsored studies is much higher. Possibly because, in many cases, sponsored studies were conducted to establish a dose-response relationship. Therefore, these studies included several dose arms for the same antipsychotic (e.g., for risperidone and amisulpride), which increased the variation in terms of therapeutic efficacy, and were likely to enhance the variation of therapeutic effects (and increased heterogeneity) with respect to hostility. Studies without sponsorship used established fixed doses of antipsychotic medications, or flexible doses in an established dose range to achieve the best therapeutic effects in individual patients, which could be behind, at least in part, the lower level of heterogeneity in these studies. Finally, a number of studies only used one typical antipsychotic as a control versus multiple atypical antipsychotics or dose groups. Although the N of the typical antipsychotics is divided by the number of atypical arms, this could cause an over- or underestimation.
Moreover, some limitations of our inclusion criteria should be noted. This review only included studies investigating the medication for 4 weeks or more. We did not look at the acute effect of antipsychotic drugs in emergency situations, as these effects may differ from those of maintenance treatment. These studies mostly focus on the first day of treatment and include, for example, the use of short-acting olanzapine IM, short-acting aripiprazole IM, loxapine inhaled, sublingual asenapine and antihistamines. As a recent review towards medication for psychosis-induced aggression or agitation concluded there is only a small amount of evidence and of poor quality [39], future research could compare the effects of medications in emergency situations and long-term treatment. Finally, only studies with hostility scores as outcome measure are included and not the studies using physical violent incidents as an outcome measure, as there were too few of those. However, our results seem to be compatible with previous research studying the anti-aggressive effects of typical and atypical antipsychotics in a population of violent patients, showing a superiority for atypical drugs and specifically clozapine [10, 28–31].
The strengths of this study, however, are the large number of patients used in the main analysis and the quantification of effect sizes.
In conclusion, we confirmed previous claims of superiority for atypical antipsychotics as compared to typical antipsychotics in treating/reducing hostility but found only a small effect with high heterogeneity. When we restricted our meta-analysis to only studies that were not industry sponsored, the effect remained largely the same, but heterogeneity disappeared. This suggests that there is indeed a small yet significant advantage. When we restricted our analysis to clozapine versus typical medication, the effect size almost doubled and heterogeneity largely disappeared. Finally, when differentiating between high and low dosed antipsychotics, the high dosed studies had a significantly greater effect on hostility compared with low-dose antipsychotics. Although this effect could be partially caused by the clozapine studies in the high-dose groups, this possibly indicates that higher dosed atypical antipsychotics are most effective to deal with violent behavior from patients with psychosis-spectrum disorder in daily practice. These results are important for clinicians to help their shared decision making with patients when choosing maintenance treatment, as next to efficacy for psychosis and tolerability, safety for the patient and their environment is an important outcome.
Acknowledgements
IS has received funding from the Dutch Research Organization (ZonMW, HAMLETT study 848041003). The remaining authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Witt Katrina, van Dorn Richard, Fazel Seena. Risk Factors for Violence in Psychosis: Systematic Review and Meta-Regression Analysis of 110 Studies. PLoS ONE. 2013;8(2):e55942. doi: 10.1371/journal.pone.0055942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fazel Seena, Gulati Gautam, Linsell Louise, Geddes John R., Grann Martin. Schizophrenia and Violence: Systematic Review and Meta-Analysis. PLoS Medicine. 2009;6(8):e1000120. doi: 10.1371/journal.pmed.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Large MM, Nielssen O. Violence in first-episode psychosis: a systematic review and meta-analysis. Schiz Res. 2011;125:209–20. doi: 10.1016/j.schres.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Hodgins S, Riaz M. Violence and phases of illness: differential risk and predictors. Eur Psychiatry. 2011;26:518–24. doi: 10.1016/j.eurpsy.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Volavka J, Citrome L. Pathways to aggression in schizophrenia affect results of treatment. Schizophr Bull. 2011;36:921–9. doi: 10.1093/schbul/sbr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor PJ. Psychosis and violence: stories, fears and reality. Can J Psychiatry. 2008;53:647–59. doi: 10.1177/070674370805301004. [DOI] [PubMed] [Google Scholar]
- 7.Hodgins S. Violent behavior among people with schizophrenia: a framework for investigations of causes, and effective treatment, and prevention. Philos Trans R Soc. 2008;363:2505–18. doi: 10.1098/rstb.2008.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frogley C, Taylor D, Dickens G, Picchioni M. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15:1351–71. doi: 10.1017/S146114571100201X. [DOI] [PubMed] [Google Scholar]
- 9.Glazer WM, Dickson RA. Clozapine reduces violence and persistent aggression in schizophrenia. J Clin Psychiatry. 1998;59:8–14. doi: 10.4088/JCP.v59n0103. [DOI] [PubMed] [Google Scholar]
- 10.Spivak B, Mester R, Wittenberg N, Maman Z, Weizman A. Reduction of aggressiveness and impulsiveness during clozapine treatment in chronic neuroleptic-resistant schizophrenic patients. Clin Neuropharmacol. 1997;20:442–6. doi: 10.1097/00002826-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Aleman A, Kahn RS. Effects of the atypical antipsychotic risperidone on hostility and aggression in schizophrenia: a meta-analysis of controlled trials. Eur Neuro. 2001;11:289–93. doi: 10.1016/s0924-977x(01)00097-9. [DOI] [PubMed] [Google Scholar]
- 12.Buckley PF. The role of typical and atypical antipsychotic medications in the management of agitation and aggression. J Clin Psychiatry. 1999;60:52–60. [PubMed] [Google Scholar]
- 13.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 14.Hedlund JL, Vieweg BW. The Brief Psychiatric Rating Scale (BPRS): a comprehensive review. J Oper Psychiatry. 1980;11:48–65. [Google Scholar]
- 15.Knezevic Vladimir, Mitrovic Dragan, Drezgic-Vukic Svetlana, Knezevic Jelena, Ivezic Aleksandar, Siladji-Mladenovic Djendji, Golubovic Boris. Prevalence and Correlates of Aggression and Hostility in Hospitalized Schizophrenic Patients. Journal of Interpersonal Violence. 2016;32(2):151–163. doi: 10.1177/0886260515585537. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Lindenmayer JP, Bernstein-Hyman R, Grochowski S. Five-factor model of schizophrenia. J Nerv Ment Dis. 1994;182:631–8. doi: 10.1097/00005053-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Chengappa KNR, Goldstein JM, Greenwood M, John V, Levine J. A post hoc analysis of the impact on hostility and agitation of quetiapine and haloperidol among patients with schizophrenia. Clin Ther. 2003;25:530–41. doi: 10.1016/S0149-2918(03)80094-2. [DOI] [PubMed] [Google Scholar]
- 19.Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods. 1996;1:170–7. doi: 10.1037/1082-989X.1.2.170. [DOI] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgings T, Rothstein HR. Introduction to meta-analysis. Chapter 12. Chichester, UK: Wiley; 2009. [Google Scholar]
- 21.Borenstein M, Higgings JPT, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges L, Higgings J, Rothstein H. Comprehensive meta analysis version. Englewood, NJ: Biostat Inc; 2005. p. 2. [Google Scholar]
- 23.Gardner DM, Murphy AL, O’Donnel H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic drugs. Am J Psychiatry. 2010;167:686–93. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 24.Min SK, Rhee CS, Kim CE, Kang DY. Risperidone versus haloperidol in the treatment of chronic schizophrenic patients: a parallel group double-blind comparative trial. Yonsei Med J. 1993;34:179–90. doi: 10.3349/ymj.1993.34.2.179. [DOI] [PubMed] [Google Scholar]
- 25.Peuskens J. Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Br J Psychiatry. 1995;166:712–26. doi: 10.1192/bjp.166.6.712. [DOI] [PubMed] [Google Scholar]
- 26.Citrome L, Volavka J, Czobor P, Brook S, Loebel A, Mandel FS. Efficacy of ziprasidone against hostility in schizophrenia: post hoc analysis of randomized, open-label study data. J Clin Psychiatry. 2006;67:4. doi: 10.4088/JCP.v67n0415. [DOI] [PubMed] [Google Scholar]
- 27.Volavka J, Czobor P, Derks E, Bitter I, Libiger J, Kahn RS, et al. Efficacy of antipsychotic drugs against hostility in the European first-episode schizophrenia trial (EUFEST) J Clin Psychiatry. 2011;72:955–61. doi: 10.4088/JCP.10m06529. [DOI] [PubMed] [Google Scholar]
- 28.Krakowski MI, Czobor P, Citrome L, Bark N, Cooper MA. Atypical antipsychotics in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63:622–9. doi: 10.1001/archpsyc.63.6.622. [DOI] [PubMed] [Google Scholar]
- 29.Krakowski MI, Czobor P, Nolan KA. Atypical antipsychotics, neurocognitive deficits and aggression in schizophrenic patients. J Clin Psychopharmacol. 2008;28:485–93. doi: 10.1097/JCP.0b013e3181855cd6. [DOI] [PubMed] [Google Scholar]
- 30.Swanson JW, Schwartz MS, Elbogen EB. Effectiveness of atypical antipsychotic medications in reducing violent behavior among persons with schizophrenia in community-based treatment. Schizophr Bull. 2004;30:1. doi: 10.1093/oxfordjournals.schbul.a007065. [DOI] [PubMed] [Google Scholar]
- 31.Volavka J, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome L. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone or haloperidol. J Clin Psychopharmacol. 2004;24:225–8. doi: 10.1097/01.jcp.0000117424.05703.29. [DOI] [PubMed] [Google Scholar]
- 32.Brieden T, Ujeyl M, Naber D. Psychopharmacological treatment of aggression in schizophrenic patients. Pharmacopsychiatry. 2002;35:83–9. doi: 10.1055/s-2002-31523. [DOI] [PubMed] [Google Scholar]
- 33.Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: multiple-treatments meta-analysis. Lancet. 2013;382:14. doi: 10.1016/S0140-6736(13)62175-3. [DOI] [PubMed] [Google Scholar]
- 34.Correll CU, Yu X, Xiang Y, Kane JM, Masand P. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29:92–107. [PubMed] [Google Scholar]
- 35.Gallitano-Mendel A, Wozniak DF, Pehek EA, Milbrandt J. Mice lacking the immediate early gene Egr3 respond to the anti-aggressive effects of clozapine yet are relatively resistant to its sedating effects. Neuropsychopharmacology. 2008;33:1266–75. doi: 10.1038/sj.npp.1301505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silver H. Fluvoxamine as an adjunctive agent in schizophrenia. CNS Drug Rev. 2001;7:283–304. doi: 10.1111/j.1527-3458.2001.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo CH, Tsai GE, Liao CH, Wang MY, Chang JP, Tsuang HC, et al. Emotional management and 5-HT2A receptor gene variance in patients with schizophrenia. Biol Psychol. 2010;83:79–83. doi: 10.1016/j.biopsycho.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Volavka J, Citrome L. Heterogeneity of violence in schizophrenia and implications for long-term treatment. Int J Clin Pract. 2008;62:1237–45. doi: 10.1111/j.1742-1241.2008.01797.x. [DOI] [PubMed] [Google Scholar]
- 39.Gillies D, Sampson S, Beck A, Rathbone J. Benzodiazepines for psychosis-induced aggression or agitation. Cochrane Database of Systematic Reviews 2013. [DOI] [PubMed]
- 40.Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, et al. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–6. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- 41.Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer JP, McEvoy J, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52:1510–4. doi: 10.1176/appi.ps.52.11.1510. [DOI] [PubMed] [Google Scholar]
- 42.Claghorn J, Honingfeld G, Abuzzahab F, Wang R, Steinbook R, Tuason V, et al. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7:377–84. doi: 10.1097/00004714-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Czobor P, Volavka J, Meibach R. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol. 1995;15:243–9. doi: 10.1097/00004714-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Delcker A., Schoon M., Oczkowski B., Gaertner H. Amisulpride Versus Haloperidol in Treatment of Schizophrenic Patients - Results of a Double-Blind Study. Pharmacopsychiatry. 1990;23(03):125–130. doi: 10.1055/s-2007-1014494. [DOI] [PubMed] [Google Scholar]
- 45.Gaebel W, Riesbeck M, Wölwer W, Klimke A, Eickhoff M, von Wilmsdorff M, et al. Maintenance treatment with risperidone of low-dose haloperidol in first-episode schizophrenia: 1-year results of a randomized controlled trial within the German research network on schizophrenia. J Clin Psychiatry. 2007;68:1763–74. doi: 10.4088/JCP.v68n1116. [DOI] [PubMed] [Google Scholar]
- 46.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. Arch Gen Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 47.Kane JM, Marder SR, Schooler NR, Wirshing WC, Umbricht D, Baker RW, et al. Clozapine and haloperidol in moderately refractory schizophrenia. Arch Gen Psychiatry. 2001;58:965–72. doi: 10.1001/archpsyc.58.10.965. [DOI] [PubMed] [Google Scholar]
- 48.Kinon BJ, Roychowdhury SM, Milton DR, Hill AL. Effective resolution with olanzapine of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia. J Clin Psychiatry. 2001;62:17–21. doi: 10.4088/JCP.v62n0204. [DOI] [PubMed] [Google Scholar]
- 49.Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American Trials. J Clin Psychiatry. 1997;58:538–46. doi: 10.4088/JCP.v58n1205. [DOI] [PubMed] [Google Scholar]
- 50.Marder SR, Glynn SM, Wirshing WC, Wirshing DA, Ross D, Widmark C, et al. Maintenance treatment of schizophrenia with risperidone or haloperidol: 2-year outcomes. Am J Psychiatry. 2003;150:1405–12. doi: 10.1176/appi.ajp.160.8.1405. [DOI] [PubMed] [Google Scholar]
- 51.Müller MJ, Wetzel H, Eich FX, Rein W, Puech A, Benkert O. Dose-related effects of amisulpride on five dimensions of psychopathology in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2002;22:554–60. doi: 10.1097/00004714-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Volavka J, Czobor P, Citrome L, Van Dorn RA. Effectiveness of antipsychotic drugs against hostility in patients with schizophrenia in the Clinial Antipsychotics Trials of Intervention Effectiveness (CATIE) study. CNS Spectr. 2014;19:374–81. doi: 10.1017/S1092852913000849. [DOI] [PMC free article] [PubMed] [Google Scholar]