Abstract
BACKGROUND
The safety and efficacy of dapoxetine for the treatment of premature ejaculation (PE) is still controversial. Thus, we decided to conduct a meta-analysis using trial sequential analysis (TSA) to determine the sufficiency of conclusions.
OBJECTIVE
Evaluate the efficacy and safety of dapoxetine in the treatment of patients with PE and assess the reliability of the findings.
DESIGN
Meta-analysis of randomized controlled trials (RCTs).
METHODS
Electronic databases including PUBMED, EMBASE, Cochrane Library, CNKI and Wanfang data were reviewed up to July 2017. RCTs evaluating the efficacy of dapoxetine in patients with PE and reporting intravaginal ejaculatory latency time (IELT), patient global impression of change (PGIC) and/or adverse events (AEs) were included.
MAIN OUTCOME MEASURES
Mean differences between trials in efficacy for IELT, and risk ratios for PGIC and treatment-emergent AEs.
SAMPLE
8 RCTs.
RESULTS
For IELT and PGIC, significant effects were found for all doses of dapoxetine versus placebo, and similar results were obtained in subgroups of the 30-mg dose versus 60-mg dose. There were also statistically different dose-related effects on AEs. Trial sequential analysis showed that the result of our meta-analysis was confirmed and further trials are unnecessary.
CONCLUSIONS
The evidence suggests that dapoxetine may be a safe and effective drug for patients with PE.
REGISTRATION
Not registered, no published protocol.
Premature ejaculation (PE) is the most commonly reported form of sexual dysfunction in men, with a prevalence varying from 19% to about 30% in the male population.1 PE refers to an inability to delay ejaculation or having less perceived control over ejaculation, based on the guidelines for PE of The International Society for Sexual Medicine (ISSM) published in 2014.2 To some extent, the quality of life for patients and their partners, including the sexual satisfaction of both partners, sexual confidence, and interpersonal relationships, are influenced by PE.3,4 Above all, PE is an important factor affecting the overall quality-of-life that cannot be ignored, particularly due to its high prevalence. Treatments recommended for PE include behavioral psychotherapy, drug therapy or a combination. Dapoxetine is one of the most widely used oral medications, though not always the most acceptable to the patient.5–7
Although previous reviews have evaluated the efficacy and safety of dapoxetine in the treatment of PE,8–10 all used pooled data on patient-reported global impression of change (PGIC) except for which included trials included patients with concomitant premature ejaculation and erectile dysfunction that may have biased the results.11 However, whether dapoxetine is efficacious and safe for the treatment of PE is still controversial. Motivated by these considerations, the goal of this analysis was to systematically evaluate the efficacy and safety profile of dapoxetine for men with PE, and use trial sequential analysis (TSA) to determine the sufficiency and conclusion of the available evidence in this meta-analysis.9
METHODS
Search strategy and information sources
Our meta-analysis adhered to the statement of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).12 The study was not previously registered so a protocol was not published. We screened literature from PubMed (July 2017), EMBASE (July 2017), Cochrane Library databases (July 2017), China National Knowledge Infrastructure (July 2017) (www.cnki.net), and Wanfang database (July 2017) (www.wanfangdata.com.cn) with the search terms “dapoxetine” OR “Priligy” (trade name for dapoxetine) AND “premature ejaculation (PE)” without language restrictions. Furthermore, we also searched the references from included reviews for further studies.
Study selection and data extraction
The study selection was independently performed by two investigators (L.J. and L.D.Z.). Any discrepancy between the two investigators was resolved by discussions or a third opinion (J.F.W.). First, we screened the titles and abstracts to exclude the obviously irrelevant reports, then made a selection based on a review of the full text for the remaining reports. For all potential studies, the inclusion criteria were as follows: (1) the age of patients ≥18 years; the patients were diagnosed with PE; the patients were treated with oral dapoxetine on-demand (1–3 hours before anticipated sexual intercourse); (2) the treatment intervention was dapoxetine versus placebo or another drug intervention;9 (3) and study design was a randomized controlled trial (RCT). Studies were excluded if: (1) studies were quasi-RCTs or not RCTs; (2) the patients were diagnosed with other andrologic conditions in terms of sexual function;9 and (3) patients were treated with a daily fixed oral dose; (4) and the studies included interventions other than dapoxetine.9
The data was extracted from the eligible studies independently by two reviewers using a standard form containing the following items: author and year of publication, number of participants, intervention, treatment duration and outcomes. The primary outcome was intravaginal ejaculatory latency time (IELT).9 Secondary outcomes were patient global impression of change (PGIC) and treatment-emergent adverse events (AEs).9 Especially, PGIC also referred to the clinical global impression of change in some studies, which was rated on a seven-point scale (became much worse, worse, slightly worse, no change, slightly better, better, or much better after treatment).9 In this study, the level of the improvement for PGIC was defined as at least “better”.
Quality assessment
The seven following items from the Cochrane Handbook for Systematic Reviews of Interventions were used by two reviewers to assess the quality of the included studies: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting and (7) other biases.13
Statistical analysis
Risk ratios (RR) with 95% CIs were calculated for PGICs and AEs. The data are plotted as Forest plots. The I2 statistic and the Mantel-Haenszel chi-squared test were used to evaluate the heterogeneity among the studies, and an I2 >50% was regarded as significant heterogeneity. 14 When heterogeneity among studies existed, a random-effects model was used; otherwise a fixed effects model was applied. A sensitivity analysis and subgroup analysis were also performed to test the results of the study. For all statistical results, P value less than .05 was considered to indicate a statistically significant difference. Meta-analysis was performed using Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Trial sequential analysis
Repeated updates of meta-analyses with new RCTs increases random errors, which may lead to overestimation of intervention effects and spurious results (false positives).15,16 To control for increased random error, trial sequential analysis (TSA) generates monitoring boundaries and estimates an optimal sample size in cumulative meta-analyses, which create thresholds for declaring significance.17,18 When a new study is included in a meta-analysis, significance is tested and confidence intervals are estimated. Cumulative Z-curves representing the findings of the data are created. Furthermore, when the Z-curves surpass the trial sequential monitoring boundary, the level of evidence is sufficient and further trials are deemed futile. TSA also determines boundaries for the benefit and harm and the boundary for futility. If one of the boundaries or the optimal sample size in cumulative meta-analyses are reached, the conclusion might be considered confirmed and no further trials are needed. Conversely, if no boundaries are reached, more trials are needed to clarify the conclusion. 19–21 For our TSA, a two-sided trial sequential monitoring boundary type was used. The required information size (RIS) was calculated with α=0.05, β=0.20. The control event proportions were calculated from the present meta-analysis and a relative risk reduction of 20% was calculated from included studies. The software TSA version 0.9 beta (Copenhagen Trial Unit) was used for these analyses (Copenhagen Trial Unit, Centre for Clinical Intervention Research, www.ctu.dk/tsa). An update to TSA version 0.9.5.10 beta applied only to Figures 4, 5A, and 5B and these figures were subsequently updated. A regeneration of the graphs using the new software showed no changes in the graphs.
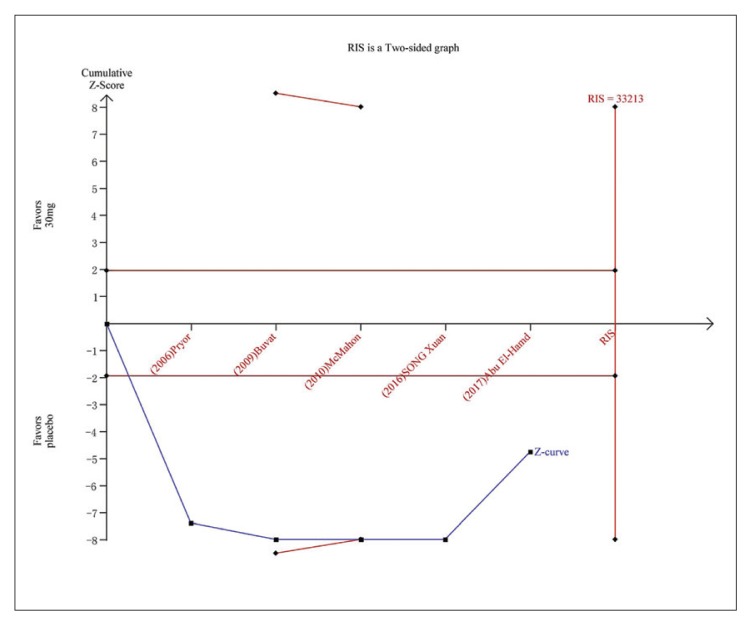
Figure 4.
Trial sequential analysis for IELT for dapoxetine 30 mg versus placebo.The continuous blue line represents the Z curve, the continuous red horizontal lines represent the conventional boundary. The red non-horizontal lines represent the trial sequential monitoring boundary. The required information size was calculated as 33213. The Z curve has crossed the conventional boundary for harm and trial sequential monitoring boundary for harm, indicating the conclusion is sufficient and no more trials are needed.
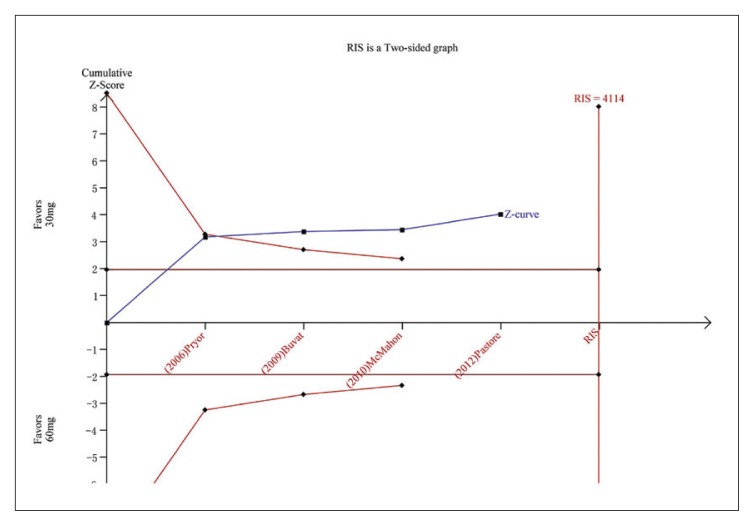
Figure 5A.
Trial sequential analysis for IELT for dapoxetine 30 mg versus dapoxetine 60 mg. The Z curve and boundaries are as in Figure 4. The required information size was calculated as 4114. The Z curve has crossed the conventional boundary for benefit and trial sequential monitoring boundary for benefit, indicating sufficient power to draw a definitive conclusion and no more trials are needed.
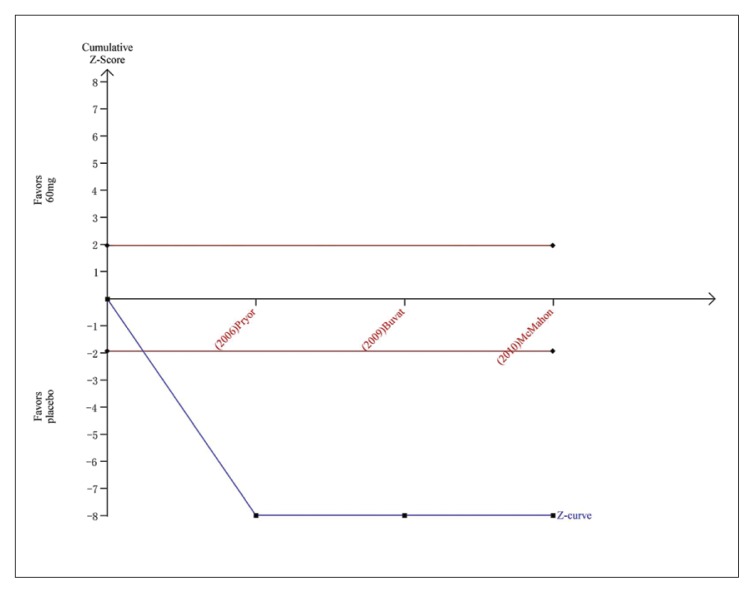
Figure 5B.
Trial sequential analysis for IELT for dapoxetine 60 mg versus placebo. The Z curve and boundaries are as in Figure 4. The RIS was not renderable because the first information fraction exceeded 100% of the RIS. The Z curve has crossed the conventional boundary for harm and required information size, showing that the conclusion is sufficient and no more trials are needed.
RESULTS
Search results and reporting quality
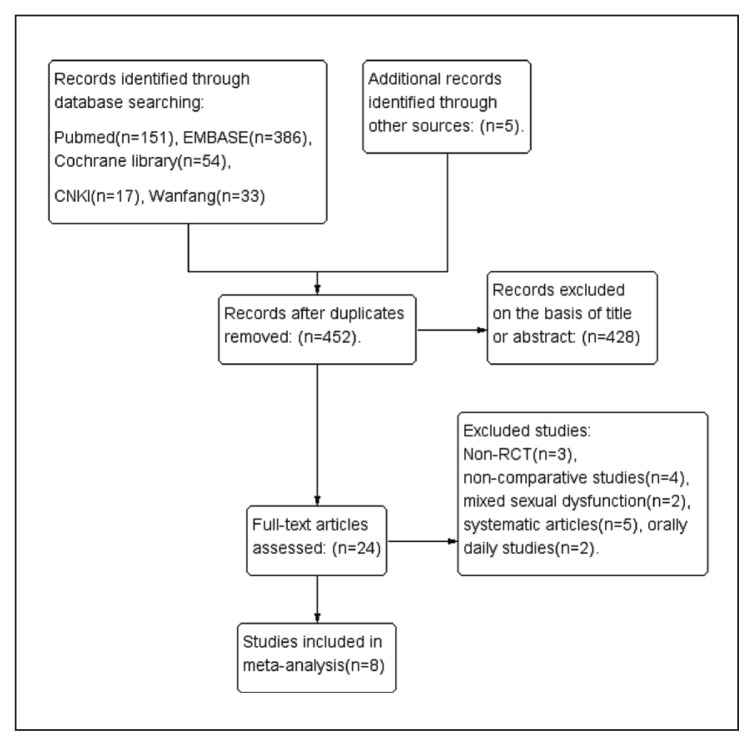
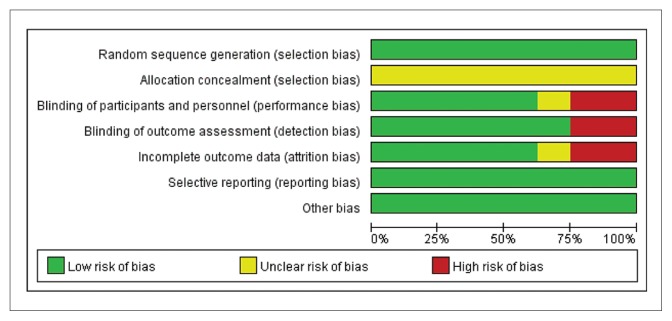
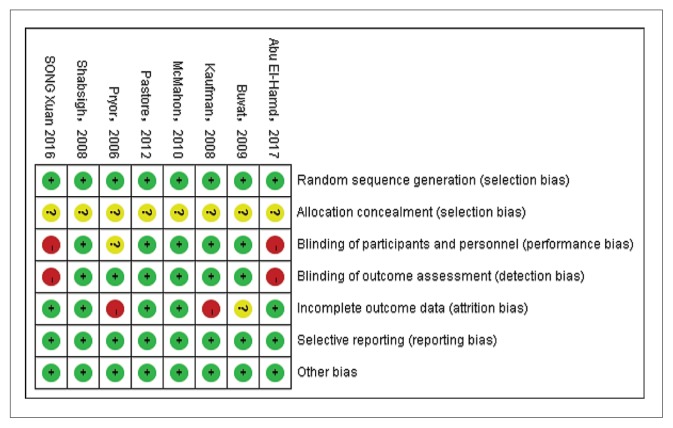
After searching the databases and removing duplicates, we obtained 24 articles for full-text evaluation. Of these, 16 articles were excluded leaving 8 studies that were included in this meta-analysis (Figure 1). The characteristics and patient demographic data are summarized in Table 1. Four single-center studies22–25 and four multicenter studies7,26–28 were identified. Five studies enrolled participants who met the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) criteria for PE and the others met the ISSM criteria. All participants reported their PE as at least “moderate”. All selected studies were designed as RCTs and clearly described the sequence generation. A double-blind was used in five studies, and the others were single-blind. Two studies described allocation concealment and four studies applied intent-to-treat analysis. The assessment of risk of bias of each methodological component was conducted by the Cochrane risk bias tool. Figure 2A and 2B present the summary of risk of bias.
Figure 1.
Flow diagram for selection of studies included in the meta-analysis.
Table 1.
| Studies | Dapoxetine dose/n | Control/n | Duration (weeks) | Outcomes measure |
|---|---|---|---|---|
|
| ||||
| Pryor, 200627 | 30 mg, 60 mg/874, 870 | Placebo/870 | 12 | IELT, PGIC, AEs |
| Kaufman, 200828 | 60 mg/432 | Placebo/221 | 9 | PGIC, AEs |
| Shabsigh, 200822 | 30 mg, 60 mg/800, 769 | Placebo/772 | 12 | IELT, PGIC, AEs |
| Buvat, 200926 | 30 mg, 60 mg/388, 389 | Placebo/385 | 24 | IELT, PGIC, AEs |
| McMahon, 201031 | 30 mg, 60 mg/354, 356 | Placebo/357 | 12 | IELT, PGIC, AEs |
| Pastore, 201223 | 30 mg/8 | 60 mg/7 | 12 | IELT, PGIC, AEs |
| AbuEl-Ham, 201725 | 30 mg/30 | Placebo/30 | 6 | IELT, AEs |
| Song Xuan, 201624 | 30 mg/46 | Placebo/42 | 4 | IELT, AEs |
Total number of patients, 7231.
Figure 2A.
The summary of risk of bias by proportion of judgements.
Figure 2B.
The summary of risk of bias by individual studies.
Primary outcome: intravaginal ejaculatory latency time
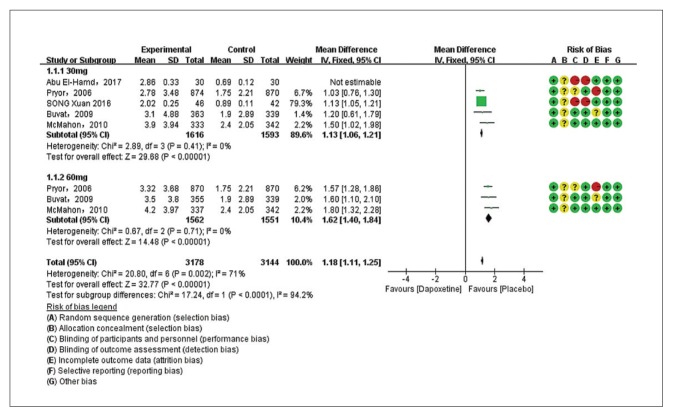
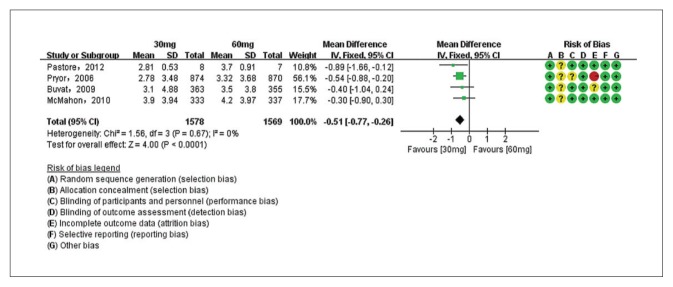
Five studies evaluating dapoxetine versus placebo were enrolled in the analysis of IELT.7,24–27 The pooled estimate presented an MD of 1.18 minutes (95% CI 1.11–1.25; P<.00001; P for heterogeneity=.002, I2=71%) (Figure 3A) in favor of participants who received dapoxetine. The I2 was 71% indicating that heterogeneity existed in the pooled studies, and a subgroup analysis, performed to decrease the heterogeneity, showed a statistically significant increase in IELT in both the 30-mg group (fixed effects model, MD 1.13min, 95% CI 1.06–1.21; P<.00001; P for heterogeneity=. 41, I2=0%) and 60-mg group (MD 1.62 min, 95% CI 1.40–1.84; P<.00001; P for heterogeneity=.71, I2=0%). The subgroup analysis of the dapoxetine group treated with 60 mg or 30 mg both indicated a significant improvement compared with placebo. Sensitivity analysis indicated that the study conducted by Abu El-Hamd was the source of the heterogeneity.25 After removing the outlier study, the result was stable. In addition, four studies7,23,26,27 compared the IELT between dopaxetine 30-mg and dopaxetine 60-mg groups, which was significantly different (Figure 3B). Sensitivity analysis showed that the result was stable. For the two groups (30 mg versus placebo and 30 mg versus 60 mg), TSA showed that the Z-curves crossed both the conventional boundary and the trial sequential monitoring boundary. Thus, the conclusion is reliable and further trials are not needed (Figure 4 and 5A). In regard to 60 mg versus placebo, the RIS was not renderable because the first information fraction exceeded 100% of the RIS, so we think the level of evidence was reached (Figure 5B).
Figure 3A.
Estimates for mean difference in IELT for dapoxetine (30 mg and 60 mg subgroup) and placebo group (favoring placebo means that IELT was 1.18 minutes shorter with placebo).
Figure 3B.
Estimates for mean difference IELT for dapoxetine 30 mg group and 60 mg group after removal of outlier (the IELT was shorter with the 30 mg dose).
The secondary outcome: patient global impression of change
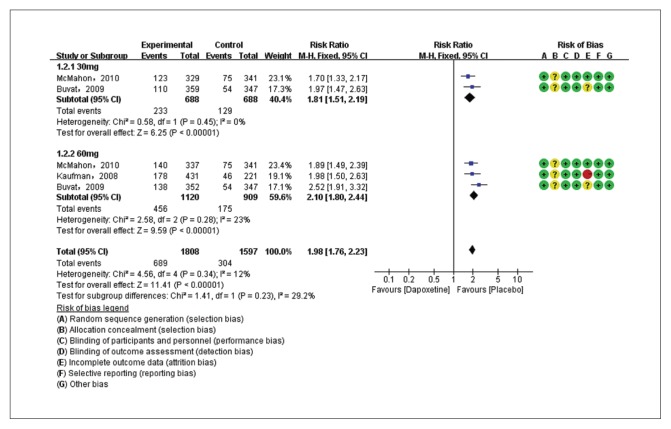
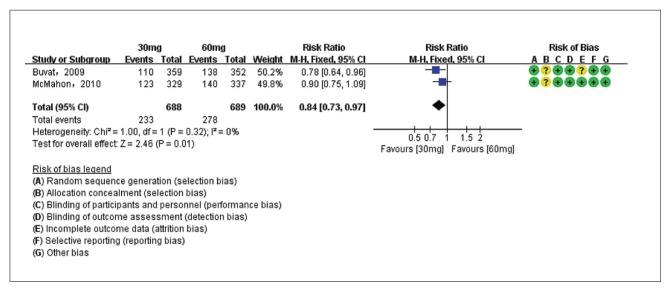
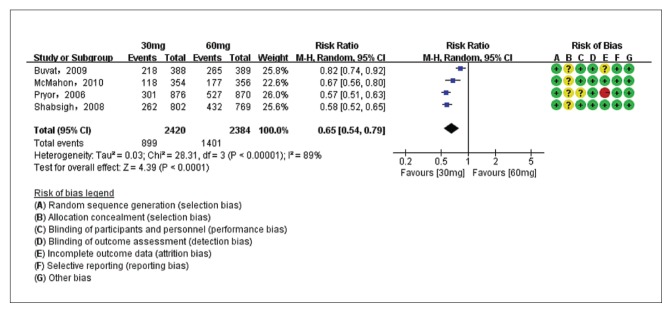
The PGIC of dapoxetine 30 mg versus placebo groups were compared in two studies7,26 with 1376 patients, and the PGIC of dapoxetine 60 mg versus placebo subgroups were compared in three studies7,26,28 with 2029 patients. Pooled analysis showed that the PGIC in the dapoxetine group was significantly higher than in the placebo group. The overall RR was 1.98 (95% CI1.76–2.23; P<.00001; P for heterogeneity=.34, I2=12%) (Figure 6A). The subgroup analysis showed that the dapoxetine (for both 30 mg and 60 mg groups) was significantly different from the placebo group in PGIC (RR: 1.81, 95% CI: 1.51–2.19, P<.00001; and RR: 2.10, 95% CI:1.80–2.44, P<.00001). In addition, the analysis of two studies7,26 comparing dapoxetine 30 mg with dapoxetine 60 mg for PGIC indicated that dapoxetine 60 mg was significantly associated with a greater improvement in PGIC than dapoxetine 30 mg (RR: 0.84, 95% CI: 0.73–0.97; P=.01) (Figure 6B).4 Sensitivity analysis showed that the results were stable for the above three groups. Besides, when applying TSA, the Z-curve crossed both the conventional boundary and the trial sequential monitoring boundary, and thus the conclusion was reliable and further trials are not needed (Figure 5C, 5D, 5E).
Figure 6A.
Estimates for risk ratio for PGIC for dapoxetine (30 mg and 60 mg subgroup) and placebo group (dapoxetine increased the PGIC by 1.98 times compared to placebo).
Figure 6B.
Estimates for risk ratio for PGIC for dapoxetine 30 mg group and 60 mg group (dapoxetine 60 mg increased PGIC versus 30 mg).
Figure 5C.
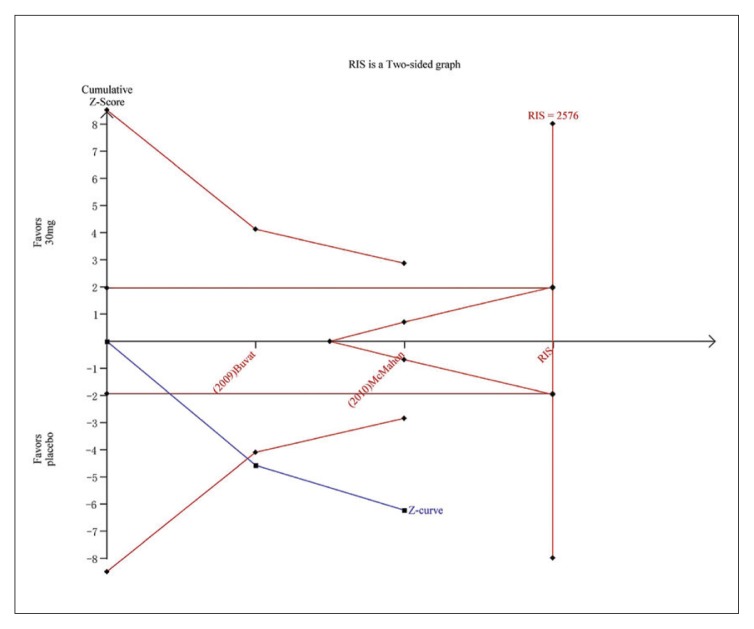
Trial sequential analysis for PGIC for dapoxetine 30 mg versus placebo. The Z curve and boundaries are as in Figure 4. The two red lines that intersect the horizontal coordinate line represent the futility boundary. The required information size was calculated as 2576. The Z curve has crossed the conventional boundary for harm and trial sequential monitoring boundary for harm, indicating sufficient power to draw a definitive conclusion and no more trials are needed.
Figure 5D.
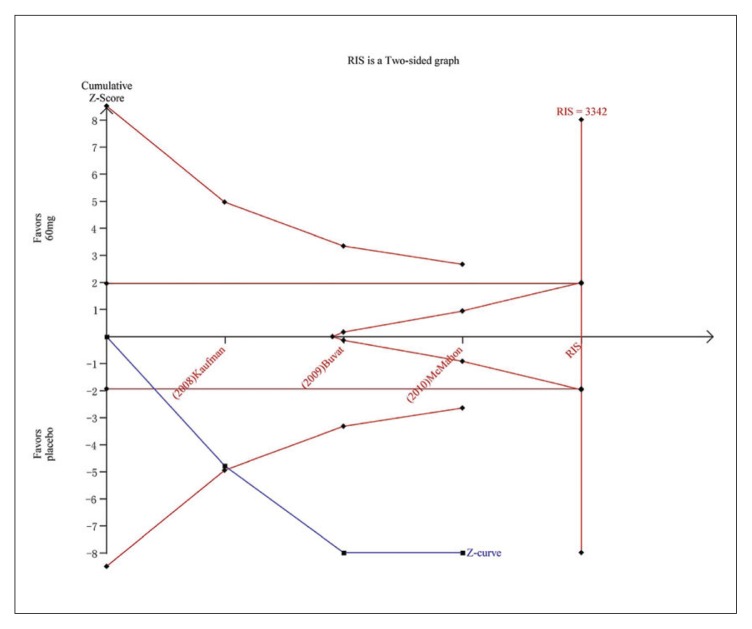
Trial sequential analysis for PGIC for dapoxetine 60 mg versus placebo. The Z curve and boundaries are as in Figure 4. The two red lines that intersect the horizontal coordinate line represent the futility boundary. The required information size was calculated as 3342. The Z curve has crossed the conventional boundary for harm and trial sequential monitoring boundary for harm, indicating sufficient power to draw a definitive conclusion and no more trials are needed.
Figure 5E.
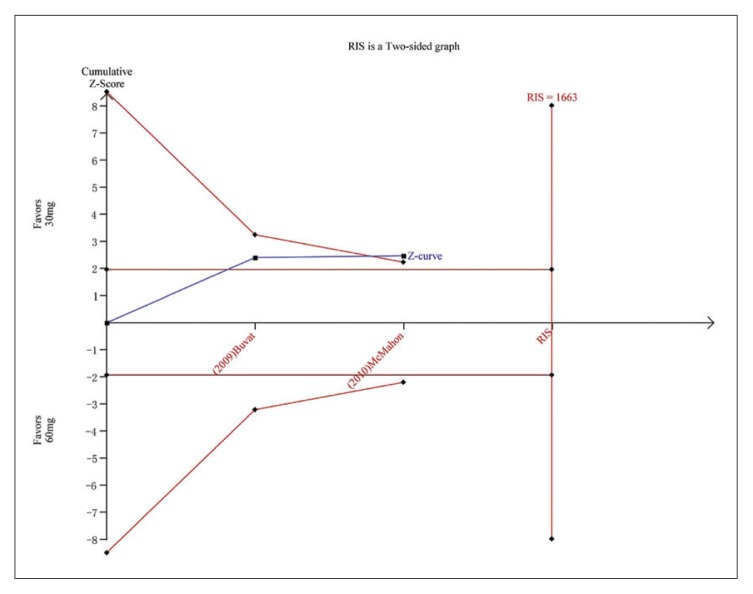
Trial sequential analysis for PGIC for dapoxetine 30 mg versus dapoxetine 60 mg. The Z curve and boundaries are as in Figure 4. The required information size was calculated as 1663. The Z curve has across the conventional boundary for benefit and trial sequential monitoring boundary for benefit, indicating sufficient power to draw a definitive conclusion and no more trials are needed.
The secondary outcome: adverse events
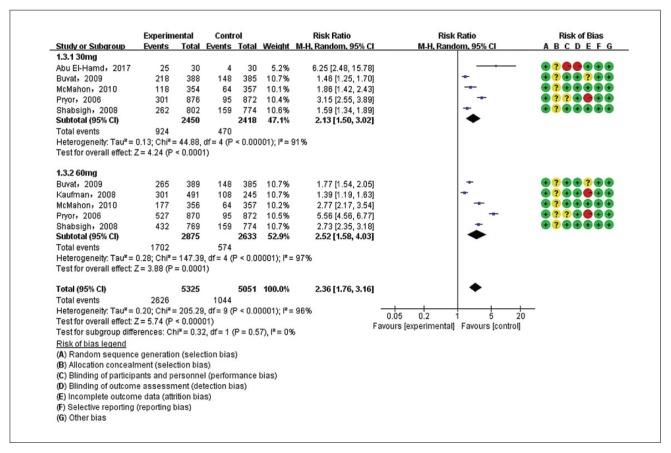
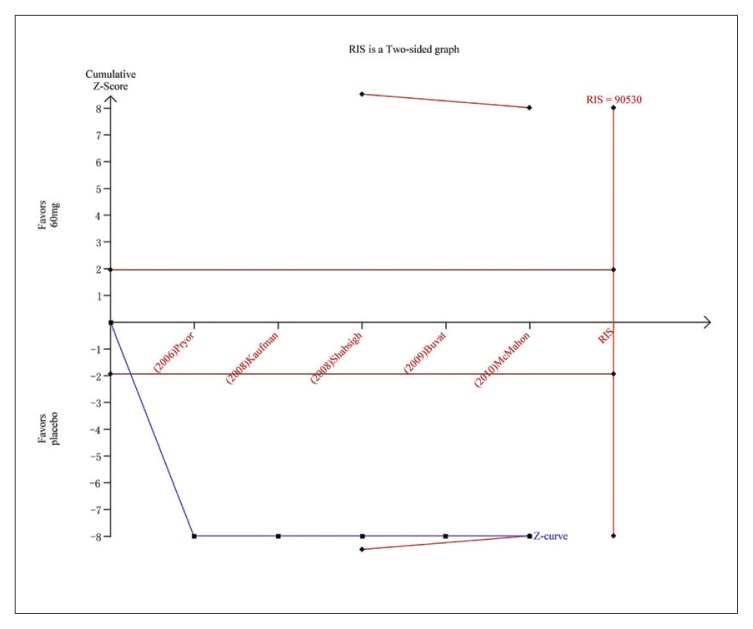
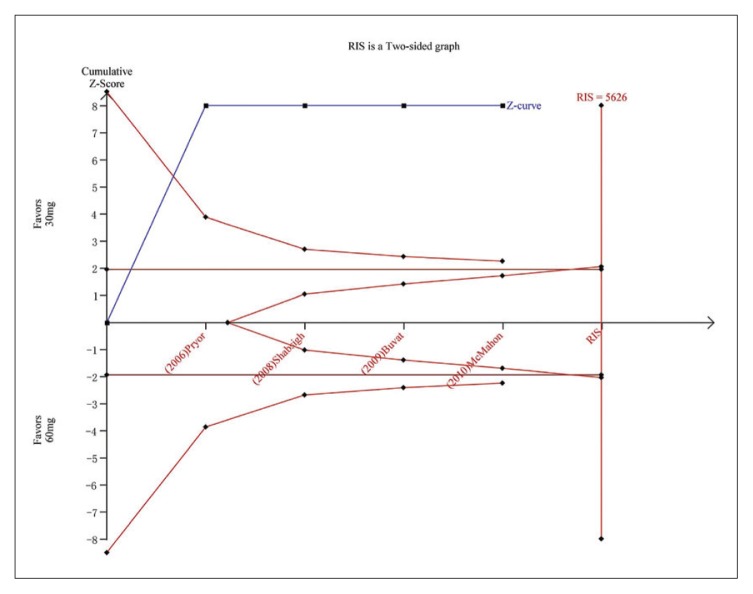
Five studies7,22,23,25,26 were included in the analysis of AE incidence for dapoxetine 30 mg versus placebo and five studies7,22,23,26,28 for dapoxetine 60 mg versus placebo. The pooled analysis suggested an RR of 2.36 (95% CI 1.76–3.16; P<.00001) (Figure 7A) in favor of the dapoxetine (30 mg and 60 mg) groups. The subgroup analysis showed that the AE incidence of dapoxetine (for both 30 mg and 60 mg groups) was significantly higher than the placebo group (RR: 2.13, 95% CI: 1.50–3.02, P<.00001; and RR: 2.52, 95%CI: 1.58–4.03, P<.00001, respectively). In addition, an analysis of four studies7,22,26,27 comparing dapoxetine 30 mg with dapoxetine 60 mg for AEs indicated that the AE incidence in dapoxetine 60 mg group was significantly higher than dapoxetine 30 mg group (RR: 0.65, 95% CI: 0.54–0.79; P<.00001) (Figure 7B). For the above three groups, sensitivity analysis showed that the results were stable. TSA on AEs in above three groups showed that the cumulative Z-curve crossed both the conventional boundary and the trial sequential monitoring boundary, establishing sufficient and conclusive evidence (Figure 5F, 5G, 5H).
Figure 7A.
Estimates for the risk ratio for AEs for dapoxetine (30 mg and 60 mg subgroup) and placebo.
Figure 7B.
Estimates for the risk ratio for AEs for dapoxetine 30 mg group and 60 mg group.
Figure 5F.
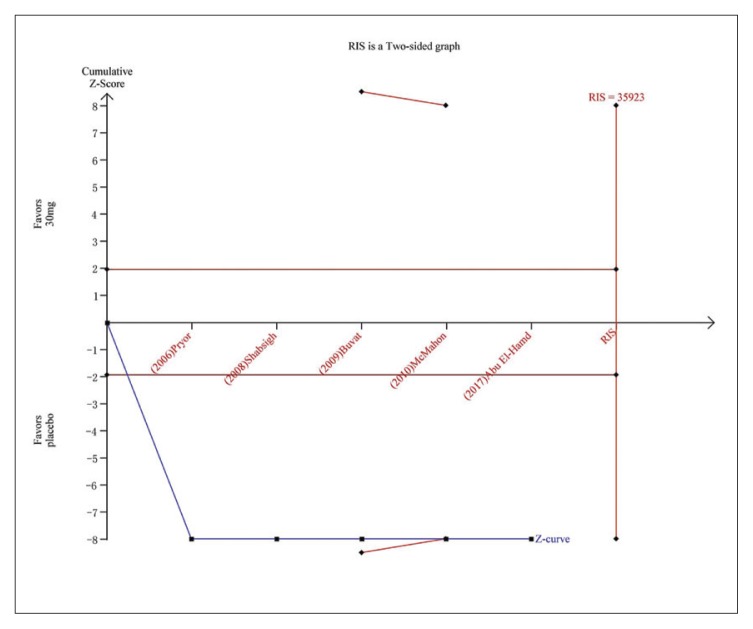
Trial sequential analysis for AEs for dapoxetine 30 mg versus placebo. The Z curve and boundaries are as in Figure 4. The required information size was calculated as 35923. The Z curve has crossed the conventional boundary for harm and trial sequential monitoring boundary for harm, indicating sufficient power to draw a definitive conclusion and no more trials are needed.
Figure 5G.
Trial sequential analysis for AEs for dapoxetine 60 mg versus placebo. The Z curve and boundaries are as in Figure 4. The Z curve and boundaries are as in Figure 4. The required information size was calculated as 90530. The Z curve has crossed the conventional boundary for harm and trial sequential monitoring boundary for harm, indicating sufficient power to draw a definitive conclusion and no more trials are needed.
Figure 5H.
Trial sequential analysis for AEs for dapoxetine 30 mg versus dapoxetine 60 mg. The Z curve and boundaries are as in Figure 4. The two red lines that intersect the horizontal coordinate line represent the futility boundary. The required information size was calculated as 5626. The Z curve has crossed the conventional boundary for benefit and trial sequential monitoring boundary for benefit, indicating sufficient power to draw a definitive conclusion and no more trials are needed.
Publication bias
Because of the small number of studies in this review, approaches for assessing publication bias are not validated and may not truly reflect publication bias. Thus, assessment of publication bias was not performed.29,30
DISCUSSION
Our meta-analysis found that dapoxetine significantly improved the conditions of PE compared to placebo and this was further validated by TSA. Contrary to previous meta-analyses that included patients in different settings, we focused on patients treated with dapoxetine on-demand and patients evaluated by the PGIC in PE with the level of at least “better”. Such measures contribute to decreased heterogeneity and may result in a more reliable result. At the start of our meta-analysis, a precise search strategy, quality assessment criteria, data abstraction and analysis were clearly defined. Therefore, studies of patients with concomitant erectile dysfunction,31 studies including patients receiving dapoxetine daily32,33 and studies with repeated data were excluded.34 No articles from the gray literature were found after an extensive search strategy, while some valuable unpublished information maybe still exist. Ultimately, eight RCTs were identified for inclusion in the present meta-analysis.
An increase in IELT is the main goal of PE therapy. The present meta-analysis suggested that IELT significantly increased with dapoxetine (30 mg and 60 mg) versus placebo. In addition, the outcome of the meta-analysis comparing dapoxetine 60 mg with 30 mg on-demand orally showed that there was a significant increase in IELT. Thus, 60-mg dapoxetine increased IELT more obviously than 30 mg on-demand for PE. Moreover, dapoxetine (30 mg and 60 mg) significantly improved PGIC compared with placebo; the 60-mg group had an advantage over the 30-mg group in PGIC. The outcomes in our meta-analysis, including IELT and PGIC evaluations, showed significant efficacy in improving PE. However, even if each subgroup was characterized by low heterogeneity, this result should be interpreted with caution because of the diversity in population in terms of the demographic characteristics, geographic regions, races, and baseline IELT.
Our analysis indicated that the incidence of AEs was more frequent with dapoxetine than with placebo. 4 The most frequently reported AEs were nausea, dizziness, diarrhea, insomnia, and headache. However, dapoxetine was well tolerated with a low incidence of AEs. Furthermore, most AEs occurred with the first dose,31–37 and all of these AEs occurred more frequently with dapoxetine 60 mg than 30 mg. Thus, the recommended starting dose is 30 mg. The dose may be escalated to 60 mg if the treatment effect is insufficient and the 30-mg dose is well tolerated. During this process, although some AEs were uncommon, cases of orthostatic hypotension and syncope (including loss of consciousness) were also reported in the dapoxetine arm, especially within 3 hours of the first dose. Thus, special attention should be given to related AEs.31,34,38
To increase the robustness of the present meta-analysis, we performed TSA to control the risk of random error due to repetitive testing. To our knowledge, this is the first study to assess the efficacy and safety of dapoxetine with the TSA method. TSA shows that the evidence of this meta-analysis was sufficient to confirm our conclusions about IELT, PGIC and AEs. Therefore, no further studies are needed. However, some limitations existed in the present meta-analysis. Though conference articles and clinical trial information had been screened, unpublished manuscripts and data may exist. Incorrect classification of patients (lifelong vs acquired PE) and inconsistent criteria of the diagnosis of PE both can lead to bias. In addition, some studies were sponsored by industry.7,22,25–27 Whether the studies used intention-to-treat analysis or not may impact the result of the included studies, which may lead to differences in outcomes. Thus, the risk of potential heterogeneity was inevitable although subgroup analysis and sensitivity analysis were conducted to minimize these effects.
In conclusion, the present meta-analyses supports the notion that dapoxetine (30 mg or 60 mg) yielded favorable results in IELT and PGIC compared with placebo. Moreover, 60 mg had an advantage over 30-mg dapoxetine. Dapoxetine was well tolerated, and generally safe. Use of TSA allows us to make firm conclusions for the IELT, PGIC and AEs.
Footnotes
Funding: None.
CONFLICT OF INTEREST: No relationship with manufacturer of drug.
REFERENCES
- 1.Bai Y, Pu C, Han P, Li J, Yuan H, Tang Y[7], et al. Selective serotonin reuptake inhibitors plus phosphodiesterase 5 inhibitors for premature ejaculation: a systematic review and meta-analysis. Urology. 2015 Oct;86(4):758–64. doi: 10.1016/j.urology.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Althof SE, McMahon CG, Waldinger MD, Serefoglu EC, Shindel AW, Adaikan PG, et al. An update of the International Society of Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation (PE) J Sex Med. 2014 Jun;11(6):1392–422. doi: 10.1111/jsm.12504. [DOI] [PubMed] [Google Scholar]
- 3.Symonds T, Roblin D, Hart K, Althof S. How does premature ejaculation impact a man’s life? J Sex Marital Ther. 2003 Oct-Dec;29(5):361–70. doi: 10.1080/00926230390224738. [DOI] [PubMed] [Google Scholar]
- 4.De Hong C, Ren LL, Yu H, Qiang W. The role of dapoxetine hydrochloride on-demand for the treatment of men with premature ejaculation. Sci Rep. 2014 Dec 1;4:7269. doi: 10.1038/srep07269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Althof SE, Abdo CH, Dean J, Hackett G, McCabe M, McMahon CG, et al. International Society for Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation. J Sex Med. 2010 Sep;7(9):2947–69. doi: 10.1111/j.1743-6109.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- 6.Mondaini N, Fusco F, Cai T, Benemei S, Mirone V, Bartoletti R. Dapoxetine treatment in patients with lifelong premature ejaculation: the reasons of a “Waterloo”. Urology. 2013 Sep;82(3):620–4. doi: 10.1016/j.urology.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 7.McMahon C, Kim SW, Park NC, Chang CP, Rivas D, Tesfaye F, et al. Treatment of premature ejaculation in the Asia-Pacific region: results from a phase III double-blind, parallel-group study of dapoxetine. J Sex Med. 2010 Jan;7(1 Pt 1):256–68. doi: 10.1111/j.1743-6109.2009.01560.x. [DOI] [PubMed] [Google Scholar]
- 8.Yue FG, Dong L, Hu TT, Qu XY. Efficacy of Dapoxetine for the Treatment of Premature Ejaculation: A Meta-analysis of Randomized Clinical Trials on Intravaginal Ejaculatory Latency Time, Patient-reported Outcomes, and Adverse Events. Urology. 2015 Apr;85(4):856–61. doi: 10.1016/j.urology.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Yuan H, Bai Y, Pu C, Tang Y, Dong Q, et al. Dapoxetine for Premature Ejaculation: An Updated Meta-Analysis of Randomized Controlled Trials. Clin Ther. 2014 Dec 1;36(12):2003–14. doi: 10.1016/j.clinthera.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Castiglione F, Albersen M, Hedlund P, Gratzke C, Salonia A, Giuliano F. Current Pharmacological Management of Premature Ejaculation: A Systematic Review and Meta-analysis. Eur Urol. 2016 May;69(5):904–16. doi: 10.1016/j.eururo.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Russo A, Capogrosso P, Ventimiglia E, La Croce G, Boeri L, Montorsi F, et al. Efficacy and safety of dapoxetine in treatment of premature ejaculation: an evidence-based review. Int J Clin Pract. 2016 Sep;70(9):723–33. doi: 10.1111/ijcp.12843. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.CHH JPT, GS Cochrane handbook for systematic reviews of interventions version 5.1.0. updated March 2011. The Cochrane Collaboration. 2011. www.cochrane-handbook.org.
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borm GF, Donders AR. Updating meta-analyses leads to larger type I errors than publication bias. J Clin Epidemiol. 2009 Aug;62(8):825–830. doi: 10.1016/j.jclinepi.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis-a simulation study. PLoS One. 2011;6(10):e25491. doi: 10.1371/journal.pone.0025491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017 Mar 6;17(1):39. doi: 10.1186/s12874-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imberger G, Thorlund K, Gluud C, Wetterslev J. False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis:an empirical review. BMJ Open. 2016 Aug 12;6(8):e011890. doi: 10.1136/bmjopen-2016-011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. 2009 Feb;38(1):276–86. doi: 10.1093/ije/dyn179. [DOI] [PubMed] [Google Scholar]
- 20.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive-Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009 Feb;38(1):287–98. doi: 10.1093/ije/dyn188. [DOI] [PubMed] [Google Scholar]
- 21.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008 Jan;61(1):64–75. doi: 10.1016/j.jclinepi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Shabsigh R, Patrick DL, Rowland DL, Bull SA, Tesfaye F, Rothman M. Perceived control over ejaculation is central to treatment benefit in men with premature ejaculation: results from phase III trials with dapoxetine. BJU Int. 2008 Sep;102(7):824–8. doi: 10.1111/j.1464-410X.2008.07845.x. [DOI] [PubMed] [Google Scholar]
- 23.Pastore AL1, Palleschi G, Leto A, Pacini L, Iori F, Leonardo C, et al. A prospective randomized study to compare pelvic floor rehabilitation and dapoxetine for treatment of lifelong premature ejaculation. Int J Androl. 2012 Aug;35(4):528–33. doi: 10.1111/j.1365-2605.2011.01243.x. [DOI] [PubMed] [Google Scholar]
- 24.Song X, Tang W, Wang DL, Pu J. A randomized controlled clinical study on treatment of primary premature ejaculation with dapoxetine. Zhong Guo Xin Yao Yu Lin Chuang Za Zhi[China] 2016;35(4):268–72. [Google Scholar]
- 25.Abu El-Hamd M, Abdelhamed A. Comparison of the clinical efficacy and safety of the on-demand use of paroxetine, dapoxetine, sildenafil and combined dapoxetine with sildenafil in treatment of patients with premature ejaculation: A randomised placebo-controlled clinical trial. Andrologia. 2017 May 12;00:e12829. doi: 10.1111/and.12829. [DOI] [PubMed] [Google Scholar]
- 26.Buvat J, Tesfaye F, Rothman M, Rivas DA, Giuliano F. Dapoxetine for the treatment of premature ejaculation: Results from a randomized, double-blind, placebo-controlled phase 3 trial in 22 countries. Eur Urol. 2009 Apr;55(4):957–67. doi: 10.1016/j.eururo.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Pryor JL, Althof SE, Steidle C, Rosen RC, Hellstrom WJG, Shabsigh R. Efficacy and tolerability of dapoxetine in the treatment of premature ejaculation: Integrated analysis of two randomized,double-blind, placebo-controlled trials. Lancet. 2006;368(9539):929–37. doi: 10.1016/S0140-6736(06)69373-2. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman JM, Rosen RC, Mudumbi RV, Tesfaye F, Hashmonay R, Rivas D. Treatment benefit of dapoxetine for premature ejaculation: Results from a placebo-controlled phase III trial. BJU Int. 2009 Mar;103(5):651–8. doi: 10.1111/j.1464-410X.2008.08165.x. [DOI] [PubMed] [Google Scholar]
- 29.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006 Sep 16;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Schulz KF, Altman DG CONSORT. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. Med Res Methodol. 2001;1:2. doi: 10.1186/1471-2288-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon CG, Giuliano F, Dean J, Hellstrom WJ, Bull S, Tesfaye F, et al. Efficacy and Safety of Dapoxetine in Men with Premature Ejaculation and Concomitant Erectile Dysfunction Treated with a Phosphodiesterase Type 5 Inhibitor: Randomized, Placebo-Controlled, Phase III Study. J Sex Med. 2013 Sep;10(9):2312–25. doi: 10.1111/jsm.12236. [DOI] [PubMed] [Google Scholar]
- 32.Safarinejad MR. Comparison of Dapoxetine Versus Paroxetine in Patients With Premature Ejaculation: A Double-blind, Placebo controlled, Fixed-dose, Randomized Study. Clin Neuropharmacol. 2006 Sep-Oct;29(5):243–52. doi: 10.1097/01.WNF.0000228210.12194.46. [DOI] [PubMed] [Google Scholar]
- 33.Safarinejad MR. Safety and Efficacy of Dapoxetine in the Treatment of Premature Ejaculation: A Double-Blind, Placebo-Controlled, Fixed-Dose, Randomized Study. Neuropsychopharmacology. 2008 May;33(6):1259–65. doi: 10.1038/sj.npp.1301500. [DOI] [PubMed] [Google Scholar]
- 34.McMahon CG, Althof SE, Kaufman JM, Buvat J, Levine SB, Aquilina JW, et al. Efficacy and Safety of Dapoxetine for the Treatment of Premature Ejaculation: Integrated Analysis of Results from Five Phase 3 Trials. J Sex Med. 2011 Feb;8(2):524–39. doi: 10.1111/j.1743-6109.2010.02097.x. [DOI] [PubMed] [Google Scholar]
- 35.Mirone V, Arcaniolo D, Rivas D, Bull S, Aquilina JW, Verze P PAUSE study team. Results from a prospective observational study of men with premature ejaculation treated with dapoxetine or alternative care: the pause study. Eur Urol. 2014 Apr;65(4):733–9. doi: 10.1016/j.eururo.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 36.McCarty E, Dinsmore W. Dapoxetine: an evidence-based review of its effectiveness in treatment of premature ejaculation. Core Evid. 2012;7:1–14. doi: 10.2147/CE.S13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee WK1, Lee SH, Cho ST, Lee YS, Oh CY, Yoo C, et al. Comparison between ondemand dosing of dapoxetine alone and dapoxetine plus mirodenafil in patients with lifelong premature ejaculation: Prospective, randomized, double-blind, placebo-controlled, multicenter study. J Sex Med. 2013 Nov;10(11):2832–41. doi: 10.1111/jsm.12287. [DOI] [PubMed] [Google Scholar]
- 38.Verze P, Cai T, Magno C, Sabella F, Cucchiara V, Palmieri A, et al. Comparison of Treatment Emergent Adverse Events in Men With Premature Ejaculation Treated With Dapoxetine and Alternate Oral Treatments: Results From a Large Multinational Observational Trial. J Sex Med. 2016 Feb;13(2):194–99. doi: 10.1016/j.jsxm.2015.12.017. [DOI] [PubMed] [Google Scholar]