Abstract
BACKGROUND
The clinical relevance of androgen receptors (ARs) expressed in breast cancer cells and the suggested prognostic impact has been an area of active research. The prevalence rate of AR expression in breast cancer has never been reported among Jordanian patients.
OBJECTIVE
Determine the expression rate of ARs among invasive ductal breast cancer cases of different stages and molecular subtypes. Also, analyze the relationship between AR expression and clinicopathologic and immunohistochemical criteria, and assess the impact of AR expression on survival.
DESIGN
Retrospective medical record review.
SETTING
Tertiary care hospital in Amman, Jordan.
PATIENTS AND METHODS
Our study comprised only of cases of invasive ductal breast carcinoma of no special type among females from records during a 10-year period between 2006 and 2015. Immunohistochemical staining was considered positive if more than 10% of tumor nuclei showed positive staining.
MAIN OUTCOME MEASURES
The expression rate of ARs and the association of the expression rate with the clinicopathologic features of invasive breast cancer.
SAMPLE SIZE
293.
RESULTS
Immunohistochemical staining for AR revealed positive staining in 180 (61.4%) cases, including approximately 50% of triple-negative breast cancer cases. AR positivity correlated with estrogen receptor (ER) status (P=.007) and smaller T size (P=.014). However, no significant association was found with any of the other variables. AR expression was positively associated with overall survival (P=.022) in general and in ER-positive cases (P=.012). However, in the multivariate Cox regression model, AR was not independently associated with survival.
CONCLUSIONS
These results were consistent with international reports showing a significant relationship of AR expression with ER status. In addition, AR expression was significantly associated with smaller tumor size. Although AR status was not independently associated with survival, our data suggest AR is a good prognostic factor.
LIMITATIONS
Some clinical data were missing.
Breast cancer is the most common malignancy worldwide and the leading cause of cancer-related deaths in women.1 In Jordan, it is the most common malignancy afflicting women, accounting for 37% of newly diagnosed cancer cases, and is the most common cause of cancer-related deaths per the most recent report provided by the Jordanian Ministry of Health.2 In a recently published study of 752 patients with breast cancer, 74.3% of breast cancer cases were classified as invasive ductal carcinoma of no special type (IDC-NST), constituting the most common type of breast cancer in Jordan.3 Most of these tumors were moderately differentiated. Approximately one-third were localized at time of diagnosis, whereas 40% were regionally disseminated, leading to low survival rates among patients.4
The etiology of breast cancer involves a combination of genetic, environmental, and hormonal factors that collectively alter normal breast tissue, resulting in neoplastic transformation.5 Hormonal factors act through complex downstream signaling molecules transmitted through sex steroid receptors and growth factors. The expression of estrogen receptor (ER), progesterone receptor (PR), and the epidermal growth factor receptor-2 (HER-2) in breast cancer has been extensively studied. Targeted therapy against these receptors is now a validated modality of treatment in conjunction with surgery, radiotherapy, and chemotherapy.6,7
Another receptor of the nuclear steroid hormone family is the androgen receptor (AR). It functions as an intracellular transcription factor.8 Binding of specific ligands to the receptor induces conformational changes, dimerization, and subsequent receptor translocation into the nucleus. The dimer then binds to its hormone receptor elements (HRE) within the DNA, resulting in a series of regulatory transcriptional events that function differently in respect to tissue type.9,10
Recently, the clinical relevance of this receptor being expressed in breast cancer cells, its role in neoplastic transformation, and suggested prognostic impact has been an area of active research. Many studies reported AR expression in 60% to 80% of breast cancer cases but lower and higher percentages have been described, and these differences can be explained by demographic variations as well as the use of different cutoff points for AR expression intensity.11–15 Many authors have reported significant associations between AR expression by cancer cells and improved overall survival and disease-free survival.16–21 In addition, targeted therapy blocking AR receptors is being developed, and researchers are trying to better understand the molecular signaling pathways by which androgens alter the proliferation of cancer cells. It may be the only endocrine therapy available to a subset of patients with the aggressive triple negative tumors that lack the expression of ER, PR, and HER-2/neu, and hence, are unable to respond to targeted therapy.22 Several clinical trials have also illustrated the activity of anti-androgen therapy for the treatment of AR-positive triple negative breast cancers (TNBC).23,24
The prevalence rate of AR expression in breast cancer has never been reported among Jordanian patients. Our study aimed to evaluate the prevalence rate of AR expression in breast cancer cases diagnosed at Jordan University hospital over a 10-year period. We also studied the correlation of AR expression with other clinicopathologic parameters, such as the menopausal status, tumor grade, ER, PR, HER-2/neu expression, T stage, and the presence of distant metastases, in addition to the impact of AR expression on patient survival.
PATIENTS AND METHODS
After the Institutional Review Board approval was obtained at Jordan University Hospital, hematoxylin- and eosin-stained tumor slides, paraffin blocks, and their corresponding ER, PR, and HER-2/neu for all documented cases of IDC-NST of the breast were retrieved from the archives of the Histopathology Department at Jordan University Hospital (JUH). These cases were diagnosed during the 10-year period between 2006 and 2015 and comprised modified radical mastectomy specimens from patients with no previous history or prior neoadjuvant chemotherapy. Clinical information of all cases, including menopausal status and the presence or absence of lymph node involvement or distant metastases, was gathered from patient files.
Morphologic evaluation
One 4-μm-thick section from each submitted paraffin-embedded tissue block was stained with hematoxylin and eosin to verify the presence of IDC and the adequacy of fixation. Cases were classified based on the 2012 WHO classification of breast tumors25 and staged according to the updated 7th edition of the American Joint Committee on Cancer (AJCC).26 The modified Bloom-Richardson-Elston (BRE) grading system was used and categorized the cases into low, intermediate, and high grades.
Immunohistochemistry
Immunohistochemical (IHC) staining of AR was performed on 4-μm-thick sections that were added to poly- L-lysine-coated slides that underwent conventional deparaffinization (in incubator for 30 minutes at 70°C), followed by hydration. Antigen retrieval (20 min; 10 mmol/EDTA buffer, pH 8.0) was done in a microwave, followed by inhibition of endogenous peroxidase activity (hydrogen peroxidase for 5 min). Protein blocker (normal serum) was applied for 5 to 10 minutes, then tapped off, and the excess was wiped away without rinsing. Immunostaining was performed using mouse anti-AR primary antibody (clone AR441) at a 1:50 dilution. The antibody was purchased from Genova Diagnostics (Asheville, NC, USA) and diluted (1:50). This was followed by incubation with a secondary antibody (Super Enhancer) for 20 to 30 minutes. Then, a tertiary antibody (HRP-Polymer) was applied for another 20 to 30 minutes. Addition of chromogen substrate (Leica Biosystems RE 7105, RE 7143) was performed manually by an experienced technician. With each staining run, normal prostatic tissue was used as a positive control. Nuclear staining of AR was considered positive when more than 10% of tumor cell nuclei were stained.
Statistical analysis
Categorical and numerical data were statistically analyzed using IBM SPSS, version 17.0. The Kaplan-Meier method was used to analyze survival. Statistical significance was established when P value was equal to or less than .05.
RESULTS
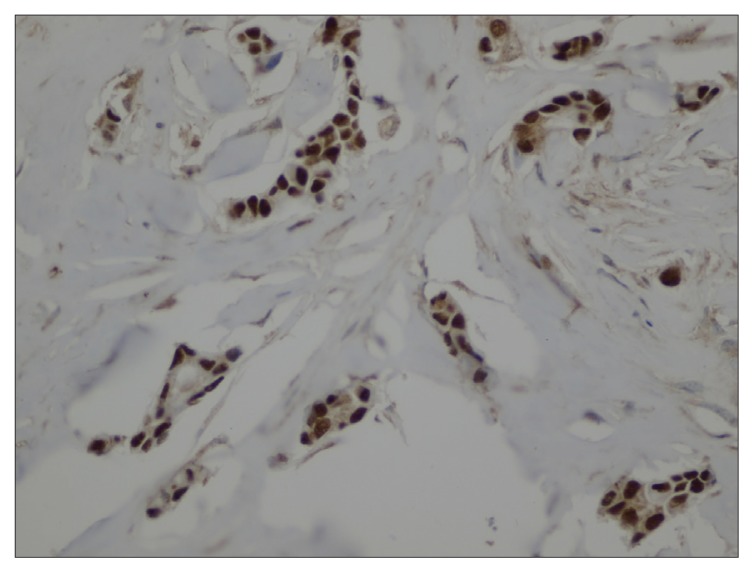
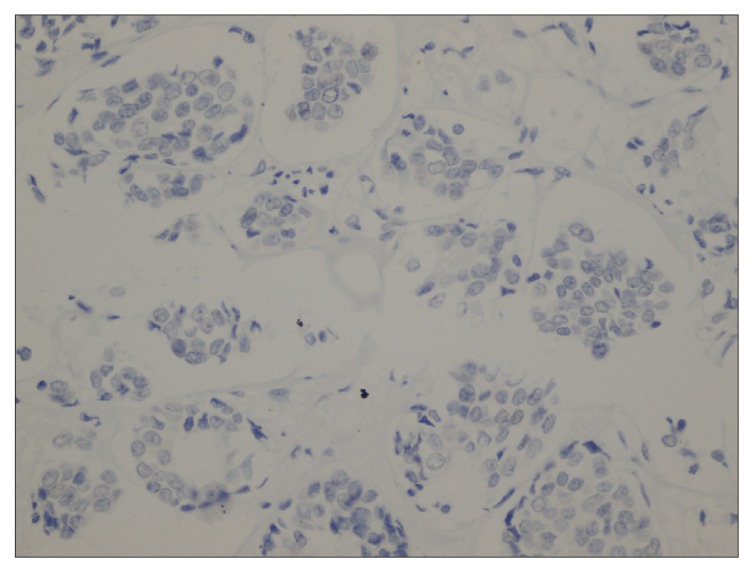
We identified 293 cases of IDC-NST that ranged between 22 and 79 years of age, with a mean age of 50 years, and almost half were premenopausal (Table 1). In addition, approximately 50% of the tumors were of T2 size, relative to almost 27% with T1, 12% with T3, and 7% with T4. Positive lymph node involvement was indicated for 64.5% of cases, and 15.7% were metastatic. As for receptor biomarkers, staining for ER, PR and HER-2/neu was 83.4%, 75.8%, and 22.5% of cases, respectively. Some data was missing and excluded from the analysis (Table 1). Positive staining for AR was detected in 61.4% of cases. AR expression was significantly associated with ER status (P=.007), in which 65.3% of AR-positive cases were also ER-positive relative to 44.7% of cases. In addition, AR-positive cases tended to be of significantly smaller tumor size. For example, 70.8% of T1 cases were AR-positive, whereas 67.7% of T4 cases were AR-negative (P=.014. Interestingly, AR expression was higher in tumors without lymph node involvement and in non-metastatic tumors, although this did not reach statistical significance. Figures 1A and 1B show cases of positive and negative nuclear staining for AR.
Table 1.
Association of AR expression in breast cancer cases with clinicopathological and immunohistochemical features (n=293).a
| Variable | No. of cases (%) | AR expression | P value | ||
|---|---|---|---|---|---|
| Positive n (%) | Negative n (%) | ||||
| 293 (100%) | 180 (61.4%) | 113 (38.6%) | |||
|
| |||||
| Age (years) | <40 | 32 (10.9%) | 21 (65.6%) | 11 (34.4%) | .875 |
| 40–55 | 125 (42.7%) | 76 (60.8%) | 49 (39.2%) | ||
| >55 | 136 (46.4%) | 83 (61.0%) | 53 (39.0%) | ||
| Menopausal status | Pre | 149 (50.9%) | 90 (60.4%) | 59 (39.6%) | .402 |
| Post | 144 (49.1%) | 90 (62.5%) | 54 (37.5%) | ||
| Primary tumor size (T) (n=265, 90.4%) | T1 | 72 (27.2%) | 51 (70.8%) | 21 (29.2%) | .014 |
| T2 | 144 (54.3%) | 87 (60.4%) | 57 (39.6%) | ||
| T3 | 31 (11.7%) | 15 (48.4%) | 16 (51.6%) | ||
| T4 | 18 (6.8%) | 6 (33.3%) | 12 (66.7%) | ||
| Lymph node involvement (n=256, 87.4%) | Negative | 91 (35.5%) | 59 (64.8%) | 32 (35.2%) | .158 |
| Positive | 165 (64.5%) | 95 (57.6%) | 70 (42.4%) | ||
| Metastasis (n=153, 52.2%) | Negative | 129 (84.3%) | 77 (59.7%) | 52 (40.3%) | .150 |
| Positive | 24 (15.7%) | 11 (45.8%) | 13 (54.2%) | ||
| TNM stage group (n=136, 46.4%) | I | 18 (13.2%) | 14 (77.8%) | 4 (22.2%) | .074 |
| II | 56 (41.2%) | 29 (51.8%) | 27 (48.2%) | ||
| III | 38 (27.9%) | 26 (68.4%) | 12 (31.6%) | ||
| IV | 24 (17.7%) | 11 (45.8%) | 13 (54.2%) | ||
| Histological grade (n=285, 97.3%) | Grade 1 | 35 (12.3%) | 23 (65.7%) | 12 (34.3%) | .809 |
| Grade 2 | 153 (53.7%) | 92 (60.1%) | 61 (39.9%) | ||
| Grade 3 | 97 (34.0%) | 58 (59.8%) | 39 (40.2%) | ||
| Lymphovascular invasion (n=282, 69.3%) | Positive | 121 (42.9%) | 71 (58.7%) | 50 (41.3%) | .425 |
| Negative | 161 (57.1%) | 102 (36.4%) | 59 (63.6%) | ||
| ER status (n=283, 96.6%) | Positive | 236 (83.4%) | 154 (65.3%) | 82 (34.7%) | .007 |
| Negative | 47 (16.6%) | 21 (44.7%) | 26 (55.3%) | ||
| PR status (n=273, 93.2%) | Positive | 208 (76.2%) | 131 (63.0%) | 77 (37.0%) | .232 |
| Negative | 65 (23.8%) | 37 (56.9%) | 28 (43.1%) | ||
| HER2/neu status (n=275, 93.9%) | Positive | 62 (22.5%) | 38 (61.3%) | 24 (38.7%) | .968 |
| Equivocal | 13 (4.7%) | 8 (61.5%) | 5 (38.5%) | ||
| Negative | 200 (72.7%) | 126 (63.0%) | 74 (37.0%) | ||
| TNBC (n=281, 95.3%) | Negative | 250 (89%) | 159 (63.6%) | 91 (34.4%) | .075 |
| Positive | 31 (11%) | 15 (48.4%) | 16 (51.6%) | ||
Some information was not available for certain criteria and, hence percentages are calculated per cases with available data and missing data were excluded from analysis occurred at random and did not bias the results. Statistical analyses by Pearson’s chi-squared test.
Figure 1A.
Case of IDC-NST with positive nuclear staining for AR (400×).
Figure 1B.
Case of IDC-NST with negative nuclear staining for AR (400×).
In addition, there was no significant association between the expression of AR with other clinicopathological features of the tumors, including menopausal status (P=.402) and histologic grade (P=.809) (Table 1). In addition, positive AR expression was not associated with expression of either PR (P=.232) or HER-2/neu overexpression (P=.968). Approximately 48% of triple-negative breast cancer cases were AR-positive with a lack of association with AR status (P=.075).
The data were analyzed further to correlate AR expression with clinicopathological and immunohistochemical features of breast cancer cases according to ER status. As shown in Table 2, AR expression was significantly associated only with smaller tumor size (P=.043) in patients with ER-positive but not ER-negative tumors. ER expression with clinicopathological and and immunohistochemical factors such as age, menopausal status, TNM stage, PR and HER2/neu status were also correlated (Table 3). Only in tumors lacking the expression of AR was the expression of ER associated with tumors of lower histological grade (P=.031) and in menopausal patients (P=.027). Regardless of the AR expression status, ER was significantly associated with expression of PR and absence of HER2/neu expression (P<.001 for each).
Table 2.
Association of AR expression cases with clinicopathological and immunohistochemical features of breast cancer cases according to ER expression status.
| ER-positive tumors | ER-negative tumors | |||||
|---|---|---|---|---|---|---|
| AR-positive | AR-negative | P value | AR-positive | AR-negative | P value | |
|
| ||||||
| Age (years) | ||||||
| <40 | 17 (70.8) | 7 (29.2) | .665 | 3 (60) | 2 (40) | .692 |
| 40–55 | 65 (67.0) | 32 (33.0) | 10 (40) | 15 (60) | ||
| >55 | 72 (62.6) | 43 (37.4) | 8 (47.1) | 9 (52.9) | ||
| Menopausal status | ||||||
| Pre | 75 (67.0) | 36 (37.9) | .350 | 13 (41.9) | 18 (58.1) | .413 |
| Post | 79 (63.7) | 45 (36.3) | 8 (50.0) | 8 (50.0) | ||
| Primary tumor size (T) | ||||||
| T1 | 45 (76.3) | 14 (23.7) | 4 (40.0) | 6 (60.0) | ||
| T2 | 74 (61.7) | 46 (38.3) | .043 | 12 (52.2) | 11 (47.8) | .364 |
| T3 | 12 (52.2) | 11 (47.8) | 3 (37.5) | 5 (62.5) | ||
| T4 | 6 (42.9) | 8 (57.1) | 0 (0.0) | 3 (100) | ||
| Lymph node involvement | ||||||
| Negative | 53 (70.7) | 22 (29.3) | .096 | 5 (35.7) | 9 (64.3) | .409 |
| Positive | 80 (60.6) | 52 (39.4) | 13 (44.8) | 16 (55.2) | ||
| Metastasis | ||||||
| Negative | 71 (62.8) | 42 (37.2) | .100 | 6 (40.0) | 9 (60.0) | .669 |
| Positive | 10 (45.5) | 12 (54.5) | 1 (50.0) | 1 (50.0) | ||
| TNM stage group | ||||||
| I | 14 (87.5) | 2 (12.5) | 0 (0.0) | 2 (100) | ||
| II | 27 (54.0) | 23 (46.0) | .035 | 2 (33.3) | 4 (66.7) | .327 |
| III | 23 (67.6) | 11 (32.4) | 3 (75.0) | 1 (25.0) | ||
| IV | 10 (45.4) | 12 (54.6) | 1 (50) | 1 (50) | ||
| Histological grade | ||||||
| Grade 1 | 21 (70.0) | 9 (30.0) | .540 | 2 (50.0) | 2 (50.0) | .242 |
| Grade 2 | 77 (61.1) | 49 (38.9) | 12 (57.1) | 9 (42.9) | ||
| Grade 3 | 49 (67.1) | 24 (32.9) | 7 (31.8) | 15 (68.2) | ||
| Lymphovascular invasion | ||||||
| Positive | 59 (62.1) | 37 (37.4) | .398 | 9 (40.9) | 13 (59.1) | .999 |
| Negative | 92 (68.1) | 43 (31.9) | 9 (40.9) | 13 (59.1) | ||
| PR status | ||||||
| Positive | 128 (64.0) | 72 (36.0) | .295 | 3 (37.5) | 5 (62.5) | .999 |
| Negative | 21 (75.0) | 7 (25.0) | 16 (43.2) | 21 (56.8) | ||
| HER2/neu status | ||||||
| Positive | 23 (79.3) | 6 (20.7) | .103 | 15 (46.9) | 17 (53.1) | .500 |
| Equivocal | 7 (87.5) | 1 (12.5) | 1 (20.0) | 4 (80.0) | ||
| Negative | 121 (63.4) | 70 (36.6) | 4 (50.0) | 4 (50.0) | ||
Data are number (percentage). Statistical analyses by Pearson’s chi-squared test.
Table 3.
Association of ER expression with clinicopathological and immunohistochemical features of breast cancer cases according to AR expression status.
| AR-positive tumors | AR-negative tumors | |||||
|---|---|---|---|---|---|---|
| ER-positive | ER-negative | P value | ER-positive | ER-negative | P value | |
|
| ||||||
| Age (years) | ||||||
| <40 | 17 (85.0) | 3 (15.0) | .741 | 7 (77.8) | 2 (22.2) | .235 |
| 40–55 | 65 (86.7) | 10 (13.3) | 32 (68.1) | 15 (31.9) | ||
| >55 | 72 (90) | 8 (10) | 43 (82.7) | 9 (17.3) | ||
| Menopausal status | ||||||
| Pre | 75 (85.2) | 13 (14.8) | .184 | 37 (67.3) | 18 (32.7) | .027 |
| Post | 79 (90.8) | 8 (9.2) | 45 (84.9) | 8 (15.1) | ||
| Primary tumor size (T) | ||||||
| T1 | 45 (91.8) | 4 (8.2) | 14 (70.0) | 6 (30.0) | ||
| T2 | 74 (86.0) | 12 (14.0) | .443 | 46 (80.7) | 11 (19.3) | .657 |
| T3 | 12 (80.0) | 3 (20.0) | 11 (68.8) | 5 (31.2) | ||
| T4 | 6 (100) | 0 (0) | 8 (72.7) | 3 (2.9) | ||
| Lymph node involvement | ||||||
| Negative | 53 (91.4) | 5 (8.6) | .235 | 22 (71.0) | 9 (29.0) | .364 |
| Positive | 80 (86.0) | 13 (14.0) | 52 (76.5) | 16 (23.5) | ||
| Metastasis | ||||||
| Negative | 71 (92.2) | 6 (7.8) | .621 | 42 (82.4) | 9 (17.6) | .345 |
| Positive | 10 (90.9) | 1 (9.1) | 12 (92.3) | 1 (7.7) | ||
| TNM stage group | ||||||
| I | 14 (100) | 0 (0.0) | 2 (50) | 2 (50) | ||
| II | 27 (93.1) | 2 (6.9) | .615 | 23 (85.2) | 4 (14.8) | .173 |
| III | 23 (88.5) | 3 (11.5) | 11 (91.7) | 1 (8.3) | ||
| IV | 10 (90.9) | 1 (9.1) | 12 (92.3) | 1 (7.7) | ||
| Histological grade | ||||||
| Grade 1 | 21 (91.3) | 2 (8.7) | .757 | 9 (81.8) | 2 (18.2) | .031 |
| Grade 2 | 77 (86.5) | 12 (13.5) | 49 (84.5) | 9 (15.5) | ||
| Grade 3 | 49 (87.5) | 7 (12.5) | 24 (61.5) | 15 (38.5) | ||
| Lymphovascular invasion | ||||||
| Positive | 59 (86.8) | 9 (13.2) | .448 | 36 (73.5) | 13 (26.5) | .821 |
| Negative | 92 (91.1) | 9 (8.9) | 43 (76.8) | 13 (23.2) | ||
| PR status | ||||||
| Positive | 128 (97.7) | 3 (2.3) | <.01 | 72 (93.5) | 5 (6.5) | <.01 |
| Negative | 21 (56.8) | 16 (43.2) | 7 (25.0) | 21 (75.0) | ||
| HER2/neu status | ||||||
| Positive | 23 (60.5) | 15 (39.5) | <.001 | 6 (26.1) | 17 (73.9) | <.001 |
| Equivocal | 7 (87.5) | 1 (12.5) | 1 (20.0) | 4 (80.0) | ||
| Negative | 121 (96.8) | 4 (3.2) | 70 (94.6) | 4 (5.4) | ||
Data are number (percentage). Statistical analyses by Pearson’s chi-squared test.
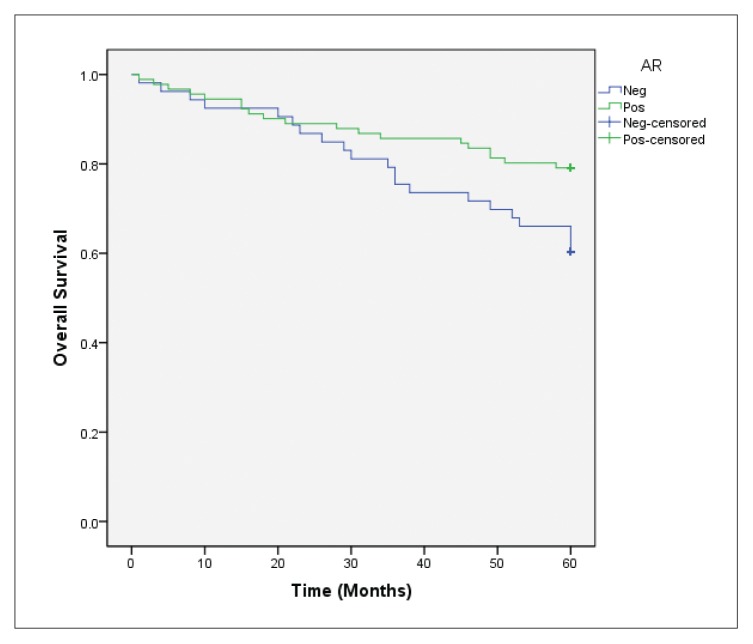
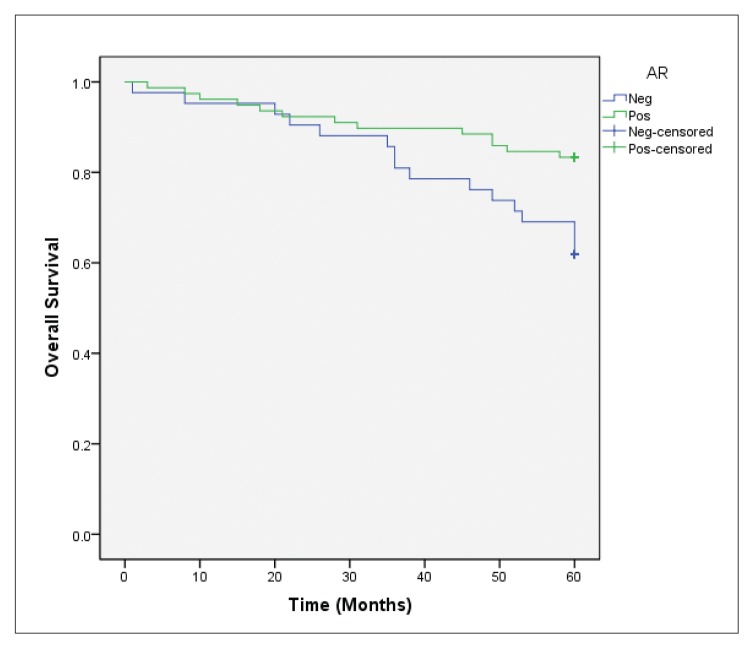
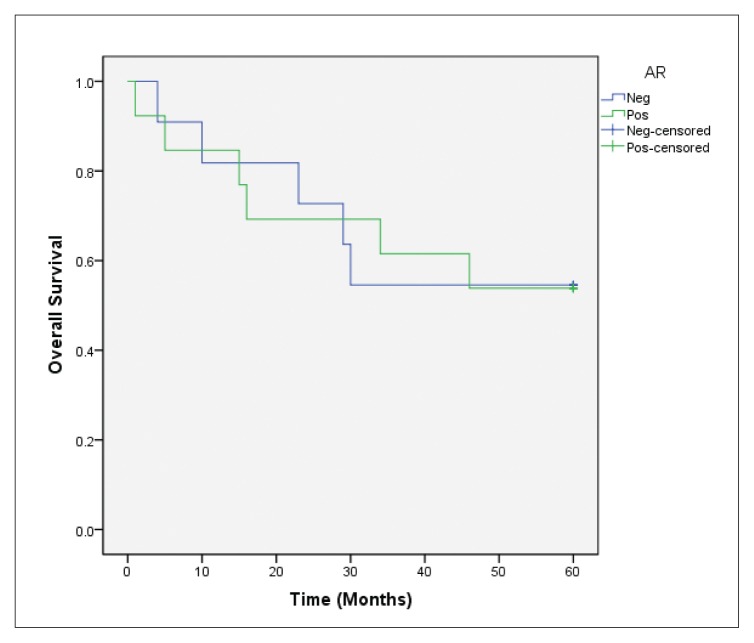
Forty deaths occurred among 144 patients who completed the 5-year follow-up. In the overall survival analysis, a significantly favorable prognosis (P=.022) was observed in AR-positive cases in comparison to AR-negative cases (Figure 2A). After stratifying these cases according to ER status, AR expression was also positively associated with overall survival (P=.012) in the ER-positive group (Figure 2B), but not in ER-negative tumor cases (P=.987) (Figure 2C). In the multivariate Cox regression models for overall survival, statistical significance of AR expression disappeared when adjusting for the clinicopathological parameters previously discussed (data not shown).
Figure 2A.
Overall survival according to AR expression in general (P=.022 for AR-positive vs AR-negative cases).
Figure 2B.
Overall survival according to AR expression in the ER-positive group (P=.012 for AR-positive vs AR-negative cases).
Figure 2C.
Overall survival according to AR expression in the ER-negative group (P=.987 for AR-positive vs AR-negative cases).
DISCUSSION
The prevalence of AR expression, its biological role, and prognostic significance in breast cancer are well established.16,27–30 For example, a significant association between AR expression with decreased recurrence rate among all breast cancer types and better overall survival in ER-positive tumors has been reported.17 Other studies have suggested considering AR expression as an independent prognostic factor from tumor size, stage, and grade.31–33 In a study analyzing 678 cases of breast cancer, 396 of which were of the non-basal triple negative subtype, AR expression was associated with better overall patient survival and was inversely associated with tumor grade.34 Thus, the determination of AR expression appears to be a promising approach to achieve better understanding of breast cancer. This study aimed to determine the prevalence rate of AR expression in breast cancer in association with immunohistochemical and clinicopathological criteria.
Studies have reported a prevalence rate ranging between 40% and 80%.17,19,35,36 In 2010, Castellano et al reported an expression rate of 70.9% among 859 ER-positive tumors.32 Another study by Agrawal et al demonstrated an AR expression rate in 43.7% of 96 cases of predominantly ER-positive invasive breast carcinomas. 19 In Korea, the percentage was close to 58% among 931 patients.26 Higher rates of expression reaching 80% were reported by two other studies.37,38 Our study demonstrates an expression rate of 61.4%, which is in agreement with other studies.
We also studied the association between AR receptor expression and other hormones. AR was expressed in 88% of ER-positive tumors in comparison to only 12% of ER-negative tumors. Many studies have reported that AR expression was higher in ER-positive tumors compared to ER-negative tumors.18,37–39,41 These included two studies from Egypt.39,41 AR was expressed among 95% of ER-positive tumors in comparison to only 10% of estrogen-negative tumors in another study.40 This positive relationship was also linked with a more favorable prognosis among ER-positive patients.11
We found no association between AR and PR or HER-2/neu expression status (P=.968 and P=.226, respectively). In contrast, AR expression correlated with PR positivity and HER-2/neu negativity in many studies. 18,31,41,42 The low sample number could be a factor in our findings.
We found that 48.4% of TNBCs expressed AR, which was higher than the 30% reported by Farag et al from Egypt.43 These tumors are generally more aggressive than their ER-positive counterparts, with higher rates of relapse in the early stages and decreased overall survival rates.44,45 International studies reported variable expression rates.46–48 A more recent study reported an expression rate of 24.8% among TNBCs, and an even lower expression rate in African American women.34
A significant relationship was found between AR expression and the histologic grades of tumors. Many studies have found that AR expression correlates with a lower overall tumor grade.26,36,37,41 Lower grade tumors tend to have better prognosis, and hence, the expression of AR can serve as a good prognostic indicator. We found no significant association between tumor grade and AR expression. However, AR was expressed more frequently in grade I tumors (65.7%) as compared to grade III tumors (59.8%).
No significant association was observed between menopausal status and AR expression in our study. In an Egyptian study, a significantly increased expression of AR in postmenopausal patients was found.41 Patients whose tumors expressed AR were more likely to have a longer disease-free survival than those with AR-negative tumors.49 Similar results were reported in another study in which researchers found a significant association between high AR expression and distant metastasis-free survival among 250 cases of invasive breast carcinoma.18 Although we found no significant association between AR expression and the presence of distant metastasis or lymph node metastasis, AR-positive tumors tended to be negative for both criteria. It is possible that inclusion of more samples could reveal a positive association.
A functional relationship has previously been reported between ER and AR whereby AR has an antagonistic effect to ER.33 In fact, the potential therapeutic effects of aromatase inhibitors may be due to both a reduction in estrogen levels and an increase in inhibitory AR signaling pathways.50 In support of the latter observation is a large retrospective study that demonstrated a prognostic and predictive role of AR in the subset of ER-positive tumors.32 However, in ER-negative tumors, the situation is different as there is no benefit of endocrine therapy, and tumors have a poor prognosis.51 The lack of ER expression in breast cancer cells switches the inhibitory effect of AR to that of an oncogenic role, at least in cell lines.52 Taken together, AR expression could be an additional significant factor for endocrine therapy for ER-positive cancers. For that reason, we analyzed the prognostic significance of AR according to ER expression status and vice versa. In ER-positive tumors, AR expression was significantly associated only with smaller tumor size as reported in a previous study.16 However, there was no significant correlation with other clinicopathologic parameters, such as lymph nodal involvement, distant metastases, tumor histologic grade, and menopausal status. On the other hand, in patients with ER-negative cancer, AR expression was not statistically related to any clinicopathologic parameter in parallel with the latter study.16
Regardless of the AR expression status, ER status is significantly associated with expression of PR and absence of HER2/neu expression. A study on the expression of ARs in primary breast cancer and the relationship of ER, AR, and HER2/neu expression showed that AR was expressed significantly in correlation with HER-2/ neu overexpression in ER-negative tumors, but not in ER-positive tumors.48
We showed an important implication of AR in prognosis. In univariate survival analyses, AR was generally a significant factor for overall survival outcome. This was also found in the ER-positive subgroup. However, the prognostic significance of AR disappeared in multivariate analyses. These findings were consistent with previous reports.16,32,40 One limitation of this study was that some patient files had missing data. In conclusion, AR expression appears to be a prognostic factor, as it is associated with lower tumor grade and well-differentiated histologic type. This observation is consistent with what has been reported previously. These findings substantiate previous findings that AR is a good prognostic indicator. The high prevalence of AR expression in breast cancer is consistent with studies that support the use of anti-androgen therapy as beneficial in increasing survival. More studies among Jordanian breast cancer patients are needed to assess the actual rate of AR expression, not only in a single histologic subtype, but in other subtypes as well.
Footnotes
Funding: Scientific Research Fund, Ministry of Higher Education and Scientific Research
CONFLICT OF INTEREST: None.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA CANCER J CLIN. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Melhem JM, Abbadi AA. Advanced Breast Cancer: Response and Responsibility. Jor Med J. 2010;41(4) [Google Scholar]
- 3.Obeidat F, Ahram M, Al Khader A, Battah K, Alchalabi M, Melhem JM, Suleiman A. Clinical and histopathological features of breast cancer in Jordan: Experience from a tertiary care hospital. J Pak Med Assoc. 2017;67:1206. [PubMed] [Google Scholar]
- 4.Sayaideh A, Nimri O, Arqoub K, Zaghal MA. Cancer incidence in Jordan 2012. Jordan: Non-Communicable Diseases Directorate, Jordan Cancer Registry. Ministry of Health; 2012. [Google Scholar]
- 5.Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas. 2001;38:103–13. doi: 10.1016/s0378-5122(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 6.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 7.Lumachi F, Luisetto G, Basso MMS, Basso U, Brunello A, Camozzi V. Endocrine therapy of breast cancer. Curr Med Chem. 2011;18:513–22. doi: 10.2174/092986711794480177. [DOI] [PubMed] [Google Scholar]
- 8.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem Rev. 2005;105:3352–70. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 10.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 11.Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, Marotti JD, Hankinson SE, Colditz GA, Tamimi RM. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17:1867–74. doi: 10.1158/1078-0432.CCR-10-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Amicis F, Thirugnansampanthan J, Cui Y. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2010;121:1–11. doi: 10.1007/s10549-009-0436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H. Androgen receptors frequently are expressed in breast carcinomas. Cancer. 2003;98:703–711. doi: 10.1002/cncr.11532. [DOI] [PubMed] [Google Scholar]
- 14.Peters KM, Edwards SL, Nair SS, French JD, Bailey PJ, Salkield K, Stein S, Wagner S, Francis GD, Clark SJ, Brown MA. Androgen receptor expression predicts breast cancer survival: the role of genetic and epigenetic events. BMC Cancer. 2012;12:132. doi: 10.1186/1471-2407-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soreide JA, Lea OA, Varhaug JE, Skarstein A, Kvinnsland S. Androgen receptors in operable breast cancer: relation to other steroid hormone receptors, correlations to prognostic factors and predictive value for effect of adjuvant tamoxifen treatment. Eur J Surg Oncol. 1992;18:112–118. [PubMed] [Google Scholar]
- 16.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, Kim SI, Park BW, Lee KS. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. 2011;22:1755–62. doi: 10.1093/annonc/mdq678. [DOI] [PubMed] [Google Scholar]
- 17.Qu Q, Mao Y, Fei XC, Shen KW. The impact of androgen receptor expression on breast cancer survival: a retrospective study and meta-analysis. PloS One. 2013;8:e82650. doi: 10.1371/journal.pone.0082650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokunaga E, Hisamatsu Y, Taketani K, Yamashita N, Akiyoshi S, Okada S, Tanaka K, Saeki H, Oki E, Aishima S, Oda Y. Differential impact of the expression of the androgen receptor by age in estrogen receptor–positive breast cancer. Cancer med. 2013;2:763–73. doi: 10.1002/cam4.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal A, Ziolkowski P, Grzebieniak Z, Jelen M, Bobinski P, Agrawal S. Expression of Androgen Receptor in Estrogen Receptor–positive Breast Cancer. Appl Immunohistochem Mol Morphol. 2016;24:550. doi: 10.1097/PAI.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozovic-Spasojevic I, Zardavas D, Brohée S, Ameye L, Fumagalli D, Ades F, De Azambuja E, Bareche Y, Piccart M, Paesmans M, Sotiriou C. The prognostic role of androgen receptor in patients with early stage breast cancer: A meta-analysis of clinical and gene expression data. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0979. clincanres-0979. [DOI] [PubMed] [Google Scholar]
- 21.Kayahan M, Idiz UO, Gucin Z, Erözgen F, Memmi N, Müslümanoglu M. Cinical Significance of Androgen Receptor, CK-5/6, KI-67 and Molecular Subtypes in Breast Cancer. J Breast Health. 2014;10:201. doi: 10.5152/tjbh.2014.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garay JP, Park BH. Androgen receptor as a targeted therapy for breast cancer. Am J Cancer Res. 2012;2:434. [PMC free article] [PubMed] [Google Scholar]
- 23.Shah PD, Gucalp A, Traina TA. The role of the androgen receptor in triple-negative breast cancer. Womens Health. 2013;9:351–60. doi: 10.2217/whe.13.33. [DOI] [PubMed] [Google Scholar]
- 24.Bonnefoi H, Grellety T, Tredan O, Saghatchian M, Dalenc F, Mailliez A, L’haridon T, Cottu P, Abadie-Lacourtoisie S, You B, Mousseau M. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12–1) Ann Oncol. 2016;27:812–8. doi: 10.1093/annonc/mdw067. [DOI] [PubMed] [Google Scholar]
- 25.Lakhani SR, EI Schnitt SJ. WHO Classification of Tumours of the Breast. 4th edn. France Lyon: IARC Press; 2012. [Google Scholar]
- 26.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 27.Tsang JY, Ni YB, Chan SK, Shao MM, Law BK, Tan PH, Gary MT. Androgen receptor expression shows distinctive significance in ER positive and negative breast cancers. Ann Surg Oncol. 2014;21:2218–28. doi: 10.1245/s10434-014-3629-2. [DOI] [PubMed] [Google Scholar]
- 28.Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen Receptor Expression in Estrogen Receptor–Negative Breast Cancer: Immunohistochemical, Clinical, and Prognostic Associations. Am J Clin Pathol. 2003;120:725–31. doi: 10.1309/42F0-0D0D-JD0J-5EDT. [DOI] [PubMed] [Google Scholar]
- 29.Isola JJ. Immunohistochemical demonstration of androgen receptor in breast cancer and its relationship to other prognostic factors. J Pathol. 1993;170:31–5. doi: 10.1002/path.1711700106. [DOI] [PubMed] [Google Scholar]
- 30.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, Rimm DL, Liu XS, Brown M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20:119–31. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aleskandarany MA, Abduljabbar R, Ashankyty I, Elmouna A, Jerjees D, Ali S, Buluwela L, Diez-Rodriguez M, Caldas C, Green AR, Ellis IO. Prognostic significance of androgen receptor expression in invasive breast cancer: transcriptomic and protein expression analysis. Breast Cancer Res Treat. 2016;159:215–27. doi: 10.1007/s10549-016-3934-5. [DOI] [PubMed] [Google Scholar]
- 32.Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, Durando A, Donadio M, Bussolati G, Coates AS, Viale G. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010;124:607–17. doi: 10.1007/s10549-010-0761-y. [DOI] [PubMed] [Google Scholar]
- 33.Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM. Androgen receptor inhibits estrogen receptor-α activity and is prognostic in breast cancer. Cancer Res. 2009;69:6131–40. doi: 10.1158/0008-5472.CAN-09-0452. [DOI] [PubMed] [Google Scholar]
- 34.Gasparini P, Fassan M, Cascione L, Guler G, Balci S, Irkkan C, Paisie C, Lovat F, Morrison C, Zhang J, Scarpa A. Androgen receptor status is a prognostic marker in non-basal triple negative breast cancers and determines novel therapeutic options. PLoS One. 2014;9:e88525. doi: 10.1371/journal.pone.0088525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, Seruga B, Tannock IF, Ocana A, Amir E. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2013;106:djt319. doi: 10.1093/jnci/djt319. [DOI] [PubMed] [Google Scholar]
- 36.Mishra AK, Agrawal U, Negi S, Bansal A, Mohil R, Chintamani C, Bhatnagar A, Bhatnagar D, Saxena S. Expression of androgen receptor in breast cancer & its correlation with other steroid receptors & growth factors. Indian J Med Res. 2012;135:843. [PMC free article] [PubMed] [Google Scholar]
- 37.Qi JP, Yang YL, Zhu H, Wang J, Jia Y, Liu N, Song YJ, Zan LK, Zhang X, Zhou M, Gu YH. Expression of the androgen receptor and its correlation with molecular subtypes in 980 Chinese breast cancer patients. Breast cancer: Basic Clin Res. 2012;6 doi: 10.4137/BCBCR.S8323. BCBCR-S8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun XJ, Zuo K, Tang SX, Lu HF, Shui RH, Yu BH, Xu XL, Cheng YF, Tu XY, Bi R, Yang WT. Correlation between androgen receptor expression and surrogate molecular subtypes in invasive breast carcinoma. Zhonghua Bing Li Xue Za Zhi. 2017;46:476–80. doi: 10.3760/cma.j.issn.0529-5807.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Alshenawy HA. Prevalence of androgen receptors in invasive breast carcinoma and its relation with estrogen receptor, progesterone receptor and Her2/neu expression. J Egypt Natl Canc Inst. 2012;24:77–83. doi: 10.1016/j.jnci.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 41.Samaka RM, Younes SF. Androgen Receptor Expression in Breast Carcinoma of Egyptian Patients. J Clin Diagn Res. 2016;10:EC17. doi: 10.7860/JCDR/2016/23364.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa Y, Hai E, Matsumoto K, Ikeda K, Tokunaga S, Nagahara H, Sakurai K, Inoue T, Nishiguchi Y. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. 2008;13:431–5. doi: 10.1007/s10147-008-0770-6. [DOI] [PubMed] [Google Scholar]
- 43.Farag K, Elfarargy OM, El Shorbagy S, Salem RA, Balata SA, Abdel-latif RM, Harb OA, Megahed OA, Gertallah LM. Prevalence of Androgen Receptors expression in Triple Negative Breast Cancer Patients and its Correlation with Clinicopathological Criteria: Our Institutes experience. Menopause. 2017;4:11–1. [Google Scholar]
- 44.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 45.Lambertini M, Santoro L, Del Mastro L, Nguyen B, Livraghi L, Ugolini D, Peccatori FA, Azim HA. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev. 2016;49:65–76. doi: 10.1016/j.ctrv.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 46.McGhan LJ, McCullough AE, Protheroe CA, Dueck AC, Lee JJ, Nunez-Nateras R, Castle EP, Gray RJ, Wasif N, Goetz MP, Hawse JR. Androgen receptor-positive triple negative breast cancer: a unique breast cancer subtype. Ann Surg Oncol. 2014;21:361–7. doi: 10.1245/s10434-013-3260-7. [DOI] [PubMed] [Google Scholar]
- 47.Thike AA, Chong LY, Cheok PY, Li HH, Yip GW, Bay BH, Tse GM, Iqbal J, Tan PH. Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod Pathol. 2014;27:352. doi: 10.1038/modpathol.2013.145. [DOI] [PubMed] [Google Scholar]
- 48.Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, Park BW, Lee KS. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2009;21:488–92. doi: 10.1093/annonc/mdp510. [DOI] [PubMed] [Google Scholar]
- 49.Jiang H-S, Kuang X-Y, Sun W-L, Xu Y, Zheng Y-Z, Liu Y-R, Lang G-T, Qiao F, Hu X, Shao Z-M. Androgen receptor expression predicts different clinical outcomes for breast cancer patients stratified by hormone receptor status. Oncotarget. 2016;7:41285–41293. doi: 10.18632/oncotarget.9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macedo LF, Guo Z, Tilghman SL, Sabnis GJ, Qiu Y, Brodie A. Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res. 2006;66:7775–82. doi: 10.1158/0008-5472.CAN-05-3984. [DOI] [PubMed] [Google Scholar]
- 51.Rakha EA, Reis-Filho JS, Ellis IO. Combinatorial biomarker expression in breast cancer. Breast Cancer Res Treat. 2010;120:293–308. doi: 10.1007/s10549-010-0746-x. [DOI] [PubMed] [Google Scholar]
- 52.Robinson JL, MacArthur S, Ross-Innes CS, Tilley WD, Neal DE, Mills IG, Carroll JS. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J. 2011;30:3019–27. doi: 10.1038/emboj.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]