Abstract
Background
We investigated how STI risk perception relates to behavioural STI risk and STI healthcare (sexual health clinic attendance/chlamydia testing) in the British population.
Methods
Natsal-3, a national probability-sample survey undertaken 2010–12, included 8397 sexually-active 16–44 year-olds. Participants rated their risk of STIs (excluding HIV) given their current sexual lifestyle. Urine from a randomly-selected sub-sample of participants (n = 4550) was tested for prevalent STIs (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis).
Findings
Most men (64% (95% CI: 62–66)) and women (73% (72–74)) rated themselves as not at all at risk of STIs, 30% (29–32) men and 23% (22–25) women self-rated as not very much, and 5% (5–6) men and 3% (3–4) women as greatly/quite a lot at risk. Although those reporting STI risk behaviours were more likely to perceive themselves as at risk, > 70% men and > 85% women classified as having had unsafe sex in the past year, and similar proportions of those with a prevalent STI, perceived themselves as not at all or not very much at risk. Increased risk perception was associated with greater STI healthcare-use (past year), although not after adjusting for sexual behaviour, indicating in a mediation analysis that risk perception was neither necessary or sufficient for seeking care Furthermore, 58% (48–67) men and 31% (22–41) women who had unsafe sex (past year) and rated themselves as greatly/quite a lot at risk had neither attended nor tested.
Interpretation
Many people at risk of STIs in Britain underestimated their risk, and many who correctly perceived themselves to be at risk had not recently accessed STI healthcare. Health promotion needs to address this mismatch and ensure that people access healthcare appropriate to their needs.
Research in context
Evidence before this study
Decades of research into behavioural economics, cognitive psychology, and neuroscience have shaped our understanding of how people perceive risk, and why, for any particular threat, this may not always tally with an evidence-based view of risk favoured by scientists and decision-makers. This ‘perception gap’ has been demonstrated across different aspects of health and safety, with the direction and extent of the gap varying by topic and population. We searched PubMed and Google Scholar (February 2018) for the terms ‘STI risk perception’ and ‘STI perceived risk’ to review the literature relating to perceived risk of sexually transmitted infections. In selecting articles, we focussed on STIs other than HIV and on studies from high-income countries, given the potential for differences in risk perception between HIV and other STIs and across population groups. These studies suggested low levels of STI risk perception, both in the general population and among high-risk sub-groups, however no recent nationally-representative data were available on STI risk perception in the general population in Britain.
Added value of this study
This is the first study to quantify the risk perception gap for STIs in a nationally-representative sample of the British population aged 16–44 years. The combination of behavioural, risk perception, service use, and biological (urine samples tested for STIs) data enables an in-depth examination of the gap between STI risk perception and actual risk, and of the relationship between behaviour, perception, and healthcare-use. We found that although increased STI risk perception was associated with greater reporting of risk behaviours, large proportions of those at risk, including those with a prevalent STI detected in their urine, did not perceive themselves to be at risk. Furthermore, many of those who reported risk behaviours and did perceive themselves to be at risk had not attended sexual health clinics or been tested for chlamydia, and mediation analysis suggested STI risk perception was not on the causal pathway between STI risk behaviour and engagement with STI healthcare. These findings suggest the need for other triggers to encourage STI testing and overcome barriers to accessing care among those at risk.
Implications of all the available evidence
This study identifies falsely optimistic views of personal STI risk among those at behavioural risk of, and those with, prevalent STIs in the British population, which could have important implications for individual and population sexual health. This risk perception gap is consistent with that found in other areas of public health and, together with the evidence that risk perception may be neither sufficient nor necessary for engagement with STI healthcare, supports the use of health promotion approaches which emphasise relevant behaviours of both individuals and their partners such as partner change and unprotected sex and consider diverse influences on behaviour including social, emotional and cognitive processes.
Alt-text: Unlabelled Box
1. Introduction
Several models of behaviour change propose that recognising oneself to be at risk is a key requirement for action, alongside other factors including self-efficacy, social norms, availability of a course of action to reduce risk, and cues to behaviour change [1]. However, across multiple areas of health a ‘perception gap’ has been found between people's risk perceptions and their actual risk [2]. Risk perception is influenced by many factors other than knowledge, including personality characteristics [3], emotional responses [4], how much benefit is obtained as a result of taking the risk [5], mental shortcuts (‘heuristics’) and biases including optimism bias, where people overestimate the personal likelihood of experiencing positive events and underestimate negative events [5], [6]. Ropeik argues that subjectivity in individuals' processing of risk information is inescapable, and the expectation of scientific, policy, and public health communities that people should make rational fact-based decisions is naïve [2]. Nevertheless, the perception gap can lead to behavioural choices that damage the health of individuals and populations, and must be understood and managed, as is increasingly acknowledged in risk communication and health policy [5], [7].
As sexually transmitted infections (STIs) are often asymptomatic, testing of those without symptoms is an important component of treatment and control, therefore understanding motivations to engage with STI healthcare, including the potential role of risk perception, is critical. Actual risk of acquiring an STI depends on individual and partner behaviours and STI prevalence in one's sexual network. The fact that individuals rarely have complete information on these factors, combined with other influences on risk perception as described above may lead to an under- or over-estimation of individual STI risk, which in turn could influence sexual behaviour and healthcare-seeking. We have previously shown that in Britain, substantial proportions of those at risk had not accessed sexual health clinics or chlamydia testing despite these services being free at the point of care [8], suggesting either a lack of risk perception or other barriers to accessing care. We have also previously shown a mismatch between HIV risk perception and indicators of HIV risk among the British population [9], however these findings may not be generalisable to other STIs due to real and perceived differences in how prevalent the infections are, which population groups are affected, and the consequences of infection.
Although several studies internationally have found low STI risk perception, including among high-risk populations [10], [11], [12], [13], the only recent British data come from convenience samples of young people and may not represent the population more generally [14], [15]. We investigate the relationship between STI risk perception and sexual behaviour and prevalent STIs to assess the risk perception gap using data from Britain's most recent probability sample survey, the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3), meaning the data are broadly representative of the British population aged 16–44 years. We also investigate associations between risk perception and STI healthcare-use, including assessment of the extent to which risk perception may lead those with risk behaviours to attend services/test for STIs, based on the hypothesis that risk perception is one mechanism but that there may be others. These analyses aim to inform and refine health promotion messaging and identify opportunities for improving healthcare pathways.
2. Methods
2.1. Participants and Procedures
Natsal-3 is a stratified probability-sample survey of 15,162 men and women aged 16–74 years in Britain, conducted in 2010–2012. Full details of the methods and demographic characteristics of participants have been reported elsewhere [16]. The response rate was 57.7% (of all known eligible or estimated eligible addresses). Participants were interviewed using computer-assisted face-to-face (CAPI) and self-completion (CASI) questionnaires. We present data on STI risk perception among 8357 participants (3391 men, 4966 women) aged 16–44 years reporting at least one sexual partner in the past year (‘sexually-active’). The 16–44 year age group was selected to focus on those at greater risk of STIs [17], [18], however we also include an appendix with estimates for all participants aged 16–74 years.
2.2. Measures
Towards the end of the interview, participants were asked a CAPI question about perceived risk of STIs (excluding HIV) using a showcard listing response options assigned letter codes to promote privacy (panel). This followed a similar question about perceived HIV risk [9]. CASI questions about sexual behaviour with opposite- and same-sex partners included: number of partners, number of partners without a condom, and sexual practices. Detailed CASI questions were asked about up to three most recent sexual partners in the past 5 years, including month/year of first and most recent occasion of sex, condom use at first and most recent sex, whether any of these partnerships overlapped (concurrency). Participants were considered to have had ‘unsafe sex’ in the past year if they reported condomless sex with a new partner and/or ≥ 2 sexual partners and no condom use at all (excluding those reporting only oral sex in the past year). This measure was created to align with clinical recommendations for sexual health checks among those who have had unprotected sex with a new partner [19] and epidemiological data showing increased risk of STIs with multiple partners [18]. CASI questions about STI healthcare-use included whether the participant had ever attended a sexual health clinic (GUM clinic) or tested for chlamydia in any setting (and if so when). Non-response to CAPI and CASI questions was low (typically 1–3%), non-response to the STI risk perception question was < 0.5%.
Panel: STI risk perception question wording.
[Interviewer hands participant showcard with response options]
People are also at risk of getting other sexually transmitted infections. What do you think about the risks to you, personally, with your present lifestyle of getting a sexually transmitted infection that is not HIV?
Just tell me the letter that corresponds to your answer.
-
1.
(H) Greatly at risk
-
2.
(B) Quite a lot
-
3.
(W) Not very much
-
4.
(S) Not at all at risk
Alt-text: Unlabelled Box
A randomly-selected sub-sample of participants aged 16–44 (n = 4550) were invited to provide urine samples which were tested for STIs including Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, and Trichomonas vaginalis; full details of the urine protocol have been reported elsewhere [16], [18]. We present STI risk perception among 59 men and 114 women testing positive for ≥ 1 of these (‘prevalent STI’). As these STIs are treatable with antibiotics within a short timeframe, it was assumed that most participants with prevalent STIs had not yet been diagnosed and were unaware of the infection.
2.3. Statistical Analysis
Analyses were carried out using Stata v14 (StatCorp, 2015. Stata Statistical Software: Release 14. College Station, Texas, USA) accounting for stratification, clustering, and weighting of the sample. Data were weighted to account for differential probabilities of selection to the survey and to address non-response bias by age, sex and region. Additional weights were applied for analysis of the urine data to account for selection and non-response to the urine sample.
We present descriptive analyses (percentages and 95% confidence intervals (CIs)) of risk perception by age group and sex. Multinomial regression was used to examine associations between sexual behaviour and STI risk perception to allow inclusion of risk perception as a categorical, rather than binary, outcome. Age-adjusted relative risk ratios were calculated (with age entered into the model as a continuous variable) for rating oneself as greatly/quite a lot at risk (combined due to small numbers) or not very much at risk, both compared with not at all at risk. We examined the perception gap by describing STI risk perception among those reporting unsafe sex in the past year, and those with a prevalent STI detected in their urine, as measures of actual risk.
Finally, we conducted mediation analysis [20] to investigate the hypothesis that risk perception is on the causal pathway between STI risk behaviours and STI healthcare-use. According to this approach, the following criteria should be met to provide evidence for this hypothesis: 1) STI risk behaviours should be associated with STI healthcare-use (demonstrated in our previous work) [8], 2) STI risk behaviours should be associated with STI risk perception (assessed using multinomial regression analysis described above), 3) STI risk perception should be associated with healthcare-use after adjustment for risk behaviours, 4) adjusting for STI risk perception should reduce (partial mediation) or remove (complete mediation) the association between risk behaviours and healthcare-use.
Criteria 3) and 4) were assessed via two multivariable logistic regression models (dependent variable = STI healthcare-use): model 1 included risk behaviours only, whereas model 2 additionally included risk perception. Age was included in all models as a continuous variable and an a priori important confounder. Rather than include all behavioural variables in these models, a selection process was used to identify a restricted set of behavioural variables for inclusion, based on exploratory analysis showing potential collinearity between behavioural variables (indicated by increased standard errors). Behavioural variables were prioritised for entry into the model based on conceptual importance (e.g. unsafe sex was prioritised as it incorporates both partner change and condom use), strength of evidence (p-value) and strength of association (odds ratio) in age-adjusted analyses. Entry to the model was predicated on a p-value < 0.15 in age-adjusted analysis, retention was based on a p-value < 0.1. Given the risk of multi-collinearity, standard errors for all variables were checked with the addition of each variable to the model for indications of instability. Analyses were conducted separately for men and women, given differences in the epidemiology of STIs [18], gender differences in the experience and reporting of sexual behaviours [21], and the ‘sexual scripts’ which shape these behaviours [22], as well as differences in the reasons for [23], and type of sexual health service used [8], and potential differences in the role of risk perception in STI healthcare-use. The final model 1 included only participants for which risk perception data were available to ensure the comparison between the models was not biased by missing data.
We hypothesised that the effect of STI risk behaviours on risk perception would be of medium size (OR = 2; Cohen's d of 0.38), and the effect of risk perception on clinic attendance would be small (OR = 1.2; Cohen's d of 0.10) and that risk perception would only partially mediate the effect of risk behaviour on clinic attendance. Under these assumptions, a sample size of around 2680 would be required for 80% power to detect total mediation [24]. We therefore have good power to detect total mediation with our sample size of approximately 3300 men and 4900 women, even under more conservative assumptions. Sample sizes required to detect partial mediation with 80% power are substantially less.
2.4. Ethics
Natsal-3 was approved by the Oxfordshire Research Ethics Committee A (reference: 09/0604/27). Verbal informed consent was obtained from participants for the interview, with written informed consent for the urine samples.
2.5. Data Sharing
Natsal-3 data are available via the UK Data Archive [25].
2.6. Role of the Funding Source
The sponsors of the study played no role in the study design, data collection, data analysis, interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. STI Risk Perception by Age and Sex
Among all sexually-active men aged 16–44 years, 64.2% rated themselves as not at all at risk of STIs, 30.4% as not very much at risk, 4.3% as quite a lot at risk, and 1.2% as greatly at risk (Table 1). Women were more likely to rate themselves as not at all at risk: 73.1% rated themselves as not at all at risk, 23.2% as not very much, 2.9% as quite a lot and 0.8% as greatly (Table 1; p-value for difference between men and women < 0.001). Risk perception was strongly associated with age, with perceived risk greater among younger men and women (Supplementary Table 1).
Table 1.
STI risk perception among those with prevalent STIs, those reporting unsafe sex, and all those sexually-active aged 16–44 years, by sex.
| Men |
Women |
||||||
|---|---|---|---|---|---|---|---|
| Those with prevalent STIa | Those reporting unsafe sexb, past year | All sexually active menc | Those with prevalent STIa | Those reporting unsafe sexb, past year | All sexually active womenc | ||
| Not at all at risk | % | 43.5 | 39.2 | 64.2 | 58.7 | 51.0 | 73.1 |
| 95% CI | (29.2–59.0) | (35.1–43.5) | (62.3–66.1) | (46.8–69.6) | (47.4–54.5) | (71.7–74.5) | |
| Not very much | % | 29.4 | 47.0 | 30.4 | 29.5 | 37.9 | 23.2 |
| 95% CI | (17.8–44.5) | (42.8–51.1) | (28.6–32.2) | (20.1–41.0) | (34.6–41.3) | (22.0–24.6) | |
| Quite a lot | % | 26.0 | 10.1 | 4.3 | 10.6 | 9.3 | 2.9 |
| 95% CI | (16.8–43.6) | (8.0–15.6) | (3.6–5.0) | (5.6–19.2) | (7.3–11.8) | (2.3–3.5) | |
| Greatly at risk | % | 1.1 | 3.8 | 1.2 | 1.2 | 1.8 | 0.8 |
| 95% CI | (0.1–7.3) | (2.5–5.6) | (0.8–1.6) | (0.2–8.3) | (1.1–3.0) | (0.6–1.2) | |
| Total | 100% | 100% | 100% | 100% | 100% | 100% | |
| Denominators (unwt, wt) | 59, 50 | 769, 664 | 3391, 3486 | 114, 66 | 1075, 625 | 4966, 3473 | |
Denominator: those aged 16–44 years. unwt = unweighted; wt = weighted.
Prevalent STIs detected in urine: Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, or Trichomonas vaginalis.
Unsafe sex defined as reported either no condom used at first occasion of sex with a new partner and/or sex with 2 + partners and no condom used with any partner (past year), excluding those who had only oral sex in the past year.
Sexually-active defined as at least one sexual partner in the past year.
3.2. Risk Perception by Sexual Behaviour and Prevalent STIs
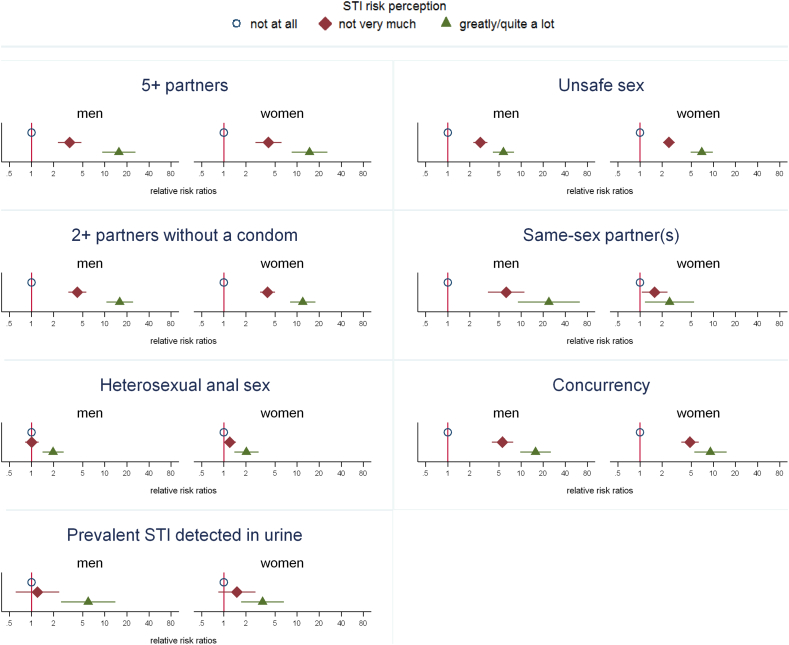
Behavioural and partnership factors known to be related to increased STI risk were strongly associated with rating oneself as greatly/quite a lot at risk, and also associated, but less strongly so, with rating oneself as not very much at risk (both in relation to rating oneself as not at all at risk) (Fig. 1, Supplementary Table 2). For example, men reporting 5 or more sexual partners in the past year were 16 times as likely to rate themselves as greatly/quite a lot at risk as those with fewer than 5 partners and 4 times as likely to rate themselves as not very much at risk (corresponding age-adjusted relative risk ratios for women: 15 and 4 respectively).
Fig. 1.
Age-adjusted relative risk ratios (with 95% confidence interval) for rating oneself as greatly/quite a lot or not very much at risk, compared with not at all at risk, by sexual behaviours and presence of STIs in urine in the past year (sexually-active men and women aged 16–44 years).
Notes for this figure: Denominator is those aged 16–44 reporting at least one sexual partner in the past year. Graphs show age-adjusted relative risk ratios (with 95% confidence interval) for each outcome, comparing those rating themselves as not very much at risk or greatly/quite a lot at risk with those rating themselves as not at all at risk. ‘Unsafe sex’ = reported either no condom used at first occasion of sex with a new partner and/or sex with 2 + partners and no condom used with any partner (past year), excluding those who had only oral sex in the past year. Prevalent STI in urine: Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, or Mycoplasma genitalium. Denominators for prevalent STI in urine are smaller than for behavioural variables as urine was only requested from a sub-sample of participants. For full data, including denominators, see Supplementary Table 2.
Despite these strong associations, the absolute proportion of those reporting risk behaviours but not rating themselves as at risk was very high. For example, among those classed as having ‘unsafe sex’ in the past year (comprising approximately 1 in 5 sexually-active 16–44-year-olds), 39.2% (95% CI: 35.1%–43.5%) of men and 51.0% (47.4%–54.5%) of women rated themselves as not at all at risk of STIs (Table 1). 59 men and 114 women had a prevalent STI detected in their urine sample at the time of interview (Chlamydia trachomatis in 36 men and 62 women; Neisseria gonorrhoeae in 2 men and 3 women; Mycoplasma genitalium in 24 men and 48 women; Trichomonas vaginalis in 7 women). Among these, 43.5% (29.2%–59.0%) of men and 58.7% (46.8%–69.6%) of women rated themselves as not at all at risk of STIs, and a further 29.4% (17.8%–44.5%) and 29.5% (20.1%–41.0%) respectively rated themselves as not very much at risk (Table 1). However, of those with a prevalent STI, 63.7% (50.2%–75.3%) of men and 41.8% (31.4%–52.9%) reported having had unsafe sex in the past year.
3.3. STI Risk Perception and STI Healthcare-use
Overall, 19.1% (17.8%–20.5%) of sexually-active men and 28.9% (27.5%–30.3%) of sexually-active women reported STI healthcare-use in the past year in terms of reporting sexual health clinic attendance and/or chlamydia testing (Supplementary Table 3). There was strong evidence that increased risk perception was associated with greater STI healthcare-use with 42.0% (35.3%–49.1%) of men and 53.7% (46.1%–61.1%) of women who rated themselves as greatly/quite a lot at risk having attended/tested in the past year, compared with 13.5% (12.1%–15.1%) of men and 23.8% (22.4%–25.4%) of women who rated themselves as not at all at risk (Table 2). Given previously reported findings that risk behaviour is also strongly associated with STI service use [8], we tested the hypothesis that STI risk perception is on the causal pathway between risk behaviour and STI healthcare-use. However, we found that STI risk perception was no longer associated with STI healthcare-use after adjustment for STI risk behaviours (p = 0.26 for men, p = 0.32 for women; Table 2; mediation analysis criterion 3). Furthermore, adjustment for STI risk perception made little difference to associations between STI risk behaviours and STI healthcare-use, as assessed by changes in the odds ratios between model 1 and model 2 (mediation analysis criterion 4). In addition, substantial proportions of those reporting both risk behaviour and perceiving themselves to be at risk did not report STI healthcare-use. For example, among those classed as having unsafe sex and rating themselves as greatly/quite a lot at risk (n = 121 men and 121 women), 57.8% (47.7%–67.3%) of men and 31.1% (22.3%–41.4%) of women had neither attended a sexual health clinic nor tested for chlamydia in the past year.
Table 2.
Multivariable analysis of the association between STI risk perception and sexual behaviours and STI healthcare-use (testing for chlamydia/attending a sexual health clinic, past year).
| (a) Sexually-active men aged 16–44 years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Denominators |
Clinic attendance / chlamydia test |
Age-adjusted logistic regression |
Model 1: Adjusted for age & risk behaviours (n = 3378) |
Model 2: OR adjusted for age, risk behaviours, risk perception (n = 3378) |
||||||||
| unwt | wt | % | 95% CI | OR | 95% CI | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Perceived risk of STIs | 0.26 | |||||||||||
| Not at all | 1985 | 2238 | 13.5% | [12.1%, 15.1%] | 1.00 | 1.00 | ||||||
| Not very much | 1166 | 1058 | 26.8% | [23.9%, 29.9%] | 1.56 | (1.27–1.93) | 1.14 | (0.90–1.43) | ||||
| Greatly/quite a lot | 240 | 190 | 42.0% | [35.3%, 49.1%] | 3.00 | (2.15–4.20) | 1.36 | (0.92–2.01) | ||||
| Number of partners, past year | < 0.001 | < 0.001 | ||||||||||
| 1 | 2332 | 2624 | 12.4% | [11.1%, 13.8%] | 1.00 | 1.00 | 1.00 | |||||
| 2 | 437 | 370 | 33.1% | [28.0%, 38.6%] | 2.19 | (1.65–2.90) | 2.14 | (1.62–2.83) | 2.02 | (1.51–2.70) | ||
| 3–4 | 358 | 292 | 39.4% | [33.5%, 45.6%] | 3.12 | (2.25–4.33) | 2.94 | (2.10–4.10) | 2.74 | (1.93–3.89) | ||
| 5 + | 258 | 197 | 53.4% | [46.0%, 60.7%] | 5.05 | (3.65–6.97) | 4.32 | (3.11–6.00) | 3.92 | (2.75–5.61) | ||
| Same-sex partners, past year | 0.001 | 0.002 | ||||||||||
| No | 3292 | 3408 | 18.5% | [17.1%, 19.9%] | 1.00 | 1.00 | 1.00 | |||||
| Yes | 113 | 92 | 45.6% | [34.8%, 56.9%] | 3.84 | (2.21–6.67) | 2.73 | (1.53–4.88) | 2.55 | (1.42–4.59) | ||
| Heterosexual anal sex, past year | 0.01 | 0.01 | ||||||||||
| No | 2684 | 2824 | 17.6% | [16.2%, 19.2%] | 1.00 | 1.00 | 1.00 | |||||
| Yes | 717 | 671 | 25.8% | [22.4%, 29.4%] | 1.47 | (1.19–1.81) | 1.36 | (1.09–1.71) | 1.35 | (1.08–1.69) | ||
| b) Sexually-active women aged 16–44 years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Denominators |
Clinic attendance / chlamydia test |
Age-adjusted logistic regression |
Model 1: Adjusted for age & risk behaviours (n = 4948) |
Model 2: OR adjusted for age, risk behaviours, risk perception (n = 4948) |
||||||||
| unwt | wt | % | 95% CI | OR | 95% CI | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Perceived risk of STIs | 0.32 | |||||||||||
| Not at all | 3461 | 2539 | 23.8% | [22.4%, 25.4%] | 1.00 | 1.00 | ||||||
| Not very much | 1295 | 807 | 41.0% | [37.8%, 44.3%] | 1.59 | (1.34–1.89) | 1.16 | (0.96–1.40) | ||||
| Greatly/quite a lot | 210 | 127 | 53.7% | [46.1%, 61.1%] | 2.35 | (1.67–3.31) | 1.11 | (0.76–1.62) | ||||
| Number of partners, past year | < 0.001 | < 0.001 | ||||||||||
| 1 | 3844 | 2836 | 22.1% | [20.7%, 23.5%] | 1.00 | 1.00 | 1.00 | |||||
| 2 | 508 | 296 | 51.0% | [46.0%, 56.1%] | 2.64 | (2.06–3.38) | 2.00 | (1.49–2.70) | 1.95 | (1.45–2.62) | ||
| 3–4 | 391 | 208 | 63.5% | [58.1%, 68.6%] | 4.00 | (2.97–5.39) | 2.92 | (2.05–4.17) | 2.78 | (1.95–3.97) | ||
| 5 + | 219 | 130 | 70.2% | [61.5%, 77.6%] | 4.98 | (3.25–7.64) | 3.56 | (2.23–5.68) | 3.39 | (2.11–5.44) | ||
| Unsafe sex, past year | < 0.001 | < 0.001 | ||||||||||
| No | 3871 | 2833 | 23.4% | [22.0%, 24.9%] | 1.00 | 1.00 | 1.00 | |||||
| Yes | 1086 | 631 | 53.6% | [50.1%, 57.1%] | 2.96 | (2.46–3.57) | 1.73 | (1.38–2.17) | 1.72 | (1.37–2.16) | ||
| Number of partners without a condom, past year | 0.03 | 0.03 | ||||||||||
| 0 | 735 | 501 | 24.0% | [20.6%, 27.8%] | 1.00 | 1.00 | 1.00 | |||||
| 1 | 3620 | 2635 | 13.8% | [12.3%, 15.3%] | 1.32 | (1.06–1.66) | 1.32 | (1.06–1.66) | 1.32 | (1.05–1.67) | ||
| 2 + | 576 | 318 | 41.3% | [36.4%, 46.4%] | 4.82 | (3.58–6.48) | 1.50 | (1.04–2.17) | 1.50 | (1.04–2.16) | ||
unwt = unweighted; wt = weighted; OR = odds ratio from logistic regression.
Unsafe sex = reported either no condom used at first occasion of sex with a new partner and/or sex with 2 + partners and no condom used with any partner (past year); excludes those who had only oral sex in the past year.
Model 1 excludes those with missing data for STI risk perception to enable complete case comparison with model 2 (n = 13 men, n = 18 women).
4. Discussion
4.1. Summary of Key Findings
Using data from a large probability-sample survey of the British population, we found that STI risk perception was higher among population groups in whom STIs are more common, such as younger people, and those reporting behaviours associated with STI risk, suggesting some knowledge of STI risk factors among the general population. However, this was against a backdrop of very low STI risk perception overall and in fact, large proportions of those with markers of high STI risk, including those with a prevalent STI, did not perceive themselves to be at risk. Although STI risk perception was associated with having attended a sexual health clinic and/or being tested for chlamydia in the past year, mediation analysis suggested that risk perception may not be a key mechanism by which risk behaviour leads to STI healthcare-use. Indeed, substantial proportions of those who reported risk behaviours and perceived themselves to be at risk had not attended a sexual health clinic or tested for chlamydia in the past year, thus perceived risk may not be sufficient to prompt STI healthcare-use for many of those at risk.
4.2. Strengths and Limitations
The strengths and weaknesses of Natsal-3 have been described in detail elsewhere [16]. Strengths include the use of probability sampling methods to obtain a sample broadly representative of the general population in Britain when the data were collected in 2010–'12, and the detailed behavioural data, combined with demographic, attitudinal, service use and biological (STI) data. The response rate was 58%, in line with other major social surveys conducted at the time. While non-response could be a source of bias for our data, we aimed to minimise this bias as the sample was weighted to the age, sex, and regional profile of the British population at the time of the fieldwork, and after weighting had been applied, the sample was generally comparable to the British population on other demographic characteristics at that time [16]. However, the sampling strategy used for the Natsal studies means that the target population is specifically the population resident in private households in Britain and so excludes those who live in institutions whose behaviour might differ from others. While this sampling strategy is also a potential source of bias, the institutionalised population constitutes a relatively small proportion of the British population.
Natsal is a cross-sectional survey, therefore the ability to examine causal relationships is limited. The mediation analysis of the relationship between STI risk perception and healthcare-seeking should therefore be interpreted with caution, as some reverse causality is likely. For example, receiving positive or negative STI test results may directly influence risk perception and/or behaviour. Moreover it is possible that there is partial mediation through STI risk perception that we were unable to detect due to unmeasured confounding. However, the very minimal changes that adjustment for STI risk perception made to the associations between STI risk behaviours and STI healthcare-use suggests that STI risk perception is unlikely to be a substantial mediator and the results of the mediation analysis are supported by our finding that substantial proportions of those reporting both risk behaviours and high risk perception had not accessed STI services/testing in the past year. Only a single question was asked about STI risk perception, which may not capture the complexity of this concept, for example, individuals' risk perception may change over time, and people may perceive their risk to be different for different STIs. Furthermore, the placement of this question after detailed questions about sexual behaviour may have influenced participants' assessment of their risk. In addition, this question was asked in the face-to-face section of the interview, and although a showcard was used so that participants only needed to give a response code, it is plausible that responses may still be subject to social desirability bias.
We analysed associations between markers of actual risk based on individuals' self-reported risk behaviour and STI risk perception. However, actual STI risk is determined by a combination of one's own behaviour (well known to each individual), that of sexual partner(s) (which may be confounded by lack of accurate information), and the prevalence of STIs in one's sexual network (which is difficult to estimate), hence actual risk cannot be completely captured in an individual interview. Some questions on the characteristics of participants' most recent partners were asked in Natsal, and we have previously found that women whose recent sexual partners were older than them, and men whose recent sexual partners were of a different ethnic background, had higher STI risk perception after adjustment for confounders (paper in press). However, none of these are reliable enough indicators of that partner's actual risk of STIs to improve our classification of participants' overall risk.
Despite these limitations, this paper's analysis does give insight into the discrepancy between risk behaviour, STI risk perception and STI healthcare use, and the inclusion of biological data on prevalent infection provides an additional objective measure of actual risk among a sub-sample of participants. It was not possible to identify participants who are definitively ‘low risk’ given unknown information about partners' risk factors – for example, we have previously shown that 60% of women with chlamydia detected in urine only reported one sexual partner in the past year [18], [26]. This precluded examination of whether a perception gap exists in the opposite direction, whereby some people have high risk perception despite low actual risk. Finally, although the overall sample size was large, some sub-groups known to be at higher risk of STIs (e.g. men who have sex with men (MSM)) were too small to allow analyses within this sub-group.
4.3. Relation to Existing Literature
Our finding of greater risk perception in men compared to women but lower health service use highlights the importance of the many different social, environmental, and individual-level factors that result in any one behaviour. For example, women have more routine opportunities to access or be offered STI testing through attending sexual health services for contraception, chlamydia testing, and cervical smear screening which may both raise women's awareness of, and improve access to, testing. This is also evident from the latest STI surveillance data [27], which show that in 2017 there were 1,590,094 attendances at sexual health services by women vs. 1,027,472 by men. In terms of young people, an estimated 28% of young females (15–24 years) and 11% of young males were tested for chlamydia through the National Chlamydia Screening Programme in 2017 i.e. chlamydia testing access/coverage was 2.5 times higher in young women than young men.
Our findings of underestimation of personal risks are in line with those from other areas of risky health behaviours, including smoking and alcohol [28], [29], [30], and with other studies of STI risk perception, including among high-prevalence populations [10], [11], [12], [13], [14]. They are also consistent with our previously reported findings on HIV risk perception [9]. Explanations for low levels of STI risk perception among those at risk have included lack of knowledge about STIs and their prevalence [15], [31], lack of symptoms [32], and optimistic bias [32], [33]. It has also been shown that people misjudge their sexual partners' risk [34], and studies in several populations have described the use of inappropriate markers of partners' STI risk in deciding whether or not to use a condom, including partners' reputation, where they live, demeanour, physical attributes including clothing and personal hygiene, and lack of any visible STI symptoms [15], [31], [33], [35]. Evidence that people generally overestimate the risk of ‘visible’ phenomena and underestimate the risk of less visible phenomena (‘availability bias’) [36] suggests that the often invisible and stigmatised nature of STIs may contribute to underestimation of risk.
4.4. Interpretation
Low perceived risk is likely to be appropriate for most people in the general British population, given STIs are indeed relatively uncommon, for example chlamydia prevalence is estimated to be around 2–3% in those aged 16–24 years and lower among over-25s [18]. It is also encouraging that those reporting risk behaviours were more likely to perceive themselves as at risk, suggesting some understanding of risk factors for STIs. However, absolute levels of risk perception were low among those with risk behaviours or prevalent infection. The fact that STI risk perception did not appear to be a key mechanism explaining associations between individual risk behaviour and testing/clinic attendance is consistent with theories of behaviour change which point at perception of individual risk as just one element needed for change, alongside other personal, societal and environmental factors [1]. Given the limitations inherent in cross-sectional data described above, further qualitative and/or longitudinal research would be needed to better elucidate the role (if any) of risk perception in prompting STI testing among those at risk.
5. Conclusions
We have identified falsely optimistic views of personal STI risk among a substantial proportion of those at risk of STIs in the British population, which could have a negative impact on efforts to promote safe sex and STI testing, and the control of STIs. The literature on reasons for inaccurate risk perception described above suggests a number of reasons this may occur, and public health interventions aiming to reduce this perception gap should incorporate these and lessons from the wider risk communication literature and the related field of behavioural insights, including consideration of the role of social, emotional and cognitive influences on risk perception and behaviour [5], [7]. Evidence from these disciplines warns that it may not be possible to eliminate the perception gap, and our data indicate that perceived risk alone is not sufficient for an at-risk individual to engage with STI healthcare, highlighting the importance of diverse approaches to encouraging and enabling STI testing. This could include expanding initiatives such as online services and self-testing, which have the potential to offer convenient means of reaching those at risk who do not currently engage with sexual health care. These findings also support public health messaging that emphasise triggers to testing other than risk perception. For example, in England, regular testing for HIV and STIs is strongly recommended for those with recognised risk behaviours, and the National Chlamydia Screening Programme provides opportunistic screening to sexually-active young people and recommends that anyone under 25 who is sexually-active should be screened for chlamydia annually (regardless of symptoms and perceived risk), and on change of sexual partner [27]. Given the disparity in risk perception and prevalent STI identified in this study, such approaches would seem an appropriate public health measure.
Author Contributions
SC, AMJ, CHM, and PS originally conceived this article. SC conducted the statistical analysis and wrote the first draft. AMJ, CHM, and PS, initial applicants on Natsal-3, wrote the study protocol and obtained funding. AMJ, CHM, PS, SC, CT, and NF designed the Natsal-3 questionnaire, applied for ethics approval, undertook piloting, and managed the fieldwork. SC, CHM and CT managed data. All authors interpreted data, reviewed successive drafts and approved the final version of the article.
Conflicts of Interest
AMJ has been a Governor of the Wellcome Trust since 2011. All other authors declare that they have no conflicts of interest.
Funding
Medical Research Council, Wellcome Trust.
Acknowledgements
We thank the study participants and the team of interviewers, operations and computing staff from NatCen Social Research. Natsal-3 is a collaboration between University College London (London, UK), the London School of Hygiene and Tropical Medicine (London, UK), NatCen Social Research, Public Health England, and the University of Manchester (Manchester, UK). The study was supported by grants from the Medical Research Council (G0701757) and the Wellcome Trust (084840) with contributions from the Economic and Social Research Council and UK Department of Health. SC was funded to undertake independent research supported by the National Institute for Health Research (NIHR Research Methods Programme, Fellowships and Internships, NIHR-RMFI-2014-05-28). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2018.08.001.
Appendix A. Supplementary Data
Supplementary tables
References
- 1.Nutbeam D., Harries E., Wise M. 3rd edition. McGraw-Hill; North Ryde: 2010. Theory in a nutshell: a practical guide to health promotion theories.http://www.mheducation.co.uk/9780070278431-emea-theory-in-a-nutshell-a-practical-guide-to-health-promotion-theories Available from: [Google Scholar]
- 2.Ropeik D. The Perception Gap: recognizing and managing the risks that arise when we get risk wrong. Food Chem Toxicol. 2012;50:1222–1225. doi: 10.1016/j.fct.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Bouyer M., Bagdassarian S., Chaabanne S., Mullet E. Personality correlates of risk perception. Risk Anal. 2001;21:457–466. doi: 10.1111/0272-4332.213125. [DOI] [PubMed] [Google Scholar]
- 4.Buck R., Ferrer R. Emotion, warnings, and the ethics of risk communication. In: Roeser S., Hillerbrand P., Peterson M., editors. Handbook of risk theory. Delft; The Netherlands: 2012. [Google Scholar]
- 5.Ropeik D. Risk Communication: an overlooked tool for improving public health. In: Last J., Wallace R., editors. Public health and preventative medicine. 2007. http://www.dropeik.com/dropeik/pdf/text_book_chapter.pdf Available from: [Google Scholar]
- 6.Weinstein N.D. Unrealistic optimism about future life events. J Pers Soc Psychol. 1980;39:806–820. [Google Scholar]
- 7.Behavioural Insights Team Applying behavioural insight to health. 2010. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/60524/403936_BehaviouralInsight_acc.pdf Available from: [London]
- 8.Tanton C., Geary R.S., Clifton S. Sexual health clinic attendance and non-attendance in Britain: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3) Sex Transm Infect. 2018;94:267–275. doi: 10.1136/sextrans-2017-053193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifton S., Nardone A., Field N. HIV testing, risk perception, and behaviour in the British population. AIDS. 2016;30:943–952. doi: 10.1097/QAD.0000000000001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravningen K., Braaten T., Schirmer H. Self-perceived risk and prevalent chlamydia infection among adolescents in Norway: a population-based cross-sectional study. Sex Transm Infect. 2016;92:91–96. doi: 10.1136/sextrans-2014-051927. [DOI] [PubMed] [Google Scholar]
- 11.Leval A., Sundström K., Ploner A., Dahlström L.A., Widmark C., Sparén P. Assessing perceived risk and STI prevention behavior: a national population-based study with special reference to HPV. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickey M.T., Cleland C. Sexually transmitted infection risk perception among female college students. J Am Assoc Nurse Pract. 2013;25:377–384. doi: 10.1111/j.1745-7599.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- 13.Ethier K.A., Kershaw T., Niccolai L., Lewis J.B., Ickovics J.R., Ethier K.A. Adolescent women underestimate their susceptibility to sexually transmitted infections. Sex Transm Infect. 2003;79:408–411. doi: 10.1136/sti.79.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanakira E., Goyder E., Freeman J., O'Cathain A., Kinghorn G., Jakubovic M. Social and psychosocial factors associated with high-risk sexual behaviour among university students in the United Kingdom: a web-survey. Int J STD AIDS. 2015;26:369–378. doi: 10.1177/0956462414538950. [DOI] [PubMed] [Google Scholar]
- 15.Newby K.V., Wallace L.M., French D.P. How do young adults perceive the risk of chlamydia infection? A qualitative study. Br J Health Psychol. 2012;17:144–154. doi: 10.1111/j.2044-8287.2011.02027.x. [DOI] [PubMed] [Google Scholar]
- 16.Erens B., Phelps A., Clifton S. Methodology of the third British National Survey of Sexual Attitudes and Lifestyles (Natsal-3) Sex Transm Infect. 2014;90:84–89. doi: 10.1136/sextrans-2013-051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Public Health England Sexually transmitted infections and chlamydia screening in England, 2016. Health Prot Rep. 2017:11. [Google Scholar]
- 18.Sonnenberg P., Clifton S., Beddows S. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal) Lancet. 2013;382:1795–1806. doi: 10.1016/S0140-6736(13)61947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.What should I do if I think I've got an STI? - health questions - NHS Choices. https://www.nhs.uk/chq/Pages/962.aspx Available from:
- 20.Mackinnon D.P., Fairchild A.J., Fritz M.S. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercer C.H., Tanton C., Prah P. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal) Lancet. 2013;382:1781–1794. doi: 10.1016/S0140-6736(13)62035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon W., Gagnon J.H. Sexual scripts: origins, influences and changes. Qual Sociol. 2003;26:491–497. [Google Scholar]
- 23.Mapp F., Wellings K., Hickson F., Mercer C.H. Understanding sexual healthcare seeking behaviour: why a broader research perspective is needed. BMC Health Serv Res. 2017;6:462. doi: 10.1186/s12913-017-2420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritz M.S., MacKinnon D.P. Required sample size to detect the mediated effect. Psychol Sci. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson A.M., UCL . 2015. National Survey of Sexual Attitudes and Lifestyles, 2010–2012. [data collection] [Google Scholar]
- 26.Woodhall S.C., Soldan K., Sonnenberg P. Is chlamydia screening and testing in Britain reaching young adults at risk of infection? Findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3) Sex Transm Infect. 2016;92:218–227. doi: 10.1136/sextrans-2015-052013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sexually transmitted infections and screening for chlamydia in England, 2017 Health Protection Report volume 12 number 20. https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables Available from:
- 28.Bocquier A., Fressard L., Verger P., Legleye S., Peretti-Watel P. Alcohol and cancer: risk perception and risk denial beliefs among the French general population. Eur J Public Health. 2017 doi: 10.1093/eurpub/ckx024. [published online April 28] [DOI] [PubMed] [Google Scholar]
- 29.Desgraz B., Collet T.-H., Rodondi N., Cornuz J., Carole Clair. Comparison of self-perceived cardiovascular disease risk among smokers with Framingham and PROCAM scores: a cross-sectional analysis of baseline data from a randomised controlled trial. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-012063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstein N.D., Marcus S.E., Moser R.P. Smokers'unrealistic optimism about their risk. Tob Control. 2005;14:55–59. doi: 10.1136/tc.2004.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senior K., Helmer J., Chenhall R., Burbank V. ‘Young clean and safe?’ young people's perceptions of risk from sexually transmitted infections in regional, rural and remote Australia. Cult Health Sex. 2014;16:453–466. doi: 10.1080/13691058.2014.888096. [DOI] [PubMed] [Google Scholar]
- 32.Balfe M., Brugha R. What concerns do young adults in Ireland have about attending health services for STD testing? Deviant Behav. 2011;32:320–350. [Google Scholar]
- 33.Pollack L.M., Boyer C.B., Weinstein N.D. Perceived risk for sexually transmitted infections aligns with sexual risk behavior with the exception of condom nonuse. Sex Transm Dis. 2013;40:388–394. doi: 10.1097/OLQ.0b013e318283d2e5. [DOI] [PubMed] [Google Scholar]
- 34.Stoner B.P., Whittington W.L.H., Aral S.O., Hughes J.P., Handsfield H.H., Holmes K.K. Avoiding risky sex partners: perception of partners' risks v partners' self reported risks. Sex Transm Infect. 2003;79:197–201. doi: 10.1136/sti.79.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limmer M. I don't shag dirty girls. Am J Mens Health. 2016;10:128–140. doi: 10.1177/1557988314559241. [DOI] [PubMed] [Google Scholar]
- 36.Tversky A., Kahneman D. Availability: a heuristic for judging frequency and probability. In: Kahneman D., Slovic P., Tversky A., editors. Judgement under uncertainty: heuristics and biases. Cambridge University Press; 1982. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables