Abstract
β-Catenin–dependent Wnt signaling controls numerous aspects of skeletal development and postnatal bone repair. Currently available transgenic Wnt reporter mice allow for visualization of global canonical Wnt signaling activity within skeletal tissues, without delineation of cell type. This is particularly important in a bone repair context, in which the inflammatory phase can obscure the visualization of mesenchymal cell types of interest. To tackle the issue of tissue-specific Wnt signaling, we have generated and characterized a transgenic mouse strain [termed paired related homeobox 1 (Prx1)–Wnt–green fluorescent protein (GFP), by crossing a previously validated Prx1-Cre strain with a nuclear fluorescent reporter driven by T-cell factor/lymphoid enhancer factor activity (Rosa26-Tcf/Lef-LSL-H2B-GFP)]. Prx1-Wnt-GFP animals were subject to three models of long bone and membranous bone repair (displaced forelimb fracture, tibial cortical defect, and frontal bone defect). Results showed that, irrespective of bone type, locoregional mesenchymal cell activation of Wnt signaling occurs in a defined temporospatial pattern among Prx1-Wnt-GFP mice. In summary, Prx1-Wnt-GFP reporter animals allow for improved visualization, spatial discrimination, and facile quantification of Wnt-activated mesenchymal cells within models of adult bone repair.
The canonical proteins of the Wnt signaling family regulate cellular proliferation, fate, and behavior in many contexts. Canonical Wnt signaling is mediated mainly by the multifunctional β-catenin protein, a potent coactivator of transcription factors such as lymphoid enhancer factor (LEF) and T-cell factor (TCF).1 Wnt proteins are critically involved in cell-to-cell signaling during mammalian development and skeletal repair.
Currently, transgenic mouse reporter strains are used to identify cells in the Wnt pathway active at specific times, which are important to fully elucidate how these growth factors elicit diverse cellular responses. Existing reporter strains for canonical Wnt signaling are based on transgenes expressing β-galactosidase or green fluorescent protein (GFP) under the control of either the Axin2 promoter or fusion of LEF/TCF binding sites and a minimal promoter. To monitor the formation of the β-catenin/TCF transcription complex, the Tcf optimal promoter–β-galactosidase transgenic mice were made by fusing three LEF/TCF binding sites to c-fos minimal promoter.2, 3 A second reporter strain uses β-catenin–activated transgene driving the expression of nuclear β-galactosidase, designed by fusing seven TCF/LEF binding sites upstream of a 0.13-kb fragment containing the minimal promoter-TATA box of the Siamois gene.4 The Axin2 reporter strain uses stable knock-in of the LacZ frame with the endogenous start codon to demonstrate Axin2 expression, which is a target and negative regulator of the canonical Wnt signaling pathway.5
Although these reporter strains have been useful in studying Wnt/β-catenin expression, they are limited by their expression in many cell types, which makes it difficult to visualize changes in canonical Wnt signaling in a particular cell type and variation in transgene expression. Fracture repair is a complex process that may involve a brisk inflammatory response absent in embryonic development. The local influx of inflammatory cells begins in the earliest phases of a fracture hematoma with polymorphonuclear leukocytes, accompanied by granulocytes, macrophages, or other immune cell populations that interact with native stromal progenitor cells during bone healing.6, 7 Multiple lines of investigation demonstrate that the immune-osteoblastic cross talk is of clear importance in bone repair. For example, nonsteroidal anti-inflammatory drugs are well known to have a quantifiable detrimental impact on fracture healing.8 Mast cells have been recently reported as critical mediators of bone repair.9 Improved methods to visualize signaling activity in immune versus mesenchymal tissues are a necessary first step in better study of lineage cross talk in bone repair. Although these existing Wnt reporter models are useful to understand targets of canonical Wnt signaling, further differentiation that characterizes expression within cells of mesenchymal origin can help to understand the biological function of Wnt signaling within a lineage-specific inflammatory milieu.
In this study, we describe the activation of Wnt signaling within mesenchymal cells in a healing adult mouse skeleton. To address this question, our transgenic mouse has reporter expression that depends on the removal of a loxP-stop-loxP cassette by Cre-mediated recombination. When crossed with paired related homeobox 1 (Prx1)–Cre mice, this model results in a mesenchymal-specific visualization of Wnt signaling that is useful in a variety of bone defect and fracture healing models.
Materials and Methods
Generation of Transgenic Reporter Mice
The Wnt reporter strain (Rosa26-Tcf/Lef-LSL-H2B-GFP) and the derivation of this Wnt reporter strain have been described in detail previously,10 and the strain was a kind gift from the Nathans Laboratory at Johns Hopkins University (Baltimore, MD). In brief, this reporter strain was generated by knocking in the histone H2B-GFP-6xmyc fusion protein under the control of 8xTCF-LEF binding sites and a minimal promoter into the Rosa26 locus. This reporter expression also requires removal of a loxP-stop-loxP cassette by Cre-mediated recombination. Transgenic Prx1-Cre Wnt reporter mice were produced from Prx1-Cre females (Jackson Laboratory, Bar Harbor, ME; stock number 005584) bred with Wnt reporter males. Mice were heterozygous for Wnt reporter allele. Transgenic mice were identified by analysis of genomic DNA from tail biopsy specimens by PCR. The following primer sequences were used: Rosa26, 5′-CACTTGCTCTCCCAAAGTCG-3′ (forward) and 5′-TAGTCTAACTCGCGACACTG-3′ (reverse); R26WntGFP, 5′-CTCAGAACCAAATCTGGTTCCATCC-3′ (forward) and 5′-ATGATCCAGACATGATAAGATACA-3′ (reverse); and Prx1-Cre, 5′-GAACCTGATGGACATGTTCAGG-3′ (forward) and 5′-AGTGCGTTCGAACGCTAGAGCCTGT-3′ (reverse).
Animal Care
All animal experiments were performed according to the approved protocol of the Animal Care and Use Committee at Johns Hopkins University. Prx1-Wnt-GFP reporter animals were housed in a single pathogen-free ventilated cage, fed a standard rodent chow diet, and provided tap water ad libitum. For long bone defects, 12-week–old animals of mixed sex were used. For calvarial defects, 8-week–old animals of mixed sex were used. Anesthesia was performed with 2% to 3% isoflurane in 100% oxygen at a flow rate of 1 L/minute, and animals were operated upon on a warm small animal surgery station. Postoperative monitoring was performed in accordance with institutional policy. Analgesia was administered using buprenorphine (0.1 mL/25 g body weight) via i.p. injection after surgery or forelimb fracture; subsequent injections were performed twice daily for 48 hours.
Tibial Monocortical Osteotomy
Hair overlying the lower limb was shaved, and the skin was aseptically prepared using betadine scrub. A skin incision was made over the medial aspect of the proximal tibia. A 1.0-mm–diameter drill hole was performed using a high-speed dental drill. The defect was performed at the distal end of the tibial crest through the medial cortex (unicortical defect). The skin was then sutured closed. The mice were weighed and examined daily for abnormal behavior or diminished food intake. Animals were sacrificed at 3, 7, 14, and 21 days postoperatively for analysis (n = 4 mice per time point).
Ulna and Radius Fractures by Mechanical Loading–Induced Displacement
Mechanical loading was performed by axially compressing the right forelimb by placing the olecranon process and flexed carpus into specially designed fixtures, adapted from prior publications.11 A dynamic loading system (Electroforce 3200 Series II; TA Systems, New Castle, DE) was used to apply force and monitor displacement. After confirming anesthesia, a 0.3 N compressive preload was applied, then the right forelimb was cyclically loaded with loading force ranging from 4 to 5 N while displacement was monitored. Loading was terminated when peak displacement for each mouse reached the loading displacement limit (control stop) of 4.5 mm, thus ensuring the mice sustain a complete fracture of the forelimb. Animals were sacrificed 7 days after fracture (n = 3 mice).
Frontal Bone Defect Generation
After aseptic preparation of the skin overlying the skull, a 1-cm sagittal incision was performed over the midline skull to expose the frontal bone. The overlying pericranium was gently removed from the right frontal bone. A 1.8-mm full-thickness frontal bone defect was generated using a microsurgical drill using a trephine drill bit. Meticulous care was taken to protect the neighboring posterofrontal and coronal sutures and the underlying dura mater. Calvarial defect sites were irrigated by saline. Finally, the skin was sutured and the animal was monitored per established postoperative protocols. Animals were sacrificed at 3, 7, 14, and 21 days after surgery for analysis (n = 3 mice per time point).
Micro–Computed Tomography Imaging
Samples were harvested from 3 to 21 days after injury, fixed in 4% paraformaldehyde for 24 hours, and scanned using a high-resolution micro–computed tomography imaging system (SkyScan 1294; Bruker MicroCT N.V., Kontich, Belgium). Scans were obtained at an image resolution of 10 μm, with the following settings: 1 mm of aluminum filter, X-ray voltage of 65 kVP, anode current of 153 μA, exposure time of 65 milliseconds, frame averaging of 4, and rotation step of 0.3 degrees. Three-dimensional images were then reconstructed from the two-dimensional X-ray projections by implementing the Feldkamp algorithm using a commercial software package, NRecon software (2.0.4.0 SkyScan; Bruker, Luxemburg, Belgium). For the three-dimensional morphometric analyses of images, CTVox (Bruker) and CTAn software (1.13 SkyScan; Bruker) programs were used.
Histologic Analysis
After imaging, samples were transferred to 14% EDTA for decalcification for 14 to 21 days. Samples were then embedded in OCT compound and sectioned in a coronal or sagittal plane at 10-μm thickness. Hematoxylin and eosin staining was performed on sections, as previously described.12 For Wnt-reporter immunofluorescence staining, additional sections were incubated with the following primary antibodies: anti-GFP (A-21311; Thermo Fisher Scientific, Waltham, MA). The frozen slices were washed with phosphate-buffered saline (PBS) three times for 10 minutes. Slides were then permeabilized with 0.5% Triton X-100 for 10 minutes. Antigen retrieval was performed by trypsin for 10 minutes at 37°C, then blocked with 1% bovine serum albumin in PBS for 1 hour at 25°C. Primary antibodies (1:200 in PBS + 0.1% Triton X-100 + 1% bovine serum albumin) were added to each section and incubated at room temperature for 1 hour and then overnight at 4°C. Sections were counterstained with DAPI mounting medium.
For dual immunofluorescence staining, additional sections were incubated with the following primary antibodies: anti-GFP (1:200; Ab-13970; Thermo Fisher Scientific), anti-aggrecan (1:1000; Ab-3778; Thermo Fisher Scientific), anti–Col-X (Ab-58632; Thermo Fisher Scientific), or anti-osteocalcin (OCN; 1:1000; Ab-93876; Thermo Fisher Scientific). They were also incubated with the appropriate secondary antibodies (Table 1). The frozen sections were washed with PBS three times for 10 minutes. Slides were then permeabilized with 0.5% Triton X-100 for 10 minutes. Antigen retrieval was performed by trypsin for 10 minutes at 37°C, then blocked with 5% goat serum in PBS for 1 hour at 25°C. Primary antibodies diluted with 5% goat serum in PBS were added to each section and incubated overnight at 4°C. Slides were then washed in PBS three times for 10 minutes. The appropriate secondary antibodies were then added to each slide for 1 hour at room temperature. Sections were counterstained with DAPI mounting medium. All histologic sections were examined under a Zeiss 780 confocal microscope (Zeiss, Thornwood, NY).
Table 1.
Antibodies Used for Immunologic Staining
| Antigen | Manufacturer, species, catalog no. | Dilution used | Type |
|---|---|---|---|
| GFP | Thermo Fisher Scientific, rabbit, A-21311 | 1:200 | Primary |
| GFP (used for double IHC studies) | Thermo Fisher Scientific, chicken, Ab-13970 | 1:200 | Primary |
| Aggrecan | Thermo Fisher Scientific, mouse, Ab-3778 | 1:1000 | Primary |
| Collagen X | Thermo Fisher Scientific, rabbit, Ab-58632 | 1:1000 | Primary |
| Osteocalcin | Thermo Fisher Scientific, rabbit, Ab-93876 | 1:1000 | Primary |
| Goat anti-chicken IgY | Thermo Fisher Scientific, goat, A-11039 | 1:200 | Secondary |
| Goat anti-mouse IgG | Thermo Fisher Scientific, goat, Ab-150119 | 1:1000 | Secondary |
| Goat anti-rabbit IgG | Vector Laboratories (Burlingame, CA), goat, DI-1594 | 1:1000 | Secondary |
GFP, green fluorescent protein; IHC, immunohistochemistry.
Quantification of Reporter Activity and Statistical Analysis
Reporter activity was quantified using Imaris 9.0.1 (Bitplane, Zurich, Switzerland). Surface rendering was performed with the following settings: smooth surface detail of 0.625 μm and background subtraction with diameter of largest sphere of 2 μm. Means and SDs were calculated from numerical data. In figures, bar graphs represent means, whereas error bars represent 1 SD. The statistical software SPSS for Windows version 18.0 (SPSS, Armonk, NY) was used for all statistical analyses. Statistical significance was determined at the P < 0.05 level. n = 10 images obtained from n = 4 individual samples per time point.
Results
Prx1-Wnt-GFP Reporter Activity after Long Bone Injury
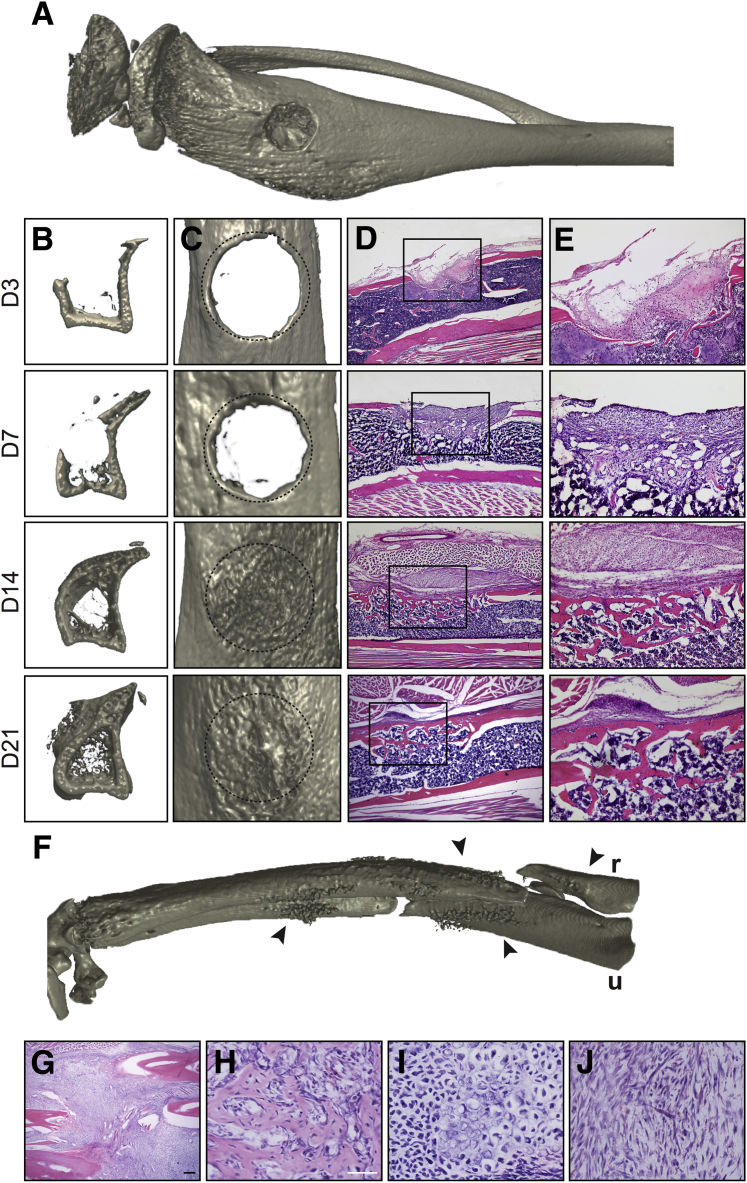
Long bone repair occurs over a well-chronicled timeline of stages, starting with early inflammation, progressing through a rapid period of bone formation through both intramembranous and endochondral pathways, and a later remodeling stage after bone union has been achieved. A monocortical tibial defect allows for visualization of all healing stages over a short time course, primarily via an intramembranous repair process (Figure 1). Herein, a circular defect of the proximal tibial metadiaphysis (Figure 1A) can be examined in axial cross section (Figure 1B) or from a top-down view (Figure 1C). The initial bone healing response is from the lateral defect edges, as seen on day 3. At day 7, more prominent ossification along the defect edges is present, along with nascent ossification of the defect site and new intramedullary and endosteal trabecular bone growth. At day 14, complete defect reossification is apparent, but with an immature and woven bone appearance when examined in a top-down view. By day 21, axial sections demonstrate continued intramedullary bone formation, whereas increased cortical remodeling imparts a smoother appearance to the defect surface. The histologic appearance of monocortical tibial defects mirrors the radiographic findings (Figure 1, D and E). At day 3 after injury, the defect site is populated by a fibroproliferative stroma with inflammatory cells, blood, and fibrin. At 7 days, a mixture of fibrous, chondroid, and osteoblastic cells is present, with early woven bone formation. At day 14, bony continuity is present, although the new bone formation is composed of thin trabeculae of a mixture of woven and lamellar bone types. At day 21, evidence of bone maturation and remodeling is seen, with increased corticalization of the defect site.
Figure 1.
Micro–computed tomography (microCT) and histologic appearance of long bone defect healing models. A–E: Tibial monocortical defect model. A: Three-dimensional microCT reconstruction, demonstrating a 1.0-mm monocortical defect in the medial tibial metaphysis. B: Axial cross-sectional reconstructions to encompass the defect site, as seen on the medial aspect of the tibia (left hand side), at days (D) 3, 7, 14, and 21 after injury. C: Top-down views of the tibial defect site, at D 3, 7, 14, and 21 after injury. Original defect margins outlined by dashed circles. D and E: Histologic appearance of tibial defect site by routine hematoxylin and eosin (H&E) staining at D 3, 7, 14, and 21 after injury (D), with corresponding high-magnification images (E). Boxed areas in D are shown at higher magnification in E. F–J: Complete displaced fracture of the forelimb model. F: Three-dimensional microCT reconstruction, demonstrating a midshaft displaced fracture of the radius (r) and ulna (u). Arrowheads indicate margins of fracture. G–J: Histologic appearance of fracture site by routine H&E staining at day 7 after fracture (G), with corresponding high-magnification images demonstrating the heterogeneity of this soft callus with foci of woven bone formation (H), mineralizing cartilage (I), and fibrous/granulation-type tissue (J). Scale bars: 200 μm (D and G); 25 μm (H–J). Original magnification: ×4 (D and G); ×10 (E); ×20 (H–J).
In contrast, a larger bony injury results in primarily endochondral repair (Figure 1, F–J). Herein, axial compression of the forelimb via mechanical loading results in a displaced complete fracture of the radius and ulna, which occurs in the midshaft of both bones (Figure 1F). At 7 days after forelimb fracture, a large soft callus is present at the fracture site (Figure 1G). High-magnification images of the fracture callus demonstrate a variety of cell types present, including foci of intramembranous ossification (Figure 1H), large areas of mineralizing cartilage (Figure 1I), and areas of fibrovascular stroma resembling granulation tissue (Figure 1J).
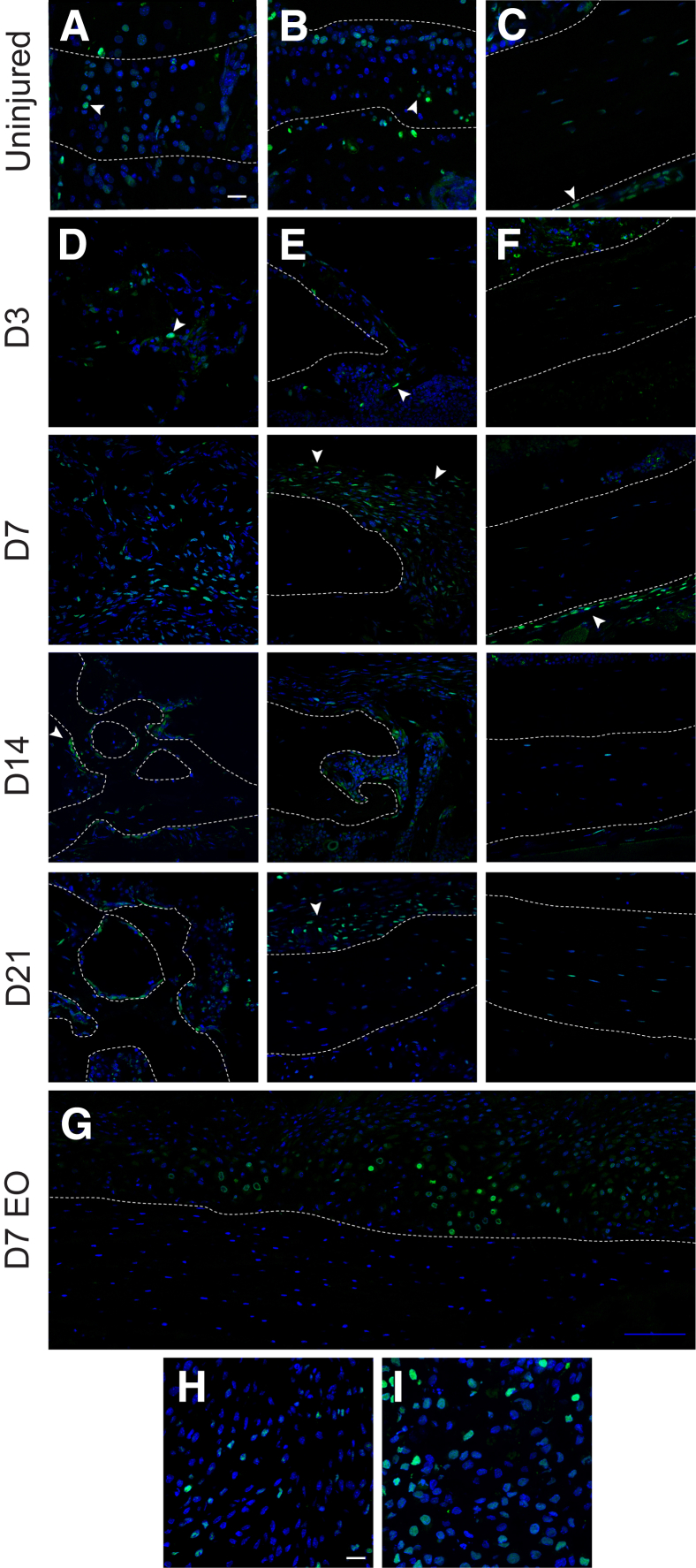
Prx1-Wnt-GFP reporter activity was next examined within either long bone injury model (Figure 2). First, Prx1-Wnt-GFP reporter activity was examined within the cortical tibial defect in comparison to the uninjured tibia (Figure 2, A–F). A survey of Prx1-Wnt-GFP activity in the uninjured tibia found GFP+ cells within the physeal chondrocytes (Figure 2A), articular chondrocytes (Figure 2B), and periosteum of the uninjured cortex (Figure 2C), as well as some reporter activity within bone lining endosteal/paratrabecular cells (not shown). In the tibial cortical defect, attention was paid to the midsubstance of the defect (Figure 2D), lateral defect edge (Figure 2E), and contralateral uninjured cortex (Figure 2F). These represented hot spots for reporter activity across the period of defect healing. Within the middefect substance (Figure 2D), scattered GFP+ stromal cells were present as early as day 3 after injury. Reporter activity markedly increased at day 7, with high numbers of GFP+ mesenchyme either with a fibroblastic morphology or associated with new formed osteoid. At days 14 and 21, bone lining osteoblasts within the ossified defect constituted the bulk of GFP+ cells, with occasional osteocytes also showing reporter activity. The defect edge was next examined (Figure 2E), a predominant site of defect reossification. At day 3, the defect edge was sharply demarcated and with only sporadic GFP+ cells present. At day 7, a marked increase in fibroblastic stromal cells was present, which was periosteal based and with a high amount of reporter activity. At day 14 and within the newly united bone shown, reporter activity was again most commonly observed among bone lining osteoblasts. At day 21, the neocortex was predominantly reporter negative, whereas the residual periosteal callus showed continued reporter activity. Although less conspicuous, Prx1-Wnt-GFP reporter activity was also seen on the uninjured contralateral cortex just adjacent to the defect site (Figure 2F). Herein, faint reporter activity was present along either the endosteum (as seen in day 3) or the periosteum (as seen in day 7), which returned to baseline at day 21 after injury. Next, attention was paid to tissue sections of the Prx1-Wnt-GFP reporter animals that underwent forelimb fracture—the more prominent endochondral model of bone repair (Figure 2, G–I). Tile scans of GFP+ reporter activity showed a clear increase in reporter activity within the soft callus in comparison to the injured native cortical bone (Figure 2G). High-magnification images demonstrated that the most obvious hot spots for Prx1-Wnt-GFP reporter activity were within either mesenchymal cells of the fibrovascular stroma (Figure 2H) or most prominently chondrocytes within the callus (Figure 2I).
Figure 2.
Prx1-Wnt-GFP reporter activity in long bone defect healing. A–C: Prx1-Wnt-GFP reporter activity within the uninjured tibia. Reporter activity is present within physeal chondrocytes (A), articular chondrocytes (B), and scattered periosteal cells (arrowheads) and some osteocytes of the uninjured tibial cortex (C). D–F: Prx1-Wnt-GFP reporter activity within the monocortical tibial defect model. Three areas within the defect site were focused on, including the middefect substance (D), defect edge (E), and contralateral cortex (F). D: Prx1-Wnt-GFP reporter activity within the middefect at days (D) 3, 7, 14, and 21 after injury. Reporter activity appears green, whereas DAPI counterstain appears blue. E: Prx1-Wnt-GFP reporter activity at the defect edge at D 3, 7, 14, and 21 after injury. F: Prx1-Wnt-GFP reporter activity in and around the uninjured (posterolateral) cortex at D 3, 7, 14, and 21 after injury. G–I: Prx1-Wnt-GFP reporter activity within the forelimb fracture model, which heals with prominent endochondral ossification (EO). Analysis performed at 7 days after fracture (D7 EO). G: Tile scan of the healing soft callus (above dashed line). H and I: High magnification of the fibrovascular stromal tissue (H) and cartilaginous components of the soft callus (I). Dashed lines indicate margins of bone, whereas arrowheads indicate representative reporter activity. Scale bars: 20 μm (A–F, H, and I); 100 μm (G). Original magnification, ×40 (A–I)
Quantification and Characterization of Prx1-Wnt-GFP Reporter Activity
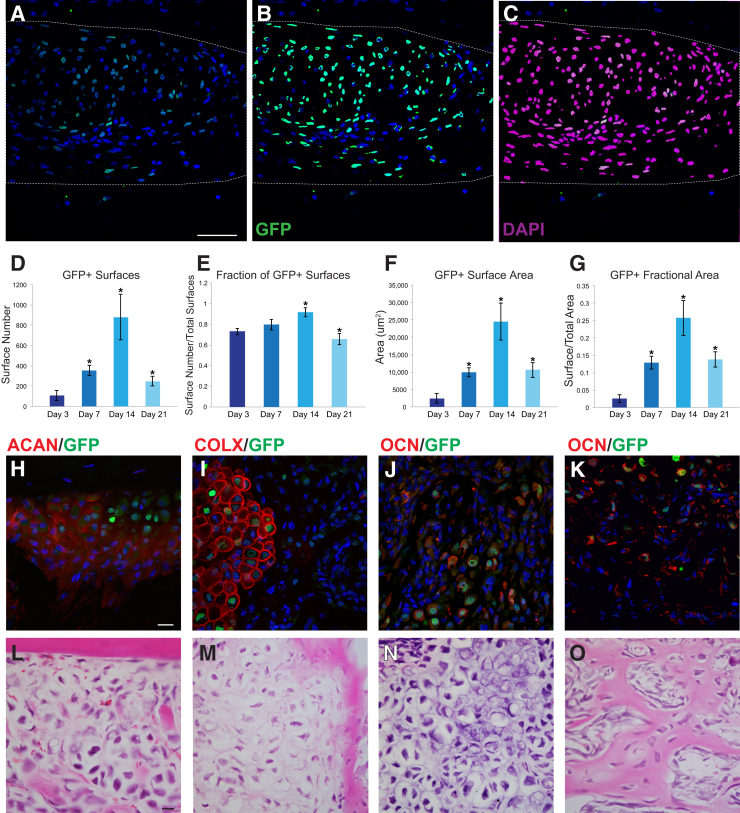
A main advantage of the Prx1-Wnt-GFP reporter system is the nuclear fluorescence that lends itself to easy and standardized quantification methods. The number, distribution, and location of GFP+ Wnt reporter signaling helps to illustrate activation of Wnt signaling within mesenchymal cells. This is demonstrated using the surface rendering tool within Imaris software. The create surface tool was used to make a solid surface best matching GFP+ expression using the interactive software histogram. An illustrative example of the defect is shown in Figure 3. A high magnification of the defect site is shown in Figure 3, A–C. The original image (Figure 3A), GFP+ surface rendering (Figure 3B), and DAPI+ surface rendering (Figure 3C) demonstrate the criteria used for quantification. Dashed lines indicate the area of interest. A dramatic increase in the number of GFP+ surfaces (cells) was observed from 3 to 14 days after injury, followed by a reduction in GFP+ surface number as reossification at day 21 (Figure 3D). When normalized to total DAPI+ surfaces (total cell number), reporter activity was detectable within 66% to 92% of total cells across time points of healing (Figure 3E). Again, reporter activity was most robustly observed at day 14 after injury (detectable in 92% of surfaces/cells). GFP+ surface area was next evaluated (Figure 3F), which is reflective of both the number of reporter positive cells as well as their individual intensity of fluorescence. GFP+ surface area again demonstrated a marked peak at 14 days after injury (Figure 3F). These trends were essentially unchanged when GFP+ surface area was normalized to the total area within the region of interest (Figure 3G).
Figure 3.
Quantification and immunohistochemical characterization of Prx1-Wnt-GFP reporter activity during long bone healing. A–G: Quantification of temporal differences in Prx1-Wnt-GFP reporter activity within the tibial monocortical defect model. A: Representative image of Prx1-Wnt-GFP reporter activity within the tibial bone defect site (day 21 shown). Reporter activity appears green, whereas DAPI counterstain appears blue. Dashed lines demarcate the region of interest. B: Imaris surface rendering of GFP+ surfaces of the same image, used to quantify the number of Prx1-Wnt-GFP reporter positive cells/surfaces. C: Imaris surface rendering of DAPI+ nuclei of the same image, used to quantify the total number of cells/surfaces, shown in purple. D: Total number of GFP+ surfaces. E: Total number of GFP+ surfaces normalized to total number of DAPI+ surfaces. F: Total GFP+ surface area. G: Total GFP+ surface area normalized to area of the region of interest. H–K: Immunohistochemical characterization of Prx1-Wnt-GFP reporter activity within long bone injury models. H–J: Within forelimb fracture model that demonstrates endochondral ossification, immunolocalization of aggrecan (ACAN; H), type X collagen (COLX; I), and osteocalcin (OCN; J). Immunohistochemical staining appears red, whereas Prx1-Wnt-GFP reporter activity appears green. DAPI counterstain appears blue. K: Within tibial defect model that demonstrates intramembranous healing, immunolocalization of OCN. L–O: Hematoxylin and eosin images are shown for sections corresponding with the above immunofluorescence images. In all cases, immunostaining performed at day 7 after injury. ∗P < 0.05 versus day 3. Scale bars = 20 μm (A–C and H–O).
The Prx1-Wnt-GFP reporter also allows for easy immunofluorescence colocalization with proteins of interest in osteochondrogenic differentiation (Figure 3, H–K). First, tissue sections of the forelimb fracture with prominent cartilage were costained with markers of endochondral ossification (Figure 3, H–J), including aggrecan, type X collagen, and OCN. Results showed a high degree of correlation between chondrocytic markers and Prx1-Wnt-GFP reporter activity, especially with the markers of chondrocyte hypertrophy type X collagen (Figure 3I) and OCN (Figure 3J). For example, quantitative analysis showed type X collagen immunoreactivity among 63.5% of GFP+ cells (±3.4%) in high-powered fields of forelimb fracture sections. Likewise, quantitative analysis showed OCN immunoreactivity among 65.8% of GFP+ cells (±12.8%) in similar high-powered fields. Similar immunohistochemical stains were performed on the cortical tibial defect, which heals without a conspicuous cartilaginous intermediate (Figure 3K). Herein, colocalization of OCN in bone lining cells with Prx1-Wnt-GFP reporter activity was also seen. Analogous to the findings within the forelimb fracture model, on average, OCN immunoreactivity was observed in 60.5% of GFP+ cells (±17.6%). Hematoxylin and eosin staining of corresponding adjacent sections of the immunohistochemical stain is shown in Figure 3, L–O. Thus, a high percentage of Prx1-Wnt-GFP reporter expression is seen in cells with markers of osteochondrogenic differentiation, particularly markers of osteocartilaginous matrix and chondrocyte hypertrophy.
Prx1-Wnt-GFP Reporter Activity after Dermal Bone Injury
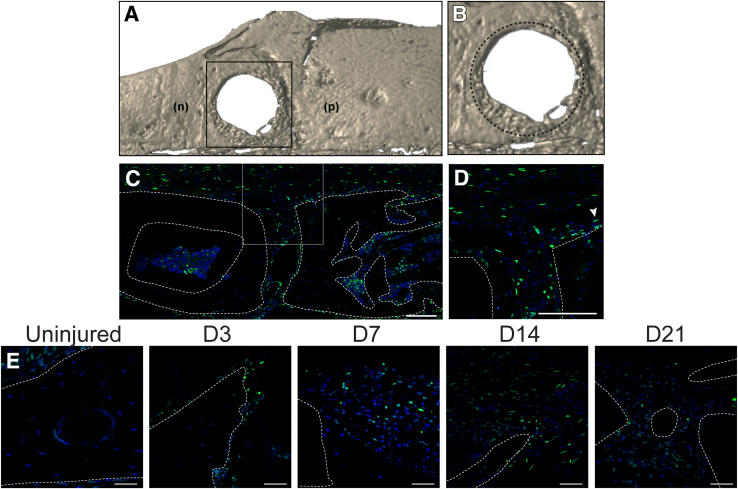
Although Prx1 is most commonly thought of as a limb bud mesenchyme reporter, Prx1 expression extends to the craniofacial skeleton as well.13 The use of the Prx1-Wnt-GFP reporter system in cranial healing—bones that are formed and heal without a cartilage intermediate—was next validated. A full-thickness frontal bone was used, which exemplifies another healing bone defect model.14 Herein, significant healing occurs over a 3-week period, as shown by micro–computed tomography reconstructions (Figure 4, A and B). To ensure Prx1-Wnt-GFP reporter activity within the skull, the uninjured sagittal suture was first examined (a previously reported domain of Wnt signaling activity15 and Prx1 expression13). As expected, robust reporter activity was present within the sagittal suture, associated periosteum, and bone lining cells within the marrow/diploic space (Figure 4, C and D). Interestingly, a similar timeline of Prx1-Wnt-GFP reporter activity was observed before and after frontal bone injury. Scattered and infrequent Prx1-Wnt-GFP reporter activity was found in the uninjured frontal bone (Figure 4E). However, after injury, the primary hot spot is found at the medial and lateral bone defect edges (Figure 4E). Herein and in keeping with the tibial injury model, isolated reporter activity was present at day 3 after injury. Stromal fibroblastic cells at days 7 and 14 showed a marked increase in GFP+ reporter activity at the defect edge. Because bone formation was apparent, Prx1-Wnt-GFP reporter activity became more restricted in localization to bone lining osteoblasts.
Figure 4.
Prx1-Wnt-GFP reporter activity in frontal bone defect healing. A and B: Micro–computed tomography reconstruction of frontal bone defect, in a top-down view with high magnification. Boxed area in A is shown at higher magnification in B. Dashed circle represents original bone defect. C: Prx1-Wnt-GFP reporter activity within the intact sagittal suture. Sagittal suture shown in coronal cross section, with parietal bone edges demarcated by dashed white lines. Boxed area is show at higher magnification in D. D: High-magnification image. D and E: Arrowhead (D) indicates representative reporter activity of Prx1-Wnt-GFP at the calvarial defect edge, before injury and at days (D) 3, 7, 14, and 21 after injury (E). The bone defect edge/bony margins are demarcated by dashed white lines. Scale bars: 100 μm (C and D); 50 μm (E). n, nasal bone; p, parietal bone.
Discussion
In summary, the Prx1-Wnt-GFP reporter system allows for isolated examination of canonical Wnt reporter signaling activity within the skeletal mesenchyme. This murine model exhibits strong and specific nuclear fluorescent reporter activity,10 with specificity for mesenchymal rather than inflammatory/hematopoietic cell types that lie in contrast to other Wnt signaling reporters.2, 3, 4, 16 In the present brief communication, the utility of Prx1-Wnt-GFP mice for quantitative and spatiotemporal Wnt signaling documentation in two models of adult bone healing was observed. Strikingly, a similar spatiotemporal pattern of Wnt signaling up-regulation was observed within both intramembranous and endochondral bone injury models.
Potential use of Prx1-Wnt-GFP reporter animals within skeletal biology exceeds bone defect repair, and this could encompass many other contexts in which Prx1 expression domains exist within the adult skeleton—including the articular cartilage,17, 18 the cranial sutures,13, 19 or long bone periosteum.20, 21 In particular, Prx1-Wnt-GFP mice may be used in the study of models of articular cartilage regeneration or degeneration, examination of Wnt signaling during normal and premature cranial suture fusion, or periosteal reactive bone formation in response to stress fracture or mechanical loading. Thus, the Prx1-Wnt-GFP mouse strain may be used to report on canonical Wnt signaling activity in a lineage-specific manner across areas in skeletal biology.
Acknowledgments
We thank the Johns Hopkins University School of Medicine Microscope Facility for technical assistance and the Nathans Laboratory at Johns Hopkins University for providing the Rosa26-Tcf/Lef-LSL-H2B-GFP strain.
L.C. and L.Z. collected data and wrote the manuscript; J.X., C.A.M., N.Y., and E.Z. collected data; A.W.J. provided financial support and approved the manuscript.
Footnotes
Supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01 AR070773 (A.W.J.) and K08 AR068316 (A.W.J.), US Army Medical Research Acquisition Activity through the Peer Reviewed Medical Research Program W81XWH-180109121 (A.W.J.), American Cancer Society Research Scholar grant RSG-18-027-01-CSM (A.W.J.), the Orthopedic Research and Education Foundation (with funding provided by the Musculoskeletal Transplant Foundation; A.W.J.), the Maryland Stem Cell Research Foundation (A.W.J.), and the Musculoskeletal Transplant Foundation (A.W.J.).
L.C. and L.Z. contributed equally to this work.
Disclosures: None declared.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.07.003.
Supplemental Data
References
- 1.Cadigan K.M., Waterman M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012;4:a007906. doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DasGupta R., Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 3.Hens J.R., Wilson K.M., Dann P., Chen X., Horowitz M.C., Wysolmerski J.J. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005;20:1103–1113. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- 4.Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A.B., Volpin D., Bressan G.M., Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van de Wetering M., Clevers H., Schlag P.M., Birchmeier W., Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kon T., Cho T.J., Aizawa T., Yamazaki M., Nooh N., Graves D., Gerstenfeld L.C., Einhorn T.A. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 7.Loi F., Cordova L.A., Pajarinen J., Lin T.H., Yao Z., Goodman S.B. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagi H.C., Jordan C.J., Barei D.P., Serrano-Riera R., Steverson B. Indomethacin prophylaxis for heterotopic ossification after acetabular fracture surgery increases the risk for nonunion of the posterior wall. J Orthop Trauma. 2014;28:377–383. doi: 10.1097/BOT.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 9.Kroner J., Kovtun A., Kemmler J., Messmann J.J., Strauss G., Seitz S., Schinke T., Amling M., Kotrba J., Froebel J., Dudeck J., Dudeck A., Ignatius A. Mast cells are critical regulators of bone fracture-induced inflammation and osteoclast formation and activity. J Bone Miner Res. 2017;32:2431–2444. doi: 10.1002/jbmr.3234. [DOI] [PubMed] [Google Scholar]
- 10.Cho C., Smallwood P.M., Nathans J. Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogenesis and blood-brain barrier regulation. Neuron. 2017;95:1056–1073.e5. doi: 10.1016/j.neuron.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez M.D., Schmid G.J., McKenzie J.A., Ornitz D.M., Silva M.J. Healing of non-displaced fractures produced by fatigue loading of the mouse ulna. Bone. 2010;46:1604–1612. doi: 10.1016/j.bone.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X., Péault B., Chen W., Li W., Corselli M., James A.W., Lee M., Siu R.K., Shen P., Zheng Z., Shen J., Kwak J., Zara J.N., Chen F., Zhang H., Yin Z., Wu B., Ting K., Soo C. The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng A. 2011;17:2497–2509. doi: 10.1089/ten.tea.2010.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilk K., Yeh S.A., Mortensen L.J., Ghaffarigarakani S., Lombardo C.M., Bassir S.H., Aldawood Z.A., Lin C.P., Intini G. Postnatal calvarial skeletal stem cells expressing PRX1 reside exclusively in the calvarial sutures and are required for bone regeneration. Stem Cell Reports. 2017;8:933–946. doi: 10.1016/j.stemcr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quarto N., Wan D.C., Kwan M.D., Panetta N.J., Li S., Longaker M.T. Origin matters: differences in embryonic tissue origin and Wnt signaling determine the osteogenic potential and healing capacity of frontal and parietal calvarial bones. J Bone Miner Res. 2010;25:1680–1694. doi: 10.1359/jbmr.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruyama T., Jeong J., Sheu T.J., Hsu W. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat Commun. 2016;7:10526. doi: 10.1038/ncomms10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Alam D., Green M., Tabatabai Irani R., Parsa S., Danopoulos S., Sala F.G., Branch J., El Agha E., Tiozzo C., Voswinckel R., Jesudason E.C., Warburton D., Bellusci S. Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2(LacZ) during murine lung development and repair. PLoS One. 2011;6:e23139. doi: 10.1371/journal.pone.0023139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taniguchi Y., Kawata M., Ho Chang S., Mori D., Okada K., Kobayashi H., Sugita S., Hosaka Y., Inui H., Taketomi S., Yano F., Ikeda T., Akiyama H., Mills A.A., Chung U.I., Tanaka S., Kawaguchi H., Saito T. Regulation of chondrocyte survival in mouse articular cartilage by p63. Arthritis Rheumatol. 2017;69:598–609. doi: 10.1002/art.39976. [DOI] [PubMed] [Google Scholar]
- 18.Blaney Davidson E.N., van de Loo F.A., van den Berg W.B., van der Kraan P.M. How to build an inducible cartilage-specific transgenic mouse. Arthritis Res Ther. 2014;16:210. doi: 10.1186/ar4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo H.S., Serra R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol. 2007;310:304–316. doi: 10.1016/j.ydbio.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murao H., Yamamoto K., Matsuda S., Akiyama H. Periosteal cells are a major source of soft callus in bone fracture. J Bone Miner Metab. 2013;31:390–398. doi: 10.1007/s00774-013-0429-x. [DOI] [PubMed] [Google Scholar]
- 21.Kawanami A., Matsushita T., Chan Y.Y., Murakami S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem Biophys Res Commun. 2009;386:477–482. doi: 10.1016/j.bbrc.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.