Abstract
Background
Pulmonary sarcomatoid carcinoma is a rare, poorly differentiated, and highly aggressive type of non-small cell lung cancer. High tumor mutational burden and PD-L1 overexpression make it an excellent candidate for immunotherapy.
Objectives and Method
We presented the case of a patient who underwent left inferior lobectomy with concurrent right paravertebral muscular metastasectomy for an infiltrative neoplastic mass, whose final diagnosis was consistent with stage IV pulmonary sarcomatoid carcinoma. He received first- and second-line chemotherapy without any benefit. Since March 2016, he has been treated with the anti-PD1 agent nivolumab with a dramatic improvement of symptoms, disappearance of a brain lesion, and partial response on other metastatic sites. He tolerated the treatment well and is still responding after 22 months from the beginning.
Results and Conclusions
In very lethal non-small cell lung cancer subtypes such as the sarcomatoid variants, high tumor burden and deteriorated general conditions should not preclude, at least in some cases, the use of immunotherapy. Anti-PD1 may also have a reliable role in disease control in the brain. Lastly, the strong rationale behind sarcomatoid histology should further prompt trials exploring immunotherapeutic approaches in this subset of non-small cell lung cancer.
Keywords: Immunotherapy, Lung cancer, Pulmonary sarcomatoid carcinoma, Brain metastasis
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare, poorly differentiated, and highly aggressive tumor entity accounting for less than 3% of non-small cell lung cancer (NSCLC) [1]. Early metastatic dissemination and resistance to conventional therapies result in a prognosis usually poorer than other lung cancers [2]. Recently, the targeting of immunologic checkpoints turned out to be a major breakthrough in oncology. Nivolumab is a fully human IgG4 PD-1 blocking antibody approved for previously treated advanced NSCLC [3, 4]. High tumor mutational burden and PD-L1 overexpression make PSC an excellent candidate for immunotherapy [5]. However, only anecdotal case reports on anti-PD1 efficacy in PSC have been described so far [6].
Case Report
On November 2015, a 74-year-old male with ECOG PS 0 and no smoking history underwent left inferior lobectomy with concurrent right paravertebral muscular metastasectomy for a large infiltrative neoplastic mass, whose final diagnosis was consistent with stage IV PSC (Table 1). Medical history also reports HCV-related hepatitis successfully treated with PEG interferon in 2005 and a melanoma of the scalp surgically removed in 2013.
Table 1.
Histopathological and molecular features of the lung cancer biopsy
| Feature/biomarker | Results |
|---|---|
| MNF-116 | –/+ |
| AE1/AE3 | –/+ |
| EMA | –/+ |
| CK7 | – |
| TTF1 | – |
| Vimentin | + (mainly in sarcomatoid component) |
| P63 | – |
| Napsin | – |
| Calretinin | –/+ |
| D240 | – |
| WT1 | –/+ |
| HMB45 | – |
| S100 | – |
| Ki67 | 40% |
| ALK (D5F3 clone) | negative |
| ROS1 rearrangements (clone D4D6) | negative |
| MET IHC and FISH | negative |
| 30-gene next-generation sequencing | no mutations detected |
At histology, the tumor was characterized by mixed non-small cell carcinoma and sarcoma. The epithelial component showed highly pleomorphic tumor cells with striking nuclear atypia, including atypical mitoses and large area of necrosis and moderate reactive inflammation. Sarcoma-like elements (spindle and giant cells) were present in about half of neoplasia. A large panel of antibodies was used in order to well characterize the tumor and rule out other tumors, firstly melanoma, given his past medical history (Table 1). Tumor samples were analyzed by 30-gene next-generation sequencing, but no mutations were detected. ALK (D5F3 clone) and ROS1 rearrangements (D4D6 clone) and MET investigated either by immunohistochemistry or fluorescence in situ hybridization were also negative. Interestingly, immunohistochemistry for PDL-1 (clones SP263 by Ventana Medical System and E1L3N by Cell Signaling) showed a strong expression both in tumoral cells (tumor proportion score ≥50%) and in inflammatory cells (Fig. 1).
Fig. 1.
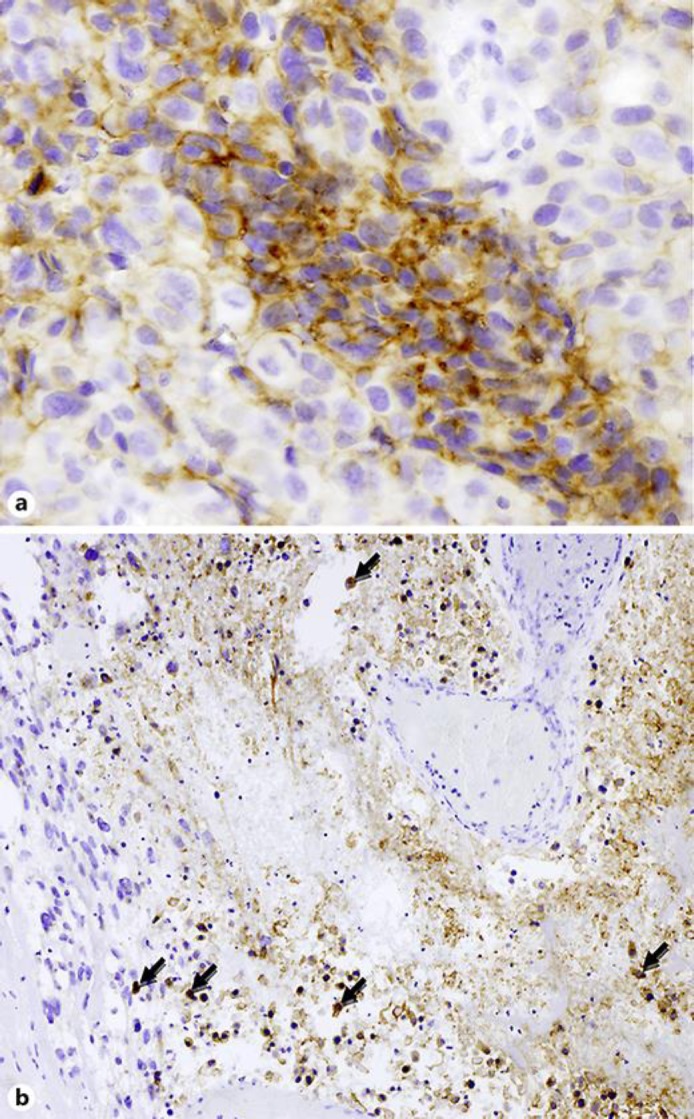
Immunohistochemistry for PDL-1 (clones SP263 by Ventana Medical System and E1L3N by Cell Signaling) showing a strong expression in both tumoral cells (a, tumor proportion score ≥50%; original magnification ×200) and inflammatory cells inside an area of tumor necrosis (b, macrophages and lymphocytes with arrows; original magnification ×100).
On January 2016, following surgery, the patient initiated a first-line chemotherapy regimen consisting of carboplatin/pemetrexed at conventional doses. After cycle 1, he experienced grade 2 nausea and vomiting and complained of abdominal right-sided pain. A computed tomography (CT) scan was then performed showing progressive disease with the appearance of lung, adrenal, liver, and lymph node metastases. A second-line chemotherapy with weekly docetaxel was then started, although the patient's general health conditions rapidly deteriorated and diffuse pain worsened requiring opioids.
After 2 cycles with docetaxel, a CT scan demonstrated the appearance of a brain lesion and an increase in size of lung, hepatic, and right paravertebral muscle lesions.
In March, the patient started receiving the anti-PD1 agent nivolumab at the recommended dose of 3 mg/kg every 2 weeks within an expanded access program. After only one administration, he experienced a dramatic improvement of symptoms associated with a rapid decrease of LDH and a progressive tapering of opioids therapy until discontinuation. A subsequent reassessment by CT scan showed disappearance of the brain lesion and partial response on other sites (Fig. 2). Since December 2016, according to the patient's request, nivolumab administration has been modified to a 3-weekly schedule. Treatment was well tolerated, except for grade 1 transient cutaneous rash, vitiligo on the hands, and a slow recovery from pneumonia successfully treated by the combination of oral steroids (prednisone maximum dose 50 mg for no more than 10 days) and antibiotics on July 2017 (Fig. 3). Currently, at the end of July 2018, 28 months after the initiation of the anti-PD1, the patient is still on therapy and further responding.
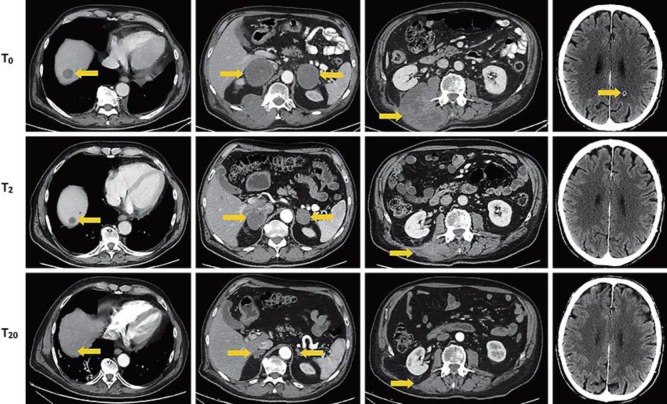
Fig. 2.
Abdomen and brain CT scans. T0 images are related to the clinical status before nivolumab (March 21, 2016), T2 images refer to the situation after 2 months (May 20, 2016), and T20 images describe the tumor after 20 months (November 16, 2017). In the left column, images refer to the CT follow-up on the hepatic lesion (arrow), then the adrenal gland lesions (arrow), and the lesion of right paravertebral muscle (arrow). In the right column, images refer to the CNS lesion (arrow) before nivolumab therapy (T0), after 4 cycles (T2), and after 34 cycles (T20).
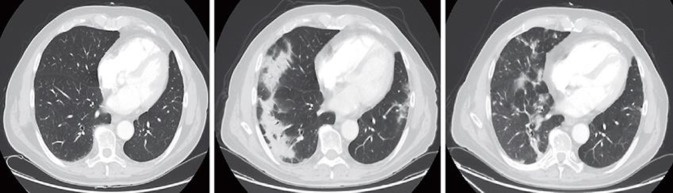
Fig. 3.
Pulmonary CT scans. Images refer to the slow recovery from pneumonia from May 11 to July 17 to November 16, 2017 (from left to right).
Discussion and Conclusion
To our knowledge, the case presented herein is adding valuable insights into the use of a checkpoint inhibitor in very lethal NSCLC subtypes, such as the sarcomatoid variants. Firstly, high tumor burden and deteriorated general conditions should not preclude the use of immunotherapy as, at least in some cases, it may result in rapid tumor shrinkage together with profound and lasting response. Secondly, anti-PD1 may have a reliable role in disease control also in the brain, despite earlier concerns about its capability of crossing the blood-brain barrier. Lastly, the strong rationale behind sarcomatoid histology should further prompt trials exploring immunotherapeutic approaches in this subset of NSCLC.
Other open questions remain to be answered. For instance, whether the prior therapy with PEG interferon could have acted as trigger for T cells thus boosting nivolumab activity and, again, whether changing the treatment schedule to 3-weekly could be a safe, effective, and cost-saving way to deliver immune checkpoint inhibitors while improving patients' distress.
Statement of Ethics
The authors have no ethical conflicts to disclose
Disclosure Statement
The authors have no conflicts of interest to declare.
Author Contributions
Massimiliano Salati, Cinzia Baldessari, Giulia Grizzi, Stefano Cascinu, Massimo Dominici, and Fausto Barbieri: medical oncologists in charge of the patient; Fiorella Calabrese and Giulio Rossi: pathologists in charge of the tumor specimen analyses; Elisa Pettorelli: data manager.
Acknowledgement
Bristol-Myers-Squibb Italy for providing nivolumab.
References
- 1.Yendamuri S, Caty L, Pine M, Adem S, Bogner P, Miller A, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery. 2012 Sep;152((3)):397–402. doi: 10.1016/j.surg.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Shum E, Stuart M, Borczuk A, Wang F, Cheng H, Halmos B. Recent advances in the management of pulmonary sarcomatoid carcinoma. Expert Rev Respir Med. 2016 Mar;10((4)):1–10. doi: 10.1586/17476348.2016.1157475. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015 Oct;373((17)):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373((2)):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrock AB, Li SD, Frampton GM, Suh J, Braun E, Mehra R, et al. Pulmonary Sarcomatoid Carcinomas Commonly Harbor Either Potentially Targetable Genomic Alterations or High Tumor Mutational Burden as Observed by Comprehensive Genomic Profiling. J Thorac Oncol. 2017 Jun;12((6)):932–42. doi: 10.1016/j.jtho.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Gounant V, Brosseau S, Naltet C, Opsomer MA, Antoine M, Danel C, et al. Nivolumab-induced organizing pneumonitis in a patient with lung sarcomatoid carcinoma. Lung Cancer. 2016 Sep;99:162–5. doi: 10.1016/j.lungcan.2016.07.010. [DOI] [PubMed] [Google Scholar]