Abstract
This commentary highlights the article by Lin et al that demonstrates the therapeutic potential of small-molecule atypical protein kinase C inhibitors in inflammatory ocular disease.
The retina is a complex and sensitive tissue responsible for converting light energy to a visual signal. Retinal neurons require both abundant metabolic substrates and a highly specialized defined microenvironment. Extensive retinal and choroidal microvascular networks are essential to supply adequate oxygen and nutrients to retinal neurons. The permeability of retinal blood vessels is tightly regulated to provide a selectively permeable barrier to maintain a microenvironment that is osmotically balanced, is immune privileged, and contains appropriate concentrations of ions and neurotransmitters. Under pathologic conditions, the permeability of the retinal microvasculature increases, resulting in blood-retinal barrier (BRB) breakdown.
Endothelial tight junctions are a critical component of the BRB. A variety of tight junction proteins, including signaling, membrane, and scaffolding proteins, are essential for maintenance of endothelial tight junctions. Vascular endothelial growth factor (VEGF) signaling phosphorylates tight junction proteins, targeting protein complexes for endosomal recycling and proteasomal degradation. Conversely, tumor necrosis factor-α (TNF-α) activates NF-κB to suppress transcription of tight junction proteins. Although VEGF and TNF-α regulate vascular permeability through distinct mechanisms and potentially at distinct stages of disease, both pathways are dependent on atypical protein kinase C (aPKC), and are inactivated by aPKC inhibition.
The aPKC isoforms (ζ and λ/ι) are a part of the PKC family that is central to diverse processes, such as development, proliferation, migration, cell survival, and cell polarity. aPKCs play a role in the innate immune response, and PKCζ is essential for TNF-α activation of NF-κB signaling and subsequent transcriptional suppression of tight junction proteins.1 When activated by VEGF signaling, aPKCs directly phosphorylate tight junction proteins, targeting these complexes for degradation.2 aPKC inhibition, therefore, alleviates both VEGF and TNF-α–mediated BRB breakdown, so it may have a therapeutic advantage over anti-VEGF or anti–TNF-α monotherapy.
A recent study, highlighted in this issue of The American Journal of Pathology, evaluates the therapeutic potential of aPKC inhibition in retinal vascular permeability driven by inflammation.3 Lin et al3 demonstrate that genetic and small-molecule aPKC inhibition represses vascular permeability and retinal edema in ischemia/reperfusion injury and in VEGF/TNF-α–mediated BRB breakdown. Furthermore, aPKC inhibition decreased the retinal innate immune response in ischemia/reperfusion, decreasing immune cell infiltration and expression of inflammatory genes.3 This study contributes to mechanistic understanding of sterile inflammation-mediated vascular permeability and demonstrates that small-molecule aPKC inhibitors have therapeutic potential in inflammatory ocular disease.
The purpose of this commentary is to provide a broad perspective on the etiology and pathologic role of BRB breakdown in ocular disease, and of the therapeutic potential of aPKC inhibition in both growth factor– and cytokine-driven vascular permeability. We furthermore discuss the therapeutic potential of aPKC inhibitors in common ocular diseases and potential applications of aPKC inhibitors that have not yet been explored. Finally, we discuss as yet unanswered questions regarding the role of inflammation and aPKC in ocular disease.
Breakdown of the Blood-Retinal Barrier
The retina is responsible for converting light to an electrical signal that is transmitted to the brain in the process of phototransduction. The retina is a complex and heterogeneous tissue, and a variety of retinal neurons are required for phototransduction. Photoreceptors are located in the posterior outer nuclear layer, and are responsible for converting light to an electrical signal. The inner nuclear layer is composed of secondary and tertiary neurons, such as bipolar, horizontal, and amacrine cells, and the ganglion cell layer located in the innermost retinal layer is composed of ganglion cells. Müller glia and astrocytes provide metabolic support to retinal neurons and facilitate communication between retinal blood vessels and neurons. Microglia function as resident immune cells in the retina, monitoring the retinal microenvironment and modulating interaction with circulating immune cells.4
Retinal neurons are metabolically demanding and, therefore, require an extensive blood supply to deliver sufficient energy substrates and oxygen. The inner retinal blood supply stems from the central retinal artery, which branches into three vascular plexuses that span the inner retina. The choroid capillary bed, located posterior to the retina, is the blood supply for photoreceptors, which are located in the outer nuclear layer.
Retinal neurons require a defined environment that is osmotically balanced, is immune privileged, and has an appropriate concentration of extracellular ions. To maintain this environment, retinal and choroidal blood vessels, together with the retinal pigment epithelium, form the BRB, which is selectively permeable and controls the retinal microenvironment under endogenous conditions.5 The BRB also prevents infiltration of circulating immune cells, maintaining the immune privilege of the retina. As demonstrated in the presently highlighted study by Lin et al,3 infiltration of immune cells is a major pathology of BRB breakdown, and exacerbates local inflammatory signaling.
The endothelial tight junction complex is an important component of the BRB. Several dozen proteins, including membrane, scaffolding, and signaling proteins, are required for maintenance of endothelial tight junctions.5 Particularly important to the BRB are occludins and claudins, which are transmembrane proteins, and zonula occludens proteins, which are scaffolding proteins.6 Both the abundance and localization of tight junction proteins are essential for proper maintenance of the blood-retinal barrier.
Under acute injury or chronic disease conditions, the BRB is weakened, facilitating vascular permeability.6 Acute changes in BRB function, for example in response to injury, are protective, allowing infiltration of immune cells to attack invading pathogens or phagocytize damaged neurons. However, chronic breakdown of the BRB is detrimental to retinal function and visual acuity. Retinal edema, particularly in the macula, compromises visual acuity. Loss of the defined retinal microenvironment compromises the function and survival of retinal neurons. Furthermore, chronic infiltration of immune cells exacerbates inflammation and resultant inflammatory pathologies. Interventions targeting inflammation, such as those revealed in the highlighted study by Lin et al,3 therefore alleviate BRB and resultant pathologies.
BRB breakdown is central to many ocular diseases, including diabetic retinopathy, ischemic retinopathy, ischemia/reperfusion injury, age-related macular degeneration, and retinal vein occlusions.7, 8, 9 The upstream mechanisms for BRB breakdown are context dependent, but the downstream effectors that facilitate breakdown of tight junctions are similar across multiple ocular diseases. Two primary regulators of BRB breakdown are vascular growth factors and inflammatory mediators, which are secreted by stressed neurons and glia, and in some cases by adherent/infiltrating immune cells.6
VEGF promotes vascular permeability and pathologic angiogenesis, and it is secreted by neurons, glia, and the RPE under hypoxic, oxidative, metabolic, or inflammatory stress. VEGF binds to VEGF receptors in endothelial cells, which activate signaling cascades that ultimately phosphorylate the tight junction proteins occludin and VE-cadherin, targeting these proteins for ubiquitination and proteasomal degradation.2, 10 Furthermore, ubiquitinated occludin modulates internalization of other tight junction proteins for endosomal recycling, further facilitating BRB breakdown.10 Accordingly, anti-VEGF therapy alleviates BRB breakdown, and it is a common treatment for diabetic macular edema, age-related macular degeneration, retinal vein occlusion, and other retinal microvascular diseases involving BRB breakdown.7, 8, 9 However, some patients are refractory to anti-VEGF therapy, presumably because BRB breakdown in these patients occurs via VEGF-independent mechanisms.
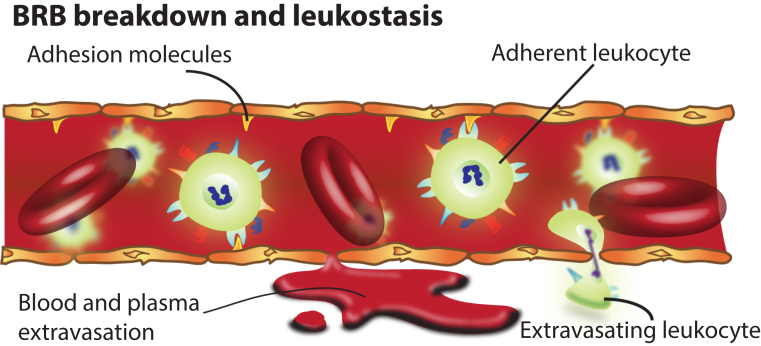
Many ocular diseases, including ischemia reperfusion, diabetic retinopathy, age-related macular degeneration, and retinal vein occlusion, are associated with an inflammatory response.6 Activated glia and stressed neurons may secrete inflammatory factors directly into the retinal microenvironment.4 Circulating leukocytes also adhere to endothelial cells via adhesion molecules and secrete inflammatory factors in the process known as leukostasis (Figure 1), which contributes to ocular disease. Leukocytes may also infiltrate the retina, especially if the BRB is already compromised. Intraocular TNF-α is elevated in ocular disease, which is known to facilitate BRB breakdown via mechanisms that are distinct from VEGF-induced vascular permeability.11 In some ocular diseases, such as diabetic retinopathy, VEGF modulates early breakdown of the BRB, whereas TNF-α regulates late-stage BRB breakdown.11
Figure 1.
Blood-retinal barrier (BRB) breakdown and leukostasis. In the diseased retina, tight junctions in endothelial cells are compromised, contributing to breakdown, which allows extravasation of blood, plasma, and immune cells into the neural retina. Stressed endothelial cells express adhesion molecules that facilitate adhesion of leukocytes in the process of leukostasis.
TNF-α decreases mRNA levels of claudin-5 and zonula occludens protein 1 through an NF-κB–dependent mechanism.1 Accordingly, glucocorticoids, which suppress inflammation, alleviate TNF-α–induced vascular permeability.1 Furthermore, anti–TNF-α therapies alleviate vascular permeability in ocular disease, particularly in patients refractory to anti-VEGF therapy.12
BRB breakdown contributes to loss of visual acuity and neuronal cell death and dysfunction in ocular disease, and it is therefore of significant therapeutic interest.6 Currently available therapeutics targeting VEGF effectively alleviate BRB breakdown in many patients.7, 8, 9 Small clinical studies suggest that anti-inflammatory and anti–TNF-α therapies may be effective in patients refractory to anti-VEGF therapy.12 However, although VEGF and TNF-α facilitate BRB through distinct mechanisms, aPKC is essential for both pathways, and may therefore be a desirable therapeutic target, as indicated by Lin et al3 in the highlighted study.
Atypical Protein Kinase C
The PKC family is ubiquitous in cell signaling, and PKC regulates diverse developmental, physiological, and pathologic events. The PKC family is divided into three subfamilies, including conventional, novel, and atypical PKCs.13 All PKC-family proteins contain a C-terminal catalytic domain and N-terminal regulatory domains. Specificity of the C-terminal domain dictates which substrates each PKC phosphorylates once activated, whereas the N-terminal regulatory domain determines upstream activation of PKCs.14
The mammalian aPKC family is composed of two isoforms, aPKCζ and aPKCι/λ. The aPKCs regulate many essential developmental and cellular processes, such as cell fate determination, cellular polarity, the cell cycle and cell signaling pathways (eg, Wnt signaling), Janus activating kinase/Stat signaling, and the hedgehog pathway.14 Interestingly, PKCζ but not aPKCι/λ is essential for endothelial cell polarity; deletion of PKCζ or transgenic expression of kinase-dead PKCζ results in essentially normal mice, whereas deletion of aPKC-ι/λ is embryonic lethal. The basis for these differential functions remains unclear, but once polarity is established, globally inhibiting aPKC activity does not disrupt cellular polarity.
aPKC as a Therapeutic Target in Ocular Disease
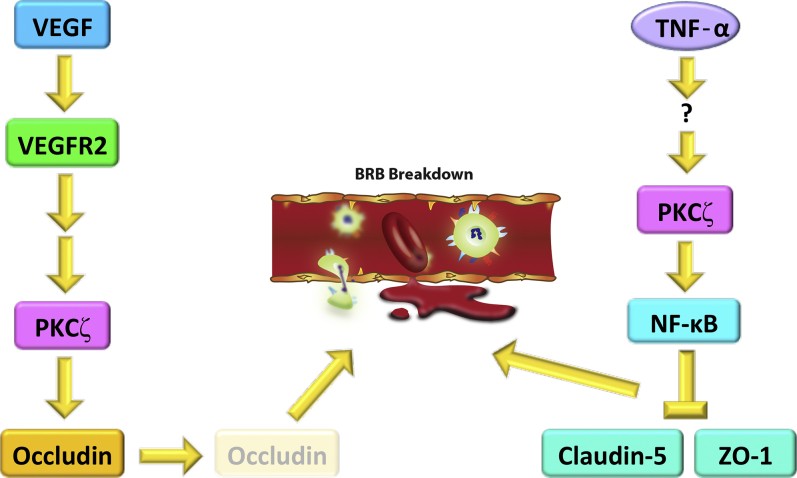
aPKC signaling is also important in the context of vascular permeability (Figure 2). VEGF regulates vascular permeability by activating hyperphosphorylation of occludins and cadherins, which are direct substrates of conventional PKCs, and potentially of aPKCs.1, 15 Contrastingly, TNF-α induces vascular permeability by activating NF-κB, which is dependent on aPKCs, but is independent of conventional or novel PKCs.1 Because VEGF and TNF-α–induced vascular permeability are both dependent on aPKC signaling, aPKC inhibition is a viable therapeutic modality for BRB breakdown. The same research team highlighted in this issue developed 2-amino-3-carboxy-4-pheynylthiopenes as novel, specific inhibitors of aPKC.16
Figure 2.
Vascular endothelial growth factor (VEGF)– and tumor necrosis factor-α (TNF-α)–mediated blood-retinal barrier (BRB) breakdown are protein kinase Cζ (PKCζ) dependent. VEGF activates VEGF receptor 2 (VEGFR2). Activation of VEGFR2 facilitates PKCζ activation. Activated PKCζ then phosphorylates the tight junction protein occludin, targeting occludin for proteasomal degradation and endosomal recycling. Occludin degradation compromises endothelial tight junctions, contributing to BRB breakdown. TNF-α activates PKCζ through as yet unknown mechanisms, which activates NF-κB signaling. NF-κB represses transcription of tight junction components claudin-5 and zonula occludens protein 1 (ZO-1), compromising endothelial tight junctions and facilitating BRB breakdown.
Breakdown of the BRB and subsequent macular edema is a major sight-threatening pathology of diabetic retinopathy, which is the leading cause of blindness in the working-age population. Both anti-VEGF and anti–TNF-α therapies are effective in diabetic retinopathy and diabetic macular edema, suggesting that aPKC may be a viable therapeutic target. Accordingly, several groups have demonstrated that PKCζ regulates BRB breakdown in diabetic retinopathy,17 and that suppressing PKCζ and/or PKCι/λ signaling alleviates BRB breakdown.18 Furthermore, the authors of the highlighted study3 have previously demonstrated that aPKC regulates both VEGF and TNF-α–mediated BRB breakdown, and that newly developed aPKC inhibitors suppress this pathology.1, 15, 19
Ischemia/reperfusion retinal injury results in breakdown of the BRB, which is regulated by VEGF,20 and potentially by TNF-α.21 Accordingly, the authors identify in the present study that aPKC inhibition and transgenic expression of kinase-dead PKCζ suppress BRB breakdown and inflammation in retinal ischemia/reperfusion injury, and inhibit recruitment of infiltrating immune cells.3 aPKC inhibition also down-regulates expression of adhesion molecules by suppressing inflammatory signaling, which may alleviate leukostasis. The authors suggest that the anti-inflammatory effects of aPKC inhibition are likely mediated through suppression of NF-κB activation, and that protective effects against BRB breakdown are likely mediated by inhibition of both the TNF-α/NF-κB axis and VEGF signaling. Accordingly, the authors demonstrate that aPKC inhibition suppresses inflammation and BRB breakdown induced by intraocular injection of VEGF and TNF-α.
The role of aPKC in microvascular pathologies of ocular disease is strongly supported in the scientific literature, and by the present study. Preliminary studies suggest that aPKC may also regulate degeneration of the neural retina and retinal pigment epithelium, potentially through TNF-α–mediated inflammatory signaling.22, 23 Accordingly, aPKC inhibitors have therapeutic potential in retinal neurodegeneration, which is a likely topic of future investigations.
Conclusions and Future Directions
Basic research studies strongly suggest that aPKC plays a central role in BRB breakdown, and that aPKC inhibition has potent therapeutic effects in multiple ocular diseases. Mechanistic studies suggest that aPKC is essential for both VEGF- and TNF-α–mediated BRB breakdown, and that aPKC inhibition suppresses inflammation-mediated BRB breakdown. However, aPKC may also mediate endothelial barrier breakdown by other permeabilizing cytokines, such as thrombin and chemokine (C-C motif) ligand 2, which are also known to regulate BRB breakdown.3 The role of aPKC in thrombin- and chemokine (C-C motif) ligand 2–mediated BRB breakdown is a topic of ongoing investigations.
Previous work has demonstrated that aPKCs modulate the inflammatory response in both endothelial cells and macrophages.1 In the present study, Lin et al3 selectively overexpress kinase-dead PKCζ in endothelial cells and myeloid cells using the Tie2/Tek promoter, which suppresses BRB breakdown and inflammation. This may be attributable to direct modulation of permeability by PKCζ in endothelial cells, or to suppression of the inflammatory response in both leukocytes and endothelial cells, inhibiting recruitment of immune cells. Future studies with cell type–specific expression of kinase-dead PKCζ will elucidate the relative contribution of PKCζ signaling in each cell type to inflammation and BRB breakdown.
Inflammation is central to many common ocular diseases, and therapeutic interventions targeting inflammation alleviate many pathologies of ocular disease, including BRB breakdown. However, the etiology of inflammation in ocular disease remains controversial. In many contexts, it is also unclear whether inflammation is causative in ocular disease, or instead is induced by the underlying ocular disease and subsequently exacerbates the pathology. Furthermore, the relative contributions of local versus systemic inflammation are not yet fully understood. These important questions are the topics of ongoing investigations, and will yield important insights into the pathology of ocular disease. Although inflammation is central to many ocular diseases, the etiology of inflammation and its relative role in disease induction versus progression are likely to be context dependent. However, therapeutic interventions targeting inflammation are highly effective, despite current gaps in knowledge regarding the etiologies of inflammation and disease. Anti-inflammatory therapies are, therefore, clinically significant, even as mechanisms for upstream induction of inflammation continue to be elucidated.
Targeting inflammation and growth factor–mediated breakdown of the BRB has led to highly effective therapeutic interventions for a variety of common blinding ocular diseases. Still, there is significant pressure to further elucidate the mechanisms and etiology of inflammation and induction of vascular growth factors. These investigations will lead to additional approaches to treatment, and potentially identify other common regulators of both inflammation and growth factor–mediated vascular permeability that can then be exploited as therapeutic targets.
The therapeutic potential of aPKC inhibition in inflammatory and neovascular ocular diseases is well supported. Therefore, further insights into the mechanisms of aPKC-mediated vascular permeability and inflammation, and of the relative contribution of aPKC signaling in specific cell types, are of significant importance. Furthermore, additional preclinical studies necessary to bring small-molecule aPKC inhibitors into clinical use will be eagerly anticipated.
Footnotes
See related article on page 2392
Supported by National Eye Institute grant 1R01EY027303, the Bright Focus Foundation, and the Massachusetts Lions Fund (K.M.C.).
Disclosures: None declared.
References
- 1.Aveleira C.A., Lin C.M., Abcouwer S.F., Ambrosio A.F., Antonetti D.A. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X., Dreffs A., Diaz-Coranguez M., Runkle E.A., Gardner T.W., Chiodo V.A., Hauswirth W.W., Antonetti D.A. Occludin S490 phosphorylation regulates vascular endothelial growth factor-induced retinal neovascularization. Am J Pathol. 2016;186:2486–2499. doi: 10.1016/j.ajpath.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin C.-M., Titchenell P.M., Keil J.M., Garcia-Ocaña A., Bolinger M.T., Abcouwer S.F., Antonetti D.A. Inhibition of atypical protein kinase C reduces inflammation-induced retinal vascular permeability. Am J Pathol. 2018;188:2392–2405. doi: 10.1016/j.ajpath.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okunuki Y., Mukai R., Pearsall E.A., Klokman G., Husain D., Park D.H., Korobkina E., Weiner H.L., Butovsky O., Ksander B.R., Miller J.W., Connor K.M. Microglia inhibit photoreceptor cell death and regulate immune cell infiltration in response to retinal detachment. Proc Natl Acad Sci U S A. 2018;115:E6264–E6273. doi: 10.1073/pnas.1719601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz-Coranguez M., Ramos C., Antonetti D.A. The inner blood-retinal barrier: cellular basis and development. Vis Res. 2017;139:123–137. doi: 10.1016/j.visres.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey T., Antonetti D.A. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal. 2011;15:1271–1284. doi: 10.1089/ars.2011.3906. [DOI] [PubMed] [Google Scholar]
- 7.Emerson M.V., Lauer A.K. Emerging therapies for the treatment of neovascular age-related macular degeneration and diabetic macular edema. BioDrugs. 2007;21:245–257. doi: 10.2165/00063030-200721040-00005. [DOI] [PubMed] [Google Scholar]
- 8.Campochiaro P.A. Anti-vascular endothelial growth factor treatment for retinal vein occlusions. Ophthalmologica. 2012;227 Suppl 1:30–35. doi: 10.1159/000337157. [DOI] [PubMed] [Google Scholar]
- 9.Antonetti D.A., Klein R., Gardner T.W. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 10.Claesson-Welsh L. Vascular permeability: the essentials. Ups J Med Sci. 2015;120:135–143. doi: 10.3109/03009734.2015.1064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H., Gandhi J.K., Zhong X., Wei Y., Gong J., Duh E.J., Vinores S.A. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arias L., Caminal J.M., Badia M.B., Rubio M.J., Catala J., Pujol O. Intravitreal infliximab in patients with macular degeneration who are nonresponders to antivascular endothelial growth factor therapy. Retina. 2010;30:1601–1608. doi: 10.1097/IAE.0b013e3181e9f942. [DOI] [PubMed] [Google Scholar]
- 13.Newton A.C. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond M.L., Prehoda K.E. Molecular control of atypical protein kinase C: tipping the balance between self-renewal and differentiation. J Mol Biol. 2016;428:1455–1464. doi: 10.1016/j.jmb.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami T., Frey T., Lin C., Antonetti D.A. Protein kinase cbeta phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes. 2012;61:1573–1583. doi: 10.2337/db11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Titchenell P.M., Showalter H.D., Pons J.F., Barber A.J., Jin Y., Antonetti D.A. Synthesis and structure-activity relationships of 2-amino-3-carboxy-4-phenylthiophenes as novel atypical protein kinase C inhibitors. Bioorg Med Chem Lett. 2013;23:3034–3038. doi: 10.1016/j.bmcl.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omri S., Behar-Cohen F., Rothschild P.R., Gelize E., Jonet L., Jeanny J.C., Omri B., Crisanti P. PKCzeta mediates breakdown of outer blood-retinal barriers in diabetic retinopathy. PLoS One. 2013;8:e81600. doi: 10.1371/journal.pone.0081600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song H.B., Jun H.O., Kim J.H., Yu Y.S., Kim K.W., Kim J.H. Suppression of protein kinase C-zeta attenuates vascular leakage via prevention of tight junction protein decrease in diabetic retinopathy. Biochem Biophys Res Commun. 2014;444:63–68. doi: 10.1016/j.bbrc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Titchenell P.M., Lin C.M., Keil J.M., Sundstrom J.M., Smith C.D., Antonetti D.A. Novel atypical PKC inhibitors prevent vascular endothelial growth factor-induced blood-retinal barrier dysfunction. Biochem J. 2012;446:455–467. doi: 10.1042/BJ20111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthusamy A., Lin C.M., Shanmugam S., Lindner H.M., Abcouwer S.F., Antonetti D.A. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. J Cereb Blood Flow Metab. 2014;34:522–531. doi: 10.1038/jcbfm.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizuka F., Shimazawa M., Egashira Y., Ogishima H., Nakamura S., Tsuruma K., Hara H. Cilostazol prevents retinal ischemic damage partly via inhibition of tumor necrosis factor-alpha-induced nuclear factor-kappa B/activator protein-1 signaling pathway. Pharmacol Res Perspect. 2013;1:e00006. doi: 10.1002/prp2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaadane I., Chahory S., Lepretre C., Omri B., Jonet L., Behar-Cohen F., Crisanti P., Torriglia A. The activation of the atypical PKC zeta in light-induced retinal degeneration and its involvement in L-DNase II control. J Cell Mol Med. 2015;19:1646–1655. doi: 10.1111/jcmm.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajapakse D., Chen M., Curtis T.M., Xu H. PKCzeta-dependent upregulation of p27kip1 contributes to oxidative stress induced retinal pigment epithelial cell multinucleation. Aging (Albany NY) 2017;9:2052–2068. doi: 10.18632/aging.101299. [DOI] [PMC free article] [PubMed] [Google Scholar]