Abstract
Background
Occult hepatitis B infection (OBI) is recognized as a risk factor for cirrhosis and hepato-cellular carcinoma. However, OBI brings together a large spectrum of patients who might harbor different characteristics and prognosis.
Methods
We analyzed the databases of a university hospital in Paris to identify OBI among patients (n = 3966) concomitantly tested for hepatitis B virus (HBV) DNA and serology during a 7-year period. OBI patients were gathered into clinical entities according to their clinical records.
Results
Forty-seven OBIs were identified (1.2%). All patients had detectable anti-HBc, isolated (n = 26) or associated with anti-HBs (n = 21). The proportion of OBIs was 3.4% for patients with isolated anti-HBc and 4.2% for patients with both anti-HBc and anti-HBs. Four clinical categories of OBI patients were identified: patients with a passed HBV infection with HBs Ag clearance (group A, 23.4%); HBV-exposed patients receiving immunosuppressive therapy (group B, 29.8%); HIV/HBV-coinfected patients with therapy discontinuation (group C, 17%); HBV-exposed patients with severe liver conditions (group D, 29.8%). Significant follow-up was available for 32 patients, showing a more deleterious prognosis in group D patients, associated more with their underlying condition than the OBI status.
Conclusions
OBI is a heterogeneous condition with various clinical implications.
Keywords: hepatitis B virus, immunosuppression, occult B infection
Chronic hepatitis B infection (CHB) is the most common cause of liver disease worldwide, leading to liver cirrhosis or hepatocellular carcinoma (HCC). CHB is defined by the persistence of HBs antigen (Ag) in the sera at least 6 months after acute infection. In untreated patients, HBs Ag positivity is associated with various levels of viral replication, according to the natural history of chronic infection and the clinical stages of the disease. However, sensitive polymerase chain reaction (PCR) assays have defined another subgroup of the CHB condition, occult hepatitis B infection (OBI), characterized by the presence of detectable viral hepatitis B virus (HBV) DNA in plasma, without any detection of HBs antigen [1, 2]
The OBI condition, which always implies the persistence of HBV in the liver, appears to be a heterogeneous entity according to the presence of key parameters such as (1) a past known HBs Ag–positive CHB; (2) detectable anti-HBc (+/- anti-HBs) antibodies; (3) mutations in specific regions of the HBs Ag coding gene; (4) other conditions such as liver disease, HIV, or hepatitis C virus (HCV) coinfection or immunosuppressive therapies [2]. This heterogeneity reflects various mechanisms that account for this atypical profile and partially explains the variation of OBI prevalence across studies. The respective sensitivity of virological assays for HBs Ag and HBV DNA detection is also of critical importance for detecting OBI. However, OBI is clearly recognized as a risk factor for cirrhosis and HCC, as well as for HBV reactivation under immunosuppressive therapy [3–5]. To better understand the clinical and virological characteristics and consequences of OBI, we estimated its prevalence and risk factors in a large retrospective observational study conducted in a mixed university teaching hospital and community-based setting.
METHODS
Study Design
The study was conducted in the Saint-Antoine University Hospital in Paris. To be included in the present analysis, participants had to have HBV screening from January 2010 to December 2016. The laboratory databases were used to retrieve results of HBV virological markers and to collect demographic and other laboratory parameters such as liver enzymes and HIV or HCV status. Through a unique identifying number allocated after anonymization, biological databases were linked to clinical databases to collect data focused on the HBV history of each patient, liver disease related or not to HBV, and ongoing immunosuppressive therapy known to reactivate HBV [6]. When available, the outcome of patients diagnosed with OBI was also analyzed.
OBI was defined by the concomitant detection of HBV DNA without detection of HBs Ag. When the positive detection of viral DNA was below 20 UI/mL (the lower quantification limit [LLQ] of the PCR method used), the plasma sample was systematically controlled on a second extraction. If the result was confirmed, the patient was classified as OBI.
As the study was purely observational and retrospective from medical databases, informed consent did not have to be individually obtained according to French ethical law. However, the databases were declared to the Conseil National Informatique et Libertés (www.cnil.fr).
Virological Methods
The Abbott Architect HBsAg Qualitative II kit was used for detection of HBs Ag, with a sensitivity of 0.02 IU/mL. The Architect immunoassay (Architect, Abbott Diagnostics, Rungis, France) was also used for the detection of anti–HBc Abs, as well as the quantification of anti–HBs Abs.
Plasma HBV-DNA viral load (VL) was quantified using a commercial PCR assay (COBAS AmpliPrep/COBAS TaqMan v2.0). The lower detection limit (LLD) of the technique is 9 IU/mL, and the LLQ is 20 IU/mL.
DNA Sequencing and Analysis
The Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS) consensus method for sequencing the overlapping Pol/S genes was used to detect potential escape mutants for HBs Ag detection [7]. This procedure was applied for all OBI patients with an HBV viral load above 500 IU/mL.
Briefly, a 630 base pair fragment covering the entire S gene starting from amino acid 33 was generated by a nested PCR and sequenced on both stands using an ABI 3500 XL Dx (Applied Biosystems, Forster City, CA). Sequence alignment and analysis used SeqScape software, v2.7.
The S and Pol open reading frames were analyzed using the Internet tools HIV grade HBV Drug Resistance Interpretation and Geno2Pheno (HBV) [8]. Every amino acid substitution in the S gene was verified, taking into account potential polymorphisms linked to HBV-infecting genotype.
Statistical Analysis
Data were analyzed using STATA (v12.1, College Station, TX), and significance was determined using a P value <.05. Data were expressed as median (interquartile range [IQR]) for continuous variables and as absolute numbers and percentages for discrete variables. The prevalence of OBI was estimated by the number of positive results within the total number of screened individuals and expressed with its confidence interval (95% CI) using the exact binomial method. The chi-square test of independence or Fisher exact test based on a 2 × 2 contingency table was used for discrete data, whereas clinical and virological characteristics were compared between OBI patients with various HBV serologic profiles using the Kruskal-Wallis test for continuous variables.
RESULTS
Global HBV Profiles
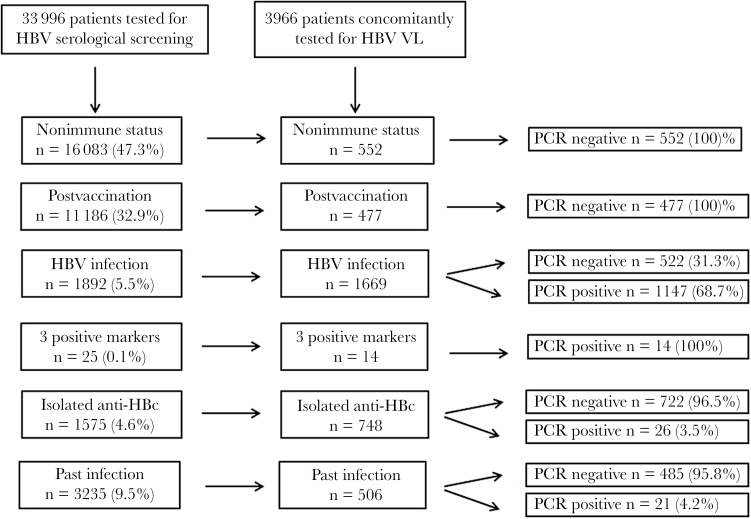
During the study period, 33 996 patients were screened for HBs antigen, anti-HBc, and anti-HBs antibodies. The serological HBV profiles, determined from the first available serum sample of each included individual, were as follows: 16 083 (47.3%) were negative for all HBV serological markers (nonimmune status); 11 186 (32.9%) had a postvaccination profile (anti-HBs positive alone), whereas 3235 (9.5%) exhibited a profile of “resolutive past infection” (negative for HBs Ag and positive for anti-HBc and anti-HBs antibodies). Additionally, 1892 (5.5%) patients had the typical profile for current HBV infection (associating the positivity of HBs Ag and anti–HBc Ab with no anti–HBs Ab). Apart from these classical profiles, we observed 25 (0.07%) patients who were positive for all 3 markers, whereas 1575 (4.6%) patients presented an isolated positivity of anti–HBc Ab.
Among these 33 996 individuals with available serology, 3966 had a concomitant quantitation of HBV VL. Figure 1 summarizes the general workflow of the study, whereas Table 1 describes the results of HBV VL according to the HBV serological profiles of the study population. None of the vaccinated or nonimmune patients displayed a positive PCR, which means that no OBI was detected among HBV-seronegative patients. On the contrary, 70% of the HBs Ag–positive patients had a detectable HBV VL at the first sample evaluated in this study. Interestingly, all the 14 patients who were positive for the 3 HBV serological markers had a positive PCR, confirming that this atypical profile indeed corresponds to an HBV infection, despite of the presence of detectable anti–HBs Abs (median [IQR], 50.5 [22–197] mIU/mL). The median HBV VL was furthermore statistically higher for this last population (median [IQR], 101 566 [171–855 000 UI/mL) compared with the HBV-infected population showing a classical profile without anti–HBs Abs (median [IQR], 923 [112–9211] UI/mL; P = .001). These data likely result from a delay in anti-HBV therapy initiation. Indeed, only 1 out of the 14 patients was receiving anti-HBV treatment at the time of sample testing.
Figure 1.
Global flowchart of the study. HBV infection-related tests were conducted from January 2010 to December 2016. Abbreviations: HBV, hepatitis B virus; PCR, polymerase chain reaction; VL, viral load.
Table 1.
HBV–Viral Load in Patients Concomitantly Tested With HBV-DNA PCR (n = 3966), According to HBV Serology
| HBV Profile | Total Patients, No. (%) | HBV Viral Load Undetectable, No. (%) | HBV Viral Load Detectable, No. (%) | HBV Viral Load, Median (IQR), UI/La |
|---|---|---|---|---|
| Nonimmune status | 552 (13.81) | 552 | 0 | NA |
| Postvaccination profile | 477 (11.93) | 477 | 0 | NA |
| Resolutive past infection | 506 (12.66) | 485 (95.8) | 21 (4.2) | 45 (23–89) |
| HBV infection | 1669 (41.7) | 522 (31.3) | 1147 (68.7) | 923 (112–9211) |
| Simultaneous positivity for HBs Ag and anti–HBs Ab | 14 (0.25) | 0 | 14 | 101 566 (171–855 000) |
| Isolated anti–HBc Ab | 748 (18.7) | 722 (96.6) | 26 (3.5) | 69 (19–207) |
Significance between HBV viral loads in HBs Ag–positive groups vs HBs Ag–negative groups was performed by Kruskal-Wallis test (not shown in table; P = .001).
Abbreviations: HBV, hepatitis B virus; NA, not applicable; PCR, polymerase chain reaction.
aOnly applicable for HBV PCR–positive patients.
Virological Characterization of OBI Profile
Among the 2283 individuals negative for HBs Ag who were tested for HBV VL, 51 were initially positive. A significant viremia above 20 UI/mL was found in 37 individuals, whereas 14 displayed a very low viremia, under the 20 UI/mL LLQ. After retest, 4 samples out of 14 had an undetectable HBV VL; therefore, 47 individuals were considered to have a confirmed OBI, leading to an estimated prevalence of OBI of 1.2% (95% CI, 0%–5.2%) in our population concomitantly evaluated for HBV serology and PCR.
These 47 subjects belong to 2 different serological groups: (1) individuals with isolated anti–HBc Abs (n = 26, 3.5%) and (2) individuals with past resolved HBV infection (n = 21, 4.2%). The HBV VL was lower in the OBI group (median [IQR], 61 [19–139] UI/mL) than in the HBs Ag–positive populations (median [IQR], 923 [112–9211] UI/mL; P = .0001).
We sequenced the overlapping HBV Pol and S gene in all OBI patients with an HBV VL above 500 UI/mL (n = 8), except 1, for whom no stored plasma was available (Table 2).
Table 2.
Description of S Mutations in OBI Patients With HBV VL Above 500 UI/mL
| Patient | HBV serological Profile | HBV VL | HBV-Genotype | Relevant S Mutations | Region | Relevant Pol Mutations |
|---|---|---|---|---|---|---|
| Patient 1 | Isolated anti-HBc | 1022 | E | T118R | MHR | None |
| Patient 2 | Isolated anti-HBc | 959 | A | P120T | MHR | None |
| Patient 3 | Isolated anti-HBc | 5787 | E | G145R | “a” determinant | None |
| Patient 4 | Isolated anti-HBc | 513 | E | M133I | “a” determinant | None |
| Patient 5 | Resolutive past infection | 5045 | B | G145S | “a” determinant | None |
| Patient 6 | Resolutive past infection | 16516 | E | F134V | “a” determinant | None |
| Patient 7 | Resolutive past infection | 1374 | E | None | NA | None |
Abbreviations: HBV, hepatitis B virus; NA, not applicable; OBI, occult hepatitis B infection; VL, viral load.
Six out of 7 exhibited potentially relevant amino acid substitutions located in the major hydrophilic region (MHR) of the S gene, 4 being more precisely located in the cysteine-rich domain “a” determinant, the major target of neutralizing antibodies and HBs Ag detection tests.
Clinical Characterization of OBI Individuals
The main clinical and biological characteristics of OBI patients are presented in Table 3. The population was predominantly male, with a median age around 50 years. The HBV VL was low (median [IQR], 61 [19–139] UI/mL) with no difference between groups. HIV coinfection was very frequent (40.9%, 18/47), with a tendency to a higher frequency in the “isolated anti-HBc” group (53.8%, 14/26) compared with the “anti-HBc/anti-HBs” group (22.2%, 4/21; P = .06). Importantly, HIV RNA viral loads were high in these coinfected patients (median, 56 833 copies/mL), reflecting the frequency of OBI among HIV-infected patients who stopped antiretroviral (ARV) therapies (n = 8) or were discovered at a late stage of HIV infection (n = 5). HCV coinfection was observed in 6.4% of OBI individuals, with no difference between the 2 serological groups.
Table 3.
Characteristics of OBI Individuals According to HBV Serological Profiles
| Total | HBV Serological Profiles | |||
|---|---|---|---|---|
| (n = 47) | HBcAb+/HBsAb- (n = 26) |
HBcAb+/HbsAb+ (n = 21) |
P a | |
| Sex ratio, male/female (% males) | 35/12 (74.5) | 20/6 (76.2) | 15/6 (71.4) | .7 |
| Age, yb | 51 (42–65) | 49 (41–62) | 57 (42–70) | .2 |
| Viral load HBV, UI/Lb | 61 (19–139) | 69 (19–207) | 45 (23–89) | .9 |
| ASTb | 34.5 (24–76) | 31 (23–46) | 79 (29–208) | .006 |
| ALTb | 25.2 (17–69) | 21 (15–31) | 49 (19–266) | .01 |
| Protrombin timeb | 84.5 (71–91) | 86 (74.5–91) | 79.5 (65–90) | .3 |
| HIV infectionc,d | 18 (40.9) | 14 (53.8) | 4 (22.2) | .06 |
| HIV-RNA viral load, copies/mLb | 56 833 (11 506–110 996) | 61 951 (15 412–208 050) | 38 734 (9695–73 807) | .8 |
| HCV infectiond,e | 3 (6.4) | 1 (3.7) | 2 (10) | .3 |
| Significant mutations in the S genef | 5 | 4 | 1 | NS |
| Asymptomatic OBI with no liver disorder | 26 | 21 | 5 | .0003 |
| Immunosuppressive therapyd | 14 (29.8) | 7 | 7 | .3 |
| First-class immunosuppressive therapy | 11 | 5 | 6 | .3 |
| Second-class immunosuppressive therapy | 1 | 1 | 0 | .5 |
| Third-class immunosuppressive therapy | 2 | 1 | 1 | .5 |
Abbreviations: ALT, alanine aminotrasferase; AST, aspartate aminotrasferase; HBV, hepatitis B virus; HCV, hepatitis C virus; OBI, occult hepatitis B infection; NS, not significant.
aSignificance between groups was determined using the Kruskal-Wallis test for continuous variables and the Pearson χ2 test or Fisher exact test for categorical variables.
bMedian (interquartile range).
cData available for 44 patients.
dNumber (%).
eData available for 35 patients.
fData available for 5 patients.
Most of the patients (44/47) did not receive any therapy active against HBV at the time of OBI diagnosis. Regarding liver assessment, 26 out of 47 were totally asymptomatic with normal liver parameters, whereas 21 OBI cases were associated with liver abnormalities and/or disease, such as acute hepatitis (n = 13), liver failure (n = 3), cirrhosis (n = 2), HCC (n = 2, including 1 without cirrhosis), and liver metastasis (n = 1). Liver assessment showed a more severe presentation in the “anti-HBc/anti-HBs” profile compared with the “isolated anti-HBc group” (P = .0003), as reflected by higher liver enzyme measurements in this group (P < .01).
By analyzing medical records, the 47 OBI cases could be associated with 4 major clinical categories (Table 4):
Table 4.
Clinical Classificationa of OBI Cases
| Total OBI Cases (n = 47) | Group A (n = 11) |
Group B (n = 14) |
Group C (n = 8) |
Group D (n = 14) |
P Value |
|---|---|---|---|---|---|
| Sex ratio, male/femaleb 35/12 (75.5) |
9/2 (81.8) | 11/3 (78.6) | 5/3 (62.5) | 10/4 (71.4) | .7 |
| Age, yc 51 (42–65) |
42 (36–51) | 66 (63–72) | 41.5 (38.5–49) | 45 (42–65) | .0001 |
| Viral load HBV, UI/Lc 61 (16–139) |
68 (24–159) | 76.5 (19–130) | 83 (19–318) | 65 (34–176) | .8 |
| ASTc 34.5 (24–76) |
30.5 (23–41.5) | 40.5 (19–70) | 32.5 (27–43.5) | 203 (46–310) | .02 |
| ALTc 25.2 (17–69) |
25.5 (19.5–33.5) | 32 (19–77) | 18 (13–23.5) | 86 (22–356) | .04 |
| Prothrombin timec 84.5 (71–91) |
85.5 (18–96) | 79 (68–88) | 89.5 (88–95) | 65 (39–86) | .03 |
| HIV infectionb 18 (40.9) |
6 (66.7) | 0 | 7 (100) | 2 (18.2) | <.0001 |
| “Isolated anti-HBc”/“resolutive past infection” profile 26/21 |
9/2 | 7/7 | 8/0 | 2/12 | .001 |
Abbreviations: ALT, alanine aminotrasferase; AST, aspartate aminotrasferase; HBV, hepatitis B virus; OBI, occult hepatitis B infection.
aDefinition of clinical categories is specified in the text.
bNumber (%).
cMedian (interquartile range).
group A: a past HBV infection, with a clearance of HBs Ag, either spontaneous or under specific antiviral therapy;
group B: the administration of immunosuppressive therapy known to induce HBV reactivation in a patient previously exposed to HBV;
group C: the interruption of ARV therapy in an HIV/HBV-coinfected patient previously controlled for HBV viral load;
group D: severe liver disease in a patient previously exposed to HBV.
Among the 11 group A patients (23.4%), HBs Ag clearance was spontaneously observed in all but 1 untreated individual with HBe Ag–negative chronic infection (formerly called “inactive carriers”). They globally had a milder level of transaminases than other categories, and two-thirds were HIV-infected. They mostly exhibited an isolated HBc Ab profile. The only subject who cleared HBs Ag under treatment was an HIV/HCV/HBV-tri-infected patient receiving tenofovir-containing antiretroviral therapy for several years.
Among the 14 group B patients (32%), 11 received drugs from the highest-risk class of immunosuppression for HBV reactivation [9, 10]. They were older than the other groups, and half of them had a resolutive past infection profile.
All 8 group C patients (16.3%) stopped ARV therapy containing dually active drugs against HIV and HBV for adherence issues. They all had an isolated anti-HBc profile.
Finally, most of the 14 group D patients (29.8%) suffered from an acute liver condition unrelated to HBV, such as drug-induced liver injury (n = 5), severe sepsis (n = 2), primary hepatitis A infection (n = 1), severe flare of auto-immune hepatitis (n = 1), or acute gallstone (n = 1). We also observed 1 case of NASH-associated cirrhosis in that category, 1 case of HCC without cirrhosis, and 1 case of liver metastasis after colon cancer. This group presented a higher level of transaminases and a lower prothrombin time level, and most had a “resolutive past infection” profile.
Outcome of Individuals With OBI
Significant follow-up (median [IQR], 468 [155–1148] days) was available for 32/47 OBI individuals (Table 5). Of these, only 2 received anti-HBV therapy at the time of OBI diagnosis, whereas 20 were secondarily treated for HBV. A significant proportion of anti-HBV therapy initiation in this context was part of ARV multitherapy, either initiated in naïve patients with recent HIV diagnosis (n = 6) or re-introduced in nonadherent patients (n = 6).
Table 5.
OBI Outcome in OBI Patients With a Significant Follow-up
| Categoriesa | Follow-up, d | HBV Serology at OBI dg | HBV VL at OBI dg | Anti-HBV at OBI dg | Anti-HBV After OBI dg | Background at OBI dg | Liver Condition | OBI Outcome |
|---|---|---|---|---|---|---|---|---|
| A | 2636 | Isolated anti-HBc | 104 | TDF | TVD | HBV/HIV coinfected | Asymptomatic OBI | Anti-HBs apparition (669 UI/L) and OBI resolution under TVD |
| A | 1816 | Isolated anti-HBc | 75 | 0 | TVD | Inactive HBV + HIV diagnosis | Asymptomatic OBI | OBI resolution under TVD |
| A | 1499 | Isolated anti-HBc | 373 | 0 | TVD | Inactive HBV + HIV diagnosis | Asymptomatic OBI | OBI resolution under TVD |
| A | 1084 | Isolated anti-HBc | 66 | 0 | 0 | Inactive HBV carrier | Asymptomatic OBI | Spontaneous OBI resolution |
| A | 387 | Isolated anti-HBc | 61 | 0 | KVX | Inactive HBV + HIV | Asymptomatic OBI | OBI resolution under KVX |
| A | 644 | Isolated anti-HBc | 496 | 0 | TVD | Inactive HBV + HIV | Asymptomatic OBI | OBI resolution under TVD |
| A | 17 | Isolated anti-HBc | 19 | 0 | 0 | Inactive HBV carrier | Asymptomatic OBI | Spontaneous OBI resolution |
| A | 938 | Isolated anti-HBc | 214 | 0 | 0 | Inactive HBV carrier | Asymptomatic OBI | Spontaneous OBI resolution |
| A | 1834 | Resolutive infection | 29 | 0 | TVD | Inactive HBV + HIV untreated | Asymptomatic OBI | OBI resolution under TVD |
| B | 1179 | Isolated anti-HBc | 26 | 0 | 0 | Myeloma under bortezomib/steroids | Asymptomatic OBI | OBI resolution after stopping IS |
| B | 1881 | Isolated anti-HBc | 513 | 0 | 0 | Prostate cancer under chemotherapy | Asymptomatic OBI | OBI resolution after stopping IS |
| B | 415 | Isolated anti-HBc | 72 | 0 | LMV | Myeloma under bortezomib/steroids | Asymptomatic OBI | OBI resolution under LMV |
| B | 1324 | Isolated anti-HBc | 207 | ETV | ETV + TVD | Liver recipient under ciclosporin | Asymptomatic OBI | OBI resolution after TVD addition |
| B | 28 | Isolated anti-HBc | 81 | 0 | TVD | Myeloma under carfilzomib/pomalidomide/ steroids | Liver failure | Death at D28 for DRESS syndrome and refractrory myeloma |
| B | 71 | Isolated anti-HBc | 130 | 0 | ETV | NHL under CHOP | Asymptomatic OBI | OBI resolution under TVD |
| B | 1117 | Resolutive infection | 5045 | 0 | TVD | Refractory idiopathic AI trombopenia under rituximab | Acute hepatitis | Liver enzyme normalization and OBI resolution under TVD |
| B | 2117 | Resolutive infection | 89 | 0 | ETV | Severe RA under tocilizumab/MTX/steroids | Asymptomatic OBI | Hepatitis flare and HBs Ag positivation at D793 after stopping ETV, resolutive after ETV reintroduction |
| B | 465 | Resolutive infection | 19 | 0 | 0 | NHL under R-CHOP | Asymptomatic OBI | Hepatitis flare with HBs Ag positivation at D 245, resolutive under ETV |
| B | 100 | Resolutive infection | 19 | 0 | ETV | Pancratic cancer under Folfirinox cure | Acute hepatitis | Anti-HBs persistance (430 UI/L) and OBI resolution under ETV |
| C | 709 | Isolated anti-HBc | 43 | 0 | TVD | HIV with ARV interruption | Asymptomatic OBI | Anti-HBs apparition (300 UI/L) and OBI resolution under TVD |
| C | 232 | Isolated anti-HBc | 400 | 0 | TVD | HIV with ARV interruption | Asymptomatic OBI | Persistance of asymptomatic OBI with adherence issue |
| C | 136 | Isolated anti-HBc | 19 | 0 | TVD | HIV with ARV interruption | OH/HBV cirrhosis | Ag HBs repositivation and cirrhosis progression with adherence issue |
| C | 68 | Isolated anti-HBc | 257 | 0 | TVD | HIV with ARV interruption | Asymptomatic OBI | OBI resolution under TVD |
| C | 874 | Isolated anti-HBc | 237 | 0 | TVD | HIV with ARV interruption | Acute hepatitis | Liver enzyme normalization and OBI resolution under TVD |
| C | 191 | Isolated anti-HBc | 19 | 0 | TVD | HIV with ARV interruption | Asymptomatic OBI | OBI resolution under TVD |
| D | 174 | Isolated anti-HBc | 1374 | 0 | 0 | AIH-associated flare | Acute hepatitis | Spontaneous hepatitis and OBI resolution |
| D | 301 | Resolutive infection | 176 | 0 | ETV | NASH cirrhosis in HIV | Ascitis decompensation | OBI resolution under TVD but death at D301 for cirrhosis |
| D | 99 | Resolutive infection | 65 | 0 | LMV+ anti-HBs | Drug-induced fulminant hepatitis | Liver failure | Liver transplatation |
| D | 430 | Resolutive infection | 34 | 0 | 0 | HCC without cirrhosis post-HBV/Delta | HCC | Spontaneous OBI resolution but cancer progression and death at d430 |
| D | 505 | Resolutive infection | 84 | 0 | STBD | Drug-induced hepatitis + HIV diagnosis | Acute hepatitis | Hepatitis and OBI resolution after stopping augmentin and STBD initiation |
| D | 14 | Resolutive infection | 1374 | 0 | 0 | Acute lysis syndrome during CLL therapy | Liver failure | Death at D14 for multi-organ failure |
| D | 360 | Resolutive infection | 19 | 0 | 0 | Severe sepsis | Acute hepatitis | OBI resolution under antibiotic therapy |
Thirty-two patients were evaluated, with a median follow-up of 448 days.
Abbreviations: AIH, autoimmune hepatitis; CLL, chronic lymphocytic leukemia; CHOP, Cyclophosphamide/Hydroxyadriamycine/Oncovin/Prednisone; Dg, diagnosis; DRESS, Drug Reaction with Eosinophilia and Systemic Symptoms; ETV: entecavir; IS, immunosuppressive therapy; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; KVX, kivexa (abacavir/lamivudine combination); LMV, lamivudine; MTX, methotrexate; NASH, nonalcoholic steatohepatitis; NHL, non-Hodgkin lymphoma; OBI, occult hepatitis B infection; OH, alcohol; R-CHOP, rituximab + CHOP; RA, rheumatoid arthritis; STBD, stribild (elvitegravir/cobicistat/emtricitabine/tenofovir); TDF, tenofovir; TVD, truvada (emtricitabine/tenofovir combination); VL, viral load.
aDefinition of clinical categories is specified in the text.
Most OBI outcomes were favorable, especially in patients from group A or group C. However, among 10 patients belonging to group B, 1 died at day 28 from an underlying disease and 2 experienced, several months after OBI diagnosis, a severe hepatic flare with HBs Ag repositivation. This occurred while patients were not receiving therapy against HBV, and both responded favorably to entecavir.
In group D, the clinical outcome was less favorable, with 3 deaths occurring among 7 patients. However, this negative progression had more to do with the underlying liver condition than the OBI diagnosis itself.
DISCUSSION
The clinical significance of OBI remains largely unknown, whereas its frequency varies across series. Using sensitive assays for HBs Ag and HBV DNA detection, we identified 47 cases of confirmed OBI, among almost 4000 patients routinely followed during a 7-year period in a university teaching hospital with outpatient clinics in Paris. This is, to our knowledge, one of the largest series of OBIs ever published, which delivers some important information.
In this series, all OBI cases were observed in patients with a positive detection of anti–HBc Abs, which is reassuring regarding the accuracy of this marker to identify previous contact with HBV. More surprisingly, almost half of the cases occurred in patients who also showed significant positivity for anti-HBs antibodies. The anti–HBs Abs titers observed in this subgroup of OBI patients were furthermore rather high (median [IQR], 155 [40–396] UI/mL), suggesting a true defect in their ability to neutralize the infection, at least for replicative capacity. These data confirm that apparent “recovery” from HBV infection implies neither immune destruction of all infected cells nor disappearance of the persistent covalently closed circular viral DNA (cccDNA) from some infected cells, thus explaining the long-life risk of HBV reactivation after HBV contact [6, 11].
Many mechanisms have been proposed to explain OBI profiles. The first is a loss of antigenicity of circulating HBs Ag caused by specific mutations in the S gene, impairing its recognition by anti-HBs antibodies. These “escape mutants” can be associated with HBV VL levels comparable to overt infections and are considered by some authors to be “false” OBI [1]. However, other mechanisms rely on a true decrease in liver expression of the HBs protein, leading to undetectable levels of HBs Ag in the bloodstream, associated with low levels of HBV replication. As for antigenicity loss, the reduced expression of HBs Ag may result from complex mutational patterns located in various regions of the HBV genome, including the pre-S, the pre-C, and the Core regions [12], but it can also be linked to a viral interference phenomenon (notably with HCV) [13] or to physiological steps of the natural history of HBV chronic infection, involving both viral and host factors [4].
Regarding HBV viral load, it was, as expected, very low in the 47 OBI cases we identified, excepted for 8 patients, in whom it was >500 UI/mL. The S gene was sequenced for 7 of these patients, showing in 6 cases potentially relevant mutations located in the MHR domain of HBs Ag. Among these mutations, 4 (T118R/P120T/M133I and G145R) are known vaccine or anti–HBs Abs escape variants [14–18] previously reported in OBI patients [19], whereas G145S and F134V are unreported substitutions located in the key amino acids 134 and 145 of the “a” determinant. Given the importance of this determinant for the tertiary antigenic conformation of the S protein [20], we cannot rule out a significant impact of these mutations on HBs Ag antigenicity. However, as important progress has been made in the detection of HBs Ag mutants by commercial assays, including the Abbott Architect assay used in this study [21], we decided to include these 6 patients in our cohort.
After comprehensive analysis of the medical record of the 47 OBI patients, we were able to classify them into 4 major clinical categories. The first category (n = 11) includes previously known HBs Ag–positive CHB patients who showed a clearance of HBs Ag, associated with persistent low viremia. These patients are mainly nontreated inactive carriers, for whom HBs Ag loss may occur after years of evolution. As has been shown after recovery of acute infection, both in animal models [22] and in humans [23], a low level of HBV viremia may persist after HBs Ag loss in chronically infected patients in plasma and/or peripheral mononuclear cells [24], especially before the appearance of anti–HBs Abs. This observation likely accounts for the highest representation of this category in the “isolated anti-HBc” OBI population, compared with the “anti-HBc/anti-HBs” population.” We assume that this OBI phase is a transient step before the complete stop of viral replication in this population [4], as suggested by the positive outcome of all patients of this group (Table 5). However, in a recent cohort of CHB patients with HBs Ag sero-clearance [25], the persistence of detectable viral DNA was associated with a higher risk of HBs sero-reversion.
The second category (n = 14) concerns previously exposed patients receiving immunosuppressive therapies, such as rituximab or high-dose steroids. The OBI profile observed in such patients may precede the appearance of HBs Ag, as it can be observed in the ramping phase of an acute infection. From these 14 patients, 2 displayed acute hepatitis at the moment of OBI diagnosis, whereas 2 others experienced, several weeks after OBI detection, a severe hepatitis flare associated with reappearance of HBs Ag. These data exemplify the importance of screening any patients receiving such therapies for HBV markers to prevent severe reactivation by appropriate prophylaxis [6, 9–11].
Apart from these 2 well-known categories, 2 others accounted for the rest of the OBI cases of this series. The third category (n = 8) is represented by a homogeneous HBV/HIV-coinfected population, where the low level of HBV viremia was observed during an interruption of the anti-HBV-containing ARV regimen, most of the time for adherence issues. This category is exclusively composed of “isolated anti-HBc” carrier patients, probably because of the delayed appearance of anti–HBs Abs following HBs Ag loss in coinfected patients under tenofovir therapy [26].
The last category represents the most surprising result of our study. Indeed, almost 30% of the OBI cases (n = 14) were observed during a severe liver disorder occurring in patients exhibiting an “anti-HBc/anti-HBs” profile. These liver conditions were most of the time acute, nonrelated to HBV, and associated with a clear increase of liver enzymes. We may assume that the low HBV viremia responsible for the OBI profile in this population results from a passive release of viral DNA from infected liver cells into the peripheral blood, even if we cannot exclude a role of immune complexes in the lack of HBs Ag reactivity. Whatever the mechanism involved, we don’t know the potential consequences of this low viremia in the progression of HBV disease in these situations. Chronic liver conditions were also observed in this category, albeit less frequently. We notably observed cases of cryptogenic cirrhosis and HCC without cirrhosis where the putative implication of HBV could be suspected, either through immune-mediated damage of hepatic parenchyma [27], ancient chromosomal integration events [28], or virus-induced modifications of cell-signaling pathways [29].
We are aware of some limitations of this work. First, OBI diagnosis was made on a single point in the HBV story of the patients, defined by the first plasma sample tested during the study period. However, the analysis of the medical records of OBI patients allowed us to expand our study beyond this single point for 32 patients in order to evaluate the long-term prognosis of OBI cases according to the clinical category they belong to. Second, we only had access to results obtained from plasma samples, without having any knowledge on what was going on in the liver of the OBI patients. This limitation obviously results from the scarcity of liver biopsies, especially in HBs Ag–negative individuals. The last limit is the inherent bias of studies based on retrospective database analysis. HBV DNA testing performed in HBs Ag–negative patients may indeed result from specific comorbidities and/or abnormal liver function tests, which prevents us from extending the OBI prevalence found in our study population to the general population.
Despite these limitations, this comprehensive description of the OBI condition delivers important messages in the management of HBV-exposed individuals. This study indeed confirms the inherent long-life persistence of HBV after infection, even in patients exhibiting significant anti-HBs antibodies, detected in almost half of our OBI cases. This implies a need to detect and/or prevent any resumption of viral replication in every HBV-exposed individual receiving immune-suppressive therapy, and also to maintain, at least in HBV/HIV-coinfected patients, effective anti-HBV therapy, even after HBs Ag loss. The clinical implications of OBI profiles belonging to the “severe liver disease” category remain to be determined, especially when the concomitant liver condition appears to be acute and independent of previous HBV exposure.
This large cohort of OBI patients shows that occult HBV infection is a heterogeneous condition that can be associated with 4 major clinical entities, with different clinical implications for each one. A better acknowledgement of viral and host factors involved in HBV pathogenesis and persistence, as well as the use of more accurate biomarkers for staging HBV disease [30], will help us to better manage this puzzling and complex OBI status.
Acknowledgments
Financial support. No funding was necessary to perform this work.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation. This work was presented at the 22nd annual meeting of the GEMHEP; December 2017; Paris, France.
References
- 1. Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008; 49:652–7. [DOI] [PubMed] [Google Scholar]
- 2. Makvandi M. Update on occult hepatitis B virus infection. World J Gastroenterol 2016; 22:8720–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi Y, Wu YH, Wu W, et al. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int 2012; 32:231–40. [DOI] [PubMed] [Google Scholar]
- 4. Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis 2002; 2:479–86. [DOI] [PubMed] [Google Scholar]
- 5. Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat 2014; 21:153–62. [DOI] [PubMed] [Google Scholar]
- 6. Pattullo V. Prevention of hepatitis B reactivation in the setting of immunosuppression. Clin Mol Hepatol 2016; 22:219–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villeneuve JP, Durantel D, Durantel S, et al. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J Hepatol 2003; 39:1085–9. [DOI] [PubMed] [Google Scholar]
- 8. Beggel B, Kalaghatgi P, Neumann-Fraune M, et al. Geno2pheno [HBV]. Saarbrücken, Germany: Max Planck Institute for Informatics; 2009. http://hbv.bioinf.mpi-inf.mpg.de/index.php. Accessed September 2018. [Google Scholar]
- 9. Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015; 148:221–44.e3. [DOI] [PubMed] [Google Scholar]
- 10. Reddy KR, Beavers KL, Hammond SP, et al. ; American Gastroenterological Association Institute American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015; 148:215–9; quiz e16–7. [DOI] [PubMed] [Google Scholar]
- 11. Xunrong L, Yan AW, Liang R, Lau GK. Hepatitis B virus (HBV) reactivation after cytotoxic or immunosuppressive therapy–pathogenesis and management. Rev Med Virol 2001; 11:287–99. [DOI] [PubMed] [Google Scholar]
- 12. Chaudhuri V, Tayal R, Nayak B, et al. Occult hepatitis B virus infection in chronic liver disease: full-length genome and analysis of mutant surface promoter. Gastroenterology 2004; 127:1356–71. [DOI] [PubMed] [Google Scholar]
- 13. Chen SY, Kao CF, Chen CM, et al. Mechanisms for inhibition of hepatitis B virus gene expression and replication by hepatitis C virus core protein. J Biol Chem 2003; 278:591–607. [DOI] [PubMed] [Google Scholar]
- 14. Lu M, Lorentz T. De novo infection in a renal transplant recipient caused by novel mutants of hepatitis B virus despite the presence of protective anti-hepatitis B surface antibody. J Infect Dis 2003; 187:1323–6. [DOI] [PubMed] [Google Scholar]
- 15. Carman WF, Korula J, Wallace L, et al. Fulminant reactivation of hepatitis B due to envelope protein mutant that escaped detection by monoclonal HBsAg ELISA. Lancet 1995; 345:1406–7. [DOI] [PubMed] [Google Scholar]
- 16. Carman WF, Zanetti AR, Karayiannis P, et al. Vaccine-induced escape mutant of hepatitis B virus. Lancet 1990; 336:325–9. [DOI] [PubMed] [Google Scholar]
- 17. Tian Y, Xu Y, Zhang Z, et al. The amino acid residues at positions 120 to 123 are crucial for the antigenicity of hepatitis B surface antigen. J Clin Microbiol 2007; 45:2971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ireland JH, O’Donnell B, Basuni AA, et al. Reactivity of 13 in vitro expressed hepatitis B surface antigen variants in 7 commercial diagnostic assays. Hepatology 2000; 31:1176–82. [DOI] [PubMed] [Google Scholar]
- 19. Zaaijer HL, Torres P, Ontañón A, et al. Multiple surface antigen mutations in five blood donors with occult hepatitis B virus infection. J Med Virol 2008; 80:1344–9. [DOI] [PubMed] [Google Scholar]
- 20. Seddigh-Tonekaboni S, Waters JA, Jeffers S, et al. Effect of variation in the common “a” determinant on the antigenicity of hepatitis B surface antigen. J Med Virol 2000; 60:113–21. [DOI] [PubMed] [Google Scholar]
- 21. Chudy M, Scheiblauer H, Hanschmann KM, et al. Performance of hepatitis B surface antigen tests with the first WHO international hepatitis B virus genotype reference panel. J Clin Virol 2013; 58:47–53. [DOI] [PubMed] [Google Scholar]
- 22. Michalak TI, Pardoe IU, Coffin CS, et al. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology 1999; 29:928–38. [DOI] [PubMed] [Google Scholar]
- 23. Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med 1996; 2:1104–8. [DOI] [PubMed] [Google Scholar]
- 24. Murakami Y, Minami M, Daimon Y, Okanoue T. Hepatitis B virus DNA in liver, serum, and peripheral blood mononuclear cells after the clearance of serum hepatitis B virus surface antigen. J Med Virol 2004; 72:203–14. [DOI] [PubMed] [Google Scholar]
- 25. Yip TC-F, Wong GL-H, Wong VW-S, et al. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J Hepatol 2018; 68:63–72. [DOI] [PubMed] [Google Scholar]
- 26. Boyd A, Lasnier E, Molina JM, et al. Liver fibrosis changes in HIV-HBV-coinfected patients: clinical, biochemical and histological effect of long-term tenofovir disoproxil fumarate use. Antivir Ther 2010; 15:963–74. [DOI] [PubMed] [Google Scholar]
- 27. Martin CM, Welge JA, Shire NJ, et al. Cytokine expression during chronic versus occult hepatitis B virus infection in HIV co-infected individuals. Cytokine 2009; 47:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raimondo G, Burk RD, Lieberman HM, et al. Interrupted replication of hepatitis B virus in liver tissue of HBsAg carriers with hepatocellular carcinoma. Virology 1988; 166:103–12. [DOI] [PubMed] [Google Scholar]
- 29. Pan J, Clayton M, Feitelson MA. Hepatitis B virus X antigen promotes transforming growth factor-beta1 (TGF-beta1) activity by up-regulation of TGF-beta1 and down-regulation of alpha2-macroglobulin. J Gen Virol 2004; 85:275–82. [DOI] [PubMed] [Google Scholar]
- 30. Zeisel MB, Lucifora J, Mason WS, et al. Towards an HBV cure: state-of-the-art and unresolved questions–report of the ANRS workshop on HBV cure. Gut 2015; 64:1314–26. [DOI] [PubMed] [Google Scholar]