Abstract
This review highlights the role of three key immune pathways in the pathophysiology of major retinal degenerative diseases including diabetic retinopathy, age‐related macular degeneration, and rare retinal dystrophies. We first discuss the mechanisms how loss of retinal homeostasis evokes an unbalanced retinal immune reaction involving responses of local microglia and recruited macrophages, activity of the alternative complement system, and inflammasome assembly in the retinal pigment epithelium. Presenting these key mechanisms as complementary targets, we specifically emphasize the concept of immunomodulation as potential treatment strategy to prevent or delay vision loss. Promising molecules are ligands for phagocyte receptors, specific inhibitors of complement activation products, and inflammasome inhibitors. We comprehensively summarize the scientific evidence for this strategy from preclinical animal models, human ocular tissue analyses, and clinical trials evolving in the last few years.
Keywords: complement, inflammasome, microglia, mononuclear phagocytes, retina
Subject Categories: Immunology, Neuroscience
Glossary
- Fate‐mapping
A method applied in developmental biology, for understanding the embryonic origin of tissues in the adult organism by investigating the correspondence between individual cells (or groups of cells) at one stage of development, and their progeny at later stages.
- Geographic atrophy (GA)
An advanced form of AMD characterized by the presence of atrophic lesions of the outer retina, resulting from loss of photoreceptors, retinal pigment epithelium (RPE), and underlying choriocapillaris.
- Humanized antibodies
Antibodies from non‐human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans in order to reduce the immunogenicity.
- Immunomodulation
Therapeutic interventions modulating the immune response to a desired level rather than suppressing it. The aim is to enhance beneficial functions while minimizing host harming processes of the immune system.
- Innate immune system
Nonspecific defense mechanisms that deliver host defense immediately or within hours of pathogen appearance or tissue insult.
- Microglia
Resident immune cells of the brain and retina that are derived from primitive myeloid progenitors originating from the yolk sac. Microglia cells are a long‐living, autonomous, and self‐renewing population and are not replenished from postnatal hematopoietic.
- Mononuclear phagocytes
Mononuclear cells include circulating blood monocytes, tissue‐resident macrophages, dendritic cells, and microglia with the ability to phagocytose.
- Non‐proliferative diabetic retinopathy (non‐PDR)
An early stage of diabetic retinopathy characterized by damage to retinal vasculature and loss of pericytes. It can further progress into PDR defined by pathological neovascular growth, vitreous hemorrhage, retinal scars, and detachment, resulting in irreversible vision loss.
- Ontogeny
All the developmental events that occur during the existence of a living organism. In cell biology, ontogeny refers specifically to developmental and differentiation processes within a cell lineage.
- Phagoptosis (primary phagocytosis)
Cell death resulting from phagocytosis of reversibly stressed cells by phagocytes, provoked by exposure of “eat‐me” signals (e.g., phosphatidylserine) and/or loss of “don't‐eat‐me” signals (e.g., polysialic acid).
- Rd1 and rd10
Two of the 16 naturally occurring mouse mutant lines that manifest degeneration of the photoreceptors. Rd1 and rd10 mice carry mutation in exon 7 and exon 13 of the beta subunit of the rod phosphodiesterase gene, respectively.
Introduction
Diabetic retinopathy (DR) and age‐related macular degeneration (AMD) are the two most frequent retinal degenerative and neovascular diseases in the developed world. While the former is an end‐stage diabetic complication and leading cause of visual impairment among working‐age adults, the latter is the most common cause of blindness in the elderly, especially among Caucasians. Around one‐third of the population is diagnosed with diabetes, with one‐tenth having vision‐threatening disease course which includes diabetic macular edema (DME) or proliferative diabetic retinopathy (PDR; Ting et al, 2016). Also alarming are the epidemiologic facts about AMD with more than 150 million people worldwide suffering from early forms and around 10 million people developing the late stages which are geographic atrophy (GA) and neovascular AMD (Wong et al, 2014). Characteristic for DR and the neovascular form of AMD is blood vessel growth from the subretinal space into the retina. The vascular network not only forms in an unregulated manner, but also becomes leaky. New vessel formation is driven by the angiogenic factor vascular endothelial growth factor (VEGF), and hence, both diseases are treated with intravitreal injections of VEGF inhibitors; however, treatment success is not guaranteed (Cummings & Cunha‐Vaz, 2008). Moreover, no treatment options are currently available for patients suffering from GA. Another group of blinding diseases lacking established therapeutic options are inherited retinal degenerations, such as retinitis pigmentosa (RP). RP is the most frequent monogenic photoreceptor degenerating disease with an estimated prevalence of 1:4,000 (Haim, 2002).
The vertebrate retina is a highly organized layered structure with more than 60 distinct cell types (Masland, 2001; Hoon et al, 2014). Both the highly active photoreceptor cells and the phagocytic retinal pigment epithelium cells (RPE) contribute to the generation of metabolic by‐products (Chiu & Taylor, 2011; Datta et al, 2017). With increasing age, there is a decline in functionality of retinal cells (Damani et al, 2011; Mitter et al, 2014). Hence, the cells are less effective in dealing with the accumulating metabolic waste (Wang et al, 2009; Mitter et al, 2014). Moreover, the decline in functionality is accompanied by a drop in efficacy, for instance, of the RPE to phagocyte shedded photoreceptor debris (Nandrot et al, 2004; Gu et al, 2012; Mazzoni et al, 2014). Throughout lifetime, this complex organ is challenged by a variety of noxious insults including hypoxia, hyperglycemia, and inherited mutations (Fritsche et al, 2016; Masuda et al, 2017). These circumstances demand constant surveillance of the retina for the detection and defense against pathologic perturbation. To meet this demand, the retina is equipped with a highly sensitive innate immune system. This immune system includes (i) surveilling microglia cells, which migrate to the site of damage and phagocyte apoptotic material (Karlstetter et al, 2015), (ii) activation of the complement system to opsonize cellular debris (Xu & Chen, 2016), and (iii) inflammasome assembly in the RPE (Doyle et al, 2012; Gao et al, 2015). When not tightly controlled, these immune pathways pose threat to the surrounding host tissue. Hence, a cross‐talk with Müller cells and retinal neurons through the release of regulatory molecules, including complement factors, chemokines, and neurotrophic factors, limits overt immune activation in the healthy retina (Harada et al, 2002; Langmann, 2007; Wolf et al, 2013). In the event of a transient imbalance in retinal physiology, rapid activation of the immune response will induce restoration of tissue homeostasis and function. However, in case of persistent insult, chronic over‐activation of the inflammatory response can lead to devastating tissue remodeling (Chen & Xu, 2015). Pro‐inflammatory factors such as reactive oxygen species (ROS), TNF‐α, and CCL2 as well as complement activators such as C1q are released into the cytosol by overly active microglia (Scholz et al, 2015a; Madeira et al, 2018). Furthermore, microglia over‐express the anaphylatoxin receptors C5aR and C3aR, while complement inhibitors such as CFH and CFI are downregulated (Zipfel & Skerka, 2009; Guillonneau et al, 2017; Madeira et al, 2018). Complement factors also act as enhanced triggers for inflammasome assembly, which leads to the activation of the pro‐inflammatory cytokines pro‐IL‐1β and pro‐IL‐18 (Nebel et al, 2017; Madeira et al, 2018). The resulting chronic inflammatory response is associated with a decline in RPE function and structure, breach of the blood–retina barrier (BRB), new vessel formation, and recruitment of choroidal macrophages (Donoso et al, 2006; Liu et al, 2013; Sato et al, 2018). Hence, these circumstances necessitate therapy approaches targeting the malfunctioning immune response. Therefore, inhibiting sustained inflammation represents a plausible therapeutic target to treat a broad range of retinal pathologies (Langmann, 2007).
Here, we comprehensively summarize the role of the three key innate immune pathways in the most common retinal degenerative diseases. Furthermore, we comment on recent developments in preclinical models targeting these pathways and summarize the current status of clinical trials.
Targeting mononuclear phagocytes in retinal degenerative diseases
Mononuclear phagocytes in the healthy and diseased retina
Cells of the mononuclear phagocyte (MP) lineage include circulating blood monocytes, tissue‐resident macrophages, dendritic cells, and microglia (Chow et al, 2011). These cells can be differentiated by their ontogeny, location, function, and phenotype (Guilliams et al, 2014). MP ontogeny is a research area with much controversy; however, fate‐mapping studies have established that unlike blood‐borne monocyte‐derived macrophages, microglia originate from primitive myeloid progenitors in the extra‐embryonic yolk sac which migrate into the CNS before the blood–brain barrier (BBB) is established (Ginhoux et al, 2010). Once the tissue is matured, the self‐renewing microglia population is maintained in the brain parenchyma and the retina throughout the entire life span where they compose the resident immune cells (Réu et al, 2017). Interestingly, in the adult retina, microglia replenish from two distinct extra‐retinal sources as shown by pharmacologic depletion using the selective CSF1R inhibitor PLX5622 (Huang et al, 2018a). Unlike brain microglia, the cells in the retina were not repopulated from nestin‐positive precursors (Huang et al, 2018b). Instead, replenished microglia in the retina displayed a dual extra‐retinal origin and long‐distance migration ability. First, the residual microglia in the optic nerve repopulate the retina along the center‐to‐periphery axis, and second, macrophages from the ciliary body and iris relocate to the periphery and migrate toward the center. Furthermore, repopulated microglia fully restore the broad functionalities of naive microglia (Huang et al, 2018a; Zhang et al, 2018). These repopulation mechanisms are mainly regulated by the neuronal chemokine CX3CL1 and its receptor (CX3CR1) in microglia (Zhang et al, 2018).
In the mature retina, microglia reside in the inner and outer plexiform layers and form a sophisticated network of non‐overlapping cells (Hume et al, 1983). Here, these cells exhibit an abundantly ramified morphology spanning the complete nuclear layers with their long protrusions (Karlstetter et al, 2015). The dynamic nature of microglia allows them to execute housekeeping functions. The most crucial role is the constant active surveillance of retinal homeostasis where they are indispensable for the immune response and synaptic pruning and transmission (Schafer et al, 2012; Wang et al, 2016a).
In order to sense the environment for endogenous or exogenous non‐physiological stimuli, microglia engage surface receptors or pattern recognition receptors (PRRs; Kettenmann et al, 2011; Kigerl et al, 2014). These surface receptors ligate complement components, cytokines, chemokines, and damage‐ or pathogen‐associated molecular patterns (DAMPs/PAMPs; Karlstetter et al, 2015). In the event of an insult, microglia sense the danger signals and respond by retracting their surveilling processes concomitant with upregulating the expression of surface receptors (Jurgens & Johnson, 2012). Furthermore, they proliferate and migrate to the site of damage, while releasing pro‐inflammatory cytokines and ROS to neutralize the damage (Ferrer‐Martin et al, 2015). Moreover, their phagocytic capacity is significantly enhanced to effectively clear debris and prevent accumulation of waste products (Kohno et al, 2014). In the healthy retina, the insult is rapidly neutralized, the damaged tissue is repaired, and a return to homeostasis is achieved with only very little retinal remodeling (Chen et al, 2012). This finite microglial activation is beneficial since the toxicity associated with the immune response is outweighed by the toxicity produced due to the noxious insult (Guillonneau et al, 2017). However, under aggravated conditions owing to genetic predispositions or high glucose levels, microglial activation persists (Gupta et al, 2003; Omri et al, 2011). Non‐resolving inflammation causes terminal damage since the increased release of neurotoxic by‐products and the lack of regenerative capacity prevent retinal recovery (Chen & Xu, 2015). Furthermore, over‐reactive microglia are not able to distinguish between stressed and apoptotic cells, and thus phagocyte viable neurons as well (phagoptosis; Brown & Neher, 2012, 2014). Under these circumstances, neurotoxic microglia accumulate at the site of damage and fail to return to their homeostatic state (Tang & Kern, 2011; Ardeljan & Chan, 2013). Additionally, by the secretion of chemokines such as CCL2, microglia attract further phagocytes, which include infiltrating monocytes and choroidal macrophages due to the leakage of the BBB and the newly formed blood vessels (Caicedo et al, 2005; Sennlaub et al, 2013). Indeed, such changes in retinal microglia morphology, location, and infiltration of macrophages are common hallmarks of AMD, DR, and hereditary retinopathies (Fig 1; Karlstetter et al, 2015; Guillonneau et al, 2017).
Figure 1. Schematic representation of microglial activity in the retina.
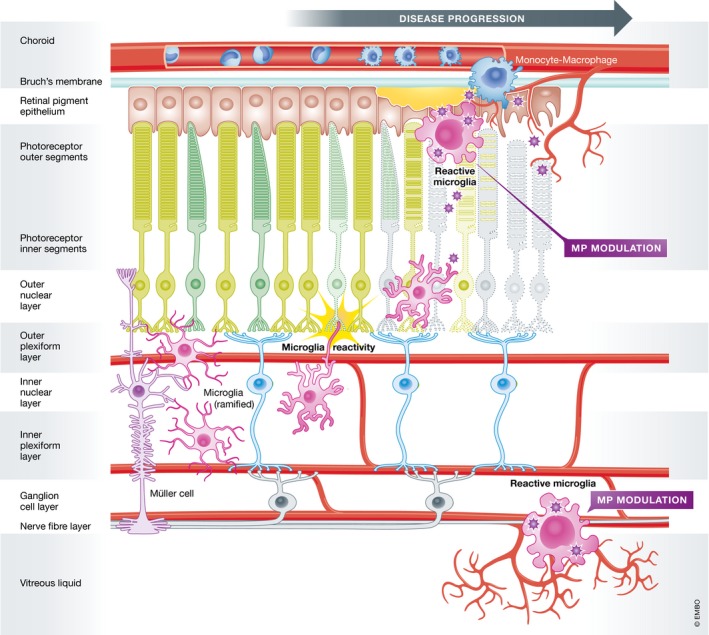
Under homeostatic conditions, resident microglia mainly populate the plexiform layers. With their long protrusions, they continuously scan their environment and phagocytose cell debris. Different insults leading to abnormal cell functions or degeneration in the RPE, the photoreceptor layer, and the ganglion cell layer rapidly alert microglia. Resident microglia migrate to the lesion sites, where they transform into amoeboid full‐blown phagocytes and recruit macrophages from the periphery. Modified from Karlstetter et al (2010).
Early aspects of DR consist of microaneurysms and intraretinal microvascular anomalies (non‐PDR), while PDR involves neovascularization and preretinal and vitreal hemorrhages (Das et al, 2015; Lechner et al, 2017b). Due to these clinical features, DR was only regarded as a vascular complication; however, recent investigations have identified inflammation as an important contributing factor in disease development (Adamis, 2002; Rangasamy et al, 2012). Elevation in systemic and local chemokine concentration is present in patients suffering from DR (Petrovic et al, 2010; Koleva‐Georgieva et al, 2011; Suzuki et al, 2011). Several authors could demonstrate increased levels of TNF‐α, IL‐8, CCL2, IL‐1β, and IL‐6 in the vitreous fluid (Demircan et al, 2006; Murugeswari et al, 2008; Boss et al, 2017). Furthermore, peripheral monocytes from DR patients secrete high amounts of IL‐1β, IL‐6, TNF‐α, IL‐8, and IL‐1ra (Hatanaka et al, 2006; Bradshaw et al, 2009). These inflammatory cytokines are associated with phagocyte reactivity and serve as chemoattractants for invading macrophages. Leakage of the retinal vasculature is mainly induced by VEGF, but also involves TNF‐α, which decreases the expression of tight junction proteins ZO‐1 and claudin‐5 (Behl et al, 2008; Aveleira et al, 2010). The increase in endothelial cell permeability then leads to further immune cell recruitment and disease manifestation. Indeed, histopathological analysis of eyes from patients with non‐PDR and PDR exhibited increased numbers of hypertrophic microglia which correlated with disease severity (Zeng et al, 2008). MPs were clustered around retinal hemorrhages and microaneurysms. In late stages, increased numbers of phagocytes were present in the ganglion cell layer, in the newly formed blood vessels, and around the optic nerve head (Zeng et al, 2008). Also, there is ample evidence that hyperglycemia induces immune cell reactivity through oxidative stress or indirectly via effects mediated by stressed retinal cells in the proximity (Du et al, 2002; Rashid et al, 2018). Oxidative stress in hyperglycemia is driven by accelerated free radical production concomitant with compromised antioxidant generation (Cameron et al, 1994; Tomlinson & Gardiner, 2008). These circumstances cause translocation of nuclear factor‐kappa B (NF‐κB), pro‐inflammatory cytokine expression, and MP activation (Nishikawa et al, 2000).
Age‐related macular degeneration, the leading cause of vision loss in the elderly, is a disease of the macula–RPE–choroid interface (Bhutto & Lutty, 2012; Ardeljan & Chan, 2013; Biesemeier et al, 2014). The RPE is essential for the maintenance and survival of photoreceptor cells by engulfing and degrading shedded photoreceptor disks and protecting the retina against light and oxidative stress (Young, 1967; Boulton, 2013; Mazzoni et al, 2014). These circumstances require a high metabolic activity rendering the RPE very susceptible to oxidative damage (Mitter et al, 2012). Oxidative stress is therefore a risk factor for AMD and hence needs rapid neutralization to ensure proper vision (Jarrett & Boulton, 2012). Indeed, the RPE engages in autophagy and mitophagy, directing ROS‐producing mitochondria to lysosomes, as defense mechanisms against ROS increase (Lee et al, 2012; Mitter et al, 2012; Ferguson & Green, 2014). Mitter et al (2014) found an age‐related increase in autophagosomes and proteins involved in autophagy in the RPE; however, these factors were significantly reduced in human AMD donor eyes. Deteriorated autophagy concomitant with increased accumulating ROS results in aggregation of damaged organelles and toxic by‐products including the photoreactive age‐pigment lipofuscin (Wang et al, 2009; Mitter et al, 2014). These deposits become apparent as drusen located in the sub‐RPE area and subretinal space during early AMD (Ishibashi et al, 1986; Abdelsalam et al, 1999; Gupta et al, 2003; Fritsche et al, 2016). Strikingly, drusen components include lipoproteins and complement factors serving as attractants and activators of MPs (Penfold et al, 1985; Johnson et al, 2000; Hageman et al, 2001; Nozaki et al, 2006; Buschini et al, 2011). Indeed, bloated phagocytic microglia were found to closely associate with drusen in AMD patients (Gupta et al, 2003). While the elimination of retinal debris by MPs is principally positive, impaired function during aging of these cells leads to sustained pro‐inflammatory environment (Streit et al, 2004; Chan‐Ling et al, 2007; Damani et al, 2011). A comparative transcriptome analysis of AMD and normal human donor eyes demonstrated a significant over‐expression of immune‐related transcripts including complement and chemokine mRNAs in all AMD samples (Newman et al, 2012). Furthermore, high levels of CCL2 and VEGF, two cytokines involved in MP recruitment and choroidal neovascularization (CNV), are present in ocular fluids from neovascular AMD patients (Fauser et al, 2015; Lechner et al, 2017a). As a consequence, accumulating subretinal microglia can directly induce death of nearby photoreceptors. This suggests that microglial reactivity is a driving force in photoreceptor demise and disease manifestation.
Unlike AMD, which is a multifactorial disease, hereditary degenerations of the human retina are mostly monogenic. The majority of the documented mutations are associated with genes expressed in photoreceptors and RPE (Karlstetter et al, 2015; RetNet, 2018). RP, the most common form of hereditary retinal degeneration, is characterized by night blindness and tunnel vision due to rod demise (Hartong et al, 2006). Even more, late stages of RP are characterized by central vision loss attributed to the secondary death of cone photoreceptors (Hartong et al, 2006). Involvement of MPs was proven by analyzing retinal sections with concentric RP demonstrating bloated microglia in the photoreceptor layer with rhodopsin‐positive inclusions (Gupta et al, 2003; Zhao et al, 2015a,b,c). The authors hypothesized that activated phagocytes release pro‐inflammatory cytotoxic factors that subsequently trigger the death of adjacent cones. The resulting pro‐inflammatory environment leads to further recruitment of MPs which, when overly activated, are unable to discriminate between dead versus stressed‐but‐viable neurons and hence engage in phagoptosis (Brown & Neher, 2012, 2014). Indeed, co‐staining of rhodopsin with apoptosis markers in mouse models of RP demonstrated rhodopsin‐positive inclusions that were mostly TUNEL‐negative (Zhao et al, 2015a,b,c). Furthermore, microglial phagocytosis in the ONL corresponded to concurrent exposure of phosphatidylserine which serves as an “eat‐me” signal in stressed rods. Also, microglia were found to upregulate lactadherin/milk fat globule‐EGF factor 8 protein (MFG‐E8), a “bridging” molecule between phagocytes and phosphatidylserine on neurons to facilitate rapid engulfment and internalization of stressed neurons (Neniskyte & Brown, 2013).
Therapeutic strategies targeting mononuclear phagocytes in preclinical models of retinal degenerative diseases
As discussed above, MP activation in the retina is initiated either through direct recognition of immune triggers, such as DAMPs/PAMPs, chemoattractants, and complement components, or indirectly by sensing an amplitude of stressors in the surrounding environment including ROS and “eat‐me” signals from dying cells. Overshooting MP reactivity often leads to tissue damage, but their depletion does not always result in tissue homeostasis (Zhao et al, 2015a,b,c). Several studies point out that microglia are indispensable for the maintenance of synaptic structures in the adult CNS. Their depletion in the mature CNS can cause deficits in learning tasks and a significant reduction in motor‐learning‐dependent synapse formation (Parkhurst et al, 2013). More importantly, phagocyte ablation in the adult retina leads to the degeneration of photoreceptor synapses in the outer plexiform layer and hence a functional deterioration in retinal light responses (Wang et al, 2016a). Therefore, effective immunomodulatory compounds should dampen the overt pro‐inflammatory response of retinal phagocytes but preserve their homeostatic functions which are vital for retinal integrity.
Mononuclear phagocytes are composed of a heterogeneous population with diverse functionalities (Hanisch, 2013). What remains inconclusive is whether the beneficial and detrimental effects carried out by reactive MPs are executed by the same population or by distinct subtypes. Distinguishing these cell populations is a challenging task. For instance, infiltrating macrophages cannot be easily separated from resident microglia in laser‐induced CNV in mice. Laser‐induced CNV is extensively applied in retinal research since it recapitulates several main features of exudative AMD (Lambert et al, 2013). The laser impact results in the rupture of Bruch's membrane, a rapid recruitment of MPs, and penetration of choroidal capillaries into the avascular retina within a few days. In contrast, alternative models for experimental CNV that involve injections of pro‐angiogenic substances have a much lower incidence of neovascularization (Shah et al, 2015). In order to specifically target resident microglia and to distinguish them from short‐lived infiltrating cells in this model, we used tamoxifen‐inducible conditional Cx3cr1CreER mice to delete the floxed gene for interferon‐α/β receptor 1 (Ifnar1; Luckoff et al, 2016). These Cx3cr1CreER:Ifnar1fl/fl mice were subjected to laser injury 4 weeks after tamoxifen injection when monocyte‐derived macrophages were already washed out and replaced. However, no differentiation between retinal microglia and potentially long‐lived tissue‐resident macrophages in the periphery could be achieved (Reyes et al, 2017). Another elegant approach for cell discrimination is to use fate‐mapping combined with endogenous genetic reporters and multiple expression markers. O'Koren et al (2016) demonstrated that retinal microglia have a unique CD45(low) CD11c(low) F4/80(low) I‐A/I‐E(−) signature which is conserved in the steady state and during retinal injury. By investigating these cells, the authors found that microglia migrate to the photoreceptor outer segments while monocyte‐derived macrophages appear throughout the entire retina (Reyes et al, 2017). For further insights into microglia heterogeneity, the reader is directed to other excellent reviews (Hanisch, 2013; Reyes et al, 2017).
Genetic mouse models combined with experimental approaches mimicking retinal degenerative diseases have greatly expanded our knowledge on the mechanisms involved in retinal MP activation (Luckoff et al, 2017). In the following section, we present main concepts for microglia‐related immunomodulation. These strategies involve the prevention and/or resolution of retinal degeneration and neovascularization by (i) targeting activating and inhibitory cell surface receptors, (ii) modulating intracellular molecules, and (iii) controlling released inflammatory mediators.
Purinergic receptors
The purines adenosine triphosphate (ATP) and adenosine serve as neuro‐ and gliotransmitters in the retina contributing to the bidirectional neuron–glia communication as well as the cross‐talk between photoreceptors and the RPE (Newman, 2006; Housley et al, 2009; Wurm et al, 2010). Physiologically, purines are tonically released in the dark; however, this release is elevated when neurons are active (Khakh & North, 2006; Uckermann et al, 2006; Niyadurupola et al, 2013). Growing evidence suggests that dysregulated purinergic signaling contributes to gliosis in the diseased retina (Sanderson et al, 2014). Degenerating cells or elevated glucose levels increase the extracellular ATP concentration, which activates the P2X7 receptor (P2X7R) on MPs and induces a chemokine release through PKC/MAP kinase pathway activation (Fig 2; Potucek et al, 2006; Costa et al, 2009; Shiratori et al, 2010; He et al, 2017). ATP stimulation evokes the release of pro‐inflammatory cytokines IL‐6, TNF‐α, and CCL2 in primary microglia, which was absent when P2X7 was deleted (Morigiwa et al, 2000; Shieh et al, 2014). In a murine model of axonal injury that culminates in the death of retinal neurons, P2X7‐deficient mice exhibited a delayed loss of retinal neurons and a decrease in phagocytic microglia (Nadal‐Nicolas et al, 2016). Moreover, intravitreal administration of the selective P2X7 antagonist A438079 delayed axotomy‐induced ganglion cell death.
Figure 2. Activation pathways and therapy targets for mononuclear phagocytes.
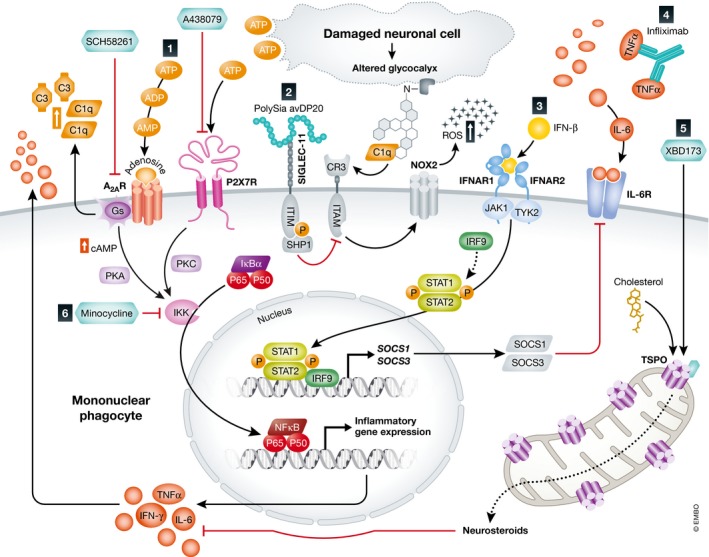
(1) The purines ATP and adenosine ligate to their receptors P2X7 and A2AR, respectively, which act through PKA/PKC signaling and thereby activate IKK. IKK aids to cleave and translocate NF‐κB into the nucleus and induce inflammatory gene expression and cytokine release. A2AR additionally potentiates the expression of the complement component C3 and the opsonin C1q. Neuroprotective effects were observed when A2AR and P2X7R were blocked using the selective inhibitors SCH58261 and A438079, respectively. (2) Intravitreal administration of polySia avDP20 compensates for desialylated neurons by binding to its receptor SIGLEC‐11 and inhibiting neurotoxic inflammation through its ITIM domain. During inflammation, desialylated neurons activate CR3 associated ITAM and thereby trigger excessive release of ROS via NOX2. (3) The IFN‐β pathway involves STAT1‐ and STAT2‐induced SOCS1/SOCS3 release which reduces cytokine expression through a negative feedback and inhibits IL‐6 signaling. (4) Neutralizing antibodies such as infliximab aid to neutralize cytosolic TNF‐α. (5) TSPO ligands such as XBD173 stimulate the production of neurosteroids which limit the release of pro‐inflammatory cytokines. (6) Minocycline is a potent inhibitor of NF‐κB signaling.
Adenosine is a neuromodulator critically involved in neurodegenerative diseases (Yu et al, 2004; Kalda et al, 2006). It modulates microglial reactivity mainly through the activation of its G‐protein‐coupled receptor A2A (A2AR; Fig 2; Cunha, 2001; Canas et al, 2009). Strikingly, agonists of A2AR potentiate LPS‐induced microglial reactivity (Saura et al, 2005). Conversely, its pharmacological inhibition results in neuroprotective effects by attenuating microglial NO production and modulating cyclooxygenase‐2 (COX2) expression in a rat model of striatal excitotoxicity (Saura et al, 2005; Minghetti et al, 2007). Similarly, inhibition of A2AR prevents microglial reactivity in mice that were subjected to intraperitoneal LPS injection (Rebola et al, 2011) and selective A2AR blockade reduces NO production in microglia (Madeira et al, 2015). In a retinal degeneration model of transient ischemia–reperfusion, intravitreal injection of the A2AR‐blocking compound SCH58261 attenuated neuronal loss by inhibiting microglial reactivity (Madeira et al, 2016). In microglia, A2AR clearly facilitates the release of cytokines and NO production, which are likely induced through increased cAMP levels and the activation of protein kinase A (PKA; Fig 2; Moreau & Huber, 1999). Subsequent activation of MAP kinases including extracellular signal‐regulated kinase (ERK) 1/2 and IkappaB kinase (IKK) then induces altered gene expression (Kyriakis & Avruch, 2001; Schulte & Fredholm, 2003; Chio et al, 2004; Dang et al, 2014). Recently, we showed that A2AR antagonism also limits complement and inflammasome activation (Madeira et al, 2018). The exposure of human microglia to RPE cell debris induced activation of the complement cascade which is strongly associated with the pathogenesis of AMD (Zipfel & Skerka, 2009; Schick et al, 2017). Inhibition of A2AR prevented this change in microglial complement activation as well as inflammasome activation in ARPE‐19 cells that were exposed to conditioned media from activated microglia treated with the A2AR blocker. Therefore, selective A2AR antagonists could serve as tools to modulate microglial activity and limit RPE inflammatory response.
CD200 receptor
CD200 is a transmembrane glycoprotein expressed on the vascular endothelium, photoreceptors, RPE, and ganglion cells. Its respective receptor is expressed on microglia which, upon ligand binding, provides a potent quiescence signal (Dick et al, 2001, 2003). Deficiency in CD200 signaling is associated with microglial proliferation and strong iNOS expression, indicating a latent pro‐inflammatory condition (Dick et al, 2003). In an animal model of uveoretinitis, CD200 deficiency caused increased numbers of microglia concomitant with increased expression of iNOS (Broderick et al, 2002). Similarly, in the murine laser model of exudative AMD, CD200R knockout animals displayed significantly increased new vessel formation and elevated levels of VEGF‐A, arginase 1, and IL‐1β (Horie et al, 2013). Conversely, intravitreal injection of the CD200R agonistic monoclonal antibody DX109 diminished microglial reactivity and macrophage infiltration, thereby suppressing pathological angiogenesis and vascular damage (Horie et al, 2013). Also, systemic administration of DX109 was effective in suppressing IFN‐γ‐mediated phagocyte activation and protected against tissue damage during experimental autoimmune uveoretinitis (Copland et al, 2007). Hence, CD200R agonists could be used to diminish pro‐angiogenic and pro‐inflammatory gene expression which prevents pathological angiogenesis.
Polysialic acid receptors
In the vertebrate CNS, polysialic acid (polySia) caps consistent of α‐8‐linked N‐acetylneuraminic acids are commonly attached to glycoproteins of healthy neurons (Schnaar et al, 2014; Hildebrandt & Dityatev, 2015). PolySia can alleviate neurotoxicity via binding to sialic acid‐binding immunoglobulin‐like lectin 11 (SIGLEC‐11), a primate lineage‐specific receptor (Angata et al, 2002; Linnartz‐Gerlach et al, 2014). Interestingly, some strains of neuroinvasive bacteria can also produce capsular polysaccharide identical to polySia in order to escape immune recognition in the human host (Troy, 1992; Schwarz et al, 2017). Ectopic expression of human SIGLEC‐11 in cultured murine microglia suppressed the LPS‐induced transcription of the pro‐inflammatory mediators IL‐1β and iNOS (Wang & Neumann, 2010). SIGLEC‐11 mediates immune inhibition through its cytosolic immunoreceptor tyrosine‐based inhibition motif (ITIM; Fig 2). Binding of polySia to its receptor leads to phosphorylation of ITIM (Linnartz & Neumann, 2013). Successive recruitment of the second messenger SHP1 dephosphorylates the intracellular immunoreceptor tyrosine‐based activation motif (ITAM), thus limiting a number of downstream pathways linked to the phagocytosis of neurites and NADPH oxidase (NOX2)‐mediated production of ROS (Fig 2; Graham et al, 2007; Hamerman et al, 2009). Under pathological conditions, immune cells secrete neuraminidases which cleave sialic acid residues on neurons (Amith et al, 2010; Pshezhetsky & Hinek, 2011; Nomura et al, 2017). Desialylated neurons are consequently opsonized by complement component C1q, which is produced and secreted by microglia (Linnartz et al, 2012; Madeira et al, 2018). Indeed, soluble sialic acid residues accumulate in serum, and C1q is found in the retina during early stages of AMD (van der Schaft et al, 1993; Goswami et al, 2003). Subsequently, the opsonized glycocalyx is recognized by complement receptor 3 (CR3) coupled to ITAM leading to phagocytosis of the neuronal structures (Fig 2; Linnartz et al, 2012). Intriguingly, blockage of CR3 prevented neurite phagocytosis by microglia, which was as seen when polySia was removed from cultured neurons by treatment with sialidases (Wang & Neumann, 2010).
These results point toward a polySia‐based therapy to target inflammation. Consistently, studies showed that nanomolar concentrations of low molecular weight polySia with average degree of polymerization of 20 (polySia avDP20) significantly reduced pro‐inflammatory gene transcription, abnormal phagocytosis, and oxidative burst in human macrophages challenged with LPS or amyloid‐β1–42 (Shahraz et al, 2015). Furthermore, we used humanized transgenic mice expressing human SIGLEC‐11, subjected them to laser injury, and treated them with intravitreal injections of polySia avDP20 (Karlstetter et al, 2017). Already low doses of polySia avDP20 significantly reduced microglial activation and vascular leakage by reducing TNF‐α and VEGF‐A levels as well as superoxide production (Fig 2). As a second mechanism of action, independent from SIGLEC‐11 signaling, higher doses of polySia avDP20 blocked alternative complement activation and reduced membrane attack formation in the diseased retina (Karlstetter et al, 2017).
Interferon‐β (IFN‐β)
Important evidence for potent immunomodulatory effects of IFN‐β on brain microglia came from gene deletion studies in experimental autoimmune encephalomyelitis (EAE) mouse models. Animals lacking either the Ifn‐β gene or its cognate interferon‐α/β receptor (Ifnar) exhibited elevated microglial reactivity concomitant with an even severe EAE disease phenotype when compared to wild‐type controls (Teige et al, 2003; Prinz et al, 2008). More importantly, IFN‐β treatment in a multiple sclerosis patient completely reversed subfoveal neovascularization and choroiditis emphasizing the therapeutic potential of IFN‐β for inflammatory and vascular diseases of the eye (Cirino et al, 2006).
We have therefore studied whether IFN‐β therapy could have beneficial immunomodulatory effects in the laser CNV model for exudative AMD. We demonstrated that systemic administration of IFN‐β not only inhibited MP reactivity and macrophage recruitment but also reduced vascular leakage and neoangiogenesis (Luckoff et al, 2016). The immune cell reactivity was evaluated by counting the total number of reactive MPs in the laser spot and their ramification status. Both parameters were significantly affected by IFN‐β treatment. Conversely, genetic deletion of Ifnar1 in mice resulted in aggravated disease after laser treatment. Similar results were obtained with a microglia‐specific conditional deletion of IFN‐β signaling (Cx3cr1CreER:Ifnar1fl/fl). Our results provide a mechanistic explanation for earlier publications where damage associated with laser photocoagulation in rabbits and monkeys was successfully treated with IFN‐β (Tobe et al, 1995; Kimoto et al, 2002). Apart from affecting microglial reactivity by blocking the production of neurotoxic superoxide radicals, IFN‐β also promotes RPE homeostasis and suppresses proliferative activity of endothelial cells (Kimoto et al, 2002; Jin et al, 2007).
Despite the clear indications of a protective IFN‐β signaling in retinal microglia, the exact molecular pathways remain poorly understood. IFN‐β signaling involves the transcription of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 by translocating the transcription factors STAT1 and STAT2 into the nucleus (Rashid et al, 2018; Fig 2). SOCS1 and SOCS3 expression is known to engage in inhibitory signals to mitigate microglial activation and prevent cell toxicity (Kimura et al, 2005; Baker et al, 2009; McCormick & Heller, 2015). Supporting evidence comes from a study where SOCS3 deficiency in myeloid cells exaggerated retinal degeneration and accelerated retinal angiogenesis in a murine model of uveoretinitis (Chen et al, 2018). In these mice, SOCS3‐deficient retinas demonstrated higher levels of pro‐inflammatory cytokines IL‐1β, TNF‐α, and IFN‐γ as well as angiogenic factors including VEGF‐A. Similarly, SOCS1 protected retinal cells from staurosporine‐ and H2O2‐induced apoptosis (Yu et al, 2011). Also, members of the SOCS family are potentially key physiological negative regulators of IL‐6 signaling in macrophages (Croker et al, 2003; Wilson, 2014; Fig 2).
TSPO ligands
Translocator protein 18 kDa (TSPO) is a highly conserved 5α‐helical transmembrane protein located on the outer mitochondrial membrane (Girard et al, 2012). Highly induced TSPO protein expression is predominantly found in activated microglia during various neuropathological conditions (Daugherty et al, 2013; Karlstetter et al, 2014; Rashid et al, 2018). Concomitantly, astrocytes and Müller cells upregulate the secretion of an endogenous TSPO ligand, diazepam binding inhibitor (DBI) protein which is sensed by microglia and serves to limit the magnitude of microglial reactivity by inducing feedback regulation (Wang et al, 2014). Finally, triakontatetraneuropeptide (TTN), the biologically active cleavage product of DBI, triggers the transformation of activated microglia to baseline quiescence (Wang et al, 2014).
Based on this concept of feedback regulation, synthetic TSPO ligands were effective immunoregulators in various animal models for neurological diseases including Alzheimer's disease, multiple sclerosis, and anxiety disorders (Rupprecht et al, 2009; Barron et al, 2013). In our study on retinal degeneration, we tested the ability of the specific TSPO ligand XBD173 to dampen microglial reactivity in the acutely white light‐damaged mouse retina (Fig 2). In this model, exposure to intense white light leads to a significant loss of photoreceptor cells and thinning of the outer nuclear layer within a few days after dark adaptation and light exposure (Wenzel et al, 2005). We found that systemic administration of XBD173 markedly limited the accumulation of amoeboid microglia in the outer retina and protected from overt cell death (Scholz et al, 2015a). Mechanistically, XBD173 efficiently suppressed pro‐inflammatory gene expression in cultured microglia and reduced neuronal cell death in microglia‐conditioned medium (Karlstetter et al, 2014). Moreover, XBD173 triggered a neuroprotective microglia phenotype in explanted organotypic mouse retinal cultures (Karlstetter et al, 2014). These effects mediated by XBD173 were prevented upon blocking the enzymatic conversion of cholesterol to pregnenolone (Fig 2), which can be converted to progesterone, a potent neurosteroid with pleiotropic neuroprotective properties (Pettus et al, 2005; Guennoun et al, 2015; Cai et al, 2018). In rd1 mice, a model for retinitis pigmentosa, oral progesterone treatment decreased gliosis and cell death leading to improved retinal function (Sanchez‐Vallejo et al, 2015). Similarly, TTN stimulation of microglia increased levels of dehydroepiandrosterone, an effective anti‐inflammatory neurosteroid (Wang et al, 2014), and the TSPO ligand Ro5‐4864 effectively reduced diabetic neuropathy through a local increase in neurosteroids (Giatti et al, 2009). These findings clearly support the concept that TSPO exerts its neuroprotective effects by modulating neuronal steroidogenesis.
Minocycline
Minocycline is a membrane‐permeable semi‐synthetic tetracycline derivative with strong neuroprotective and immunomodulatory effects (Garrido‐Mesa et al, 2013). Minocycline blocks microglial activation in response to a variety of inflammatory stimuli by inhibiting Toll‐like receptor 2 (TLR2) and TLR4 signaling (Nikodemova et al, 2006; Halder et al, 2013). TLRs induce a potent immune response upon recognition of PAMPs (Uematsu & Akira, 2006). Receptor signaling triggers cytokine production through translocation of NF‐κB into the nucleus which is essential for the defense of the host cell (Beutler, 2004). Increased expression of TLR2 and TLR4 concomitant with elevated NF‐κB levels is often found in human monocytes under conditions of hyperglycemia (Mohammad et al, 2006; Dasu et al, 2008). In the mouse retina, pre‐diabetic conditions and high‐fat diet caused TLR4‐dependent activation of microglia ad macrophages concomitant with vision loss (Lee et al, 2015). Microglia themselves can experience necroptosis, a form of inflammatory cell death, through TLR4 activation in rd1 mice, thereby exacerbating retinal inflammation and damage (Huang et al, 2017).
Minocycline potently inhibits NF‐κB transcriptional activity by blocking the degradation of IκBα (Nikodemova et al, 2006; Fig 2). Systemic minocycline therapy in light‐damaged mice reduced pro‐inflammatory cytokine release, prevented microgliosis, and preserved photoreceptor function in the retina (Zhang et al, 2004; Scholz et al, 2015b). Similarly, minocycline inhibited microglial reactivity and photoreceptor apoptosis in the rd10 mouse model of human RP (Peng et al, 2014). In a streptozotocin (STZ)‐induced rat model of DR, minocycline blocked microglial COX2 expression and prevented the release of IL‐1β and TNF‐α with concomitant reduction in caspase‐3‐mediated apoptosis (Krady et al, 2005). Recent evidence also suggests that minocycline can block the expression of PARP1, a chromatin‐associated enzyme which promotes the expression of IL‐1β and TNF‐α in glial cells, and thereby reduces retinal apoptosis (Wu et al, 2015).
Cytokine inhibition
Enhanced levels of pro‐inflammatory cytokines are involved in AMD (de Oliveira Dias et al, 2011), RP (He et al, 2015), and DR (Patel et al, 2008). Specifically, the cytokines TNF‐α, IL‐1β, and IL‐6 are significantly elevated in retinal pathologies at the time point of immune cell reactivity (Armstrong et al, 1998; Oh et al, 1999; Seddon et al, 2005; Poon et al, 2015; Zhao et al, 2015a). Therefore, scavenging of cytokines has been a valid therapeutic concept in these retinal pathologies.
Several TNF‐α‐inhibiting antibodies have been developed and tested as potential therapy options for retinal degenerations including preclinical models for AMD, glaucoma, and ischemic retinopathy (Al‐Gayyar & Elsherbiny, 2013). Secretion of TNF‐α by phagocytes stimulates VEGF production in RPE and promotes angiogenesis, hence being a candidate target for treating AMD and DR (Cousins et al, 2004; Regatieri et al, 2009). TNF‐α is also a negative regulator of the RPE transcription factor orthodenticle homeobox 2 (OTX2) which orchestrates expression of critical genes involved in proper retinal function (Mathis et al, 2017). In various rodent models, researchers demonstrated positive effects of intravitreal injections of the TNF‐α antibodies (Shi et al, 2006; Regatieri et al, 2009). Histopathological findings confirmed that CNV lesions in treated mice were smaller in size compared to the control animals (Shi et al, 2006). Furthermore, intravitreal injection of low doses of infliximab (10–40 μg) abates the cytokine availability and modulates angiogenesis (Fig 2; Regatieri et al, 2009). Studies in monkey demonstrated that intravitreal injections of adalimumab and the single‐chain antibody fragment ESBA105, both potent TNF‐α inhibitors, resulted in CNV reduction, whereas topical treatment had only weak effects (Lichtlen et al, 2010).
IL‐1β is strongly involved in neovascularization by triggering the release of angiogenic factors (Joyal et al, 2011; Horie et al, 2013; Rivera et al, 2013). Thus, IL‐1β induces a robust release of semaphoring‐3A in retinal ganglion cells and RPE in an oxygen‐induced retinopathy model (Martin et al, 2004; Joyal et al, 2011). Sema3A critically contributes to vascular decay and misguided revascularization (Rivera et al, 2013). Furthermore, excessive IL‐1β release induces P2X7R expression on monocytes, thereby triggering further IL‐1β release and retinal apoptosis (Giuliani et al, 2017). Inhibition of IL‐1β or P2X7R completely prevented the inflammation‐associated photoreceptor demise (Hu et al, 2015). Recently, Natoli et al (2017) showed that inhibition of retinal IL‐1β reduced phagocyte accumulation and photoreceptor death via downregulating chemokine expression by Müller cells and RPE in rats with focal photo‐oxidative damage.
Likewise, high levels of IL‐6 are significantly related to AMD progression and increased in mice with experimentally induced CNV (Seddon et al, 2005; Izumi‐Nagai et al, 2007). Systemic administration of the anti‐IL‐6R monoclonal antibody MR16‐1 effectively suppressed the expression of CCL2 and VEGF and reduced macrophage infiltration as well as the CNV area (Izumi‐Nagai et al, 2007). IL‐6 is known to repress Fas ligand expression in the RPE, which then leads to impaired clearance and accumulation of MPs in the subretinal space (Levy et al, 2015).
Chemokine modulation
Fractalkine or CX3CL1 is a neuronal chemokine which binds to its receptor CX3CR1 on microglia and macrophages (Geissmann et al, 2003; Wolf et al, 2013). The tightly regulated cross‐talk between neurons and microglia involving CX3CL1–CX3CR1 has an important role in immunoregulation and neuroprotection in the brain and the retina (Wolf et al, 2013; Zieger et al, 2014). Thus, CX3CR1‐deficient mice show a higher susceptibility to subthreshold light challenge leading to the accumulation of subretinal microglia, which can be prevented by keeping the animals in the dark (Combadière et al, 2007; Chinnery et al, 2012). Inhibition of CC‐motif chemokine ligand 2 (CCL2) or IL‐1β also prevented inflammatory macrophage recruitment and photoreceptor degeneration in these animals (Sennlaub et al, 2013; Eandi et al, 2016). Conversely, positive modulation of CX3CL1–CX3CR1 signaling in the diabetic mouse retina by intravitreal administration of recombinant fractalkine effectively reduced microglial proliferation (Mendiola et al, 2017).
The otherwise low expression of CCL2 in the retina is strongly enhanced under stressful conditions (Nakazawa et al, 2007; Chen et al, 2012). It is primarily secreted by activated microglia to recruit inflammatory monocytes expressing CCR2 (Mizutani et al, 2012; Sennlaub et al, 2013). The pro‐inflammatory CCL2/CCR2 axis represents a valid target for inhibition to restore immune balance. Thus, rats receiving intravitreal injection of CCL2 siRNA showed a markedly decreased phagocyte accumulation and photoreceptor apoptosis after light damage (Rutar et al, 2012). Similarly, CCR2 knockout mice had much lower photoreceptor demise after chronic blue light exposure (Hu et al, 2016), less pro‐inflammatory cells and CNV in the laser‐damage model (Robbie et al, 2016), and fewer subretinal macrophages when immunized with carboxyethylpyrrole‐modified albumin as trigger for oxidative stress (Cruz‐Guilloty et al, 2013).
The secretion of CCL3 by microglia is an early event in the pathologies of the Abca4−/− Rdh8−/− mouse model of Stargardt disease and the Mertk−/− mouse model of RP (Kohno et al, 2014). Consequently, knockout of CCL3 in these mice resulted in a milder disease form with increased retinal thickness, fewer numbers of subretinal phagocytes, and marked reduction in vascular leakage (Kohno et al, 2014).
Mononuclear phagocyte‐targeted therapy in patients with retinal pathologies
Hyper‐reflective foci
When monitoring the retina of wet AMD patients using spectral domain optical coherence tomography (SD‐OCT), small, dense particles—thereby referred to as hyper‐reflective foci (HF)—were identified (Framme et al, 2010; Altay et al, 2016). Similarly, HF were found in eyes of patients with different stages of diabetic retinopathy (De Benedetto et al, 2015; Korot et al, 2016), and a positive correlation between HF number, hard exudate size, disease severity, and inflammation has been recognized (Bolz et al, 2009; Lammer et al, 2014; Niu et al, 2017). Interestingly, the appearance and resolution of HF can be used to complement current diagnostic tools and predict disease progression and therapeutic success (Gallagher et al, 2007; Coscas et al, 2013; Abri Aghdam et al, 2015). These findings led to the assumption that HF may represent either migrating RPE cells or reactive and bloated immune cells (Framme et al, 2010; Christenbury et al, 2013; Coscas et al, 2013; Gocho et al, 2013). Pang et al compared HF with histological analyses in two donor eyes and found cholesterol crystals, indicating that HF are either RPE cells or lipid‐filled phagocytes (Ogino et al, 2012; Pang et al, 2015). Thus, the success of phagocyte‐targeting therapies could be potentially assessed by monitoring the presence and number of retinal HF using non‐invasive OCT imaging.
Clinical trials
Despite the very promising results in a wide range of preclinical studies targeting retinal phagocytes, only limited data are available from clinical trials. In a phase I/II study, Nussenblatt et al compared three immunosuppressive agents—daclizumab, rapamycin, and infliximab—in combination with anti‐VEGF therapy in AMD patients (Nussenblatt et al, 2010). The authors found that treatment with the anti‐IL‐2 receptor antibody daclizumab, as well as the mTOR inhibitor sirolimus, but not infliximab, decreased the number of anti‐VEGF intravitreal injections. However, no significant changes in visual acuity were evident. In contrast, other studies reported positive effects on vision gain following intravitreal or systemic infliximab treatment in patients with AMD and DR (Sfikakis et al, 2005, 2010; Theodossiadis et al, 2009) although some retinotoxicity was found in a small group of patients (Giganti et al, 2010).
Oral minocycline treatment was tested in five patients with DME and one patient with RP (Cukras et al, 2012; Baumgartner & Baumgartner, 2013). A 6‐month treatment with minocycline improved visual function, central macular edema, and vascular leakage in the DME patients (Cukras et al, 2012). The RP patient received minocycline together with the anti‐apoptotic drug deprenyl for 120 months and showed a slower decline in visual field as estimated from the previously documented disease course (Baumgartner & Baumgartner, 2013).
Targeting the complement system in retinal degenerative diseases
The complement pathways
The complement system, with over 30 small proteins, is a crucial component of innate immunity. Its origins can be traced back to a billion years ago when primitive proteins evolved to protect cells from pathogens. However, it was only discovered around 125 years ago as a liver‐derived heat‐labile substance circulating in the blood that “complements” antibodies in lytic killing of bacteria, fungi, and viruses.
The system becomes activated in a cascade fashion when triggered through one or more of the three major pathways: the classical pathway (CP), the mannose‐binding lectin (MBL) pathway, and the alternative pathway (AP; Fig 3). There are two critical steps for the full activation of the complement pathways: C3 cleavage and C5 cleavage by relevant convertases (Fig 3).
Figure 3. Complement activation, regulation, and immune functions.
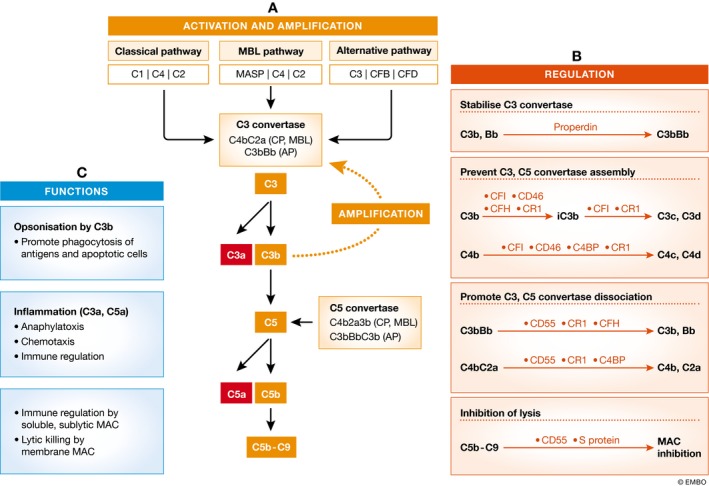
(A) The complement system can be activated by three pathways: the classical pathway (CP), mannose‐binding lectin (MBL) pathway, and the alternative pathway (AP); all lead to the cleavage of C3 and C5 and the formation of C5b‐C9. In the CP and MBL pathways, the C3 and C5 convertases are C4b2a and C4b2a3b, whereas in the AP, they are C3bBb and C3bBbC3b, respectively. Once C3 is cleaved into C3a and C3b, the C3b fragment can form C3bBb to amplify the complement activation cascade. Therefore, even if the initial activation is mediated by CP or MBL pathway, the cascade is ultimately amplified by the AP. (B) The complement activation cascades are regulated at multiple levels. Properdin is the only factor that stabilizes C3bBb and enhances complement activation. CFI, CD46, CFH, CR1, and C4BP prevent the assembly of C3 and C5 convertases by further breaking down C3b and C4b, whereas CD55, CR1, CFH, and C4BP can dissociate C3 and C5 convertases. CD59 and S protein can prevent the assembly of C5b‐C9. (C) Activation of the complement system generates C3a, C3b, and C5a fragments that are actively involved in immune responses. The soluble form of and sublytic levels of C5b‐C9 can regulate immune cell functions, whereas the membrane MACs directly kill pathogens and cells.
These convertases generate (i) C3b that may participate in opsonizing pathogens or dead cells and promoting their clearance, or form C5 convertase in the AP's feedback loop (Fig 3), (ii) C3a and C5a that can induce vasodilation, increase the permeability of small blood vessels, and induce contraction of smooth muscles (Fig 3), and (iii) C5b‐C9, also called membrane attack complex (MAC) that can modulate the immune response or affect cell cycle when released as a soluble form and at sublytic levels (Lueck et al, 2011; Lakkaraju et al, 2014) or form transmembrane channels on cell surfaces causing lysis (Fig 3).
Activation of the complement system is tightly controlled by a group of soluble and membrane‐bound regulators, including (i) C1‐inhibitor (C1INH) that inactivates C1r and C1s or MASP1 and MASP2 proteases and prevents CP‐ and MBL‐mediated complement activation, (ii) factors that accelerate the decay of convertases, such as the membrane‐bound CD55 or fluid phase factor H (CFH), and the transmembrane CD46, and (iii) CD59 that blocks the MAC assembly (Morgan & Wong et al, 1995; Zipfel & Skerka, 2009; Schmidt et al, 2016; Fig 3).
Although the main source of circulating complement components is the liver, complement proteins, receptors, and regulators are also produced and expressed locally by other cells, including immune cells and various tissue cells (Heeger & Kemper, 2012; Kolev, 2014). Locally produced complements are known to play an important role in tissue homeostasis, and thus, dysfunction or dysregulation of the system may contribute to various diseases. Recent studies have shown that the complement system can also be activated inside the cell, and intracellular complement activation is known to be involved in many important cellular functions, including intracellular pathogen recognition/elimination, cytokine production, and metabolism (Arbore et al, 2017; Kolev & Kemper, 2017; Liszewski et al, 2017).
Complement regulation in the retina
As an immune privileged tissue, the retina is segregated from systemic circulation by various barriers (e.g., BRBs), and circulating complement proteins are not able to freely move into the retinal parenchyma under physiological conditions. However, the retina itself produces a variety of complement proteins, receptors, and regulators (Anderson et al, 2010). For example, transcripts of C1qb, C1r, C2, C3, C4, CFB, and CFH were detected in the retina and RPE/choroid of human (Anderson et al, 2010) and mouse eyes (Luo et al, 2011). Complement regulatory proteins such as CD46 (Vogt et al, 2006; Fett et al, 2012), CD55 and CD59 (Vogt et al, 2006), and CFH (Chen et al, 2007) as well as complement receptors CR1 and C3aR (Fett et al, 2012) and C5aR (Vogt et al, 2006) were found in retinal neurons and RPE cells.
Complement genes in the retina (Chen et al, 2010) and RPE/choroid (Chen et al, 2008) are expressed in an age‐dependent fashion. Also, the expression of C3, C4, and CFB in mouse retina can be affected by cataract surgery (Xu et al, 2011) and irradiation (Chen et al, 2012). Recently, an age‐related accumulation of MAC was found in the choriocapillaris of healthy donor eyes (Mullins et al, 2014; Chirco et al, 2016). The role of complement proteins in retinal cell homeostasis and activation remains to be fully elucidated, and this should be an important point of consideration when targeting the complement system for treating retinal diseases.
The role of the complement system in retinal degeneration
The underlying pathologies of DR are retinal microvascular damage and neuronal degeneration. C3d and C5b‐9 have been detected in the choriocapillaris of DR eyes (Gerl et al, 2002) as well as in retinal vessels of patients suffering from type 2 diabetes for more than 9 years (Zhang et al, 2002), suggesting that the complement system may damage vascular endothelial cells through C5b‐9‐mediated lytic killing in diabetic eyes. In addition, uncontrolled complement activation may also contribute to pericyte loss in DR. Retinal pericyte‐reactive autoantibodies have been detected in patients with DR (Zhang et al, 2016) and in vitro studies have shown that the autoantibody‐initiated complement activation can induce pericyte damage and loss of function (Li et al, 2012). Increased C3a and C5a were detected in the serum (Zhang et al, 2016) and vitreous of patients with PDR (Muramatsu et al, 2013). C3a‐ and C5a‐induced inflammation may also contribute to DR pathogenesis. For example, Müller cells constitutively express C5aR and the expression can be upregulated by hyperglycemia and inflammatory stimuli such as prostaglandin E, which then results in the release of IL‐6 and VEGF, both known to be critically involved in DR pathology (Cheng et al, 2013).
The role of the complement system in the pathogenesis of AMD has been studied and reviewed extensively over the past decade (Warwick et al, 2014; Bora et al, 2015; McHarg et al, 2015). Key facts supporting the role of the complement system in the pathogenesis of AMD include the following: (i) Several complement components have been detected in drusen and AMD lesions (Anderson et al, 2002, 2010); (ii) higher plasma levels of C3a, C3d, Bb, and C5a have been observed in AMD patients (Scholl et al, 2008; Reynolds et al, 2009; Lechner et al, 2016); (iii) polymorphisms in a number of complement genes (CFH, CFB, C2, SERPING1, and C3) are genetic risk factors of AMD (Edwards, 2008; Cipriani et al, 2012); and (iv) inhibition of complement suppresses laser‐induced CNV in mice (Nozaki et al, 2006; Bora et al, 2010; Kim et al, 2013; Lipo et al, 2013). Mechanistically, CFH may inhibit CD47‐mediated resolution of subretinal inflammation and this inhibitory effect could be enhanced by the AMD associated CFH (H402) variant (Calippe et al, 2017).
A recent transcriptome study of two advanced stages of RP in dogs showed strongly increased gene expression of inflammasome and complement factors in the retina (Sudharsan et al, 2017). However, earlier studies reported reduced C3 and C4 levels and increased immune complexes in the sera from RP patients, and this reduced systemic complement activity appears to be related to poor disease prognosis (Heredia et al, 1984). The rhodopsin T17M mutation also reduces C3 secretion in RPE cells (Xiong et al, 2017), suggesting that some RP‐related genes may regulate complement expression/secretion by RPE cells. Humphries et al showed that C1q, the primary component of the classical pathway of the complement system, is a survival factor for cone cells, and C1q deficiency promoted photoreceptor death in Rho−/− mice, a mouse model of Leber's congenital amaurosis (LCA; Humphries et al, 2012). Further understanding the role of complement system activation in RP may uncover novel targets for therapy.
Modulating the complement system for the management of retinal degenerative diseases
Despite extensive research and significant advances in understanding the role of the complement system in retinal health and disease, the therapeutic value of these findings has only been tested in small groups of selected patients, such as the GA type of AMD (see below). A number of reasons may explain the slow progression in translations. First, complement dysregulation is not the primary cause of disease. The complement system only comes into play when retinal damage is evident. Therefore, modulating the complement system without addressing the initial cause of the disease may have limited impact on disease progression. Second, the physiological purpose of complement activation is likely to limit retinal damage and promote repair although excessive C3a, C5a, and C5b‐C9 may be detrimental. It is, therefore, critical to differentiate the beneficial roles from the detrimental roles of complement activation in retinal degeneration. Third, complement proteins and regulators may modulate retinal disease independent from the complement cascades. Complement‐based therapies are at different stages of clinical development for retinal diseases, particularly AMD (Xu & Chen, 2016).
Early clinical trials have proven that complement inhibitors are generally safe and well tolerated when injected intravitreally. The phase II/III studies have been conducted predominately in GA patients. For example, the latest C3‐targeted inhibitor APL‐2 has shown promising effect in the phase II study (NCT02503332), which reported a 29% reduction in the rate of GA lesion growth and 20% reduction in the group that received APL‐2 injection every other month. A greater effect was observed during the second 6 months of the study where a reduction in GA growth rate of 47 and 33% was seen in patients with monthly and every other month treatment, respectively. The phase III study is planned to begin in the second half of 2018 (Apellis‐Pharmaceuticals, 2018).
Lampalizumab (FCFD4514S) is a humanized IgG Fab fragment against CFD, thereby reducing the activation of the alternative complement pathway (Katschke et al, 2012). A phase II study (MAHALO) reported a 20% reduction in lesion area progression compared with sham control after 18‐month treatment of monthly intravitreal injections. Further subgroup analysis revealed a 44% reduction in patients who carry the CFI risk allele (rs17440077; Yaspan et al, 2017). However, further phase III studies (Spectri, NCT02247531; and Chroma, NCT02247479) failed to confirm the therapeutic effect in GA patients.
CLG561 is a fully humanized anti‐properdin antibody Fab fragment. Properdin promotes the association of C3b with CFB and provides a focal point for the assembly of C3 convertase C3bBb. A phase II study evaluating the safety and efficacy of intravitreal injections of CLG561 as a monotherapy and in combination with LFG316 in GA patients is ongoing (NCT02515942).
It must be noted that several other complement trials have ended early due to disappointing interim results. This highlights our lack of basic understanding of the mechanisms by which complement factors influence AMD. Thus, we are currently unable to address precisely when, where, and how to modulate the complement pathway in AMD and other retinal degenerative conditions.
Targeting inflammasome activation in retinal degenerative diseases
The inflammasome signaling pathway
The inflammasomes are cytosolic macromolecular signaling complexes that mediate IL‐1β and IL‐18 secretion the lytic cell death called pyroptosis. They play a crucial role in innate immunity by coordinating host immune response to invading pathogens or host‐derived danger signals. Assembly of inflammasomes is triggered by different PRRs, including nucleotide‐binding oligomerization domain‐like receptors (NLRs), absent in melanoma 2 (AIM2)‐like receptors (ALRs), or tripartite motif (TRIM) family receptors, which are capable of recognizing PAMPs and DAMPs. Assembly of the inflammasomes allows for the cleavage and activation of inflammatory caspases, which in turn cleave pro‐inflammatory cytokines pro‐IL‐1β and pro‐IL‐18 into their active forms.
Since NLRP1 was first described to form the inflammasome in 2002 (Martinon et al, 2002), members of the NLR family (NLRP1, NLRP3, and NLRC4) as well as other proteins (AIM2, pyrin) have been confirmed to initiate formation of inflammasomes (Broz & Dixit, 2016; Mathur et al, 2017). There are also other less well‐characterized PRRs, such as NLRP2, NLRP6, NLRP7, NLRP9b, NLRP12, IFN‐γ‐inducible protein 16 (IFI16), and retinoic acid‐inducible gene I (RIG‐I; also known as DDX58) which have also been reported to activate caspase‐1 (Broz & Monack, 2013; von Moltke et al, 2013; Broz & Dixit, 2016; Man & Kanneganti, 2016).
To date, the best studied and well‐characterized NLR molecule is NLRP3 (also known as NALP3, cryopyrin, CIAS1, and Pypaf1). Assembly of NLRP3 requires two signals: (i) a priming signal which activates NF‐kB, subsequently promoting the transcription of NLRP3 and pro‐IL‐1β, and (ii) an activation signal which facilitates the oligomerization of NLRP3, ASC, and procaspase‐1, resulting in the activation of NLRP3 inflammasome and secretion of mature IL‐1β and IL‐18 (Fig 4; Bauernfeind et al, 2009; Franchi et al, 2012, 2014; Juliana et al, 2012). In addition to the canonical inflammasomes, the non‐canonical inflammasome signaling pathways also exist, which target caspase‐11 in mice and caspase‐4 and caspase‐5 in humans. Shi et al (2014) have shown that caspase‐4/5/11 can directly respond to cytoplasmic LPS leading to self‐oligomerization and activation.
Figure 4. Molecular mechanisms of NLRP3 inflammasome priming and activation.
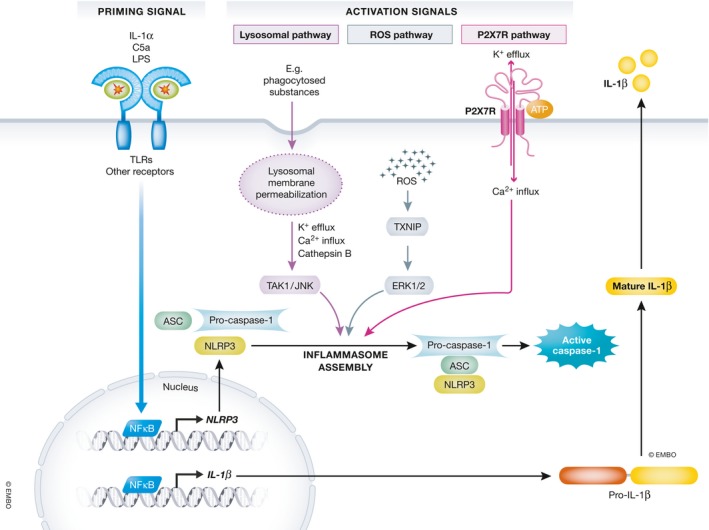
Schematic representation of the NLRP3 inflammasome pathway which requires two signals: (i) a priming signal which activates NF‐kB, subsequently promoting the transcription of NLRP3 and pro‐IL‐1β, and (ii) an activation signal which facilitates the oligomerization of NLRP3, ASC, and procaspase‐1, resulting in the activation of NLRP3 inflammasome and secretion of mature IL‐1β and IL‐18.
Clinical data on involvement of inflammasome in retinal diseases
Aberrant inflammasome activation has been implicated in multiple diseases, including retinal diseases. For instance, Tarallo et al (2012) displayed that NLRP3, IL‐1β, and IL‐18 mRNA abundance in the RPE from human eyes with GA was markedly elevated compared to normal age‐matched control eyes. Others also observed upregulation of NLRP3, pro‐IL‐1β, and pro‐IL‐18 mRNA in the macula of both GA and nAMD (Cao et al, 2016; Wang et al, 2016b). Zhao et al (2015a) analyzed the protein level of pro‐IL‐1β and IL‐1β in vitreous samples from patients with retinal diseases. The results show that pro‐IL‐1β levels in nAMD, polypoidal choroidal vasculopathy (PCV), and Eales’ disease vitreous samples were significantly elevated, and IL‐1β expression in nAMD, PCV, Eales’ disease, and RVO vitreous samples was significantly elevated when compared with the control group. Interestingly, IL‐1β levels in serum samples of PCV and nAMD were significantly decreased in the same study.
Inflammation is assumed to be involved in the generation of neovascularization in PDR (Zhou et al, 2012). Most recently, Loukovaara et al (2017) reported that NLRP3 inflammasome activation is associated with the pathogenesis of PDR. It is also demonstrated that high intraocular pressure (IOP)‐induced retinal ischemia could trigger caspase‐8 signaling to activate NLRP1 and NLRP3 inflammasomes and IL‐1β secretion via TLR4 signaling in both mouse and rat models (Chi et al, 2014). These results provide new insights into the pathogenesis and development of new therapeutic strategies for clinical treatment by linking NLRP3 inflammasome and retinal diseases.
Triggers of inflammasome activation in the retina
Oxidative stress
Increasing evidence suggests that ROS induces the activation of NLRP3 inflammasome and enhances the secretion of IL‐1β (Zhang et al, 2015; Choe & Kim, 2017). In STZ‐induced diabetic mice, rod demise was accompanied by an increase in LC3A protein, a marker for autophagosomes (Mizushima & Yoshimori, 2007; Piano et al, 2016). Similarly, ARPE‐19 cells showed signs of autophagy together with ROS release in response to high‐glucose‐induced stress (Shi et al, 2015). Inhibition of the autophagic response activated even more NLRP3 and caused IL‐1β release. The authors speculate that the cells incapable of removing ROS‐generating mitochondria may trigger NLRP3 inflammasome activation (Youle & Narendra, 2010; Shi et al, 2015). Thus, NLRP3 inflammasome activity is stimulated by ROS accumulation and counter‐balanced by autophagy (Zhou et al, 2010b).
Retinal lipofuscin contains lipid peroxidation‐ or glycoxidation‐induced end products 4‐hydroxynonenal (HNE), malondialdehyde (MDA), and advanced glycation end products (AGEs; Schutt et al, 2003). Kauppinen et al (2012) reported that HNE induced significantly increased NLRP3 mRNA levels and IL‐1β and IL‐18 production in RPE cells.
Thioredoxin (TRX)‐interacting protein (TXNIP), a TRX‐binding protein, is thought to be an endogenous inhibitor of TRX reductase activity. TXNIP dissociates from TRX at high concentrations of H2O2 and interacts with NLRP3. ROS‐dependent TXNIP–NLRP3 association was also found in monosodium urate crystals or R‐837‐treated macrophages (Zhou et al, 2010a). TXNIP can also mediate retinal inflammation, gliosis, and apoptosis in experimental diabetes (Devi et al, 2012). Zhou et al demonstrated a vital role of TXNIP in innate immunity through NLRP3 inflammasome activation and release of IL‐1β under oxidative stress. Recent studies demonstrated that ROS–TXNIP pathway mediates NLRP3 inflammasome activation in DR conditions in vitro and in vivo in rats. High glucose induces sustained upregulation of TXNIP, ROS generation, and inflammation in a Müller cell line of rats, and antioxidants or TXNIP silencing blocked IL‐1β and IL‐18 secretion in high‐glucose‐exposed human retinal microvascular endothelial cells (Devi et al, 2012; Chen et al, 2017). These results provide a potential therapeutic target for the treatment of DR.
Lysosomal membrane permeabilization
Lysosomal membrane permeabilization (LMP) is a key mechanism upstream of NLRP3 inflammasome activation, which induces subsequent cytosolic leakage of lysosomal components (Hornung et al, 2008; Stutz et al, 2009). Lysosome rupture triggers various cellular responses, including NLRP3 inflammasome activation, autophagy, and cell death (Okada et al, 2014). As lysosomes are organelles containing abundant amount of Ca2+, lysosome rupture induces Ca2+ influx from the lysosome into the cytosol activating the NLRP3 inflammasome through the CaMKII–TAK1–JNK pathway (Okada et al, 2014). TAK1 and JNK are activated in response to a soluble lysosomotropic agent L‐leucyl‐L‐leucine methyl ester (LLME) stimulus, and inhibitors of cathepsin B, cysteine proteases, or 5‐Z‐oxozeaenol (a TAK1 inhibitor) strongly attenuate the LLME‐induced activation of JNK. JNK is a family member of MAPK that responds to stress and that can regulate the activation of the NLPR3 inflammasome through ASC oligomerization. Furthermore, various LMP stimuli trigger significant K+ efflux (Munoz‐Planillo et al, 2013). Katsnelson et al (2015) reported that Ca2+ influx and K+ efflux are rapidly triggered after murine dendritic cell treatment with LLME. The lipofuscin component N‐retinylidene‐N‐retinyl‐ethanolamine (A2E) was also shown to trigger LMP (Taylor et al, 1992; Tomany et al, 2004). Brandstetter et al found that lipofuscin‐mediated phototoxicity results in LMP with cytosolic leakage of lysosomal enzymes and subsequent activation of caspase‐1 and inflammasome with secretion of IL‐1β and IL‐18 in RPE cells. NLRP3 inflammasome activation induced by LMP may contribute to AMD pathology through the release of pro‐inflammatory cytokines such as IL‐1β as well as through caspase‐1‐mediated pyroptosis (Tseng et al, 2013; Brandstetter et al, 2015b).
ATP and P2X7 receptor
P2X7R chiefly acts through the recruitment of the NLRP3 inflammasome complex (Giuliani et al, 2017). As a known and powerful activator of the NLRP3, P2X7R modulates NLRP3 expression at mRNA and protein levels, and excessive activation results in RPE cell death (Franceschini et al, 2015). During P2X7R opening, it directly allows K+ efflux and Ca2+ influx along the concentration gradient. K+ efflux is now acknowledged as a very potent stimulus for caspase‐1 activation and pro‐IL‐1β release that activates the NLRP3 inflammasome (Franchi et al, 2007; Petrilli et al, 2007; Franceschini et al, 2015). Recent evidences suggest that P2X7R and NLRP3 interact directly at discrete sub‐plasmalemmal cytoplasmic sites. P2X7R and NLRP3 can be co‐localized by confocal microscopy and co‐immunoprecipitated in both mouse microglia and mouse peritoneal macrophages (Franceschini et al, 2015).
Adenosine triphosphate promotes caspase‐1 activation, NLRP3 activation, IL‐1β and IL‐18 maturation and release, and cell death (Ferrari et al, 1997a; Perregaux et al, 2000). RPE cells and neural retina have been shown to release ATP in response to stimulation (Neal & Cunningham, 1994; Mitchell, 2001; Eldred et al, 2003; Newman, 2003; Pearson et al, 2005; Reigada & Mitchell, 2005; Reigada et al, 2005), which can act on P2X7R in the RPE cells via an autocrine or a paracrine manner (Perez et al, 1986; Xia et al, 2012). Yang et al (2011) reported that the P2X7R is expressed in both native and cultured human RPE cells and its activation induces both Ca2+ signaling and apoptosis in RPE cells. Furthermore, BzATP‐induced RPE apoptosis was blocked or significantly inhibited by P2X7R antagonists BBG, KN‐62, and oxidized ATP. Oxidized ATP, an irreversible blocker of P2X7R, abrogates ATP‐induced IL‐1β release from immune cells (Ferrari et al, 1997b). All of these above suggest that the over‐activation of P2X7R may contribute to the development of GA.
Complement components
Recently, Brandstetter et al (2015a) showed that complement component C5a is a priming signal for the NLRP3 inflammasome in RPE cells that mediates inflammasome activation by lipofuscin/blue light‐induced photo‐oxidative damage. There is also research showing that C1q represents an activation signal for the NLRP3 inflammasome, acting in a caspase‐1‐ and phagolysosome‐dependent manner in LPS‐primed mouse bone marrow‐derived macrophages and THP1 human monocytic cells (Doyle et al, 2012). In addition to C1q, C3a and MAC trigger inflammasome activation (Asgari et al, 2013; Triantafilou et al, 2013). C3a induces NLRP3 inflammasome activation and IL‐1β secretion in human monocytes by controlling the release of intracellular ATP into the extracellular space (Asgari et al, 2013). However, sublytic MAC attack generates pores on the membrane that allow Ca2+ influx, and thus increase cytosolic Ca2+ concentration, triggering NLRP3 activation and IL‐1β production (Triantafilou et al, 2013).
Amyloid‐β
Amyloid‐β (Aβ) is a component of drusen and has been suggested as pathogenic factor in AMD (Johnson et al, 2002). It is a pathogenic trigger peptide that induces inflammation and neurotoxicity in the retina. Intrinsic cytotoxicity of Aβ is due to its aggregated forms as soluble oligomers or insoluble fibrils (Gao et al, 2015). Aβ(1–40) and Aβ(1–42) are the two most common isoforms of Aβ, which are recognized to be the most relevant forms to induce neurodegeneration in amyloidosis (Zhang et al, 2012). Increasing Aβ(1–42) secretion was found in senescent ARPE‐19 cells (Glotin et al, 2008). Accumulating evidence suggests that increasing Aβ deposition with age may contribute to the development of AMD (Johnson et al, 2002; Dentchev et al, 2003; Zhao et al, 2015b). In addition to Aβ's cytotoxicity, NLRP3 inflammasome activation induced by Aβ may be responsible for RPE dysfunction. Halle et al (2008) reported that NLRP3 inflammasome activation is initiated by fibrillar Aβ‐induced lysosomal damage which increased release of lysosomal protease cathepsin B in microglia. As a trigger, Aβ stimulates RPE cells and results in accelerating the secretion of IL‐1β (Kurji et al, 2010).
Alu RNA
Alu RNA, a non‐coding RNA transcribed from Alu elements, plays a prominent role as gene modulator via genome shaping, transcriptional regulation, and mRNA alternative splicing (Hasler et al, 2007). Alu RNA accumulation secondary to DICER1 deficiency in the RPE has been implicated in GA (Tarallo et al, 2012). Kaneko et al (2011) showed that a reduction in RNase DICER1 leads to accumulation of Alu RNA transcripts in the RPE of GA patients. Delivery of a plasmid coding for Alu RNA upregulated NLRP3 and IL‐18 mRNAs in mouse RPE cells and induced ROS production in human RPE cells (Tarallo et al, 2012). These results suggest that Alu RNA triggers NLRP3 priming and mitochondrial ROS in RPE cells. ERKs promote cell death in a variety of chronic neurodegenerative states. Increased ERK1/2 phosphorylation was observed in the RPE of human eyes with GA, and Alu RNA‐induced RPE degeneration in mice is rescued by intravitreous administration of PD98059, an inhibitor of the ERK1/2‐activating kinase MEK1 (Dridi et al, 2012). Thus, RPE degeneration induced by DICER1 depletion or Alu RNA over‐expression may be mediated by ERK1/2 signaling (Dridi et al, 2012). ERK1/2 signaling also regulates angiogenesis and CNV (Hua et al, 2011; Xie et al, 2011). Therefore, ERK1/2 activation is a potential target for both atrophic and neovascular AMD.
Strategies for therapeutic modulation of inflammasome activation
IL‐1β inhibitors
IL‐1β is a key inflammatory cytokine regulated by the inflammasome, and increased levels of IL‐1β are present in DR (Kowluru et al, 2011; Liu et al, 2012) and AMD (Lavalette et al, 2011; Tarallo et al, 2012). Amelioration of IL‐1β activation prevents mitochondrial dysfunction and DNA damage (Kowluru et al, 2011). Furthermore, glucose‐induced apoptosis of retinal endothelial cells is prevented by neutralization of IL‐1β through incubating the cells with an IL‐1β antibody or IL‐1β receptor antagonist (Kowluru & Odenbach, 2004). The importance of IL‐1β in retinal diseases makes IL‐1 inhibition a therapeutic option. The IL‐1β receptor antagonist anakinra (ANA), anti‐IL‐1β antibody canakinumab (CAN), and recombinant humanized anti‐IL‐1β antibody gevokizumab (XOMA 052) have good clinical results in ocular diseases such as uveitis secondary to Behçet's disease (BD; Gul et al, 2012; Ugurlu et al, 2012; Vitale et al, 2014; Cantarini et al, 2015; Emmi et al, 2016). ANA and CAN have been shown to be an effective and safe therapeutic option for BD‐related refractory or long‐standing uveitis with a significant reduction in the rate of ocular inflammatory flare, resolution of active retinal vasculitis, preservation of visual acuity, and significant decrease in required steroid dosages (Fabiani et al, 2017). Ildefonso proposed that anti‐inflammatory genes delivered by an adeno‐associated virus (AAV) vector could be used as potential treatments for retinal inflammation (Ildefonso et al, 2015). Eyes injected with the caspase activation and recruitment domain (CARD) AAV vector had a significant decrease in both IL‐1β secretion and infiltrating cells (Ildefonso et al, 2015). Data on the efficacy of IL‐1β inhibition therapy in inflammasome‐related retinal diseases are currently lacking.
NLRP3 inhibitors
Direct inhibition of NLRP3 is an obvious approach for suppressing inflammasome activity. Recently, a small‐molecule inhibitor MCC950 (also known as CRID3), which is a diarylsulfonylurea‐based compound, was reported to be a potent and highly specific inhibitor of NLRP3, but not the AIM2, NLRC4, and NLRP1 inflammasomes (Coll et al, 2015). Coll et al (2015) reported that MCC950 could suppress both canonical and non‐canonical NLRP3 activation by preventing ASC complexes instead of blocking K+ efflux, Ca2+ flux, or NLRP3–ASC interactions. By reducing IL‐1β and IL‐18 secretion, the substance alleviated the severity of EAE and cryopyrin‐associated periodic syndromes (CAPS) in mouse models. MCC950 thus is a potential therapeutic for NLRP3‐associated diseases. It has also been reported in APP/PS1 mice, an AD model, that MCC950 suppresses inflammasome activation and IL‐1β production, stimulates Aβ phagocytosis in vitro, and reduces Aβ accumulation (Dempsey et al, 2017). With regard to the retina, MCC950 is capable of inhibiting NLRP3 inflammasome activation and apoptosis in human retinal endothelial cells (HRECs) under high‐glucose conditions, likely through downregulation of the Nek7–NLRP3 pathway (Zhang et al, 2017).
Another substance that inhibits NLRP3 is the ketone body β‐hydroxybutyrate (BHB; Youm et al, 2015). Unlike MCC950, BHB blocks only the canonical NLRP3 inflammasome activation pathway by preventing K+ efflux and reducing ASC oligomerization and speck formation (Youm et al, 2015). It reduces IL‐1β and IL‐18 production in human monocytes and attenuates caspase‐1 activation and IL‐1β secretion in mouse models of NLRP3‐associated diseases, such as Muckle–Wells syndrome, familial cold autoinflammatory syndrome, and urate crystal‐induced peritonitis. Both the MCC950 and BHB hold promise as potential novel pharmaceutical approach for treating DR, AMD, and other NLRP3‐induced ocular diseases.
Nucleoside reverse transcriptase inhibitors
Nucleoside reverse transcriptase inhibitors (NRTIs) are widely used to treat AIDS by blocking HIV replication. Fowler et al (2014) discovered that NRTIs inhibit P2X7‐mediated NLRP3 inflammasome activation independent of reverse transcriptase inhibition. Clinically relevant NRTIs such as lamivudine (3TC), stavudine (d4T), and abacavir (ABC) were shown to block caspase‐1 activation induced by Alu RNA in RPE cells (Fowler et al, 2014). Furthermore, NRTIs were efficacious in mouse models of GA and choroidal neovascularization. Intravitreous injection of the NRTIs 3TC, zidovudine (AZT), and ABC significantly suppressed laser‐induced CNV and VEGF‐A secretion in wild‐type mice but not P2rx7−/− mice (Mizutani et al, 2015). This suggests NRTIs as a possible new therapeutic approach for both dry and wet AMD.
Conclusion
In the current review, we point out the fundamental similarity between AMD, DR, and hereditary retinopathies (using the example of RP)—and possible other retinal diseases as well—namely non‐resolving and overwhelming inflammation. We pointed out three arms of inflammation which are activation of (i) mononuclear phagocytes, (ii) the complement system, and (iii) the inflammasome. All three arms are intertwined and cannot be treated exclusively without affecting each other, hence representing an attractive therapy target. Complement receptors are expressed on MPs while NLRP inflammasomes lead to IL‐1β activation and secretion contributing significantly to microglial activation and macrophage recruitment. Perturbations of immune‐suppressive capacities of the RPE, retinal neurons, and macroglia due to aging, hyperglycemia, or other defects evoked by genetic risk variants lead to non‐resolving inflammation. Hence, the proposed therapeutic options are auspicious approaches to start with.
Pending issues.
-
(i)
Experimental studies on separating retinal immune cell populations.
-
(ii)
Linking retinal microglia and macrophage phenotypes and functions with disease outcome.
-
(iii)
Identifying optimal targets in the complement cascade.
-
(iv)
Clinical studies with immunomodulatory compounds.
Conflict of interest
T.L. is named inventor on a patent application related to the use of polysialic acid for neurodegenerative diseases filed by the universities of Bonn and Cologne. All other authors declare that they have no conflict of interest.
For more information
Acknowledgements
Research in the laboratory of T.L. is supported by the DFG (LA1203/6‐2, LA1203/9‐1, LA1203/10‐1 and FOR2240), the ProRetina Foundation, and the Hans and Marlies Stock‐Foundation. Research in Dr H. Xu's laboratory is supported by Diabetes UK (11/0004230, 13/0004729), European Union's Horizon 2020 (722717), Fight for Sight (1574/1575), and Dunhill Medical Trust (R188/0211).
EMBO Mol Med (2018) 10: e8259
See the Glossary for abbreviations used in this article.
References
- Abdelsalam A, Del Priore L, Zarbin MA (1999) Drusen in age‐related macular degeneration: pathogenesis, natural course, and laser photocoagulation‐induced regression. Surv Ophthalmol 44: 1–29 [DOI] [PubMed] [Google Scholar]
- Abri Aghdam K, Pielen A, Framme C, Junker B (2015) Correlation between hyperreflective foci and clinical outcomes in neovascular age‐related macular degeneration after switching to aflibercept. Invest Ophthalmol Vis Sci 56: 6448–6455 [DOI] [PubMed] [Google Scholar]
- Adamis AP (2002) Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol 86: 363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Gayyar MM, Elsherbiny NM (2013) Contribution of TNF‐alpha to the development of retinal neurodegenerative disorders. Eur Cytokine Netw 24: 27–36 [DOI] [PubMed] [Google Scholar]
- Altay L, Scholz P, Schick T, Felsch M, Hoyng CB, den Hollander AI, Langmann T, Fauser S (2016) Association of hyperreflective foci present in early forms of age‐related macular degeneration with known age‐related macular degeneration risk polymorphisms. Invest Ophthalmol Vis Sci 57: 4315–4320 [DOI] [PubMed] [Google Scholar]
- Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, Beyaert R, Pshezhetsky AV, Szewczuk MR (2010) Neu1 desialylation of sialyl α‐2,3‐linked β‐galactosyl residues of TOLL‐like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal 22: 314–324 [DOI] [PubMed] [Google Scholar]
- Anderson JB, Smith SA, Kotwal GJ (2002) Vaccinia virus complement control protein inhibits hyperacute xenorejection. Transplant Proc 34: 1083–1085 [DOI] [PubMed] [Google Scholar]
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G et al (2010) The pivotal role of the complement system in aging and age‐related macular degeneration: hypothesis re‐visited. Prog Retin Eye Res 29: 95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angata T, Kerr SC, Greaves DR, Varki NM, Crocker PR, Varki A (2002) Cloning and characterization of human Siglec‐11. A recently evolved signaling molecule that can interact with SHP‐1 and SHP‐2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem 277: 24466–24474 [DOI] [PubMed] [Google Scholar]
- Apellis‐Pharmaceuticals (2018) http://investorsapelliscom/news-releases/news-release-details/apellis-finalizes-phase-3-clinical-trial-plans-geographic
- Arbore G, Kemper C, Kolev M (2017) Intracellular complement – the complosome – in immune cell regulation. Mol Immunol 89: 2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeljan D, Chan C‐C (2013) Aging is not a disease: distinguishing age‐related macular degeneration from aging. Prog Retin Eye Res 37: 68–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D, Augustin AJ, Spengler R, Al‐Jada A, Nickola T, Grus F, Koch F (1998) Detection of vascular endothelial growth factor and tumor necrosis factor alpha in epiretinal membranes of proliferative diabetic retinopathy, proliferative vitreoretinopathy and macular pucker. Ophthalmologica 212: 410–414 [DOI] [PubMed] [Google Scholar]
- Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Kohl J, Cook HT, Kemper C (2013) C3a modulates IL‐1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood 122: 3473–3481 [DOI] [PubMed] [Google Scholar]
- Aveleira CA, Lin C‐M, Abcouwer SF, Ambrósio AF, Antonetti DA (2010) TNF‐α signals through PKCζ/NF‐κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 59: 2872–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BJ, Akhtar LN, Benveniste EN (2009) SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol 30: 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron AM, Garcia‐Segura LM, Caruso D, Jayaraman A, Lee JW, Melcangi RC, Pike CJ (2013) Ligand for translocator protein reverses pathology in a mouse model of Alzheimer's disease. J Neurosci 33: 8891–8897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes‐Alnemri T, Wu J, Monks BG, Fitzgerald KA et al (2009) Cutting edge: NF‐kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner WA, Baumgartner AM (2013) Rationale for an experimental treatment of retinitis pigmentosa: 140‐month test of hypothesis with one patient. Med Hypotheses 81: 720–728 [DOI] [PubMed] [Google Scholar]
- Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT (2008) Diabetes‐enhanced tumor necrosis factor‐α; production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol 172: 1411–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B (2004) Inferences, questions and possibilities in Toll‐like receptor signalling. Nature 430: 257–263 [DOI] [PubMed] [Google Scholar]
- Bhutto I, Lutty G (2012) Understanding age‐related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med 33: 295–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U (2014) Choriocapillaris breakdown precedes retinal degeneration in age‐related macular degeneration. Neurobiol Aging 35: 2562–2573 [DOI] [PubMed] [Google Scholar]
- Bolz M, Schmidt‐Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C (2009) Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology 116: 914–920 [DOI] [PubMed] [Google Scholar]
- Bora NS, Jha P, Lyzogubov VV, Kaliappan S, Liu J, Tytarenko RG, Fraser DA, Morgan BP, Bora PS (2010) Recombinant membrane‐targeted form of CD59 inhibits the growth of choroidal neovascular complex in mice. J Biol Chem 285: 33826–33833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora NS, Matta B, Lyzogubov VV, Bora PS (2015) Relationship between the complement system, risk factors and prediction models in age‐related macular degeneration. Mol Immunol 63: 176–183 [DOI] [PubMed] [Google Scholar]
- Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, Juzych MS, Abrams GW, Kumar A (2017) Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci 58: 5594–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton ME (2013) Ageing of the retina and retinal pigment epithelium In Age‐related macular degeneration, Holz FG, Pauleikhoff D, Spaide RF, Bird AC. (eds), pp 45–63. Berlin, Heidelberg: Springer Berlin Heidelberg; [Google Scholar]
- Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA (2009) Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 183: 4432–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter C, Holz FG, Krohne TU (2015a) Complement component C5a primes retinal pigment epithelial cells for inflammasome activation by lipofuscin‐mediated photooxidative damage. J Biol Chem 290: 31189–31198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter C, Mohr LK, Latz E, Holz FG, Krohne TU (2015b) Light induces NLRP3 inflammasome activation in retinal pigment epithelial cells via lipofuscin‐mediated photooxidative damage. J Mol Med (Berl) 93: 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD (2002) Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol 161: 1669–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ (2012) Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem Sci 37: 325–332 [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ (2014) Microglial phagocytosis of live neurons. Nat Rev Neurosci 15: 209 [DOI] [PubMed] [Google Scholar]
- Broz P, Monack DM (2013) Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol 13: 551–565 [DOI] [PubMed] [Google Scholar]
- Broz P, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16: 407–420 [DOI] [PubMed] [Google Scholar]
- Buschini E, Piras A, Nuzzi R, Vercelli A (2011) Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol 95: 14–25 [DOI] [PubMed] [Google Scholar]
- Cai H, Zhou X, Dougherty GG, Reddy RD, Haas GL, Montrose DM, Keshavan M, Yao JK (2018) Pregnenolone‐progesterone‐allopregnanolone pathway as a potential therapeutic target in first‐episode antipsychotic‐naïve patients with schizophrenia. Psychoneuroendocrinology 90: 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Espinosa‐Heidmann DG, Piña Y, Hernandez EP, Cousins SW (2005) Blood‐derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res 81: 38–47 [DOI] [PubMed] [Google Scholar]
- Calippe B, Augustin S, Beguier F, Charles‐Messance H, Poupel L, Conart JB, Hu SJ, Lavalette S, Fauvet A, Rayes J et al (2017) Complement factor H inhibits CD47‐mediated resolution of inflammation. Immunity 46: 261–272 [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA, Archibald V, Dines KC, Maxfield EK (1994) Anti‐oxidant and pro‐oxidant effects on nerve conduction velocity, endoneurial blood flow and oxygen tension in non‐diabetic and streptozotocin‐diabetic rats. Diabetologia 37: 449–459 [DOI] [PubMed] [Google Scholar]
- Canas PM, Porciúncula LO, Cunha GMA, Silva CG, Machado NJ, Oliveira JMA, Oliveira CR, Cunha RA (2009) Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by β‐amyloid peptides via p38 mitogen‐activated protein kinase pathway. J Neurosci 29: 14741–14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarini L, Vitale A, Scalini P, Dinarello CA, Rigante D, Franceschini R, Simonini G, Borsari G, Caso F, Lucherini OM et al (2015) Anakinra treatment in drug‐resistant Behcet's disease: a case series. Clin Rheumatol 34: 1293–1301 [DOI] [PubMed] [Google Scholar]
- Cao S, Wang JC, Gao J, Wong M, To E, White VA, Cui JZ, Matsubara JA (2016) CFH Y402H polymorphism and the complement activation product C5a: effects on NF‐kappaB activation and inflammasome gene regulation. Br J Ophthalmol 100: 713–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan‐Ling T, Hughes S, Baxter L, Rosinova E, McGregor I, Morcos Y, van Nieuwenhuyzen P, Hu P (2007) Inflammation and breakdown of the blood‐retinal barrier during “physiological aging” in the rat retina: a model for CNS aging. Microcirculation 14: 63–76 [DOI] [PubMed] [Google Scholar]
- Chen M, Forrester JV, Xu H (2007) Synthesis of complement factor H by retinal pigment epithelial cells is down‐regulated by oxidized photoreceptor outer segments. Exp Eye Res 84: 635–645 [DOI] [PubMed] [Google Scholar]
- Chen H, Liu B, Lukas TJ, Neufeld AH (2008) The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age‐related macular degeneration. PLoS One 3: e2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Muckersie E, Forrester JV, Xu H (2010) Immune activation in retinal aging: a gene expression study. Invest Ophthalmol Vis Sci 51: 5888–5896 [DOI] [PubMed] [Google Scholar]
- Chen M, Zhao J, Luo C, Pandi SP, Penalva RG, Fitzgerald DC, Xu H (2012) Para‐inflammation‐mediated retinal recruitment of bone marrow‐derived myeloid cells following whole‐body irradiation is CCL2 dependent. Glia 60: 833–842 [DOI] [PubMed] [Google Scholar]
- Chen M, Xu H (2015) Parainflammation, chronic inflammation, and age‐related macular degeneration. J Leukoc Biol 98: 713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhao M, Zhao S, Lu Q, Ni L, Zou C, Lu L, Xu X, Guan H, Zheng Z et al (2017) Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: a novel inhibitory effect of minocycline. Inflamm Res 66: 157–166 [DOI] [PubMed] [Google Scholar]
- Chen M, Zhao J, Ali IHA, Marry S, Augustine J, Bhuckory M, Lynch A, Kissenpfennig A, Xu H (2018) Cytokine signaling protein 3 deficiency in myeloid cells promotes retinal degeneration and angiogenesis through arginase‐1 up‐regulation in experimental autoimmune uveoretinitis. Am J Pathol 188: 1007–1020 [DOI] [PubMed] [Google Scholar]
- Cheng L, Bu H, Portillo JA, Li Y, Subauste CS, Huang SS, Kern TS, Lin F (2013) Modulation of retinal Muller cells by complement receptor C5aR. Invest Ophthalmol Vis Sci 54: 8191–8198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Li F, Chen H, Wang Y, Zhu Y, Yang X, Zhu J, Wu F, Ouyang H, Ge J et al (2014) Caspase‐8 promotes NLRP1/NLRP3 inflammasome activation and IL‐1beta production in acute glaucoma. Proc Natl Acad Sci USA 111: 11181–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery HR, McLenachan S, Humphries T, Kezic JM, Chen X, Ruitenberg MJ, McMenamin PG (2012) Accumulation of murine subretinal macrophages: effects of age, pigmentation and CX3CR1. Neurobiol Aging 33: 1769–1776 [DOI] [PubMed] [Google Scholar]
- Chio CC, Chang YH, Hsu YW, Chi KH, Lin WW (2004) PKA‐dependent activation of PKC, p38 MAPK and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin‐6 by dibutyryl cAMP. Cell Signal 16: 565–575 [DOI] [PubMed] [Google Scholar]
- Chirco KR, Tucker BA, Stone EM, Mullins RF (2016) Selective accumulation of the complement membrane attack complex in aging choriocapillaris. Exp Eye Res 146: 393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Taylor A (2011) Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res 30: 18–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe JY, Kim SK (2017) Quercetin and ascorbic acid suppress fructose‐induced NLRP3 inflammasome activation by blocking intracellular shuttling of TXNIP in human macrophage cell lines. Inflammation 40: 980–994 [DOI] [PubMed] [Google Scholar]
- Chow A, Brown BD, Merad M (2011) Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol 11: 788–798 [DOI] [PubMed] [Google Scholar]
- Christenbury JG, Folgar FA, O'Connell R, Chiu SJ, Farsiu S, Toth CA (2013) Progression of intermediate age‐related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology 120: 1038–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani V, Matharu BK, Khan JC, Shahid H, Stanton CM, Hayward C, Wright AF, Bunce C, Clayton DG, Moore AT et al (2012) Genetic variation in complement regulators and susceptibility to age‐related macular degeneration. Immunobiology 217: 158–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino AC, Mathura JR Jr, Jampol LM (2006) Resolution of activity (choroiditis and choroidal neovascularization) of chronic recurrent punctate inner choroidopathy after treatment with interferon B‐1A. Retina 26: 1091–1092 [DOI] [PubMed] [Google Scholar]
- Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz‐Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A et al (2015) A small‐molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21: 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, Pézard A, Lavalette S, Houssier M, Jonet L, Picard E et al (2007) CX3CR1‐dependent subretinal microglia cell accumulation is associated with cardinal features of age‐related macular degeneration. J Clin Invest 117: 2920–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland DA, Calder CJ, Raveney BJ, Nicholson LB, Phillips J, Cherwinski H, Jenmalm M, Sedgwick JD, Dick AD (2007) Monoclonal antibody‐mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am J Pathol 171: 580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscas G, De Benedetto U, Coscas F, Li Calzi CI, Vismara S, Roudot‐Thoraval F, Bandello F, Souied E (2013) Hyperreflective dots: a new spectral‐domain optical coherence tomography entity for follow‐up and prognosis in exudative age‐related macular degeneration. Ophthalmologica 229: 32–37 [DOI] [PubMed] [Google Scholar]
- Costa G, Pereira T, Neto AM, Cristovao AJ, Ambrosio AF, Santos PF (2009) High glucose changes extracellular adenosine triphosphate levels in rat retinal cultures. J Neurosci Res 87: 1375–1380 [DOI] [PubMed] [Google Scholar]
- Cousins SW, Espinosa‐Heidmann DG, Csaky KG (2004) Monocyte activation in patients with age‐related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol 122: 1013–1018 [DOI] [PubMed] [Google Scholar]
- Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE et al (2003) SOCS3 negatively regulates IL‐6 signaling in vivo . Nat Immunol 4: 540–545 [DOI] [PubMed] [Google Scholar]
- Cruz‐Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E et al (2013) Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age‐related macular degeneration. Int J Inflam 2013: 503725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukras CA, Petrou P, Chew EY, Meyerle CB, Wong WT (2012) Oral minocycline for the treatment of diabetic macular edema (DME): results of a phase I/II clinical study. Invest Ophthalmol Vis Sci 53: 3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings M, Cunha‐Vaz J (2008) Treatment of neovascular age‐related macular degeneration in patients with diabetes. Clin Ophthalmol 2: 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38: 107–125 [DOI] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT (2011) Age‐related alterations in the dynamic behavior of microglia. Aging Cell 10: 263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Xu Y, Wu W, Li W, Sun Y, Yang J, Zhu Y, Zhang C (2014) Tetrandrine suppresses lipopolysaccharide‐induced microglial activation by inhibiting NF‐kappaB and ERK signaling pathways in BV2 cells. PLoS One 9: e102522 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Das A, Stroud S, Mehta A, Rangasamy S (2015) New treatments for diabetic retinopathy. Diabetes Obes Metab 17: 219–230 [DOI] [PubMed] [Google Scholar]
- Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I (2008) High glucose induces toll‐like receptor expression in human monocytes. Mech Act 57: 3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Cano M, Ebrahimi K, Wang L, Handa JT (2017) The impact of oxidative stress and inflammation on RPE degeneration in non‐neovascular AMD. Prog Retin Eye Res 60: 201–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty DJ, Selvaraj V, Chechneva OV, Liu XB, Pleasure DE, Deng W (2013) A TSPO ligand is protective in a mouse model of multiple sclerosis. EMBO Mol Med 5: 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetto U, Sacconi R, Pierro L, Lattanzio R, Bandello F (2015) Optical coherence tomographic hyperreflective foci in early stages of diabetic retinopathy. Retina 35: 449–453 [DOI] [PubMed] [Google Scholar]
- Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S (2006) Determination of vitreous interleukin‐1 (IL‐1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 20: 1366–1369 [DOI] [PubMed] [Google Scholar]
- Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O'Neill LAJ, Lynch MA (2017) Inhibiting the NLRP3 inflammasome with MCC950 promotes non‐phlogistic clearance of amyloid‐beta and cognitive function in APP/PS1 mice. Brain Behav Immun 61: 306–316 [DOI] [PubMed] [Google Scholar]
- Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL (2003) Amyloid‐beta is found in drusen from some age‐related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis 9: 184–190 [PubMed] [Google Scholar]
- Devi TS, Lee I, Huttemann M, Kumar A, Nantwi KD, Singh LP (2012) TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. Exp Diabetes Res 2012: 438238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AD, Broderick C, Forrester JV, Wright GJ (2001) Distribution of OX2 antigen and OX2 receptor within retina. Invest Ophthalmol Vis Sci 42: 170–176 [PubMed] [Google Scholar]
- Dick AD, Carter D, Robertson M, Broderick C, Hughes E, Forrester JV, Liversidge J (2003) Control of myeloid activity during retinal inflammation. J Leukoc Biol 74: 161–166 [DOI] [PubMed] [Google Scholar]
- Donoso LA, Kim D, Frost A, Callahan A, Hageman G (2006) The role of inflammation in the pathogenesis of age‐related macular degeneration. Surv Ophthalmol 51: 137–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC et al (2012) NLRP3 has a protective role in age‐related macular degeneration through the induction of IL‐18 by drusen components. Nat Med 18: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dridi S, Hirano Y, Tarallo V, Kim Y, Fowler BJ, Ambati BK, Bogdanovich S, Chiodo VA, Hauswirth WW, Kugel JF et al (2012) ERK1/2 activation is a therapeutic target in age‐related macular degeneration. Proc Natl Acad Sci USA 109: 13781–13786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Smith MA, Miller CM, Kern TS (2002) Diabetes‐induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem 80: 771–779 [DOI] [PubMed] [Google Scholar]
- Eandi CM, Charles Messance H, Augustin S, Dominguez E, Lavalette S, Forster V, Hu SJ, Siquieros L, Craft CM, Sahel JA et al (2016) Subretinal mononuclear phagocytes induce cone segment loss via IL‐1beta. eLife 5: e16490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JL (2008) The role of complement in gonococcal infection of cervical epithelia. Vaccine 26(Suppl 8): I56–I61 [DOI] [PubMed] [Google Scholar]
- Eldred JA, Sanderson J, Wormstone M, Reddan JR, Duncan G (2003) Stress‐induced ATP release from and growth modulation of human lens and retinal pigment epithelial cells. Biochem Soc Trans 31: 1213–1215 [DOI] [PubMed] [Google Scholar]
- Emmi G, Talarico R, Lopalco G, Cimaz R, Cantini F, Viapiana O, Olivieri I, Goldoni M, Vitale A, Silvestri E et al (2016) Efficacy and safety profile of anti‐interleukin‐1 treatment in Behcet's disease: a multicenter retrospective study. Clin Rheumatol 35: 1281–1286 [DOI] [PubMed] [Google Scholar]
- Fabiani C, Vitale A, Emmi G, Lopalco G, Vannozzi L, Guerriero S, Gentileschi S, Bacherini D, Franceschini R, Frediani B et al (2017) Interleukin (IL)‐1 inhibition with anakinra and canakinumab in Behcet's disease‐related uveitis: a multicenter retrospective observational study. Clin Rheumatol 36: 191–197 [DOI] [PubMed] [Google Scholar]
- Fauser S, Viebahn U, Muether PS (2015) Intraocular and systemic inflammation‐related cytokines during one year of ranibizumab treatment for neovascular age‐related macular degeneration. Acta Ophthalmol 93: 734–738 [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Green DR (2014) Autophagy and phagocytosis converge for better vision. Autophagy 10: 165–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F (1997a) Extracellular ATP triggers IL‐1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol 159: 1451–1458 [PubMed] [Google Scholar]
- Ferrari D, Wesselborg S, Bauer MK, Schulze‐Osthoff K (1997b) Extracellular ATP activates transcription factor NF‐kappaB through the P2Z purinoreceptor by selectively targeting NF‐kappaB p65. J Cell Biol 139: 1635–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer‐Martin RM, Martin‐Oliva D, Sierra‐Martin A, Carrasco MC, Martin‐Estebane M, Calvente R, Martin‐Guerrero SM, Marin‐Teva JL, Navascues J, Cuadros MA (2015) Microglial activation promotes cell survival in organotypic cultures of postnatal mouse retinal explants. PLoS One 10: e0135238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AL, Hermann MM, Muether PS, Kirchhof B, Fauser S (2012) Immunohistochemical localization of complement regulatory proteins in the human retina. Histol Histopathol 27: 357–364 [DOI] [PubMed] [Google Scholar]
- Fowler BJ, Gelfand BD, Kim Y, Kerur N, Tarallo V, Hirano Y, Amarnath S, Fowler DH, Radwan M, Young MT et al (2014) Nucleoside reverse transcriptase inhibitors possess intrinsic anti‐inflammatory activity. Science 346: 1000–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Framme C, Wolf S, Wolf‐Schnurrbusch U (2010) Small dense particles in the retina observable by spectral‐domain optical coherence tomography in age‐related macular degeneration. Invest Ophthalmol Vis Sci 51: 5965–5969 [DOI] [PubMed] [Google Scholar]
- Franceschini A, Capece M, Chiozzi P, Falzoni S, Sanz JM, Sarti AC, Bonora M, Pinton P, Di Virgilio F (2015) The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J 29: 2450–2461 [DOI] [PubMed] [Google Scholar]
- Franchi L, Kanneganti TD, Dubyak GR, Nunez G (2007) Differential requirement of P2X7 receptor and intracellular K+ for caspase‐1 activation induced by intracellular and extracellular bacteria. J Biol Chem 282: 18810–18818 [DOI] [PubMed] [Google Scholar]
- Franchi L, Munoz‐Planillo R, Nunez G (2012) Sensing and reacting to microbes through the inflammasomes. Nat Immunol 13: 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz‐Planillo R, Ozkurede U, Kim YG, Arindam C, Gale M Jr, Silverman RH, Colonna M, Akira S et al (2014) Cytosolic double‐stranded RNA activates the NLRP3 inflammasome via MAVS‐induced membrane permeabilization and K+ efflux. J Immunol 193: 4214–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg‐Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M et al (2016) A large genome‐wide association study of age‐related macular degeneration highlights contributions of rare and common variants. Nat Genet 48: 134–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MJ, Yilmaz T, Cervantes‐Castaneda RA, Foster CS (2007) The characteristic features of optical coherence tomography in posterior uveitis. Br J Ophthalmol 91: 1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Liu RT, Cao S, Cui JZ, Wang A, To E, Matsubara JA (2015) NLRP3 inflammasome: activation and regulation in age‐related macular degeneration. Mediators Inflamm 2015: 690243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido‐Mesa N, Zarzuelo A, Gálvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169: 337–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82 [DOI] [PubMed] [Google Scholar]
- Gerl VB, Bohl J, Pitz S, Stoffelns B, Pfeiffer N, Bhakdi S (2002) Extensive deposits of complement C3d and C5b‐9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci 43: 1104–1108 [PubMed] [Google Scholar]
- Giatti S, Pesaresi M, Cavaletti G, Bianchi R, Carozzi V, Lombardi R, Maschi O, Lauria G, Garcia‐Segura LM, Caruso D et al (2009) Neuroprotective effects of a ligand of translocator protein‐18 kDa (Ro5‐4864) in experimental diabetic neuropathy. Neuroscience 164: 520–529 [DOI] [PubMed] [Google Scholar]
- Giganti M, Beer PM, Lemanski N, Hartman C, Schartman J, Falk N (2010) Adverse events after intravitreal infliximab (Remicade). Retina 30: 71–80 [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard C, Liu S, Adams D, Lacroix C, Sineus M, Boucher C, Papadopoulos V, Rupprecht R, Schumacher M, Groyer G (2012) Axonal regeneration and neuroinflammation: roles for the translocator protein 18 kDa. J Neuroendocrinol 24: 71–81 [DOI] [PubMed] [Google Scholar]
- Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F (2017) The P2X7 receptor‐interleukin‐1 liaison. Front Pharmacol 8: 123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotin AL, Debacq‐Chainiaux F, Brossas JY, Faussat AM, Treton J, Zubielewicz A, Toussaint O, Mascarelli F (2008) Prematurely senescent ARPE‐19 cells display features of age‐related macular degeneration. Free Radic Biol Med 44: 1348–1361 [DOI] [PubMed] [Google Scholar]
- Gocho K, Sarda V, Falah S, Sahel J‐A, Sennlaub F, Benchaboune M, Ullern M, Paques M (2013) Adaptive optics imaging of geographic atrophy. Invest Ophthalmol Vis Sci 54: 3673–3680 [DOI] [PubMed] [Google Scholar]
- Goswami K, Nandakumar DN, Koner BC, Bobby Z, Sen SK (2003) Oxidative changes and desialylation of serum proteins in hyperthyroidism. Clin Chim Acta 337: 163–168 [DOI] [PubMed] [Google Scholar]
- Graham DB, Stephenson LM, Lam SK, Brim K, Lee HM, Bautista J, Gilfillan S, Akilesh S, Fujikawa K, Swat W (2007) An ITAM‐signaling pathway controls cross‐presentation of particulate but not soluble antigens in dendritic cells. J Exp Med 204: 2889–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Neric NJ, Crabb JS, Crabb JW, Bhattacharya SK, Rayborn ME, Hollyfield JG, Bonilha VL (2012) Age‐related changes in the retinal pigment epithelium (RPE). PLoS One 7: e38673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M (2015) Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol 146: 48–61 [DOI] [PubMed] [Google Scholar]
- Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S (2014) Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14: 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P, Sennlaub F (2017) On phagocytes and macular degeneration. Prog Retin Eye Res 61: 98–128 [DOI] [PubMed] [Google Scholar]
- Gul A, Tugal‐Tutkun I, Dinarello CA, Reznikov L, Esen BA, Mirza A, Scannon P, Solinger A (2012) Interleukin‐1beta‐regulating antibody XOMA 052 (gevokizumab) in the treatment of acute exacerbations of resistant uveitis of Behcet's disease: an open‐label pilot study. Ann Rheum Dis 71: 563–566 [DOI] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH (2003) Activated microglia in human retinitis pigmentosa, late‐onset retinal degeneration, and age‐related macular degeneration. Exp Eye Res 76: 463–471 [DOI] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF (2001) An integrated hypothesis that considers drusen as biomarkers of immune‐mediated processes at the RPE‐Bruch's membrane interface in aging and age‐related macular degeneration. Prog Retin Eye Res 20: 705–732 [DOI] [PubMed] [Google Scholar]
- Haim M (2002) The epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand 80: 1–34 [DOI] [PubMed] [Google Scholar]
- Halder SK, Matsunaga H, Ishii KJ, Akira S, Miyake K, Ueda H (2013) Retinal cell type‐specific prevention of ischemia‐induced damages by LPS‐TLR4 signaling through microglia. J Neurochem 126: 243–260 [DOI] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid‐beta. Nat Immunol 9: 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA (2009) The expanding roles of ITAM adapters FcRγ and DAP12 in myeloid cells. Immunol Rev 232: 42–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U‐K (2013) Functional diversity of microglia – how heterogeneous are they to begin with? Front Cell Neurosci 7: 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Kohsaka S, Wada E, Yoshida K, Ohno S, Mamada H, Tanaka K, Parada LF, Wada K (2002) Microglia–Müller glia cell interactions control neurotrophic factor production during light‐induced retinal degeneration. J Neurosci 22: 9228–9236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368: 1795–1809 [DOI] [PubMed] [Google Scholar]
- Hasler J, Samuelsson T, Strub K (2007) Useful ‘junk’: Alu RNAs in the human transcriptome. Cell Mol Life Sci 64: 1793–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka E, Monteagudo PT, Marrocos MSM, Campa A (2006) Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol 146: 443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang Y, Su G (2015) Recent advances in treatment of retinitis pigmentosa. Curr Stem Cell Res Ther 10: 258–265 [DOI] [PubMed] [Google Scholar]
- He Y, Taylor N, Fourgeaud L, Bhattacharya A (2017) The role of microglial P2X7: modulation of cell death and cytokine release. J Neuroinflammation 14: 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger PS, Kemper C (2012) Novel roles of complement in T effector cell regulation. Immunobiology 217: 216–224 [DOI] [PubMed] [Google Scholar]
- Heredia CD, Huguet J, Cols N, Engel P, Garcia‐Calderon PA (1984) Immune complexes in retinitis pigmentosa. Br J Ophthalmol 68: 811–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt H, Dityatev A (2015) Polysialic acid in brain development and synaptic plasticity. Top Curr Chem 366: 55–96 [DOI] [PubMed] [Google Scholar]
- Hoon M, Okawa H, Della Santina L, Wong ROL (2014) Functional architecture of the retina: development and disease. Prog Retin Eye Res 42: 44–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie S, Robbie SJ, Liu J, Wu WK, Ali RR, Bainbridge JW, Nicholson LB, Mochizuki M, Dick AD, Copland DA (2013) CD200R signaling inhibits pro‐angiogenic gene expression by macrophages and suppresses choroidal neovascularization. Sci Rep 3: 3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Bringmann A, Reichenbach A (2009) Purinergic signaling in special senses. Trends Neurosci 32: 128–141 [DOI] [PubMed] [Google Scholar]
- Hu SJ, Calippe B, Lavalette S, Roubeix C, Montassar F, Housset M, Levy O, Delarasse C, Paques M, Sahel JA et al (2015) Upregulation of P2RX7 in Cx3cr1‐deficient mononuclear phagocytes leads to increased interleukin‐1beta secretion and photoreceptor neurodegeneration. J Neurosci 35: 6987–6996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Zhang Y, Wang J, Mao P, Lv X, Yuan S, Huang Z, Ding Y, Xie P, Liu Q (2016) Knockout of Ccr2 alleviates photoreceptor cell death in rodent retina exposed to chronic blue light. Cell Death Dis 7: e2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Guerin KI, Chen J, Michan S, Stahl A, Krah NM, Seaward MR, Dennison RJ, Juan AM, Hatton CJ et al (2011) Resveratrol inhibits pathologic retinal neovascularization in Vldlr(‐/‐) mice. Invest Ophthalmol Vis Sci 52: 2809–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhou T, Sun X, Zheng Y, Cheng B, Li M, Liu X, He C (2017) Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ 25: 180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Xu Z, Xiong S, Qin G, Sun F, Yang J, Yuan TF, Zhao L, Wang K, Liang YX et al (2018a) Dual extra‐retinal origins of microglia in the model of retinal microglia repopulation. Cell Discov 4: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Xu Z, Xiong S, Sun F, Qin G, Hu G, Wang J, Zhao L, Liang YX, Wu T et al (2018b) Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat Neurosci 21: 530–540 [DOI] [PubMed] [Google Scholar]
- Hume DA, Perry VH, Gordon S (1983) Immunohistochemical localization of a macrophage‐specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol 97: 253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MM, Kenna PF, Campbell M, Tam LC, Nguyen AT, Farrar GJ, Botto M, Kiang AS, Humphries P (2012) C1q enhances cone photoreceptor survival in a mouse model of autosomal recessive retinitis pigmentosa. Eur J Hum Genet 20: 64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonso CJ, Jaime H, Biswal MR, Boye SE, Li Q, Hauswirth WW, Lewin AS (2015) Gene therapy with the caspase activation and recruitment domain reduces the ocular inflammatory response. Mol Ther 23: 875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Patterson R, Ohnishi Y, Inomata H, Ryan SJ (1986) Formation of drusen in the human eye. Am J Ophthalmol 101: 342–353 [DOI] [PubMed] [Google Scholar]
- Izumi‐Nagai K, Nagai N, Ozawa Y, Mihara M, Ohsugi Y, Kurihara T, Koto T, Satofuka S, Inoue M, Tsubota K et al (2007) Interleukin‐6 receptor‐mediated activation of signal transducer and activator of transcription‐3 (STAT3) promotes choroidal neovascularization. Am J Pathol 170: 2149–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Boulton ME (2012) Consequences of oxidative stress in age‐related macular degeneration. Mol Aspects Med 33: 399–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Kawanokuchi J, Mizuno T, Wang J, Sonobe Y, Takeuchi H, Suzumura A (2007) Interferon‐beta is neuroprotective against the toxicity induced by activated microglia. Brain Res 1179: 140–146 [DOI] [PubMed] [Google Scholar]
- Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH (2000) A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res 70: 441–449 [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH (2002) The Alzheimer's A beta ‐peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age‐related macular degeneration. Proc Natl Acad Sci USA 99: 11830–11835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D et al (2011) Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood 117: 6024–6035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliana C, Fernandes‐Alnemri T, Kang S, Farias A, Qin F, Alnemri ES (2012) Non‐transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 287: 36617–36622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW (2012) Dysregulated neuronal‐microglial cross‐talk during aging, stress and inflammation. Exp Neurol 233: 40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalda A, Yu L, Oztas E, Chen JF (2006) Novel neuroprotection by caffeine and adenosine A(2A) receptor antagonists in animal models of Parkinson's disease. J Neurol Sci 248: 9–15 [DOI] [PubMed] [Google Scholar]
- Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, Kleinman ME, Ponicsan SL, Hauswirth WW, Chiodo VA et al (2011) DICER1 deficit induces Alu RNA toxicity in age‐related macular degeneration. Nature 471: 325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Ebert S, Langmann T (2010) Microglia in the healthy and degenerating retina: insights from novel mouse models. Immunobiology 215: 685–691 [DOI] [PubMed] [Google Scholar]
- Karlstetter M, Nothdurfter C, Aslanidis A, Moeller K, Horn F, Scholz R, Neumann H, Weber BH, Rupprecht R, Langmann T (2014) Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J Neuroinflammation 11: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T (2015) Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res 45: 30–57 [DOI] [PubMed] [Google Scholar]
- Karlstetter M, Kopatz J, Aslanidis A, Shahraz A, Caramoy A, Linnartz‐Gerlach B, Lin Y, Luckoff A, Fauser S, Duker K et al (2017) Polysialic acid blocks mononuclear phagocyte reactivity, inhibits complement activation, and protects from vascular damage in the retina. EMBO Mol Med 9: 154–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katschke KJ Jr, Wu P, Ganesan R, Kelley RF, Mathieu MA, Hass PE, Murray J, Kirchhofer D, Wiesmann C, van Lookeren Campagne M (2012) Inhibiting alternative pathway complement activation by targeting the factor D exosite. J Biol Chem 287: 12886–12892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsnelson MA, Rucker LG, Russo HM, Dubyak GR (2015) K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol 194: 3937–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K (2012) Oxidative stress activates NLRP3 inflammasomes in ARPE‐19 cells–implications for age‐related macular degeneration (AMD). Immunol Lett 147: 29–33 [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U‐K, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91: 461–553 [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA (2006) P2X receptors as cell‐surface ATP sensors in health and disease. Nature 442: 527–532 [DOI] [PubMed] [Google Scholar]
- Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW (2014) Pattern recognition receptors and central nervous system repair. Exp Neurol 258: 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Meyer K, Di Bisceglie AM, Ray R (2013) Hepatitis C virus suppresses C9 complement synthesis and impairs membrane attack complex function. J Virol 87: 5858–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto T, Takahashi K, Tobe T, Fujimoto K, Uyama M, Sone S (2002) Effects of local administration of interferon‐beta on proliferation of retinal pigment epithelium in rabbit after laser photocoagulation. Jpn J Ophthalmol 46: 160–169 [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I, Kishimoto T (2005) Suppressor of cytokine signaling‐1 selectively inhibits LPS‐induced IL‐6 production by regulating JAK–STAT. Proc Natl Acad Sci USA 102: 17089–17094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno H, Maeda T, Perusek L, Pearlman E, Maeda A (2014) CCL3 production by microglial cells modulates disease severity in murine models of retinal degeneration. J Immunol 192: 3816–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev M (2014) Functional analysis of CD59 using complement‐dependent cytotoxicity assay. Methods Mol Biol 1100: 347–353 [DOI] [PubMed] [Google Scholar]
- Kolev M, Kemper C (2017) Keeping it all going‐complement meets metabolism. Front Immunol 8: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleva‐Georgieva DN, Sivkova NP, Terzieva D (2011) Serum inflammatory cytokines IL‐1beta, IL‐6, TNF‐alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (Plovdiv) 53: 44–50 [DOI] [PubMed] [Google Scholar]
- Korot E, Comer G, Steffens T, Antonetti DA (2016) Algorithm for the measure of vitreous hyperreflective foci in optical coherence tomographic scans of patients with diabetic macular edema. JAMA Ophthalmol 134: 15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Odenbach S (2004) Role of interleukin‐1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol 88: 1343–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Mohammad G, Santos JM, Tewari S, Zhong Q (2011) Interleukin‐1beta and mitochondria damage, and the development of diabetic retinopathy. J Ocul Biol Dis Infor 4: 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW (2005) Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase‐3 activation in a rodent model of diabetic retinopathy. Diabetes 54: 1559–1565 [DOI] [PubMed] [Google Scholar]
- Kurji KH, Cui JZ, Lin T, Harriman D, Prasad SS, Kojic L, Matsubara JA (2010) Microarray analysis identifies changes in inflammatory gene expression in response to amyloid‐beta stimulation of cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 51: 1151–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J (2001) Mammalian mitogen‐activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869 [DOI] [PubMed] [Google Scholar]
- Lakkaraju A, Toops KA, Xu J (2014) Should I stay or should I go? Trafficking of sub‐lytic MAC in the retinal pigment epithelium. Adv Exp Med Biol 801: 267–274 [DOI] [PubMed] [Google Scholar]
- Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart JM et al (2013) Laser‐induced choroidal neovascularization model to study age‐related macular degeneration in mice. Nat Protoc 8: 2197–2211 [DOI] [PubMed] [Google Scholar]
- Lammer J, Bolz M, Baumann B, Pircher M, Gerendas B, Schlanitz F, Hitzenberger CK, Schmidt‐Erfurth U (2014) Detection and analysis of hard exudates by polarization‐sensitive optical coherence tomography in patients with diabetic maculopathy. Invest Ophthalmol Vis Sci 55: 1564–1571 [DOI] [PubMed] [Google Scholar]
- Langmann T (2007) Microglia activation in retinal degeneration. J Leukoc Biol 81: 1345–1351 [DOI] [PubMed] [Google Scholar]
- Lavalette S, Raoul W, Houssier M, Camelo S, Levy O, Calippe B, Jonet L, Behar‐Cohen F, Chemtob S, Guillonneau X et al (2011) Interleukin‐1beta inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am J Pathol 178: 2416–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner J, Chen M, Hogg RE, Toth L, Silvestri G, Chakravarthy U, Xu H (2016) Higher plasma levels of complement C3a, C4a and C5a increase the risk of subretinal fibrosis in neovascular age‐related macular degeneration: complement activation in AMD. Immun Ageing 13: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner J, Chen M, Hogg RE, Toth L, Silvestri G, Chakravarthy U, Xu H (2017a) Peripheral blood mononuclear cells from neovascular age‐related macular degeneration patients produce higher levels of chemokines CCL2 (MCP‐1) and CXCL8 (IL‐8). J Neuroinflammation 14: 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner J, O'Leary OE, Stitt AW (2017b) The pathology associated with diabetic retinopathy. Vision Res 139: 7–14 [DOI] [PubMed] [Google Scholar]
- Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross‐talk and redox signalling. Biochem J 441: 523–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Wang PW, Yang IH, Huang HM, Chang CS, Wu CL, Chuang JH (2015) High‐fat diet induces toll‐like receptor 4‐dependent macrophage/microglial cell activation and retinal impairment. Invest Ophthalmol Vis Sci 56: 3041–3050 [DOI] [PubMed] [Google Scholar]
- Levy O, Calippe B, Lavalette S, Hu SJ, Raoul W, Dominguez E, Housset M, Paques M, Sahel JA, Bemelmans AP et al (2015) Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age‐related macular degeneration. EMBO Mol Med 7: 211–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Smith D, Li Q, Sheibani N, Huang S, Kern T, Nagaraj RH, Lin F (2012) Antibody‐mediated retinal pericyte injury: implications for diabetic retinopathy. Invest Ophthalmol Vis Sci 53: 5520–5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtlen P, Lam TT, Nork TM, Streit T, Urech DM (2010) Relative contribution of VEGF and TNF‐alpha in the cynomolgus laser‐induced CNV model: comparing the efficacy of bevacizumab, adalimumab, and ESBA105. Invest Ophthalmol Vis Sci 51: 4738–4745 [DOI] [PubMed] [Google Scholar]
- Linnartz B, Kopatz J, Tenner AJ, Neumann H (2012) Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor‐3‐mediated removal by microglia. J Neurosci 32: 946–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnartz B, Neumann H (2013) Microglial activatory (immunoreceptor tyrosine‐based activation motif)‐ and inhibitory (immunoreceptor tyrosine‐based inhibition motif)‐signaling receptors for recognition of the neuronal glycocalyx. Glia 61: 37–46 [DOI] [PubMed] [Google Scholar]
- Linnartz‐Gerlach B, Mathews M, Neumann H (2014) Sensing the neuronal glycocalyx by glial sialic acid binding immunoglobulin‐like lectins. Neuroscience 275: 113–124 [DOI] [PubMed] [Google Scholar]
- Lipo E, Cashman SM, Kumar‐Singh R (2013) Aurintricarboxylic acid inhibits complement activation, membrane attack complex, and choroidal neovascularization in a model of macular degeneration. Invest Ophthalmol Vis Sci 54: 7107–7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewski MK, Elvington M, Kulkarni HS, Atkinson JP (2017) Complement's hidden arsenal: new insights and novel functions inside the cell. Mol Immunol 84: 2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Biarnes Costa M, Gerhardinger C (2012) IL‐1beta is upregulated in the diabetic retina and retinal vessels: cell‐specific effect of high glucose and IL‐1beta autostimulation. PLoS One 7: e36949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Copland DA, Horie S, Wu W‐K, Chen M, Xu Y, Paul Morgan B, Mack M, Xu H, Nicholson LB et al (2013) Myeloid cells expressing VEGF and arginase‐1 following uptake of damaged retinal pigment epithelium suggests potential mechanism that drives the onset of choroidal angiogenesis in mice. PLoS One 8: e72935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukovaara S, Piippo N, Kinnunen K, Hytti M, Kaarniranta K, Kauppinen A (2017) NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol 95: 803–808 [DOI] [PubMed] [Google Scholar]
- Luckoff A, Caramoy A, Scholz R, Prinz M, Kalinke U, Langmann T (2016) Interferon‐beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization. EMBO Mol Med 8: 670–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckoff A, Scholz R, Sennlaub F, Xu H, Langmann T (2017) Comprehensive analysis of mouse retinal mononuclear phagocytes. Nat Protoc 12: 1136–1150 [DOI] [PubMed] [Google Scholar]
- Lueck K, Wasmuth S, Williams J, Hughes TR, Morgan BP, Lommatzsch A, Greenwood J, Moss SE, Pauleikhoff D (2011) Sub‐lytic C5b‐9 induces functional changes in retinal pigment epithelial cells consistent with age‐related macular degeneration. Eye (Lond) 25: 1074–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Chen M, Xu H (2011) Complement gene expression and regulation in mouse retina and retinal pigment epithelium/choroid. Mol Vis 17: 1588–1597 [PMC free article] [PubMed] [Google Scholar]
- Madeira MH, Elvas F, Boia R, Goncalves FQ, Cunha RA, Ambrosio AF, Santiago AR (2015) Adenosine A2AR blockade prevents neuroinflammation‐induced death of retinal ganglion cells caused by elevated pressure. J Neuroinflammation 12: 115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MH, Boia R, Elvas F, Martins T, Cunha RA, Ambrosio AF, Santiago AR (2016) Selective A2A receptor antagonist prevents microglia‐mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure‐induced transient ischemic injury. Transl Res 169: 112–128 [DOI] [PubMed] [Google Scholar]
- Madeira MH, Rashid K, Ambrosio AF, Santiago AR, Langmann T (2018) Blockade of microglial adenosine A2A receptor impacts inflammatory mechanisms, reduces ARPE‐19 cell dysfunction and prevents photoreceptor loss in vitro . Sci Rep 8: 2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Kanneganti TD (2016) Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol 16: 7–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Schlunck G, Hansen LL, Agostini HT (2004) Differential expression of angioregulatory factors in normal and CNV‐derived human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol 242: 321–326 [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL‐beta. Mol Cell 10: 417–426 [DOI] [PubMed] [Google Scholar]
- Masland RH (2001) The fundamental plan of the retina. Nat Neurosci 4: 877–886 [DOI] [PubMed] [Google Scholar]
- Masuda T, Shimazawa M, Hara H (2017) Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (Edaravone). Oxid Med Cell Longev 2017: 9208489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis T, Housset M, Eandi C, Beguier F, Touhami S, Reichman S, Augustin S, Gondouin P, Sahel J‐A, Kodjikian L et al (2017) Activated monocytes resist elimination by retinal pigment epithelium and downregulate their OTX2 expression via TNF‐α. Aging Cell 16: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Hayward JA, Man SM (2017) Molecular mechanisms of inflammasome signaling. J Leukoc Biol 103: 233–257 [DOI] [PubMed] [Google Scholar]
- Mazzoni F, Safa H, Finnemann SC (2014) Understanding photoreceptor outer segment phagocytosis: use and utility of RPE cells in culture. Exp Eye Res 126: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick SM, Heller NM (2015) Regulation of macrophage, dendritic cell, and microglial phenotype and function by the SOCS proteins. Front Immunol 6: 549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHarg S, Clark SJ, Day AJ, Bishop PN (2015) Age‐related macular degeneration and the role of the complement system. Mol Immunol 67: 43–50 [DOI] [PubMed] [Google Scholar]
- Mendiola AS, Garza R, Cardona SM, Mythen SA, Lira SA, Akassoglou K, Cardona AE (2017) Fractalkine signaling attenuates perivascular clustering of microglia and fibrinogen leakage during systemic inflammation in mouse models of diabetic retinopathy. Front Cell Neurosci 10: 303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Potenza RL, Pezzola A, Blum D, Bantubungi K, Popoli P (2007) Effects of the adenosine A2A receptor antagonist SCH 58621 on cyclooxygenase‐2 expression, glial activation, and brain‐derived neurotrophic factor availability in a rat model of striatal neurodegeneration. J Neuropathol Exp Neurol 66: 363–371 [DOI] [PubMed] [Google Scholar]
- Mitchell CH (2001) Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J Physiol 534: 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter SK, Rao HV, Qi X, Cai J, Sugrue A, Dunn WA, Grant MB, Boulton ME (2012) Autophagy in the retina: a potential role in age‐related macular degeneration. Adv Exp Med Biol 723: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W Jr, Ding J et al (2014) Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 10: 1989–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3: 542–545 [DOI] [PubMed] [Google Scholar]
- Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM, Cardona AE (2012) The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J Immunol 188: 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T, Fowler BJ, Kim Y, Yasuma R, Krueger LA, Gelfand BD, Ambati J (2015) Nucleoside reverse transcriptase inhibitors suppress laser‐induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci 56: 7122–7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MK, Morran M, Slotterbeck B, Leaman DW, Sun Y, Grafenstein HV, Hong S‐C, McInerney MF (2006) Dysregulated Toll‐like receptor expression and signaling in bone marrow‐derived macrophages at the onset of diabetes in the non‐obese diabetic mouse. Int Immunol 18: 1101–1113 [DOI] [PubMed] [Google Scholar]
- von Moltke J, Ayres JS, Kofoed EM, Chavarria‐Smith J, Vance RE (2013) Recognition of bacteria by inflammasomes. Annu Rev Immunol 31: 73–106 [DOI] [PubMed] [Google Scholar]
- Moreau JL, Huber G (1999) Central adenosine A(2A) receptors: an overview. Brain Res Brain Res Rev 31: 65–82 [DOI] [PubMed] [Google Scholar]
- Morgan J, Wong R (1995) Development of cell types and synaptic connections in the retina In Webvision: the organization of the retina and visual system, Kolb H, Fernandez E, Nelson R. (eds). Salt Lake City, UT: University of Utah Health Sciences Center; [PubMed] [Google Scholar]
- Morigiwa K, Quan M, Murakami M, Yamashita M, Fukuda Y (2000) P2 Purinoceptor expression and functional changes of hypoxia‐activated cultured rat retinal microglia. Neurosci Lett 282: 153–156 [DOI] [PubMed] [Google Scholar]
- Mullins RF, Schoo DP, Sohn EH, Flamme‐Wiese MJ, Workamelahu G, Johnston RM, Wang K, Tucker BA, Stone EM (2014) The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol 184: 3142–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz‐Planillo R, Kuffa P, Martinez‐Colon G, Smith BL, Rajendiran TM, Nunez G (2013) K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38: 1142–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu D, Wakabayashi Y, Usui Y, Okunuki Y, Kezuka T, Goto H (2013) Correlation of complement fragment C5a with inflammatory cytokines in the vitreous of patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 251: 15–17 [DOI] [PubMed] [Google Scholar]
- Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V (2008) Proinflammatory cytokines and angiogenic and anti‐angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and Eales’ disease. Retina 28: 817–824 [DOI] [PubMed] [Google Scholar]
- Nadal‐Nicolas FM, Galindo‐Romero C, Valiente‐Soriano FJ, Barbera‐Cremades M, deTorre‐Minguela C, Salinas‐Navarro M, Pelegrin P, Agudo‐Barriuso M (2016) Involvement of P2X7 receptor in neuronal degeneration triggered by traumatic injury. Sci Rep 6: 38499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Hisatomi T, Nakazawa C, Noda K, Maruyama K, She H, Matsubara A, Miyahara S, Nakao S, Yin Y et al (2007) Monocyte chemoattractant protein 1 mediates retinal detachment‐induced photoreceptor apoptosis. Proc Natl Acad Sci USA 104: 2425–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC (2004) Loss of synchronized retinal phagocytosis and age‐related blindness in mice lacking αvβ5 integrin. J Exp Med 200: 1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli R, Fernando N, Madigan M, Chu‐Tan JA, Valter K, Provis J, Rutar M (2017) Microglia‐derived IL‐1β promotes chemokine expression by Müller cells and RPE in focal retinal degeneration. Mol Neurodegener 12: 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M, Cunningham J (1994) Modulation by endogenous ATP of the light‐evoked release of ACh from retinal cholinergic neurones. Br J Pharmacol 113: 1085–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel C, Aslanidis A, Rashid K, Langmann T (2017) Activated microglia trigger inflammasome activation and lysosomal destabilization in human RPE cells. Biochem Biophys Res Commun 484: 681–686 [DOI] [PubMed] [Google Scholar]
- Neniskyte U, Brown GC (2013) Lactadherin/MFG‐E8 is essential for microglia‐mediated neuronal loss and phagoptosis induced by amyloid beta. J Neurochem 126: 312–317 [DOI] [PubMed] [Google Scholar]
- Newman EA (2003) Glial cell inhibition of neurons by release of ATP. J Neurosci 23: 1659–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA (2006) A purinergic dialogue between glia and neurons in the retina. Novartis Found Symp 276: 193–202; discussion 202–207, 233–237, 275–281 [PubMed] [Google Scholar]
- Newman AM, Gallo NB, Hancox LS, Miller NJ, Radeke CM, Maloney MA, Cooper JB, Hageman GS, Anderson DH, Johnson LV et al (2012) Systems‐level analysis of age‐related macular degeneration reveals global biomarkers and phenotype‐specific functional networks. Genome Med 4: 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Duncan ID, Watters JJ (2006) Minocycline exerts inhibitory effects on multiple mitogen‐activated protein kinases and IκBα degradation in a stimulus‐specific manner in microglia. J Neurochem 96: 314–323 [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Brownlee M (2000) The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl 77: S26–S30 [DOI] [PubMed] [Google Scholar]
- Niu S, Yu C, Chen Q, Yuan S, Lin J, Fan W, Liu Q (2017) Multimodality analysis of hyper‐reflective foci and hard exudates in patients with diabetic retinopathy. Sci Rep 7: 1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyadurupola N, Sidaway P, Ma N, Rhodes JD, Broadway DC, Sanderson J (2013) P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Invest Ophthalmol Vis Sci 54: 2163–2170 [DOI] [PubMed] [Google Scholar]
- Nomura K, Vilalta A, Allendorf DH, Hornik TC, Brown GC (2017) Activated microglia desialylate and phagocytose cells via neuraminidase, galectin‐3 and Mer tyrosine kinase. J Immunol 198: 4792–4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ et al (2006) Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA 103: 2328–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt RB, Byrnes G, Sen HN, Yeh S, Faia L, Meyerle C, Wroblewski K, Li Z, Liu B, Chew E et al (2010) A randomized pilot study of systemic immunosuppression in the treatment of age‐related macular degeneration with choroidal neovascularization. Retina 30: 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Koren EG, Mathew R, Saban DR (2016) Fate mapping reveals that microglia and recruited monocyte‐derived macrophages are definitively distinguishable by phenotype in the retina. Sci Rep 6: 20636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino K, Murakami T, Tsujikawa A, Miyamoto K, Sakamoto A, Ota M, Yoshimura N (2012) Characteristics of optical coherence tomographic hyperreflective foci in retinal vein occlusion. Retina 32: 77–85 [DOI] [PubMed] [Google Scholar]
- Oh H, Takagi H, Takagi C, Suzuma K, Otani A, Ishida K, Matsumura M, Ogura Y, Honda Y (1999) The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci 40: 1891–1898 [PubMed] [Google Scholar]
- Okada M, Matsuzawa A, Yoshimura A, Ichijo H (2014) The lysosome rupture‐activated TAK1‐JNK pathway regulates NLRP3 inflammasome activation. J Biol Chem 289: 32926–32936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Dias JR, Rodrigues EB, Maia M, Magalhaes O Jr, Penha FM, Farah ME (2011) Cytokines in neovascular age‐related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol 95: 1631–1637 [DOI] [PubMed] [Google Scholar]
- Omri S, Behar‐Cohen F, de Kozak Y, Sennlaub F, Verissimo LM, Jonet L, Savoldelli M, Omri B, Crisanti P (2011) Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: role of PKCzeta in the Goto Kakizaki rat model. Am J Pathol 179: 942–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang CE, Messinger JD, Zanzottera EC, Freund KB, Curcio CA (2015) The onion sign in neovascular age‐related macular degeneration represents cholesterol crystals. Ophthalmology 122: 2316–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR III, Lafaille JJ, Hempstead BL, Littman DR, Gan WB (2013) Microglia promote learning‐dependent synapse formation through brain‐derived neurotrophic factor. Cell 155: 1596–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JI, Saleh GM, Hykin PG, Gregor ZJ, Cree IA (2008) Concentration of haemodynamic and inflammatory related cytokines in diabetic retinopathy. Eye (Lond) 22: 223–228 [DOI] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P (2005) ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 46: 731–744 [DOI] [PubMed] [Google Scholar]
- Penfold PL, Killingsworth MC, Sarks SH (1985) Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol 223: 69–76 [DOI] [PubMed] [Google Scholar]
- Peng B, Xiao J, Wang K, So KF, Tipoe GL, Lin B (2014) Suppression of microglial activation is neuroprotective in a mouse model of human retinitis pigmentosa. J Neurosci 34: 8139–8150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JA, Greenfield AJ, Sutton R, Ferguson SJ (1986) Characterisation of phosphate binding to mitochondrial and bacterial membrane‐bound ATP synthase by studies of inhibition with 4‐chloro‐7‐nitrobenzofurazan. FEBS Lett 198: 113–118 [DOI] [PubMed] [Google Scholar]
- Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA (2000) ATP acts as an agonist to promote stimulus‐induced secretion of IL‐1 beta and IL‐18 in human blood. J Immunol 165: 4615–4623 [DOI] [PubMed] [Google Scholar]
- Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14: 1583–1589 [DOI] [PubMed] [Google Scholar]
- Petrovic MG, Korosec P, Kosnik M, Hawlina M (2010) Association of preoperative vitreous IL‐8 and VEGF levels with visual acuity after vitrectomy in proliferative diabetic retinopathy. Acta Ophthalmol 88: e311–e316 [DOI] [PubMed] [Google Scholar]
- Pettus EH, Wright DW, Stein DG, Hoffman SW (2005) Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res 1049: 112–119 [DOI] [PubMed] [Google Scholar]
- Piano I, Novelli E, Della Santina L, Strettoi E, Cervetto L, Gargini C (2016) Involvement of autophagic pathway in the progression of retinal degeneration in a mouse model of diabetes. Front Cell Neurosci 10: 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon DC, Ho YS, Chiu K, Wong HL, Chang RC (2015) Sickness: from the focus on cytokines, prostaglandins, and complement factors to the perspectives of neurons. Neurosci Biobehav Rev 57: 30–45 [DOI] [PubMed] [Google Scholar]
- Potucek YD, Crain JM, Watters JJ (2006) Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochem Int 49: 204–214 [DOI] [PubMed] [Google Scholar]
- Prinz M, Schmidt H, Mildner A, Knobeloch KP, Hanisch UK, Raasch J, Merkler D, Detje C, Gutcher I, Mages J et al (2008) Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28: 675–686 [DOI] [PubMed] [Google Scholar]
- Pshezhetsky AV, Hinek A (2011) Where catabolism meets signalling: neuraminidase 1 as a modulator of cell receptors. Glycoconj J 28: 441–452 [DOI] [PubMed] [Google Scholar]
- Rangasamy S, McGuire PG, Das A (2012) Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr J Ophthalmol 19: 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid K, Wolf A, Langmann T (2018) Microglia activation and immunomodulatory therapies for retinal degenerations. Front Cell Neurosci 12: 176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Simoes AP, Canas PM, Tome AR, Andrade GM, Barry CE, Agostinho PM, Lynch MA, Cunha RA (2011) Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J Neurochem 117: 100–111 [DOI] [PubMed] [Google Scholar]
- Regatieri CV, Dreyfuss JL, Melo GB, Lavinsky D, Farah ME, Nader HB (2009) Dual role of intravitreous infliximab in experimental choroidal neovascularization: effect on the expression of sulfated glycosaminoglycans. Invest Ophthalmol Vis Sci 50: 5487–5494 [DOI] [PubMed] [Google Scholar]
- Reigada D, Lu W, Zhang X, Friedman C, Pendrak K, McGlinn A, Stone RA, Laties AM, Mitchell CH (2005) Degradation of extracellular ATP by the retinal pigment epithelium. Am J Physiol Cell Physiol 289: C617–C624 [DOI] [PubMed] [Google Scholar]
- Reigada D, Mitchell CH (2005) Release of ATP from retinal pigment epithelial cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol 288: C132–C140 [DOI] [PubMed] [Google Scholar]
- RetNet (2018) Retinal Information Network. https://sphuthedu/RETNET/
- Réu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H et al (2017) The lifespan and turnover of microglia in the human brain. Cell Rep 20: 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes NJ, O'Koren EG, Saban DR (2017) New insights into mononuclear phagocyte biology from the visual system. Nat Rev Immunol 17: 322–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM (2009) Plasma complement components and activation fragments: associations with age‐related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci 50: 5818–5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera JC, Sitaras N, Noueihed B, Hamel D, Madaan A, Zhou T, Honore JC, Quiniou C, Joyal JS, Hardy P et al (2013) Microglia and interleukin‐1beta in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin‐3A. Arterioscler Thromb Vasc Biol 33: 1881–1891 [DOI] [PubMed] [Google Scholar]
- Robbie SJ, Georgiadis A, Barker SE, Duran Y, Smith AJ, Ali RR, Luhmann UF, Bainbridge JW (2016) Enhanced Ccl2‐Ccr2 signaling drives more severe choroidal neovascularization with aging. Neurobiol Aging 40: 110–119 [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F et al (2009) Translocator protein (18 kD) as target for anxiolytics without benzodiazepine‐like side effects. Science 325: 490–493 [DOI] [PubMed] [Google Scholar]
- Rutar M, Natoli R, Provis JM (2012) Small interfering RNA‐mediated suppression of Ccl2 in Müller cells attenuates microglial recruitment and photoreceptor death following retinal degeneration. J Neuroinflammation 9: 221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Vallejo V, Benlloch‐Navarro S, Lopez‐Pedrajas R, Romero FJ, Miranda M (2015) Neuroprotective actions of progesterone in an in vivo model of retinitis pigmentosa. Pharmacol Res 99: 276–288 [DOI] [PubMed] [Google Scholar]
- Sanderson J, Dartt DA, Trinkaus‐Randall V, Pintor J, Civan MM, Delamere NA, Fletcher EL, Salt TE, Grosche A, Mitchell CH (2014) Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res 127: 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Takeuchi M, Karasawa Y, Enoki T, Ito M (2018) Intraocular inflammatory cytokines in patients with neovascular age‐related macular degeneration before and after initiation of intravitreal injection of anti‐VEGF inhibitor. Sci Rep 8: 1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Angulo E, Ejarque A, Casado V, Tusell JM, Moratalla R, Chen JF, Schwarzschild MA, Lluis C, Franco R et al (2005) Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J Neurochem 95: 919–929 [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron 74: 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaft TL, Mooy CM, de Bruijn WC, de Jong PT (1993) Early stages of age‐related macular degeneration: an immunofluorescence and electron microscopy study. Br J Ophthalmol 77: 657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick T, Steinhauer M, Aslanidis A, Altay L, Karlstetter M, Langmann T, Kirschfink M, Fauser S (2017) Local complement activation in aqueous humor in patients with age‐related macular degeneration. Eye (Lond) 31: 810–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CQ, Harder MJ, Nichols EM, Hebecker M, Anliker M, Hochsmann B, Simmet T, Csincsi AI, Uzonyi B, Pappworth IY et al (2016) Selectivity of C3‐opsonin targeted complement inhibitors: a distinct advantage in the protection of erythrocytes from paroxysmal nocturnal hemoglobinuria patients. Immunobiology 221: 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL, Gerardy‐Schahn R, Hildebrandt H (2014) Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev 94: 461–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl HP, Charbel Issa P, Walier M, Janzer S, Pollok‐Kopp B, Borncke F, Fritsche LG, Chong NV, Fimmers R, Wienker T et al (2008) Systemic complement activation in age‐related macular degeneration. PLoS One 3: e2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R, Caramoy A, Bhuckory MB, Rashid K, Chen M, Xu H, Grimm C, Langmann T (2015a) Targeting translocator protein (18 kDa) (TSPO) dampens pro‐inflammatory microglia reactivity in the retina and protects from degeneration. J Neuroinflammation 12: 201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R, Sobotka M, Caramoy A, Stempfl T, Moehle C, Langmann T (2015b) Minocycline counter‐regulates pro‐inflammatory microglia responses in the retina and protects from degeneration. J Neuroinflammation 12: 209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB (2003) Signalling from adenosine receptors to mitogen‐activated protein kinases. Cell Signal 15: 813–827 [DOI] [PubMed] [Google Scholar]
- Schutt F, Bergmann M, Holz FG, Kopitz J (2003) Proteins modified by malondialdehyde, 4‐hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci 44: 3663–3668 [DOI] [PubMed] [Google Scholar]
- Schwarz F, Landig CS, Siddiqui S, Secundino I, Olson J, Varki N, Nizet V, Varki A (2017) Paired Siglec receptors generate opposite inflammatory responses to a human‐specific pathogen. EMBO J 36: 751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B, Rifai N (2005) Progression of age‐related macular degeneration: prospective assessment of C‐reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol 123: 774–782 [DOI] [PubMed] [Google Scholar]
- Sennlaub F, Auvynet C, Calippe B, Lavalette S, Poupel L, Hu SJ, Dominguez E, Camelo S, Levy O, Guyon E et al (2013) CCR2+ monocytes infiltrate atrophic lesions in age‐related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med 5: 1775–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfikakis PP, Markomichelakis N, Theodossiadis GP, Grigoropoulos V, Katsilambros N, Theodossiadis PG (2005) Regression of sight‐threatening macular edema in type 2 diabetes following treatment with the anti‐tumor necrosis factor monoclonal antibody infliximab. Diabetes Care 28: 445–447 [DOI] [PubMed] [Google Scholar]
- Sfikakis PP, Grigoropoulos V, Emfietzoglou I, Theodossiadis G, Tentolouris N, Delicha E, Katsiari C, Alexiadou K, Hatziagelaki E, Theodossiadis PG (2010) Infliximab for diabetic macular edema refractory to laser photocoagulation: a randomized, double‐blind, placebo‐controlled, crossover, 32‐week study. Diabetes Care 33: 1523–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RS, Soetikno BT, Lajko M, Fawzi AA (2015) A mouse model for laser‐induced choroidal neovascularization. J Vis Exp 106: e53502. 10.3791/53502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahraz A, Kopatz J, Mathy R, Kappler J, Winter D, Kapoor S, Schutza V, Scheper T, Gieselmann V, Neumann H (2015) Anti‐inflammatory activity of low molecular weight polysialic acid on human macrophages. Sci Rep 5: 16800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Semkova I, Muther PS, Dell S, Kociok N, Joussen AM (2006) Inhibition of TNF‐alpha reduces laser‐induced choroidal neovascularization. Exp Eye Res 83: 1325–1334 [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F (2014) Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192 [DOI] [PubMed] [Google Scholar]
- Shi H, Zhang Z, Wang X, Li R, Hou W, Bi W, Zhang X (2015) Inhibition of autophagy induces IL‐1beta release from ARPE‐19 cells via ROS mediated NLRP3 inflammasome activation under high glucose stress. Biochem Biophys Res Commun 463: 1071–1076 [DOI] [PubMed] [Google Scholar]
- Shieh CH, Heinrich A, Serchov T, van Calker D, Biber K (2014) P2X7‐dependent, but differentially regulated release of IL‐6, CCL2, and TNF‐alpha in cultured mouse microglia. Glia 62: 592–607 [DOI] [PubMed] [Google Scholar]
- Shiratori M, Tozaki‐Saitoh H, Yoshitake M, Tsuda M, Inoue K (2010) P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem 114: 810–819 [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL (2004) Dystrophic microglia in the aging human brain. Glia 45: 208–212 [DOI] [PubMed] [Google Scholar]
- Stutz A, Golenbock DT, Latz E (2009) Inflammasomes: too big to miss. J Clin Invest 119: 3502–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudharsan R, Beiting DP, Aguirre GD, Beltran WA (2017) Involvement of innate immune system in late stages of inherited photoreceptor degeneration. Sci Rep 7: 17897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Nakazawa M, Suzuki K, Yamazaki H, Miyagawa Y (2011) Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol 55: 256–263 [DOI] [PubMed] [Google Scholar]
- Tang J, Kern TS (2011) Inflammation in diabetic retinopathy. Prog Retin Eye Res 30: 343–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S et al (2012) DICER1 loss and Alu RNA induce age‐related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 149: 847–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HR, West S, Munoz B, Rosenthal FS, Bressler SB, Bressler NM (1992) The long‐term effects of visible light on the eye. Arch Ophthalmol 110: 99–104 [DOI] [PubMed] [Google Scholar]
- Teige I, Treschow A, Teige A, Mattsson R, Navikas V, Leanderson T, Holmdahl R, Issazadeh‐Navikas S (2003) IFN‐beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol 170: 4776–4784 [DOI] [PubMed] [Google Scholar]
- Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP (2009) Intravitreal administration of the anti‐tumor necrosis factor agent infliximab for neovascular age‐related macular degeneration. Am J Ophthalmol 147: 825–830, 830.e821 [DOI] [PubMed] [Google Scholar]
- Ting DS, Cheung GC, Wong TY (2016) Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 44: 260–277 [DOI] [PubMed] [Google Scholar]
- Tobe T, Takahashi K, Ohkuma H, Uyama M (1995) The effect of interferon‐beta on experimental choroidal neovascularization. Nippon Ganka Gakkai Zasshi 99: 571–581 [PubMed] [Google Scholar]
- Tomany SC, Cruickshanks KJ, Klein R, Klein BE, Knudtson MD (2004) Sunlight and the 10‐year incidence of age‐related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol 122: 750–757 [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Gardiner NJ (2008) Glucose neurotoxicity. Nat Rev Neurosci 9: 36 [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Hughes TR, Triantafilou M, Morgan BP (2013) The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci 126: 2903–2913 [DOI] [PubMed] [Google Scholar]
- Troy FA II (1992) Polysialylation: from bacteria to brains. Glycobiology 2: 5–23 [DOI] [PubMed] [Google Scholar]
- Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D'Amore PA, Ksander BR (2013) NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age‐related macular degeneration. Invest Ophthalmol Vis Sci 54: 110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckermann O, Wolf A, Kutzera F, Kalisch F, Beck‐Sickinger AG, Wiedemann P, Reichenbach A, Bringmann A (2006) Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: activation by neuropeptide Y. J Neurosci Res 83: 538–550 [DOI] [PubMed] [Google Scholar]
- Uematsu S, Akira S (2006) Toll‐like receptors and innate immunity. J Mol Med 84: 712–725 [DOI] [PubMed] [Google Scholar]
- Ugurlu S, Ucar D, Seyahi E, Hatemi G, Yurdakul S (2012) Canakinumab in a patient with juvenile Behcet's syndrome with refractory eye disease. Ann Rheum Dis 71: 1589–1591 [DOI] [PubMed] [Google Scholar]
- Vitale A, Rigante D, Caso F, Brizi MG, Galeazzi M, Costa L, Franceschini R, Lucherini OM, Cantarini L (2014) Inhibition of interleukin‐1 by canakinumab as a successful mono‐drug strategy for the treatment of refractory Behcet's disease: a case series. Dermatology 228: 211–214 [DOI] [PubMed] [Google Scholar]
- Vogt SD, Barnum SR, Curcio CA, Read RW (2006) Distribution of complement anaphylatoxin receptors and membrane‐bound regulators in normal human retina. Exp Eye Res 83: 834–840 [DOI] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH (2009) Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to Drusen formation and age‐related macular degeneration. PLoS One 4: e4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Neumann H (2010) Alleviation of neurotoxicity by microglial human Siglec‐11. J Neurosci 30: 3482–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang X, Zhao L, Ma W, Rodriguez IR, Fariss RN, Wong WT (2014) Macroglia‐microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J Neurosci 34: 3793–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao L, Zhang J, Fariss RN, Ma W, Kretschmer F, Wang M, Qian HH, Badea TC, Diamond JS et al (2016a) Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. J Neurosci 36: 2827–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hanus JW, Abu‐Asab MS, Shen D, Ogilvy A, Ou J, Chu XK, Shi G, Li W, Wang S et al (2016b) NLRP3 upregulation in retinal pigment epithelium in age‐related macular degeneration. Int J Mol Sci 17: E73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick A, Khandhadia S, Ennis S, Lotery A (2014) Age‐related macular degeneration: a disease of systemic or local complement dysregulation? J Clin Med 3: 1234–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Remé CE (2005) Molecular mechanisms of light‐induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res 24: 275–306 [DOI] [PubMed] [Google Scholar]
- Wilson HM (2014) SOCS proteins in macrophage polarization and function. Front Immunol 5: 357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y, Yona S, Kim KW, Jung S (2013) Microglia, seen from the CX3CR1 angle. Front Cell Neurosci 7: 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY (2014) Global prevalence of age‐related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta‐analysis. Lancet Glob Health 2: e106–e116 [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen Y, Wu Q, Jia L, Du X (2015) Minocycline inhibits PARP1 expression and decreases apoptosis in diabetic retinopathy. Mol Med Rep 12: 4887–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm A, Lipp S, Pannicke T, Linnertz R, Krugel U, Schulz A, Farber K, Zahn D, Grosse J, Wiedemann P et al (2010) Endogenous purinergic signaling is required for osmotic volume regulation of retinal glial cells. J Neurochem 112: 1261–1272 [DOI] [PubMed] [Google Scholar]
- Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH (2012) Neurons respond directly to mechanical deformation with pannexin‐mediated ATP release and autostimulation of P2X7 receptors. J Physiol 590: 2285–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kamei M, Suzuki M, Matsumura N, Nishida K, Sakimoto S, Sakaguchi H, Nishida K (2011) Suppression and regression of choroidal neovascularization in mice by a novel CCR2 antagonist, INCB3344. PLoS One 6: e28933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, Yu Y, Zhou X, Xia X, Jiang H (2017) Rhodopsin T17M mutant inhibits complement C3 secretion in retinal pigment epithelium via ROS induced downregulation of TWIST1. J Cell Biochem 118: 4914–4920 [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV, Lois N (2011) Cataract surgery induces retinal pro‐inflammatory gene expression and protein secretion. Invest Ophthalmol Vis Sci 52: 249–255 [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M (2016) Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol 787: 94–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Elner SG, Clark AJ, Hughes BA, Petty HR, Elner VM (2011) Activation of P2X receptors induces apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci 52: 1522–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaspan BL, Williams DF, Holz FG, Regillo CD, Li Z, Dressen A, van Lookeren Campagne M, Le KN, Graham RR, Beres T et al (2017) Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age‐related macular degeneration. Sci Transl Med 9: eaaf1443 [DOI] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP (2010) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD et al (2015) The ketone metabolite beta‐hydroxybutyrate blocks NLRP3 inflammasome‐mediated inflammatory disease. Nat Med 21: 263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW (1967) The renewal of photoreceptor cell outer segments. J Cell Biol 33: 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF (2004) Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med 10: 1081–1087 [DOI] [PubMed] [Google Scholar]
- Yu CR, Mahdi RR, Oh HM, Amadi‐Obi A, Levy‐Clarke G, Burton J, Eseonu A, Lee Y, Chan CC, Egwuagu CE (2011) Suppressor of cytokine signaling‐1 (SOCS1) inhibits lymphocyte recruitment into the retina and protects SOCS1 transgenic rats and mice from ocular inflammation. Invest Ophthalmol Vis Sci 52: 6978–6986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Green W, Tso MM (2008) Microglial activation in human diabetic retinopathy. Arch Ophthalmol 126: 227–232 [DOI] [PubMed] [Google Scholar]
- Zhang J, Gerhardinger C, Lorenzi M (2002) Early complement activation and decreased levels of glycosylphosphatidylinositol‐anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes 51: 3499–3504 [DOI] [PubMed] [Google Scholar]
- Zhang C, Lei B, Lam TT, Yang F, Sinha D, Tso MO (2004) Neuroprotection of photoreceptors by minocycline in light‐induced retinal degeneration. Invest Ophthalmol Vis Sci 45: 2753–2759 [DOI] [PubMed] [Google Scholar]
- Zhang H, Ma Q, Zhang YW, Xu H (2012) Proteolytic processing of Alzheimer's beta‐amyloid precursor protein. J Neurochem 120(Suppl 1): 9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang JH, Chen XY, Hu QH, Wang MX, Jin R, Zhang QY, Wang W, Wang R, Kang LL et al (2015) Reactive oxygen species‐induced TXNIP drives fructose‐mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal 22: 848–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li Y, Payne J, Srivastava S, Fan X, Fung J, Li X, Kern TS, Lin F (2016) Presence of retinal pericyte‐reactive autoantibodies in diabetic retinopathy patients. Sci Rep 6: 20341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lv X, Hu Z, Ye X, Zheng X, Ding Y, Xie P, Liu Q (2017) Protection of Mcc950 against high‐glucose‐induced human retinal endothelial cell dysfunction. Cell Death Dis 8: e2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhao L, Wang X, Ma W, Lazere A, Qian H‐h, Zhang J, Abu‐Asab M, Fariss RN, Roger JE et al (2018) Repopulating retinal microglia restore endogenous organization and function under CX3CL1‐CX3CR1 regulation. Sci Adv 4: eaap8492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, Qian H, Parkhurst CN, Gan WB, Wong WT (2015a) Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med 7: 1179–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Bai Y, Xie W, Shi X, Li F, Yang F, Sun Y, Huang L, Li X (2015b) Interleukin‐1beta level is increased in vitreous of patients with neovascular age‐related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV). PLoS One 10: e0125150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Gao J, Van J, To E, Wang A, Cao S, Cui JZ, Guo JP, Lee M, McGeer PL et al (2015c) Age‐related increases in amyloid beta and membrane attack complex: evidence of inflammasome activation in the rodent eye. J Neuroinflammation 12: 121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J (2010a) Thioredoxin‐interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11: 136–140 [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J (2010b) A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221 [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang S, Xia X (2012) Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res 37: 416–420 [DOI] [PubMed] [Google Scholar]
- Zieger M, Ahnelt PK, Uhrin P (2014) CX3CL1 (fractalkine) protein expression in normal and degenerating mouse retina: in vivo studies. PLoS One 9: e106562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C (2009) Complement regulators and inhibitory proteins. Nat Rev Immunol 9: 729–740 [DOI] [PubMed] [Google Scholar]


