Abstract
Papillary renal cell carcinoma (P-RCC) is the second most common type of malignant renal epithelial tumor, and can be subclassified into type 1, which demonstrates simple cuboidal low-grade epithelium, and type 2, which demonstrates pseudostratified high grade epithelium with abundant eosinophilic cytoplasm. Despite this clinically useful subclassification, P-RCCs exhibit considerable histomorphologic diversity, with many cases having features differing from classically described type 1 and type 2 tumors. To our knowledge, there has been no recent study which has methodically evaluated the histomorphologic features of a series of P-RCCs. To address this, we evaluated a cohort of P-RCCs diagnosed between 1997 and 2004 with long term clinical follow-up data (n=56). Histomorphologic features previously described in the spectrum of type 1 and type 2 P-RCCs were recorded for each tumor, including nuclear grade, complete tumor capsule, and cytoplasmic eosinophilia, as well as several other features. The current TNM staging (AJCC 7th edition) was assigned to all cases. Histomorphologic features were diverse, demonstrating classic type 1 P-RCC and classic type 2 P-RCC morphology, and several tumors with non-classical features. Four patients in this cohort had distant metastasis. The primary tumor was equally divided between type 1 (2 cases) and type 2 (2 cases) morphology in the cases with metastasis. All P-RCC cases with metastases demonstrated presence of high nuclear grade and high tumor stage in the primary tumor. Cluster analysis using staging parameters and histomorphologic features divided tumors into two primary clusters. All primary tumors associated with metastasis were in the same cluster.
Keywords: Papillary renal cell carcinoma, tumor stage, nuclear grade
Introduction
Papillary renal cell carcinoma (P-RCC), when appropriately categorized, is a kidney tumor that has less aggressive clinical behavior than conventional clear cell renal cell carcinoma [1]. It was sub-classified into type 1 and type 2 tumors by Delahunt and Eble in 1997[2], a schema currently recognized by the World Health Organization (WHO)[3]. In this schema, type 1 P-RCCs demonstrate papillary structures lined by simple cuboidal epithelium, have foamy macrophages within papillary cores, and frequently have low grade nuclei. In contrast, type 2 P-RCCs demonstrate papillary structures lined by pseudostratified epithelium, have cells with abundant eosinophilic cytoplasm, and show large high grade nuclei. Foamy macrophages are rarely identified. Several studies have demonstrated that type 2 P-RCCs tumors metastasize and cause patient death more frequently than type 1 tumors[4–6].
However, other studies have highlighted that P-RCCs may have morphologic features differing from the classically described type 1 and type 2 tumors. For example, Kunju et al. and Lefevre et al., describe series of low-grade P-RCCs with simple cuboidal epithelium and abundant eosinophilic cytoplasm, which exhibit indolent clinical behavior[7,8]. In an outcome study of P-RCCs, Allory et al. found that subtyping was problematic in 35% of cases; 9% of cases were thus not classified as type 1 or type 2, but were instead designated as an oncocytoid type[6]. Furthermore, most studies recognize that type 1 P-RCCs may demonstrate high nuclear grade, despite being classically associated with low grade and relatively indolent behavior[1,4–6,9–11].
Hence, to expand our understanding of P-RCCs, we sought to fully characterize the morphologic features of a series of renal neoplasms diagnosed as P-RCC at our institution for which follow-up data was available. We evaluated a pure series of PRCCs for numerous histomorphologic features previously described as differing between tumors with type 1 and type 2 morphologies. We then evaluated the relationship between these features using cluster analysis, and evaluated which histomorphologic features were associated with poor outcome, determined as the presence of distant metastasis.
Materials and Methods
Case selection
This study was conducted with approval from the institutional review board of University of Michigan Hospital and Health Systems. A series of 67 tumors previously diagnosed as P-RCC between 1997 and 2004 was evaluated. All available slides from these cases were re-reviewed (blinded) to confirm the diagnosis of P-RCC by current diagnostic criteria; staging was assigned per the AJCC staging manual, 7th edition. Eleven tumors were excluded from this study because they did not fill current WHO diagnostic criterion for P-RCC (that is, cases demonstrating a papillary architecture but not fulfilling the WHO definition), giving 56 P-RCCs for evaluation[3,12]. Slides for histomorphologic review were available for all cases. Patient charts were reviewed for time of follow-up and presence of distant metastasis.
Evaluation of histomorphologic features
Multiple morphologic features were evaluated for each case. Since Fuhrman nuclear grading is widely understood and practically feasible, we used this grading schema to assess tumor grade. Cases were assigned Fuhrman nuclear grade according to the area demonstrating highest grade, as was originally described by Fuhrman et al. [13]. Percentage of cells demonstrating high Fuhrman nuclear grade (3 or 4) was assigned as 0%, 1–50%, or 51–100%, as has been previously described for clear cell renal cell carcinoma [14]. The recently described nucleolar scoring system by the International Society of Urological Pathology (ISUP) was also assigned for each tumor[15]. ISUP grading was performed on a separate occasion from Fuhrman grading, in a manner blinded to the originally assigned Fuhrman grade.
Tumor architecture was evaluated, with assignment of a specified architecture given to a case if any area in the tumor demonstrated the given architecture. Evaluated architecture types included classic (fibrovascular cores lined by tumor cells), tubular (canaliculi or tubule formations), and solid[16]. Presence of glomeruloid growth pattern as described previously [2] was evaluated in all cases. Cases were designated as cystic if the tumor resided in a prominent cystic space (of sufficient prominence to potentially be seen as a cystic mass on imaging or gross examination), or if dominant, large cysts were identified within the tumor.
Tumors were designated as type 2 P-RCC in cases demonstrating true cellular pseudostratification and high grade nuclei, as originally described[2]. Type 1 tumors were assigned in cases demonstrating simple cuboidal epithelium, irrespective of grade or other histomorphologic features. Cases were designated as having perinucleolar clearing if a small but distinct clear halo around the nucleolus was identified; this differs from the prominent clear halo surrounding a viral inclusion like nucleolus seen in hereditary leiomyomatosis and renal cell carcinoma syndrome (HLRCC) associated renal cell carcinoma.
Peripheral rimming was assigned to cases demonstrating a tumor capsule with a single layer of tumors cells on its inner lining, which appeared separate from the main tumor mass. Complete capsule was assigned if the tumor capsule was not breached by tumor, and no tumor infiltration beyond the tumor capsule was identified. Infiltration into renal parenchyma was designated if tumor was seen to involve renal parenchyma in an invasive fashion, which could not be completely accounted for by tangential sectioning. Cytoplasmic eosinophilia was assigned to cases demonstrating abundant eosinophilic cytoplasm, a finding also referred to as oncocytic. The presence and percentage of cells demonstrating eosinophilic cytoplasm were noted. Clear cytoplasm was assigned to cases with any area of tumor cells showing optically clear cytoplasm. Cases were designated as having edematous papillae if papillae were expanded widely by tumor edema. The presence of the following was also recorded: psammomatous calcification, foamy macrophages within papillae, hemosiderin laden macrophages, tumoral necrosis (and percentage of tumor demonstrating necrosis), tumor hyalinization, and presence of separate papillary adenomas.
Statistical analysis
Univariate analysis was performed to compare histomorphological features of tumors with metastatic disease to those without metastatic disease, using Fisher’s exact test for binary variables, and an exact nonparametric Wilcox test for continuous and ordinal variables. Univariate analysis was performed similarly to evaluate pathologic staging parameters between metastatic and non-metastatic cases. Multivariate analysis comparing outcome (metastasis vs. no metastasis) to histomorphological features and pathologic staging features was performed using a Cox proportional hazards model, fitted with one variable at a time. Best model selection was performed using Akaike information criterion (AIC) and the likelihood ratio test. Statistical analysis was performed using R version 3.0.1.
Cluster analysis was performed using histomorphological features and pathologic staging parameters. Agglomerative hierarchical clustering was used, utilizing Euclidean distance and complete linkage. Clustering and heatmap generation were done using the gplots package in the R programming language.
Results
Of the 67 cases identified, 56 were confirmed as P-RCC per current WHO diagnostic criteria, in 53 patients. Three patients each had two spatially distinct tumors. In two patients these were diagnosed at different time points, while in one patient both tumors were diagnosed at a single time point. There was a near equal distribution of patients who had undergone partial nephrectomy (52%; 29/56 patients) and radical nephrectomy (48%; 27/56). Median follow-up time was 44 months (interquartile range 96 months – lower 7 months, upper 103 months). Follow-up time of at least 36 months was available for 54% (30/56) of cases.
Type 1 P-RCC was the most common subtype, accounting for 80% (45/56) of cases. Type 2 P-RCC accounted for 18% (10/56), and mixed type 1 and type 2 P-RCC morphology was seen in a single case (2%; 1/56). For the sake of simplicity, this mixed case was treated as a type 2 P-RCC in all analyses. Areas of classic papillary architecture were identified in 84% (47/56), tubular architecture was identified in 57% (32/56), and solid architecture was identified in 50% of cases (28/56). Glomerulations were seen in 20% of cases (11/56).
All cases in this series demonstrated either Fuhrman grade 2 (50%, 28 cases) or grade 3 (50%, 28 cases) nuclei. Amongst the type 1 P-RCCs, 62% (28/45) demonstrated Fuhrman grade 2 nuclei, while the remaining 38% (17/45) demonstrated Fuhrman grade 3 nuclei (38%, 17/45). All type 2 P-RCCs were Fuhrman grade 3. Of all cases, 30% (17/56) demonstrated >50% of tumor cells with Fuhrman grade 3 nuclei, representing 60% (17/28) of tumors assigned Fuhrman nuclear grade 3. Using the more recently suggested ISUP grading schema[15], all cases demonstrated either ISUP grade 2 (55%, 31 cases) or ISUP grade 3 nuclei (45%, 25 cases). Amongst the type 1 P-RCCs, 56% (25/45) were ISUP grade 2. All type 2 P-RCCs were ISUP grade 3. Assigned Fuhrman and ISUP grades were the same in 80% of cases (45 cases). Seven cases assigned Fuhrman grade 2 were assigned ISUP grade 3, while four cases assigned Fuhrman grade 3 were assigned ISUP grade 2. A breakdown of histomorphological features of type 1 and type 2 tumors is presented in Table 1.
Table 1:
Histomorphologic features in type 1 and type 2 P-RCCs
| Type 1 (n=45) | Type 2 (n=11) | |
|---|---|---|
| High Fuhrman nuclear grade (grade 3) | 17 (38%) | 11 (100%) |
| Percent high Fuhrman nuclear grade | ||
| >50% | 7 (16%) | 10 (91%) |
| <50% | 10 (22%) | 1* (9%) |
| 0% | 28 (62%) | 0 |
| High ISUP grade (grade 3) | 20 (45%) | 11 (100%) |
| Eosinophilic cytoplasm | 14 (31%) | 11 (100%) |
| Percent eosinophilic cytoplasm in cases with eosinophilic cytoplasm– mean (range) | 55% (10–100%) | 61% (20–100%) |
| Psammoma bodies | 15 (33%) | 2 (18%) |
| Foamy macrophages | 40 (89%) | 5 (45%) |
| Hemosiderin laden macrophages | 27 (60%) | 9 (82%) |
| Peripheral rimming | 44 (98%) | 10 (91%) |
| Complete capsule | 41 (91%) | 9 (82%) |
| Perinucleolar clearing | 0 | 4 (36%) |
| Necrosis | 22 (49%) | 6 (55%) |
| Classic | 36 (80%) | 11 (100%) |
| Solid | 26 (58%) | 2 (18%) |
| Tubular | 28 (62%) | 4 (36%) |
| Glomerulations | 10 (22%) | 1 (9%) |
Mixed type 1 and type 2 P-RCC
Areas demonstrating abundant eosinophilic cytoplasm were present in 45% (25/56) of cases; mean percentage of cells with eosinophilic cytoplasm in such cases was 60% (range 10–100%). Fuhrman nuclear grade 3 areas strongly tended to have eosinophilic cytoplasm in both type 1 and type 2 tumors, as 75% of cases with Fuhrman grade 3 nuclei (21/28 cases) had areas with abundant eosinophilic cytoplasm, while 14% of cases with Fuhrman grade 2 nuclei (4/28 cases) had eosinophilic cytoplasm (p<0.01). Papillary subtype also associated with eosinophilic cytoplasm, as all type 2 PRCCs had areas with eosinophilic cytoplasm. In contrast, 32% (14/44) of type 1 PRCCs had areas with eosinophilic cytoplasm (p<0.001). Of these, 71% (10/14) had Fuhrman grade 3 nuclei.
Histomorphological and staging variables in tumors with and without distant metastasis are detailed in Table 2. All tumors with distant metastasis demonstrated >50% tumor cells with high Fuhrman nuclear grade and were pT3 or greater. While 50% (2/4) with distant metastasis had type 2 morphology, the remaining 50% (2/4) had type 1 morphology. Univariate analysis demonstrated a statistically significant association between metastasis and tumors showing >50% of tumor cells with high Fuhrman nuclear grade. Statistically significant associations with metastasis were not seen with papillary subtype, ISUP grade, or Fuhrman nuclear grade alone. Statistically significant associations were seen between distant metastasis and younger patient age, the absence of foamy macrophages, lack of peripheral rimming of capsule by tumor cells, and incomplete tumor capsule. Higher pathologic tumor stage was also associated with distant metastasis in a statistically significant manner, as were the factors that comprise pathologic tumor stage, including renal vein involvement, perirenal fat involvement, hilar fat involvement, and tumor size. Examples of P-RCCs with diverse morphologies are presented in Figure 1. Clinical and pathologic data on metastatic cases are presented in Table 3.
Table 2.
Histomorphologic features and pathologic staging parameters in non-metastatic and metastatic cases, univariate analysis.
| Variable | Non-metastatic cases; percent of cases with given variable or mean (SD) (n=52) | Metastatic cases; percent of cases with given variable or mean (SD) (n=4) | p-value |
|---|---|---|---|
| pT stage | |||
| pT1 | 81% | 0% | |
| pT2 | 13% | 0% | |
| pT3 | 6% | 75% | |
| pT4 | 0% | 25% | 2.99E-05 |
| Perinephric fat involvement | |||
| Yes | 4% | 50% | |
| No | 96% | 50% | 0.0222 |
| Renal vein involvement | |||
| Yes | 0% | 25% | |
| No | 100% | 75% | 1.44E-04 |
| Hilar fat involvement | |||
| Yes | 4% | 50% | |
| No | 96% | 50% | 0.0222 |
| Age at diagnosis, years – mean (SD) | 61.6 (12.4) | 51 (7.39) | 0.0415 |
| Gender | |||
| Female | 35% | 50% | |
| Male | 65% | 50% | 0.611 |
| Papillary Subtype | |||
| Type 1 | 81% | 50% | |
| Type 2 | 19% | 50% | 0.198 |
| Fuhrman nuclear grade | |||
| Grade 2 | 54% | 0% | |
| Grade 3 | 46% | 100% | 0.111 |
| Percent high grade (Fuhrman grade 3) | |||
| 0% | 54% | 0% | |
| <50% | 21% | 0% | |
| >50% | 25% | 100% | 0.00677 |
| ISUP grade | |||
| Grade 2 | 48% | 0% | |
| Grade 3 | 52% | 100% | 0.120 |
| pN Stage | |||
| pNX | 89% | 50% | |
| pN0 | 9% | 0% | |
| pN1 | 2% | 50% | 0.118 |
| Tumor size, cm – mean (SD) | 4.53 (2.95) | 9.95 (6.23) | 0.0176 |
| Psammoma bodies | |||
| Yes | 33% | 0% | |
| No | 67% | 100% | 0.303 |
| Foamy macrophages | |||
| Yes | 85% | 25% | |
| No | 15% | 75% | 0.0211 |
| Hemosiderin laden macrophages | |||
| Yes | 67% | 25% | |
| No | 33% | 75% | 0.125 |
| Peripheral rimming | |||
| Yes | 100% | 50% | |
| No | 0% | 50% | 0.0039 |
| Complete capsule | |||
| Yes | 94% | 25% | |
| No | 6% | 75% | 0.00276 |
| Necrosis | |||
| Yes | 48% | 75% | |
| No | 52% | 25% | 0.611 |
| Necrosis percent – mean (SD) | 9.87% (21.5) | 37.5% (33) | 0.0788 |
| Cystic architecture | |||
| Yes | 21% | 50% | |
| No | 79% | 50% | 0.227 |
| Classic architecture | |||
| Yes | 83% | 100% | |
| No | 17% | 0% | 1.0 |
| Solid architecture | |||
| Yes | 54% | 0% | |
| No | 46% | 100% | 0.111 |
| Tubular architecture | |||
| Yes | 60% | 25% | |
| No | 40% | 75% | 0.303 |
| Glomeruloid bodies | |||
| Yes | 21% | 0% | |
| No | 79% | 100% | 0.575 |
| Hyalinization | |||
| Yes | 25% | 0% | |
| No | 75% | 100% | 0.563 |
| Edematous papillae | |||
| Yes | 40% | 75% | |
| No | 60% | 25% | 0.303 |
| Eosinophilic cytoplasm | |||
| Yes | 42% | 75% | |
| No | 58% | 25% | 0.314 |
| Percentage of cell with eosinophilic cytoplasm – mean (SD) | 22.3% (35.6) | 67.5% (45.7) | 0.0744 |
| Clear cytoplasm | |||
| Yes | 60% | 50% | |
| No | 40% | 50% | 1.0 |
| Separate papillary adenomas | |||
| Yes | 14% | 0% | |
| No | 86% | 100% | 1.0 |
Figure 1:
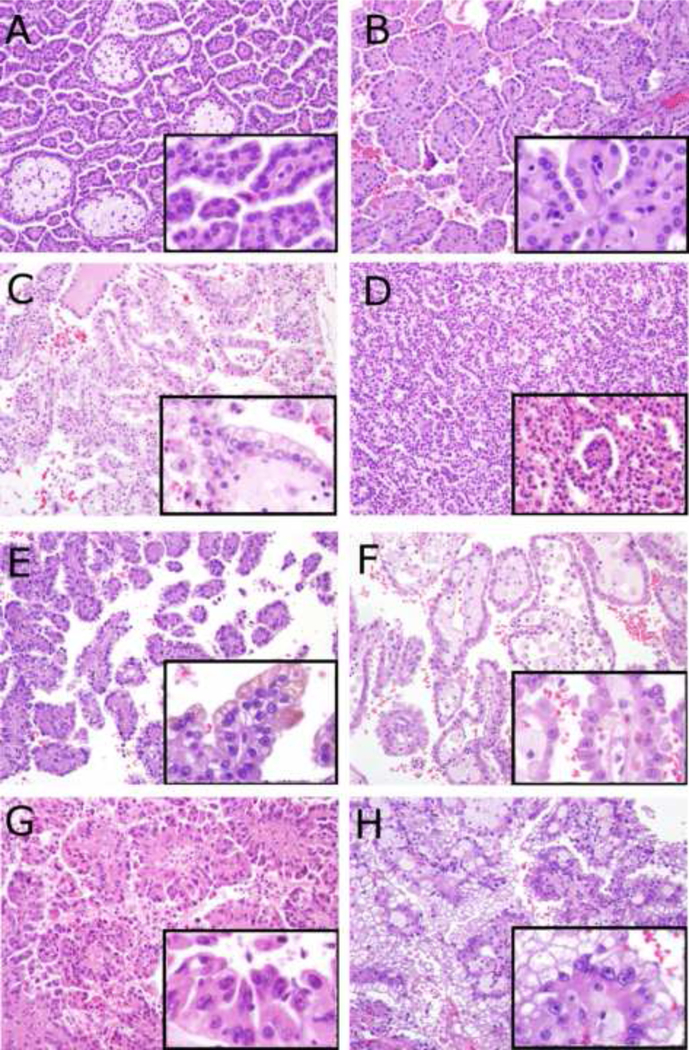
Microphotographs - larger photographs 200× magnification, smaller inset photographs 400× magnification of the same tumor. A – Classic type 1 papillary renal cell carcinoma with low grade (Fuhrman grade 2) nuclei, amphophilic cytoplasm, papillary architecture, and foamy macrophages within papillae. B – Type 1 papillary renal cell carcinoma with low grade (Fuhrman grade 2) nuclei and abundant eosinophilic cytoplasm. C – Type 1 papillary renal cell carcinoma with Fuhrman grade 2 nuclei and optically clear cytoplasm. D – Type 1 papillary renal cell carcinoma with Fuhrman grade 2 nuclei and glomerulations. E – Type 1 papillary renal cell carcinoma with higher grade (Fuhrman grade 3) nuclei. F – Type 1 papillary renal cell carcinoma with higher grade (Fuhrman grade 3) nuclei and abundant eosinophilic cytoplasm. G – Classic type 2 papillary renal cell carcinoma with higher grade (Fuhrman grade 3) nuclei, true pseudostratification, and abundant eosinophilic cytoplasm. H – Representative image from a Type 2 papillary renal cell carcinoma which demonstrated papillary areas with cells with eosinophilic cytoplasm (not shown in H) intermixed with other areas where the cytoplasm was more optically clear (which are demonstrated here).
Table 3.
Clinico-pathological features of tumors with metastatic disease.
| Age at diagnosis, years | Gender | Time to metastasis from initial diagnosis, months | Papillary subtype | >50% cells Fuhrman nuclear grade 3 | ISUP Grade | pT stage | pN stage | Eosinophilic cytoplasm | Complete capsule | Peripheral rimming | Site(s) of distant metastasis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 54 | Male | 5 | Type 1 | Yes | 3 | pT4 | pN1 | Yes | No | No | Bone-Humerus |
| 54 | Male | Metastasis present at time of at initial diagnosis | Type 2 | Yes | 3 | pT3 | pN1 | Yes | No | No | Lung |
| 56 | Female | 25 | Type 1 | Yes | 3 | pT3 | pNX | No | No | Yes | Bone - cervical vertebra |
| 40 | Female | Metastasis present at time of at initial diagnosis | Type 2 | Yes | 3 | pT3 | pNX | Yes | Yes | Yes | Liver, retroperitoneal lymph nodes, bone – lumbar vertebra |
Best model selection using AIC information criterion and the likelihood ratio test (borderline significance) left only one variable in the model (as the single most important), which was pathologic stage (pT; hazard ratio=6.5, p=0.02, Cox proportional hazard model).
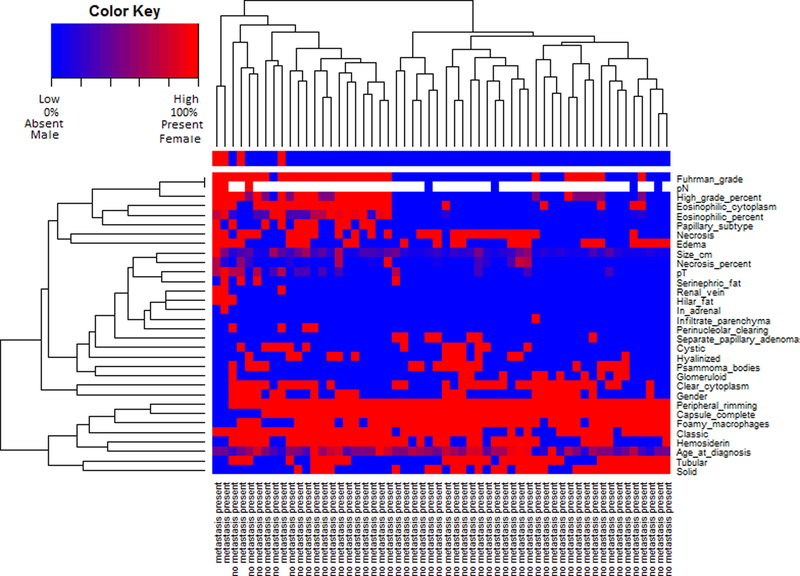
Cluster analysis using tumor staging and histomorphological variables is presented in Figure 2. Fuhrman nuclear grade, percentage of high grade cells, eosinophilic cytoplasm, papillary subtype, necrosis, papillae edema, and pN stage clustered together. Peripheral rimming, complete tumor capsule, foamy macrophages, classic tumor architecture, hemosiderin, tubular architecture, and solid architecture clustered together. Tumors divided into two broad clusters. The smaller cluster (left of Figure 2) was enriched in tumors with high Fuhrman nuclear grade, type 2 morphology, abundant eosinophilic cytoplasm, and pN1 disease. The great majority of tumors with stage pT3 or greater were in this cluster (86%, 6/7 cases). Tumors in this cluster tended to demonstrate classic architecture and have no areas with solid architecture. All four cases with distant metastases were in this cluster. The other, larger cluster (right of Figure 2) was enriched in tumors with low Fuhrman nuclear grade, type 1 morphology, peripheral rimming, complete tumor capsule, foamy macrophages, and areas with solid tumor architecture. Tumors in this cluster tended to lack eosinophilic cytoplasm, or show a small percentage of cell with eosinophilic cytoplasm if such areas were identified. These tumors also tended to be low stage, with only a single case with stage pT3. No case in this cluster was associated with distant metastasis.
Figure 2:
Heatmap including histomorphological features of papillary renal cell carcinomas and pathologic staging variables. For binary variables, red indicates the feature was present, and blue indicates the feature was absent. For percentage variables, red indicates 100%, blue indicates 0%, and varying shades of blue, red, and purple indicate percentage per the color key. For ordinal variables, red indicates the highest category, and blue indicates the lowest category, with varying color shades indicating higher category per the color key. For numeric variables, red indicates the highest value, blue indicates the lowest value, and varying shades indicate higher values per the color key. For gender, red indicates female and blue male. White indicates data for the variable was not available.
Discussion
In the current study, we performed an exhaustive and comprehensive assessment of several architectural and cytological features of P-RCCs previously described in this tumor type. As described in our results, P-RCCs exhibited a wide spectrum of morphologies with often overlapping features between type 1 and type 2 P-RCC. Our study also reflects observations from a pure cohort of P-RCCs, as we utilized strict WHO criteria for classifying these tumors and excluded tumors that had papillary architecture but did not fit the WHO definition of P-RCC[3,12].
Mancilla-Jimenez et al. were one of the first to recognize P-RCC as a distinct renal cell carcinoma (RCC) type with unique clinicopathological features[17]. In this original study, P-RCCs were found to have better survival, and were frequently lower pathologic tumor stage at the time of diagnosis, compared to non-papillary RCCs. Numerous later studies corroborated these findings, solidifying P-RCC as an RCC with relatively indolent behavior compared to conventional clear cell RCC [1,16,18–25]. Detailed morphologic descriptions later demonstrated histomorphologic features, such as nuclear grade, were predictive of behavior of P-RCCs[26]. Subtyping of P-RCCs into type 1 and type 2 tumors was subsequently proposed by Delahunt and Eble[2]. In their classic description, type 1 tumors consisted of papillae and tubular structures lined by small cells with pale cytoplasm and small oval nuclei with inconspicuous nucleoli; they frequently showed papillary edema, foamy macrophages in papillary cores, and psammoma bodies. In contrast, type 2 tumors consisted of papillae lined by large cells with abundant eosinophilic cytoplasm demonstrating pseudostratification. These had large spherical nuclei with prominent nucleoli, and uncommonly showed psammoma bodies, edematous papillae, or foamy macrophages in papillary cores. This original study showed type 2 tumors were more commonly high stage (pT3 and pT4) than type 1 tumors.
Despite these original descriptions, considerable morphologic heterogeneity has been described. For example, Kunju et al. describe a series of low grade P-RCCs with abundant eosinophilic cytoplasm and indolent clinical behavior[7]. Tumors in this study had foamy macrophages in papillae in the minority of cases. Allory et al. describe necrosis and psammoma bodies in both type 1 and type 2 P-RCCs[6]. High nuclear grade has also been well described in type 1 P-RCCs in many studies[1,4–6,10,11], in contrast to the low nuclear grade classically described. P-RCCs with optically clear cytoplasm[27] and solid growth pattern[28] have also been described. Furthermore, it has been shown that classic type 1 and type 2 morphologies may be seen in the same tumor[5]. Thus, although classification of tumors with classic features of either type 1 or type 2 P-RCCs is relatively straight forward, many cases show features differing from these classic descriptions, including cases with morphology overlapping between type 1 and type 2 tumors.
The prognostic value of P-RCC subtyping and Fuhrman nuclear grade has been well-studied but remains contentious. In a study by Delahunt et al. on a series of 66 P-RCCs with at least 5 years follow up, 38% of patients with type 1 P-RCC compared to 50% of patients with type 2 P-RCCs had died at last follow up [4]. Tumor subtype in this study demonstrated statistical significance on Cox multivariate regression for survival (including tumor stage), while Fuhrman nuclear grade did not. Allory et al. and Pignot el al. similarly showed papillary subtype was significantly associated with survival in studies involving 43 and 130 patients with P-RCC, respectively[5,6]. Similar to the study by Delahunt et al., both studies showed papillary subtype, but not Fuhrman nuclear grade, was predictive of survival on multivariate analysis.
In contrast to the above studies, others have demonstrated Fuhrman nuclear grade to be a more significant predictor of outcome than P-RCC subtype. In the largest study of a series of P-RCCs to date, Sukov et al. (n=395; including 252 type 1 and 143 type 2 tumors) demonstrated that while both Fuhrman nuclear grade and P-RCC subtype were predictive of death from RCC on univariate analysis (Fuhrman nuclear grade HR 4.0, p<0.001; P-RCC subtype HR 2.2, p=0.01), only Fuhrman nuclear grade retained statistical significance on multivariate analysis including tumor stage (Fuhrman nuclear grade HR 2.8, p=0.001; P-RCC subtype HR 0.86, p=0.66)[11]. Klatt et al. and Siki-Paotonu et al. similarly showed grade to be more predictive of outcome than subtype on multivariate analysis in large series[9,10].
The present study demonstrated that for P-RCC the presence of >50% of tumor cells showing high Fuhrman nuclear grade was predictive of tumor metastasis, while papillary subtype, Fuhrman nuclear grade alone, and ISUP grade alone were not predictive of metastasis. While others have shown that nuclear grade is predictive of tumor behavior, the present study is the first to our knowledge to show that the fraction of tumor cells with high grade nuclei is predictive of outcome in P-RCC. It has been shown in clear cell RCC that the percentage of cells showing high nuclear grade is predictive of survival[14]; our finding in P-RCC is thus precedented in another RCC subtype. While neither Fuhrman nuclear grade nor ISUP grade showed statistically significant associations with metastasis in the present study, both approached significance (p=0.11 and 0.12, respectively) using exact statistical tests required for studies, such as ours, with a low number of events (metastasis in our case). Thus, although the p values do not reach statistical significance, we do not consider our results to contradict previous studies showing the predictive value of nuclear grade, as statistical significance would very likely be attained with a larger cohort.
Cluster analysis of histomorphological variables is a novel contribution of the current study. The tumors clustered into two main groups. The first was enriched in tumors with features classically associated with type 2 P-RCC, namely high Fuhrman nuclear grade, abundant eosinophilic cytoplasm, and type 2 morphology, although numerous type 1 tumors were also identified in this cluster. The majority (86%) of tumors with stage pT3 or higher were in this cluster. All metastatic tumors were also identified in this cluster. The second cluster contained only type 1 P-RCCs, and likewise was enriched in tumors with features classically associated with type 1 tumors. Several tumors in this cluster had features classically associated with type 2 P-RCCs, however, including cytoplasmic eosinophilia and high Fuhrman nuclear grade. Only a single case with stage pT3 was in this cluster.
Although cluster analysis demonstrated trends in tumor morphology, tumors in our study demonstrated considerable histopathologic diversity. For example, we identified tumors with high Fuhrman nuclear grade, type 1 histology, and no eosinophilic cytoplasm. One such tumor metastasized to a cervical vertebra. We also identified an indolent tumor with low Fuhrman nuclear grade and 100% of cells with abundant eosinophilic cytoplasm. This tumor exhibited indolent behavior. Another tumor demonstrated complete encapsulation and peripheral rimming of capsule by tumor cells, yet also demonstrated high Fuhrman nuclear grade, type 2 morphology, and abundant eosinophilic cytoplasm. This RCC demonstrated renal vein involvement and metastasis to multiple different organs. Additional non-classical findings included tumors with type 2 morphology and areas of clear cytoplasm, and an indolent tumor with low grade nuclei, type 1 morphology, and 80% tumor necrosis. Common findings in all four tumors with distant metastasis in the current study were high grade (Fuhrman and ISUP grade 3), >50% of tumors cells with Fuhrman nuclear grade 3, and a pT3 or greater pathologic tumor stage.
In summary, the present study demonstrates P-RCCs encompass a broad histomorphological spectrum, and may demonstrate classic morphology of type 1, classic morphology of type 2 P-RCC, or highly diverse combinations of features described in either tumor type. Features classically associated with type 2 P-RCC tended to cluster together, as did features classically associated with type 1 P-RCC. Despite this histomorphological diversity, high pathologic tumor stage and high percentage of tumor cells with high Fuhrman nuclear grade were the greatest predictors of metastatic disease.
Acknowledgments
Funding sources:
This study required no direct funding. R.M. and A.M.C. are supported by the Prostate Cancer Foundation. A.M.C. is partly supported by the Alfred A. Taubman Institute, the American Cancer Society, the Howard Hughes Medical Institute, and a Doris Duke Charitable Foundation Clinical Scientist Award. AT is supported by CTSA grant 2UL1TR000433.
Footnotes
Disclosures: None
References
- 1.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–24. [DOI] [PubMed] [Google Scholar]
- 2.Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol Off J U S Can Acad Pathol Inc. 1997;10:537–44. [PubMed] [Google Scholar]
- 3.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs. IARC Press; 2004. [Google Scholar]
- 4.Delahunt B, Eble JN, McCredie MR, Bethwaite PB, Stewart JH, Bilous AM. Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol. 2001;32:590–5. [DOI] [PubMed] [Google Scholar]
- 5.Pignot G, Elie C, Conquy S, et al. Survival analysis of 130 patients with papillary renal cell carcinoma: prognostic utility of type 1 and type 2 subclassification. Urology. 2007;69:230–5. [DOI] [PubMed] [Google Scholar]
- 6.Allory Y, Ouazana D, Boucher E, Thiounn N, Vieillefond A. Papillary renal cell carcinoma. Prognostic value of morphological subtypes in a clinicopathologic study of 43 cases. Virchows Arch Int J Pathol. 2003;442:336–42. [DOI] [PubMed] [Google Scholar]
- 7.Kunju LP, Wojno K, W olf JS Jr, Cheng L, Shah RB. Papillary renal cell carcinoma with oncocytic cells and nonoverlapping low grade nuclei: expanding the morphologic spectrum with emphasis on clinicopathologic, immunohistochemical and molecular features. Hum Pathol. 2008;39:96–101. [DOI] [PubMed] [Google Scholar]
- 8.Lefèvre M, Couturier J, Sibony M, et al. Adult papillary renal tum or with oncocytic cells: clinicopathologic, immunohistochemical, and cytogenetic features of 10 cases. Am J Surg Pathol. 2005;29:1576–81. [DOI] [PubMed] [Google Scholar]
- 9.Klatte T, Anterasian C, Said JW, et al. Fuhrman grade provides higher prognostic accuracy than nucleolar grade for papillary renal cell carcinoma. J Urol. 2010;183:2143–7. [DOI] [PubMed] [Google Scholar]
- 10.Sika-Paotonu D, Bethwaite PB, McCredie MRE, William Jordan T, Delahunt B. Nucleolar grade but not Fuhrman grade is applicable to papillary renal cell carcinoma. Am J Surg Pathol. 2006;30:1091–6. [DOI] [PubMed] [Google Scholar]
- 11.Sukov WR, Lohse CM, Leibovich BC, Thompson RH, Cheville JC. Clinical and pathological features associated with prognosis in patients with papillary renal cell carcinoma. J Urol. 2012;187:54–9. [DOI] [PubMed] [Google Scholar]
- 12.Tickoo SK, Reuter VE. Differential diagnosis of renal tumors with papillary architecture. Adv Anat Pathol. 2011;18:120–32. [DOI] [PubMed] [Google Scholar]
- 13.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63. [DOI] [PubMed] [Google Scholar]
- 14.Serrano MF, Katz M, Yan Y, Kibel AS, Humphrey PA. Percentage of high-grade carcinoma as a prognostic indicator in patients with renal cell carcinoma. Cancer. 2008;113:477–83. [DOI] [PubMed] [Google Scholar]
- 15.Delahunt B, Cheville JC, Martignoni G, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37:1490–504. [DOI] [PubMed] [Google Scholar]
- 16.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tum or size. J Urol. 2003;170:2217–20. [DOI] [PubMed] [Google Scholar]
- 17.Mancilla-Jimenez R, Stanley RJ, Blath RA. Papillary renal cell carcinoma: a clinical, radiologic, and pathologic study of 34 cases. Cancer. 1976;38:2469–80. [DOI] [PubMed] [Google Scholar]
- 18.Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281–91. [DOI] [PubMed] [Google Scholar]
- 19.Bard RH, Lord B, Fromowitz F. Papillary adenocarcinoma of kidney. II. Radiographic and biologic characteristics. Urology. 1982;19:16–20. [DOI] [PubMed] [Google Scholar]
- 20.Beck SDW, Patel MI, Snyder ME, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11:71–7. [DOI] [PubMed] [Google Scholar]
- 21.Boczko S, Fromowitz FB, Bard RH. Papillary adenocarcinoma of kidney: a new perspective. Urology. 1979;14:491–5. [DOI] [PubMed] [Google Scholar]
- 22.Leibovich BC, Lohse CM, Crispen PL, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183:1309–15. [DOI] [PubMed] [Google Scholar]
- 23.Margulis V, Tamboli P, Matin SF, Swanson DA, Wood CG. Analysis of clinicopathologic predictors of oncologic outcome provides insight into the natural history of surgically managed papillary renal cell carcinoma. Cancer. 2008;112:1480–8. [DOI] [PubMed] [Google Scholar]
- 24.Mydlo JH, Bard RH. Analysis of papillary renal adenocarcinoma. Urology. 1987;30:529–34. [DOI] [PubMed] [Google Scholar]
- 25.Teloken PE, Thompson RH, Tickoo SK, et al. Prognostic impact of histological subtype on surgically treated localized renal cell carcinoma. J Urol. 2009;182:2132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, Schultz DS. Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am J Surg Pathol. 1997;21:621–35. [DOI] [PubMed] [Google Scholar]
- 27.Mai KT, Faraji H, Desantis D, Robertson SJ, Belanger EC, Levac J. Renal cell carcinoma with mixed features of papillary and clear cell cytomorphology: a fluorescent in situ hybridization study. Virchows Arch Int J Pathol. 2010;456:77–84. [DOI] [PubMed] [Google Scholar]
- 28.Renshaw AA, Zhang H, Corless CL, Fletcher JA, Pins MR. Solid variants of papillary (chromophil) renal cell carcinoma: clinicopathologic and genetic features. Am J Surg Pathol. 1997;21:1203–9. [DOI] [PubMed] [Google Scholar]