Abstract
Background & Aims:
Screening patients with cirrhosis for hepatocellular carcinoma (HCC) has been recommended. We conducted a matched case–control study within the US Veterans Affairs (VA) healthcare system to determine whether screening by abdominal ultrasonography (USS) and/or by measuring serum level of alpha fetoprotein (AFP) is associated with reduced cancer-related mortality in patients with cirrhosis.
Methods:
We defined cases (n=238) as patients with cirrhosis who died of HCC from January 1, 2013 through August 31, 2015 and had been in VA care with a diagnosis of cirrhosis for ≥4 years prior to the diagnosis of HCC. We matched each case to 1 control (n=238), defined as a patient with cirrhosis who did not die of HCC and had been in VA care for ≥4 years prior to the date of his matched case’s HCC diagnosis. Controls were matched to cases by year of cirrhosis diagnosis, race/ethnicity, age, sex, etiology of cirrhosis, MELD score, and VA medical center. We identified all USS and serum AFP tests performed within 4 years before the date of HCC diagnosis in cases or the equivalent index date in controls, and determined via chart extraction (blinded to case or control status) whether these tests were performed for screening.
Results:
There were no significant differences between cases and controls in the proportions of patients who underwent screening USS (52.9% vs 54.2%), screening measurement of serum AFP (74.8% vs 73.5%), either a screening USS or measurement of serum AFP (81.1% vs 79.4%), or both (46.6% vs 48.3%) within 4 years before the index date, with or without adjusting for potential confounders. There was also no difference in receipt of these screening tests within 1, 2, or 3 years prior to the index date.
Conclusions:
In a matched case–control study of the VA healthcare system, we found that screening patients with cirrhosis for HCC by USS, measurement of serum AFP, either test, or both was not associated with decreased HCC-related mortality. We encourage additional case–control studies to evaluate the efficacy of screening for HCC in other healthcare systems, in which available records are sufficiently detailed to enable identification of the indication for USS and AFP tests.
Keywords: Surveillance, Survival, liver cancer, liver transplantation
Graphical abstract
Introduction
Patients with cirrhosis have a high risk of hepatocellular carcinoma (HCC), ranging from 1% to 8% per year1. Most professional liver societies recommend screening cirrhotic patients with abdominal ultrasonography (USS) with or without concomitant serum alpha fetoprotein testing every 6 months2–4, but many non-liver societies do not endorse HCC screening5, 6. The rationale for HCC screening in patients with cirrhosis is that screening tests such as USS or serum AFP may identify patients with HCC at an early stage when they have potentially curative or life-prolonging treatment options including liver transplantation, radiofrequency ablation or surgical resection. However, it remains unclear whether HCC screening reduces cancer-related mortality in patients with cirrhosis, which should be the primary endpoint of HCC screening – rather than early stage migration or increased frequency of receipt of potentially curative treatments.
Two randomized controlled trials of HCC screening have been performed7, 8. However, these trials reached conflicting conclusions about screening effectiveness, and their methodology has been criticized9. Also, their results do not necessarily apply to North American and European patients with cirrhosis in the current era, because the trials were conducted in China from 1989 to 1997 among patients with chronic hepatitis B virus (HBV) infection. HBV-related HCC can occur in the absence of cirrhosis and important advances in the treatment of HCC have taken place since these studies were conducted.
Many observational studies have been performed comparing survival in patients diagnosed with HCC via screening to those who presented with symptomatic HCC. These studies were summarized in two systematic reviews10, 11 which concluded that the interpretation of these observational studies was limited due to selection, verification, leadtime and length-time biases.
Ideally, the effectiveness of HCC screening would be evaluated by a study that randomizes patients with cirrhosis to screening versus no screening. However, as concluded by the authors of the American Association for the Study of Liver Diseases (AASLD) HCC guidelines12 and demonstrated by problems in patient recruitment encountered in a pilot study13, it is unlikely that such randomized trials of HCC screening will be feasible in the United States (US), where HCC screening has become the de facto standard of care. Nonetheless, concerns have been raised that HCC surveillance has been adopted in the US without sufficient data to demonstrate its efficacy.14, 15
As an alternative to randomized controlled trials (RCT), case-control studies have the potential to evaluate the effectiveness of cancer screening in an efficient manner16–18. To test for an effect of screening on cancer-related mortality, previous receipt of the screening test (e.g. abdominal USS or serum AFP testing) is compared in patients with cirrhosis who died of HCC (cases) and in a matched sample of patients with cirrhosis who did not die of HCC (controls). A lower likelihood of screening prior to diagnosis during the time when the malignancy is occult but potentially detectable by means of the screening modality among those who died of cancer would provide evidence in support of a protective effect of screening on mortality. Thus, if HCC screening were effective, we would expect patients who died of HCC to be less likely to have been screened than patients with cirrhosis who have not died of HCC. By selecting patients with fatal, rather than incident, cancers as the case subjects, this case-control paradigm addresses the impact of screening on cancer-related mortality and is not susceptible to length-time or lead-time bias. The odds ratio in a bias-free casecontrol study of screening would be a valid estimate of the risk ratio that might be obtained from a RCT16.
The case-control study design has been used previously to evaluate screening effectiveness for malignancies other than HCC, such as colorectal cancer,19, 20 breast cancer,21 esophageal cancer,22 cervical cancer,23 prostate cancer,24 and melanoma25. We performed a matched case-control study to evaluate the extent to which screening for HCC with USS or serum AFP is associated with reduced HCC-related mortality among cirrhotic patients in the US Veterans Affairs (VA) healthcare system, the largest integrated healthcare system in the US.
Methods
Overall Study Design: matched case-control study of screening effectiveness.
We defined cases as VA patients with cirrhosis who died of HCC between 01/01/2013 and 8/31/2015 and had at least four years of follow-up time enrolled in the VA from the date of cirrhosis diagnosis to the date of HCC diagnosis. We matched each case to one control, defined as a VA patient with cirrhosis who did not die of HCC, was not diagnosed with HCC as of the date of their case’s HCC diagnosis and was in VA care at least four years prior to the date of his matched case’s HCC diagnosis (Figure 1). Cases were compared to controls with respect to abdominal USS or serum AFP tests performed for HCC screening during the four years prior to the diagnosis of HCC in the cases or the equivalent index date in the matched controls. A smaller proportion of cases than controls receiving HCC surveillance would suggest an association between HCC surveillance and decreased HCC-related mortality.
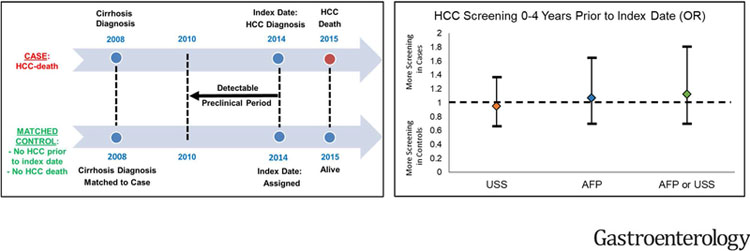
Figure 1. Schematic representation of the case-control study design, illustrating the criteria used to match cases (fatal HCC) to controls, the index date and detectable preclinical phase (DPP).
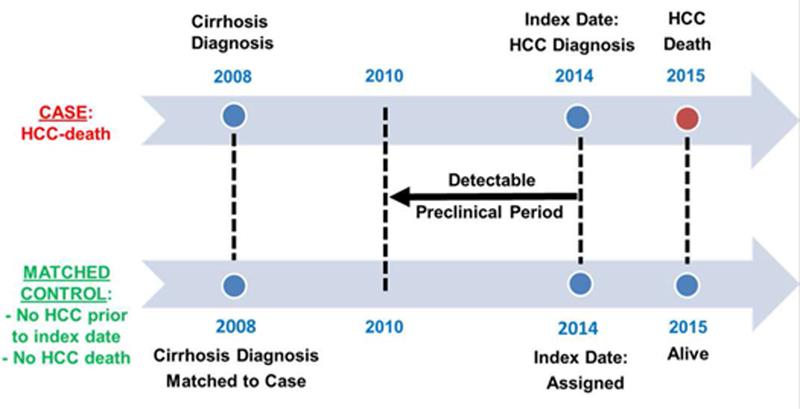
The DPP comprised an identical period of calendar years for the case and control within each matched pair (e.g. 2010–2014 in the example below), during which both case and control were in VA care at the same VA facility
* Index Date: Date of HCC diagnosis or earliest date that patients showed symptoms, laboratory abnormalities or imaging findings suspicious for HCC.
† DPP (detectable preclinical phase), is the time period prior to the index date during which we documented the occurrence of screening ultrasonography or serum AFP
ǂ Matching by age, gender, race, etiology of cirrhosis, MELD score at the time of cirrhosis diagnosis, date of cirrhosis diagnosis and VA facility
Data sources: the national VA Corporate Data Warehouse (CDW) and Medical Chart Extraction.
The VA uses a single, nationwide, comprehensive electronic healthcare information network. Data from this network reside on the Corporate Data Warehouse (CDW), a national, continually-updated data repository developed specifically to facilitate research26. We extracted data on all pharmacy prescriptions, demographics, inpatient and outpatient visits, problem lists, procedures, vital signs, diagnostic tests, and laboratory tests for patients with a diagnosis of cirrhosis who were in VA care in or prior to 2015. These CDW data were used only to identify potential cases and controls for this study. Once potential cases and controls were identified from the CDW, their electronic medical records were accessed using the Compensation and Pension Record Interchange (CAPRI), an electronic interface providing online access to Veterans’ medical records at all VA facilities in the country. CAPRI was used to obtain radiology reports, pathology reports, and inpatient and outpatient progress notes. These detailed records were electronically copied onto a specifically designed REDCap27 database. The extracted records were then reviewed by a physician-investigator blinded as to case-control status.
Identification of Cases: Patients with Fatal HCC
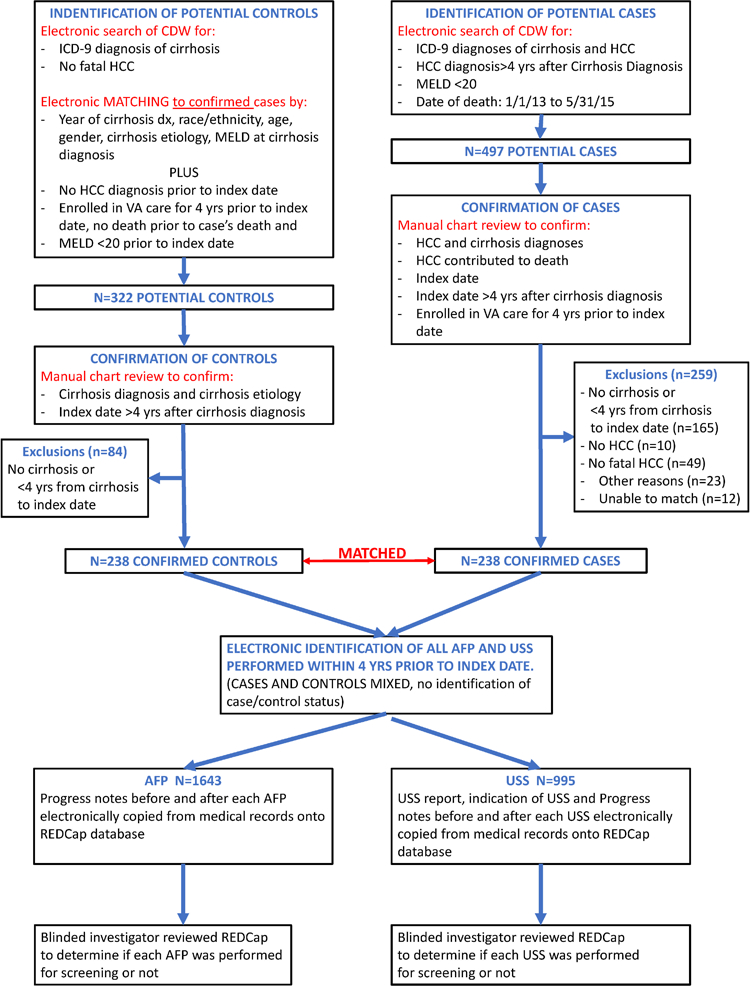
Identification and confirmation of cases was a two-step process (Figure 2). First, potential cases were identified electronically from CDW as patients with a diagnosis of cirrhosis based on appropriate International Classification of Diseases, 9th edition (ICD-9) codes (Supplemental Table 1) recorded at least twice28–34 who were diagnosed with HCC at least 4 years after the diagnosis of cirrhosis, died between 01/01/2013 and 5/31/2015, and had Model for End Stage Liver Disease (MELD) score<20 at all times prior to HCC diagnosis. We used the presence of ICD-9 code 155.0 (primary liver cancer) recorded at least twice for this preliminary identification of HCC, as in previous studies.30, 31, 34–38 A four-year period was chosen to allow enough time for screening to plausibly have an influence on HCC-related mortality. The time interval 2013–2015 was selected because it was the most recent at the time the study was initiated, such that the most “current” treatments would be available to patients diagnosed with HCC. Patients with MELD ≥20 were excluded because screening is not recommended in patients with advanced liver dysfunction (unless they are listed for liver transplantation). Including such patients might have biased the results in the direction of not finding an association between screening and reduced cancer-related mortality. Second, the medical records of potential cases were accessed at all VA facilities nationally through CAPRI by a physician-investigator blinded to screening status, in order to confirm the diagnosis of cirrhosis and HCC, identify the patients in whom HCC contributed to the patient’s death, and determine the index date. The diagnosis of cirrhosis was based on clinical features of portal hypertension due to liver disease (ascites, hepatic encephalopathy, varices), characteristic laboratory features (e.g. decreased platelets, prolonged prothrombin time, increased serum bilirubin, hypoalbuminemia), imaging characteristics (e.g. nodular liver, portosystemic collaterals), liver biopsy, and/or diagnosis documented by a gastroenterologist or hepatologist. The diagnosis of HCC was defined by the following national AASLD criteria that were in effect at the time our study was conducted12, 39: 1. Liver nodules ≥10 mm which were hypervascular in the arterial phase with washout in the portal venous or delayed phase in either 4-phase multidetector computerized tomography (CT) scan or dynamic contrast enhanced magnetic resonance imaging (MRI); 2. Liver nodules that fulfilled Liver Imaging Reporting and Data System (LIRADS) 5 criteria or 3. Liver lesions with histology consistent with HCC on biopsy.
Figure 2. Flow chart demonstrating the identification/confirmation of cases, identification/confirmation of controls and matching to cases, and identification of USS and serum AFP tests performed prior to the index date.
For patients confirmed to have HCC, the physician-investigator determined whether HCC definitely contributed to the patient’s death, which was defined as presence of metastatic HCC, multifocal HCC (>3 lesions), local or vascular invasion by HCC, large volume HCC (>6 cm), serum alpha fetoprotein (AFP) >1000, or death due to complications from HCC treatment in patients who did not have an obvious alternative cause of mortality. Only patients confirmed to have HCC in whom HCC “definitely” contributed to death were included as cases in the study.
The diagnostic definition of HCC and the criteria used to define that HCC contributed to the patient’s death were determined and validated prior to study initiation by a pilot study of a different set of 50 cases of fatal HCC reviewed independently by two of the investigators. There was excellent inter-rater agreement (97.5% agreement, kappa 0.94, p<0.001) between two investigators in assigning whether HCC definitely contributed to the patient’s death using the above criteria.
Identification of Matched Controls.
Identification and confirmation of matched controls was a two-step process (Figure 2). First, we electronically identified from CDW all patients with a diagnosis of cirrhosis28–34, defined using the same ICD-9 codes as for cases, who did not die of HCC and were not diagnosed with HCC prior to their matched case’s index date. We matched one control to each case by the following characteristics, which are strongly associated with both fatal HCC and the likelihood of screening: 1. Year of cirrhosis diagnosis; 2. Race/Ethnicity (categorized as White non-Hispanic, Black non-Hispanic, Hispanic, Other); 3. Age (within 2 years); 4. Gender; 5. Primary etiology of cirrhosis [hepatitis C (HCV), alcoholic liver disease, nonalcoholic fatty liver disease or other as we previously published40 – see Supplemental Table 2 for definitions]; 6. MELD score at the time of cirrhosis diagnosis (within 2 points); and 7. VA facility in which the diagnosis of cirrhosis was made. Controls had to be enrolled in VA care for the four years prior to the index date and alive at the time of their matched case’s death. Controls who had a MELD score ≥20 at any time prior to the index date of their matched case were excluded (just as were the cases). Second, the medical records of potential controls were accessed at all VA facilities nationally through CAPRI by a physician-investigator who was blinded to screening status in order to confirm the diagnosis and etiology of cirrhosis.
Definition of the Index Date.
The index date for cases was defined as: the date of HCC diagnosis (i.e. the earliest date of a multiphasic CT/MRI or tissue biopsy diagnostic for HCC); or the earliest date that patients reported symptoms (e.g. weight loss, abdominal pain), imaging findings (e.g. suspicious liver nodule on screening USS), or laboratory abnormalities suspicious for HCC (e.g. elevated serum AFP), whichever came first. The index date was determined for each case by review of the medical records by a physician-investigator. By definition, an ultrasound scan or serum AFP test performed after the index date could not have been a screening test. For example, if a screening USS showed a suspicious liver nodule and a serum AFP was subsequently ordered, the index date was the date of the USS and the serum AFP was not a screening test. Each control was assigned the same index date as his or her matched case. Therefore, for each case-control pair we evaluated an identical calendar period prior to the index date for presence of screening USS or AFP (Figure 1). Cases or controls with index dates occurring less than 4 years after the diagnosis of cirrhosis were excluded, since this would not have allowed us to examine screening histories for the full duration of a maximum hypothesized 4-year detectable preclinical phase of HCC.
Determination of screening USS and serum AFP in cases and controls
Each abdominal USS performed within 4 years prior to the index date was identified electronically by an analyst blinded to case/control status. The ultrasound report (which included the recorded indication for performing the ultrasound), and the ordering provider’s progress notes before and after the ultrasound, were electronically copied from the medical records onto a REDCap database by a trained research assistant as a separate record for each USS. A physician-investigator blinded to case-control status reviewed this information on REDCap and categorized each USS as having been performed “definitely” for screening, “probably” for screening, “probably not” for screening or “definitely not” for screening. The definitions of these categories are shown in Supplemental Table 3.
This process was separately performed for each serum AFP test obtained within 4 years prior to the index date. The progress notes of the ordering provider before and after the AFP result were copied from the electronic medical records and a blinded physician-investigator categorized each AFP as having been performed “definitely”, “probably”, “probably not”, or “definitely not” for screening using the criteria shown in Supplemental Table 4.
The principal investigator additionally reviewed any records that were difficult to categorize by the physician-investigator, as well as a random 10% sample of all records.
The criteria for adjudicating the screening status of USS and serum AFPs were determined by an independent chart extraction by two investigators of a different set of 50 cases and 50 controls before the study was initiated. There was excellent agreement between the two investigators for the criteria used in the study (94.2% agreement, kappa 0.90, p<0.001).
Our primary analysis considered only USS or serum AFP tests performed “definitely” for screening, but a sensitivity analysis also included those performed “probably” for screening.
Statistical Analysis
Cases were compared to their matched controls with respect to receipt of abdominal USS or serum AFP performed for screening within 0–1, 0–2, 0–3, or 0–4 years prior to the index date modeled as binary (yes/no) variables, using conditional logistic regression. This period, the detectable preclinical phase (DPP), is the period from the earliest time at which the cancer is potentially detectable using the screening modality under study to the time at which the cancer would present clinically in the absence of screening. Sheu et al. estimated the DPP for HCC by estimating the time it would take for HCCs to grow from 1cm (the minimum size potentially detectable by USS) to 10cm (a size generally expected to cause symptoms), as 3.2 years for tumors with a median growth rate, which had a doubling time of 117 days41. Based on this, we chose 4 years as the upper limit of the DPP, that is, we estimated that a small HCC that could be detectable now by USS will take a maximum of 4 years before presenting with clinical symptoms41. The maximal DPP is thought to provide the least biased estimate of any true association between receipt of screening and reduced cancerrelated mortality42. However, it has also been shown that when different periods are analyzed yielding different odds ratios, the lowest odds ratio (i.e. the one which indicates the greatest survival benefit for screening) is likely to be the least biased42. For these reasons, we analyze screening tests performed within 4 years prior to the index date (i.e. close to the estimated maximal DPP) as our primary analysis, but also analyzed screening tests performed within 1, 2 or 3 years before the index date. We did not analyze 6 months prior to index date, as this short interval would be heavily biased towards showing a higher rate of screening for the cases than the controls (i.e. erroneously making it appear as if screened patients are more likely to die of HCC).
Cases and controls were not compared with respect to the number of screening tests during the DPP, because even in the absence of effective therapy of screen-detected cancers, the cases would be expected to have been screened fewer times than the controls, assuming that the screening test is sensitive in identifying the tumor, producing a spuriously low odds ratio associated with multiple (or “regular”) screening17, 18. If a case with occult liver cancer undergoes a screening test, the cancer may be identified and a second (or third) test will never take place. However, controls (the large majority of whom do not have liver cancer) have the capacity to be screened more than once during the time interval under consideration.
Conditional logistic regression models were adjusted for age, etiology of cirrhosis (HCV, NAFLD, ALD and Other), MELD score at cirrhosis diagnosis, race/ethnicity, year of cirrhosis diagnosis, diabetes, alcohol use disorders, body mass index, eradication of HCV by antiviral treatment and receipt of abdominal computed tomography (CT) or magnetic resonance imaging (MRI) during the period of interest. Models that evaluated the effectiveness of screening serum AFP were additionally adjusted for receipt of screening USS while models that evaluated the effectiveness of screening USS were additionally adjusted for receipt of screening serum AFP.
We evaluated the following binary screening variables in different conditional logistic regression models:
Screening USS versus no screening USS
Screening serum AFP versus no screening AFP
Screening USS or serum AFP versus no screening with either USS or serum AFP.
Screening with both USS and serum AFP versus screening with only USS
Screening with both USS and serum AFP versus screening with only AFP
Screening with both USS and serum AFP versus screening with none
Power Calculations
Extrapolating from a prior VA study34, we estimated that the proportion of controls with a screening serum AFP or a screening USS during a 4-year period in our study would be approximately 70%. Using the method of Dupont specifically for power calculations in matched case-control studies43, we calculated a priori that 238 cases matched to 238 controls would provide more than 90% power to detect a 14% difference in screening between cases and controls and more than 80% power to detect a 12% difference between cases and controls (e.g. 70% screening for controls and 58% screening for cases).
Results
Aiming for a sample size of 238 pairs of cases and matched controls, we initially identified electronically a random sample of 600 potential cases and 1800 potential matched controls. After reviewing the charts of 497 out of these 600 potential cases in random sequence, we excluded 10 patients who did not have HCC, 49 patients in whom HCC did not definitely contribute to patient death, 165 patients who did not have cirrhosis or had less than 4 years’ interval between the diagnosis of cirrhosis and the index date, 23 patients for other reasons (care elsewhere [n=4], insufficient documentation [n=8], unclear cause of death [n=8], unclear HCC diagnosis [n=3]) and 12 patients who could not be matched to a control that fulfilled all matching criteria, leaving 238 cases in the current analysis (Figure 2). After reviewing the charts of 322 potential controls electronically matched to these cases, we excluded 84 who did not have cirrhosis or had less than 4 years’ interval between the diagnosis of cirrhosis and the index date leaving 238 controls in the current analysis, each matched to a single case.
Characteristics of Cases and Controls
As expected by the matching scheme, cases and controls were the same in terms of their age at diagnosis of cirrhosis (54.6 years), age at index date (62.5 years), racial/ethnic distribution, year of cirrhosis diagnosis, year of index date, time interval between cirrhosis diagnosis and index date, MELD score at the time of cirrhosis diagnosis and primary etiology of cirrhosis (Table 1). All cases and controls were men, reflecting the predominantly male VA population (by chance no women met all the inclusion criteria for cases). In most patients, the primary etiology of cirrhosis was HCV infection (80%) or alcoholic liver disease (13%). A majority of the patients were white (73.5%) followed by Black (15.1%) and Hispanic (10.1%) race/ethnicity. Patients had a mean MELD score of 9 at the time of cirrhosis diagnosis. HCV infection had been cured by antiviral treatment prior to the index date in 13.7% of controls and 8.4% of cases.
Table 1.
Characteristics of cases and their matched controls
| Controls N=238 |
Cases N=238 |
|
|---|---|---|
| Male, % | 100 | 100 |
| Age at diagnosis of cirrhosis, mean (yrs) | 54.5 | 54.6 |
| Age at index date, mean (yrs) | 61.9 | 62.0 |
| Year of cirrhosis diagnosis, % | ||
| Prior to 2003 | 42 | 42 |
| 2003–2005 | 27 | 27 |
| 2006–2008 | 27 | 27 |
| 2009–2011 | 5 | 5 |
| Time interval between diagnosis of cirrhosis and index date, yrs | 7.9 | 7.9 |
| Index Date Year, % | ||
| <=2012 | 63 | 63 |
| 2013 | 24 | 24 |
| 2014 | 11 | 11 |
| 2015 | 2.1 | 2.1 |
| Race/Ethnicity, % | ||
| White, non-Hispanic | 74 | 74 |
| Black, non-Hispanic | 15 | 15 |
| Hispanic | 10 | 10 |
| Other | 1.3 | 1.3 |
| Primary etiology of liver disease, % | ||
| Hepatitis C Virus | 80 | 80 |
| Alcoholic Liver Disease | 13 | 13 |
| Nonalcoholic Fatty Liver Disease | 2.9 | 2.9 |
| Other | 4.2 | 4.2 |
|
Sustained virologic response to HCV achieved prior to index date
(among those with HCV), % |
14 | 8.4 |
| BMI, mean (Kg/m2) | 29 | 29 |
| MELD Score, mean | 9.1 | 9.0 |
| Diabetes, % | 23 | 23 |
| Alcohol Use Disorders, % | 48 | 61 |
| CT or MRI prior to index date, % | ||
| 0–2 yrs prior to index date | 44 | 55 |
| 0–3 yrs prior to index date | 53 | 62 |
| 0–4 yrs prior to index date | 62 | 71 |
Characteristics of HCC among the cases
In the majority of cases, HCC was diagnosed by appropriate multiphasic CT or MRI (85.6%), while 28.8% had histological diagnosis (Table 2). At the time of diagnosis, 16.0% had vascular invasion, 8.0% had metastatic disease and 51.3% were within Milan criteria. A large proportion of patients received locoregional treatments, including 42.4% trans-arterial chemoembolization and 12.7% radiofrequency ablation while 28.4% were treated with sorafenib and only 2.1% underwent surgical resection. The criteria that were used to determine that the presence of HCC contributed to the patient’s death most commonly included large volume HCC (45.0%), multifocal HCC (32.4%), local or vascular invasion (26.9%) or metastasis (20.6%).
Table 2. Characteristics of HCC among cases (fatal HCC).
| Cases N (%) |
|
|---|---|
| Method of HCC diagnosis* | |
| Imaging (CT/MRI) | 204(86) |
| Histology | 69(29) |
| Stage of HCC at Diagnosis | |
| Maximum dimension of largest tumor(cm), mean(SD) |
4.5(3.4) |
| Number of tumors, mean(SD) | 2.1(1.6) |
| Number of tumors (%) | |
| 1 | 125(53) |
| 2–3 | 72(30) |
| ≥4 | 41(17) |
| Size of largest tumor (%) | |
| 0–3 cm | 92(39) |
| 3 to <5 cm | 75(32) |
| 5 to <6 cm | 16(6.7) |
| 6 to <7 cm | 15(6.3) |
| ≥7 cm | 40(17) |
| Within Milan Criteria (%)† | 122(51) |
| Beyond Milan Criteria (%) | 116(49) |
| Vascular Invasion, % | 38(16) |
| Metastasis, % | 19(8) |
| Treatment of HCC* | |
| Liver transplantation | 0(0.0) |
| Surgery (partial hepatectomy) | 5(2.1) |
| Systemic chemotherapy (sorafenib) | 69(29) |
| Trans-arterial chemoembolization | 101(42) |
| Radiofrequency ablation | 30(13) |
| Y-90 radioembolization | 7(2.9) |
| Percutaneous ethanol injection | 3(1.3) |
| Cryoablation | 1(0.4) |
| Other Treatment | 11(4.6) |
| Any one of the above treatments | 159(67) |
| HCC Contributed to patient’s death* | |
| Metastatic HCC | 49(21) |
| Multifocal HCC (>3 lesions) | 77(32) |
| Local or vascular invasion by HCC | 64(27) |
| Large Volume HCC (>6cm or AFP>1000) |
107(45) |
| Death due to complications of HCC treatment |
6(2.5) |
The categories for “method of HCC diagnosis”, “treatment of HCC” and “HCC contributed to patient’s death” are NOT mutually exclusive.
Milan Criteria: One tumor <5 cm or 2–3 tumors each of which is < 3cm
Association Between Screening and HCC-related Mortality
During the 4-year period prior to the index date, cases underwent 492 USS and 795 serum AFP tests (including 284 and 635, respectively, performed “definitely for screening”) while controls underwent a similar number of 503 USS and 848 serum AFP tests (including 287 and 641, respectively, performed “definitely for screening”)- Table 3.
Table 3.
Distribution of ultrasound scans and serum AFP tests during the 0–4 years prior to index date
| Controls | Cases | |
|---|---|---|
| USS | ||
| All USS | 503 | 492 |
| Definitely screening | 287(57.1%) | 284 (57.7%) |
| Probably screening | 6 (1.1%) | 8 (1.6%) |
| Probably not screening | 4 (0.8%) | 2 (0.4%) |
| Definitely not screening | 206 (41.0%) | 198 (40.2%) |
| AFP | ||
| All AFP | 848 | 795 |
| Definitely screening | 641 (75.6%) | 635 (79.9%) |
| Probably screening | 10 (1.2%) | 2 (0.3%) |
| Probably not screening | 0 (0.0%) | 0 (0.0%) |
| Definitely not screening | 197 (23.2%) | 158 (19.9%) |
There was no difference between cases and controls in the proportion who underwent screening USS (52.9% vs 54.2%, odds ratio (OR) 0.95, 95% CI 0.66–1.37), screening serum AFP (74.8% vs 73.5%, OR 1.07, 95% CI 0.70–1.65), or either screening USS or AFP (81.1% vs 79.4%, OR 1.12, 95% CI 0.70–1.81) within 4 years prior to the index date (Table 4).
Table 4.
Comparison of cases and controls with respect to occurrence of screening† ultrasound (USS), screening AFP or either USS or AFP at given time intervals prior to the index date.
| Controls N=238 n (%) |
Cases N=238 n (%) |
Odds Ratio* (95% CI) |
Adjusted** Odds Ratio (95% CI) |
|
|---|---|---|---|---|
|
0–4 years prior to
index date |
||||
| USS | 129 (54.2%) | 126 (52.9%) | 0.95 (0.66–1.37) | 0.95 (0.63–1.43) |
| AFP | 175 (73.5%) | 178 (74.8%) | 1.07 (0.70–1.65) | 1.08 (0.67–1.75) |
| USS or AFP | 189 (79.4%) | 193 (81.1%) | 1.12 (0.70–1.81) | 1.11 (0.68–1.82) |
|
0–3 years prior to
index date |
||||
| USS | 117 (49.2%) | 112 (47.1%) | 0.92 (0.63–1.32) | 0.91 (0.60–1.37) |
| AFP | 164 (68.9%) | 168 (70.6%) | 1.09 (0.73–1.63) | 1.13 (0.72–1.77) |
| USS or AFP | 177 (74.4%) | 182 (76.5%) | 1.13 (0.73–1.74) | 1.14 (0.72–1.79) |
|
0–2 years prior to
index date |
||||
| USS | 95 (39.9%) | 91 (38.2%) | 0.93 (0.63–1.36) | 0.93 (0.60–1.43) |
| AFP | 145 (60.9%) | 151 (63.4%) | 1.13 (0.76–1.69) | 1.18 (0.76–1.83) |
| USS or AFP | 160 (67.2%) | 165 (69.3%) | 1.12 (0.74–1.68) | 1.12 (0.73–1.73) |
|
0–1 years prior to
index date |
||||
| USS | 62 (26.1%) | 70 (29.4%) | 1.20 (0.79–1.81) | 1.20 (0.77–1.86) |
| AFP | 109 (45.8%) | 121 (50.8%) | 1.24 (0.85–1.80) | 1.22 (0.82–1.82) |
| USS or AFP | 127 (53.4%) | 143 (60.1%) | 1.33 (0.92–1.94) | 1.40 (0.95–2.08) |
Only tests performed “definitely for screening” were included in this analysis
Odds ratio of screening in cases relative to controls. An odds ratio <1 would be indicative of an association between HCC surveillance and decreased HCC-related mortality. Although this odds ratio is unadjusted, cases and controls were matched for age, gender, race, etiology of cirrhosis, MELD score at the time of cirrhosis diagnosis, date of cirrhosis diagnosis and VA facility.
Adjusted for age, etiology of cirrhosis, MELD score at cirrhosis diagnosis, race/ethnicity, year of cirrhosis diagnosis, diabetes, alcohol use disorders, body mass index, eradication of HCV by antiviral treatment and receipt of abdominal CT or MRI during the period of interest. Also, the USS analysis was adjusted for screening for serum AFP and the serum AFP analysis was adjusted for screening by USS.
There was also no difference in receipt of these screening tests within 1, 2 or 3 years prior to the index date. After adjustment for potential confounders, there was no association between screening with USS or AFP and HCC-related mortality (Table 4). Receipt of screening with both USS and AFP was not associated with HCC-related mortality compared to receipt of USS alone, AFP alone, or no screening at any of the intervals studied (1, 2, 3, or 4 years, Supplemental Table 5). When we analyzed USS and serum AFP tests that were done either definitely or probably for screening (instead of only tests done definitely for screening), we again found no association between screening and HCC-related mortality (Supplemental Table 6 and 7).
Discussion
HCC screening with USS or serum AFP or both was not associated with decreased risk of HCC-related mortality in this matched case-control study based on recent data from a national healthcare system in the United States.
Consensus on HCC screening recommendations is lacking among professional societies. Most liver societies such as the AASLD2, the European Association for the Study of the Liver (EASL)3 and the Asian Pacific Association for the Study of the Liver (APASL)4 have recommended USS every six months with or without concomitant serum AFP for HCC surveillance among patients with cirrhosis. In contrast, non-liver societies have not endorsed HCC screening due to lack of high-quality data. The U.S. Preventive Services Task Force has not adopted an HCC practice guideline, the American Cancer Society makes no recommendation on HCC screening5 and the National Cancer Institute found no evidence that screening decreases mortality from HCC but it could potentially result in harm6.
It remains unclear whether HCC screening reduces HCC-related mortality. Although two RCTs have been performed in HBV-infected patients in China prior to 1997, their results do not apply to cirrhotic patients in the current era in the US and have been criticized for methodological limitations9. The remaining studies are observational, including 39 aggregated in two meta-analyses9, 10 and one large VA study published subsequently44. However, these studies have not compared HCC-related mortality in screened versus unscreened patients. Rather, they have compared survival after the diagnosis of HCC in patients whose HCC was diagnosed because of screening versus those in whom HCC presented with symptoms. This study design is inherently susceptible to lead-time bias that may lead to overestimation of the benefits of screening. Several studies attempted to adjust for lead-time bias by estimating the “sojourn time”45 (the period during which the tumor is asymptomatic but screen detectable) using estimates of tumor growth rate or doubling time. The conclusions of these studies vary dramatically depending on the estimates of tumor doubling time and sojourn time used to adjust for lead-time bias46, 47. Additionally, this study design is limited by length-time bias (aggressive tumors are more likely to present symptomatically and less likely to be diagnosed by screening than less aggressive tumors) and by selection bias (patients who underwent screening were a selected subset of all cirrhotic patients who may have improved survival by having access to better overall care). Finally, most of these studies did not adjust for MELD score, a critical determinant of survival in patients with HCC.
Our methodology addresses many of the limitations of prior studies examining HCC screening. We employed a matched case-control study design to evaluate HCC screening effectiveness that is not susceptible to lead-time or length-time bias. Because cases are defined as patients with cirrhosis and fatal HCC, this study design yields estimates of the impact of screening on HCC-related mortality and approximates the results that would be expected from a randomized controlled trial16. We evaluated a large number of cases and matched controls who were derived randomly from a national healthcare system that provides care to 8 million Veterans in 180 medical centers across the entire United States; thus, our findings are typical of community-based settings. All VA patients have uniform access to medical care, limiting bias due to differential access to HCC screening and HCC treatments. Potential cases and controls were individually verified by chart review using pre-specified criteria. Cases were limited to patients who died in a very recent time period (2013–2015), such that current treatments for HCC would be available to potentially affect HCC-related mortality. Controls were matched to cases for important characteristics that affect receipt of screening or death from HCC, while additional potential confounders were adjusted for. Blinding to the case/control status of patients was maintained for the analyst who identified all USS and serum AFP tests performed in the 4 years prior to the index date, the research assistant who copied relevant reports from the electronic medical records onto our REDCap database and the physician-investigator who determined whether USS or serum AFP tests were done for screening. The medical records related to each of the 995 USS and 1643 AFP tests performed in the 4-year period prior to the index date were reviewed by a physician-investigator to determine whether the USS or AFP were performed for screening. We excluded cases and controls who had a MELD score ≥20 prior to the index date, since screening is not recommended in patients with decompensated cirrhosis (unless they are listed for liver transplantation) because they are unlikely to benefit from HCC treatment.
Our study had several potential limitations. It was conducted in a male population of US veterans, which might limit the generalizability of our study to women. However, we are not aware of studies showing that the test characteristics of screening USS or serum AFP or the outcomes of HCC treatment are different in men versus women with cirrhosis. Nonetheless, it would be useful to replicate our case-control study in a different healthcare system whose records would allow the accurate identification of the indication for USS and AFP testing since, to our knowledge, this is the only available case-control study of HCC screening effectiveness. The main limitation of case-control studies of cancer screening effectiveness is the potential for misclassifying as screening tests some tests that were actually done to evaluate symptoms or signs of cancer in the cases. It is possible that in our study some USS or serum AFP tests were performed in cases in order to evaluate suspected HCC, with the basis for the suspicion not specifically mentioned in the medical record. We would have misclassified these as “screening” tests, leading to falsely high odds ratio and thus a falsely low estimate of screening effectiveness. This is less a concern for USS, for which the indication has to be recorded in the report (which was available to us), but potentially more of a concern in the case of serum AFP tests, for which the ordering provider’s progress notes were the main source of information on test indication.
Two conditions are necessary for HCC screening to result in a reduction in HCC-related mortality. Firstly, screening USS or serum AFP must be able to detect HCC at an earlier stage than it would otherwise present as a result of symptoms, signs or incidental imaging. Secondly, treatment must be available for this early-stage disease that yields superior outcomes relative to treatment of disease detected in the absence of screening. The lack of effectiveness of HCC screening in our study could be related to failure in one or both of these conditions. Multiple studies suggest that HCCs detected by screening USS or serum AFP have, on average, an earlier stage at diagnosis than HCCs detected by symptoms, signs or incidental imaging34, 47–50. However, this does not prove that screening leads to earlier detection. Another explanation is that screening is more likely to identify the slow-growing tumors, which have lower stage, and more likely to miss the fast-growing tumors, which are then identified at a higher stage by symptoms. It is possible that the HCCs most likely to lead to death are HCCs least likely to be identified by current screening modalities at an early stage. Whether early treatment for HCC in patients with cirrhosis leads to a reduction in case-fatality is questionable. Patients who receive locoregional treatments or surgical resection remain at risk of developing recurrent HCC, new HCC and progressive liver dysfunction due to their underlying cirrhosis. Liver transplantation is the only treatment that can cure the cancer as well as the underlying cirrhosis and should confer a survival benefit. However, only a small minority of patients with HCC undergo liver transplantation. In 2012, 24,696 incident cases of HCC were reported in the United States Cancer Statistics registry51 (which, if anything, underestimates the total number of HCC cases), while only 1733 (7%) liver transplants were performed for HCC in the US52. Pragmatic RCTs currently under way, that randomize patients to HCC surveillance outreach with patient education and patient navigation services versus “opportunistic” surveillance53, may address the impact of surveillance of early detection and receipt of treatment, but are not designed to study cancer-related mortality.
It is unlikely that the lack of screening-related survival benefit in our study was due to untimely diagnostic and confirmatory tests for HCC or unavailability of HCC treatments in the VA system. Firstly, even among these fatal cases, 51.3% were diagnosed within Milan criteria, a much greater proportion than that of unselected patients with HCC in the national SEER registry diagnosed within Milan criteria (36.4% in 2003–2006 and 46.3% in 2013–2014)54. Secondly, we found that even among these fatal cases, who had very advanced HCC at presentation, a substantial proportion (66.8%) received a cancer-specific treatment. The fact that none of the cases received liver transplantation is not indicative of unavailability of liver transplantation, but rather a result of the fact that only carefully selected patients who are not expected to die of HCC undergo liver transplantation and hence liver transplant recipients did not contribute to the fatal cases in our study. It is unlikely that the lack of screening-related survival benefit was due to patients having advanced cirrhosis, which may discourage screening, preclude certain HCC treatments, or dictate patient survival irrespective of the presence of HCC, because patients had MELD score <20 at all times prior to the index date and a mean MELD score of 9 at the time of cirrhosis diagnosis.
In summary, we found no evidence that screening with USS or serum AFP reduces HCC-related mortality in patients with cirrhosis. This suggests that either these screening tests or the currently available treatments, or both, are suboptimal and need to be improved.
Supplementary Material
Acknowledgments
Declaration of Funding Sources
The study was funded by a NIH/NCI grant R01CA196692 and VA CSR&D grant I01CX001156 to GNI and NSW.
Role of Funding Source
The funding source played no role in study design, collection, analysis or interpretation of data.
Declaration of Personal Interests
None
Authors’ Contributions and Authorship Statement
All authors approved the final version of the manuscript
George Ioannou is the guarantor of this paper.
Andrew Moon: Abstraction of medical charts, study design, statistical analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript.
Feng Su: Study design, critical revision of the manuscript.
Ga-Young Jin: Creation of REDCap database, accessing electronic medical records.
Lauren Beste: Study concept and design, acquisition of data, statistical analysis and interpretation of data, critical revision of the manuscript.
Noel Weiss: Study concept and design, statistical analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtaining funding.
Samuel Ho: Study concept and design, interpretation of the data, critical revision of the manuscript.
Elliott Lowy: Acquisition of data, statistical analysis and interpretation of data.
Kristin Berry: Study design, analysis of data, interpretation of data, critical revision of manuscript.
George Ioannou: Study concept and design, acquisition of data, abstraction of medical charts, statistical analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtaining funding.
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- AFP
alpha fetoprotein
- CAPRI
Compensation and Pension Record Interchange
- CDW
Corporate Data Warehouse (Veterans Affairs)
- CT
computed tomography
- DPP
detectable preclinical phase
- HCC
hepatocellular carcinoma
- HBV
hepatitis B
- HCV
hepatitis C
- ICD-9
International Statistical Classification of Diseases, Injuries and Causes of Death, 9th edition
- LI-RADS
Liver Imaging Reporting and Data System
- MELD
Model for End Stage Liver Disease
- MRI
magnetic resonance imaging
- USS
ultrasound scan
- US
United States
- VA
Veterans Affairs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- 2.Heimbach J, Kulik LM, Finn R, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver and European Organisation For Research Treatment Of Cancer (EASL-EORTC) clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- 4.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society. Can Liver Cancer Be Found Early? In: American Cancer Society, ed. Volume 2018 Atlanta, GA: American Cancer Society,, 2016. [Google Scholar]
- 6.National Cancer Institute. Liver (Hepatocellular) Cancer Screening - Health Professional Version Available at: https://www.cancer.gov/types/liver/hp/liver-screening-pdq Last accessed: 01/09/2018.
- 7.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–22. [DOI] [PubMed] [Google Scholar]
- 8.Chen JG, Parkin DM, Chen QG, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen 2003;10:204–9. [DOI] [PubMed] [Google Scholar]
- 9.Kansagara D, Papak J, Pasha AS, et al. Screening for Hepatocellular Carcinoma in Chronic Liver Disease: A Systematic Review. Ann Intern Med 2014;161:261–269. [DOI] [PubMed] [Google Scholar]
- 10.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med 2014;161:261–9. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poustchi H, Farrell GC, Strasser SI, et al. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology 2011;54:1998–2004. [DOI] [PubMed] [Google Scholar]
- 14.Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med 2012;156:387–9. [DOI] [PubMed] [Google Scholar]
- 15.Kelley MJ. Surveillance for hepatocellular carcinoma. Ann Intern Med 2011;155:274; author reply 275. [DOI] [PubMed] [Google Scholar]
- 16.Morrison AS. Screening in chronic disease New York, NY: Oxford University Press, 1992. [Google Scholar]
- 17.Weiss NS, Etzioni R. Estimating the influence of rescreening interval on the benefits associated with cancer screening: approaches and limitations. Epidemiology 2002;13:713–7. [DOI] [PubMed] [Google Scholar]
- 18.Weiss NS, Dhillon PK, Etzioni R. Case-control studies of the efficacy of cancer screening: overcoming bias from nonrandom patterns of screening. Epidemiology 2004;15:409–13. [DOI] [PubMed] [Google Scholar]
- 19.Selby JV, Friedman GD, Quesenberry CP Jr., et al. Effect of fecal occult blood testing on mortality from colorectal cancer. A case-control study. Ann Intern Med 1993;118:1–6. [DOI] [PubMed] [Google Scholar]
- 20.Selby JV, Friedman GD, Quesenberry CP Jr., et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 1992;326:653–7. [DOI] [PubMed] [Google Scholar]
- 21.Newcomb PA, Weiss NS, Storer BE, et al. Breast self-examination in relation to the occurrence of advanced breast cancer. J Natl Cancer Inst 1991;83:260–5. [DOI] [PubMed] [Google Scholar]
- 22.Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology 2013;145:312–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rustagi AS, Kamineni A, Weinmann S, et al. Cervical screening and cervical cancer death among older women: a population-based, case-control study. Am J Epidemiol 2014;179:1107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman GD, Hiatt RA, Quesenberry CP Jr., et al. Case-control study of screening for prostatic cancer by digital rectal examinations. Lancet 1991;337:1526–9. [DOI] [PubMed] [Google Scholar]
- 25.Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst 1996;88:17–23. [DOI] [PubMed] [Google Scholar]
- 26.Veterans Affairs Corporate Data Warehouse Available at: http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm Last accessed on 12/19/16.
- 27.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther 2008;27:274–82. [DOI] [PubMed] [Google Scholar]
- 29.Kramer JR, Giordano TP, Souchek J, et al. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol 2005;100:56–63. [DOI] [PubMed] [Google Scholar]
- 30.Ioannou GN, Splan MF, Weiss NS, et al. Incidence and Predictors of Hepatocellular Carcinoma in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2007;5:938–945. [DOI] [PubMed] [Google Scholar]
- 31.Ioannou GN, Bryson CL, Weiss NS, et al. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 2013;57:249–57. [DOI] [PubMed] [Google Scholar]
- 32.Ioannou GN, Beste LA, Green PK. Similar Effectiveness of Boceprevir and Telaprevir Treatment Regimens for Hepatitis C Virus Infection, Based on a Nationwide Study of Veterans. Clin Gastroenterol Hepatol 2013. [DOI] [PubMed] [Google Scholar]
- 33.Ioannou GN, Scott JD, Yang Y, et al. Rates and predictors of response to anti-viral treatment for hepatitis C virus in HIV/HCV co-infection in a nationwide study of 619 patients. Aliment Pharmacol Ther 2013;38:1373–84. [DOI] [PubMed] [Google Scholar]
- 34.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med 2011;154:85–93. [DOI] [PubMed] [Google Scholar]
- 35.El-Serag HB, Johnson ML, Hachem C, et al. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 2009;136:1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanwal F, Hoang T, Kramer JR, et al. Increasing Prevalence of HCC and Cirrhosis in Patients With Chronic Hepatitis C Virus Infection. Gastroenterology 2011;140:1182–1188 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology 2013;57:1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Serag HB, Kanwal F, Davila JA, et al. A New Laboratory Based Algorithm to Predict Development of Hepatocellular Carcinoma in Patients with Hepatitis C and Cirrhosis. Gastroenterology 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61:1056–65. [DOI] [PubMed] [Google Scholar]
- 40.Beste LA, Leipertz SL, Green PK, et al. Trends in Burden of Cirrhosis and Hepatocellular Carcinoma by Underlying Liver Disease in US Veterans, 2001–2013. Gastroenterology 2015;149:1471–1482 e5. [DOI] [PubMed] [Google Scholar]
- 41.Sheu JC, Sung JL, Chen DS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology 1985;89:259–66. [DOI] [PubMed] [Google Scholar]
- 42.Etzioni RD, Weiss NS. Analysis of case-control studies of screening: impact of misspecifying the duration of detectable preclinical pathologic changes. Am J Epidemiol 1998;148:292–7. [DOI] [PubMed] [Google Scholar]
- 43.Dupont WD. Power calculations for matched case-control studies. Biometrics 1988;44:1157–68. [PubMed] [Google Scholar]
- 44.Mittal S, Kanwal F, Ying J, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol 2016;65:1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffy SW, Nagtegaal ID, Wallis M, et al. Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. Am J Epidemiol 2008;168:98–104. [DOI] [PubMed] [Google Scholar]
- 46.Cucchetti A, Trevisani F, Pecorelli A, et al. Estimation of lead-time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J Hepatol 2014;61:333–41. [DOI] [PubMed] [Google Scholar]
- 47.El-Serag HB, Kramer JR, Chen GJ, et al. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut 2011;60:992–7. [DOI] [PubMed] [Google Scholar]
- 48.Giannini E, Arzani L, Borro P, et al. Does surveillance for hepatocellular carcinoma in HCV cirrhotic patients improve treatment outcome mainly due to better clinical status at diagnosis? Hepatogastroenterology 2000;47:1395–8. [PubMed] [Google Scholar]
- 49.Kemp W, Pianko S, Nguyen S, et al. Survival in hepatocellular carcinoma: impact of screening and etiology of liver disease. J Gastroenterol Hepatol 2005;20:873–81. [DOI] [PubMed] [Google Scholar]
- 50.Leykum LK, El-Serag HB, Cornell J, et al. Screening for hepatocellular carcinoma among veterans with hepatitis C on disease stage, treatment received, and survival. Clin Gastroenterol Hepatol 2007;5:508–12. [DOI] [PubMed] [Google Scholar]
- 51.White DL, Thrift AP, Kanwal F, et al. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017;152:812–820 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.U.S. Department of Health and Human Services. Organ Procurement and Transplantation Network. National Data Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ Last accessed 02/13/18.
- 53.Texas Hepatocellular Carcinoma Consortium (THCCC) Project 5. ClinicalTrials.gov Identifier: NCT02582918. https://clinicaltrials.gov/ct2/show/NCT02582918?term=Texas+Hepatocellular+Carcinoma+Consortium &rank=1. Last accessed: 05/06/2018.
- 54.Robinson A, Tavakoli H, Liu B, et al. Advanced Hepatocellular Carcinoma (HCC) Tumor Stage at Diagnosis in the 1945–1965 Birth Cohort Reflects Poor Utilization of HCC Screening. Hepatology Communications 2018;In Press 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


