Abstract
The thalamocortical (TC) relay neuron of the dorsoLateral Geniculate Nucleus (dLGN) has borne its imprecise label for many decades in spite of strong evidence that its role in visual processing transcends the implied simplicity of the term “relay”. The retinogeniculate synapse is the site of communication between a retinal ganglion cell and a TC neuron of the dLGN. Activation of retinal fibers in the optic tract causes reliable, rapid, and robust postsynaptic potentials that drive postsynaptics spikes in a TC neuron. Cortical and subcortical modulatory systems have been known for decades to regulate retinogeniculate transmission. The dynamic properties that the retinogeniculate synapse itself exhibits during and after developmental refinement further enrich the role of the dLGN in the transmission of the retinal signal. Here we consider the structural and functional substrates for retinogeniculate synaptic transmission and plasticity, and reflect on how the complexity of the retinogeniculate synapse imparts a novel dynamic and influential capacity to subcortical processing of visual information.
Keywords: Retinogeniculate synapse, Short-term plasticity, Developmental refinement, Visual circuit, Synaptic transmission
More than a relay
The retinogeniculate synapse has been an invaluable workhorse for neuroscience research. In recent years, it has served as a powerful model for understanding the molecular and circuit-level mechanisms that influence normal development and disease (Bishop et al., 1959; Sengpiel & Kind, 2002; Kastner et al., 2006; Guido, 2008; Kano & Hashimoto, 2009; Hong & Chen, 2011; Stephan et al., 2012; Kaplan, 2014). The ability to independently label Retinal Ganglion Cells (RGCs) from opposite eyes has enabled the identification of cellular and molecular mechanisms of axon mapping, arbor pruning, and synapse elimination that drive the refinement of retinotopic maps and eye-specific lamination (Wong, 1999; Luo & O’Leary, 2005; Huberman et al., 2008a; Feller, 2009; Kano & Hashimoto, 2009; Hong & Chen, 2011). In vitro, the easily accessible bundle of RGC axons in the optic nerve provides a convenient means of selectively activating presynaptic inputs in studies of synaptic physiology and plasticity, and is also an excellent system for uncovering mechanisms of axon regeneration (Guido, 2008; Hong & Chen, 2011; Benowitz et al., 2017). In vivo, the retinogeniculate synapse has been a prominent model system for the study of sub-cortical visual processing. Numerous studies have capitalized on the ease of manipulating visual stimulation, combined with the ability to simultaneously monitor the activity patterns of inputs and outputs of the thalamus to reliably demonstrate that the transfer of information from the retina to the visual cortex is the major function of the retinogeniculate synapse (Sherman, 2005; Usrey & Alitto, 2015; Weyand, 2016).
Despite the tenacity of the term “relay” to describe the function of the dLGN, the retinogeniculate synapse does not simply transfer a copy of RGC activity patterns to the cortex. Over the past few decades, a number of retinogeniculate attributes have been shown to play a role in modifying visual information before conveying it to the cortex (Blitz et al., 2004; Sherman, 2007; Usrey & Alitto, 2015; Weyand, 2016). Simultaneous recording of the firing patterns of RGC inputs and TC target neurons in vivo has shown that the reliability of action potential generation in TC neurons depends on the local activity context, such as neuromodulatory signaling or the membrane potential. These factors can dictate or modulate the firing pattern (“tonic” or “burst”) of a TC neuron (McCormick & Bal, 1994; Usrey et al., 1998; Sherman & Guillery, 2002; Wang et al., 2007). In some cases, a TC neuron is much more likely to generate a response to the second of a pair of visually-driven potentials arriving in quick succession (separated by less than 30 ms, Mastronarde, 1987; von Krosigk et al., 1993; Usrey et al., 1998; Levine & Cleland, 2001; Rowe & Fischer, 2001; Carandini et al., 2007; Weyand, 2007; Sincich et al., 2007, 2009; Alitto et al., 2011). This integrative function increases the amount of information encoded in thalamic spiking (Wang et al., 2010). In other cases, a single RGC impulse can result in a post-synaptic burst of multiple action potentials (Usrey et al., 1998; Blitz & Regehr, 2003).
Recent work is revealing new receptive field complexity and plasticity in the dLGN that further demonstrates significant thalamic processing of visual information en route to the cortex, at least in some species. Stimulus orientation selectivity is one salient example of a complex feature encoded in subpopulations of dLGN neurons in a variety of species: mouse (Marshel et al., 2012; Piscopo et al., 2013; Scholl et al., 2013; Zhao et al., 2013) and rabbit (Levick et al., 2010; Hei et al., 2014), with weaker orientation or direction bias occurring in the cat (Hubel & Wiesel, 1961; Daniels et al., 1977; Levick & Thibos, 1980; Vidyasagar & Urbas, 1982; Soodak et al., 1987; Shou & Leventhal, 1989; Thompson et al., 1994), squirrel (Zaltsman et al., 2015), and primate (Lee et al., 1979; Smith et al., 1990; Cheong et al., 2013). Complex feature selectivity persists in TC neurons after inactivation of the primary visual cortex, suggesting that the dLGN may compute orientation or direction selectivity rather than inherit it from cortical feedback (cat Vidyasagar & Urbas, 1982; mouse Zhao et al., 2013; Scholl et al., 2013). Furthermore, cat TC neurons have a higher stimulus contrast sensitivity than their individual inputs, suggesting TC neurons can functionally integrate information from multiple RGC inputs (Rathbun et al., 2016). The presence of binocularly innervated dLGN neurons in mice, cats, and primates further supports the possibility of convergence of multiple RGCs onto single geniculate neurons in mice, cat, and primate dLGN (Sanderson et al., 1971; Howarth et al., 2014; Zeater et al., 2015; Rompani et al., 2017). Here, we explore synaptic mechanisms and structural attributes that could support the diversity of features and functions now emerging in studies of the dLGN. In this review, we describe the structural properties of RGC axons and TC neurons, together with their biophysical features and plasticity mechanisms. These properties may combine to impart novel and adaptive functionality to the dLGN, and to dynamically regulate information flow between retina and cortex.
Retinogeniculate synaptic structure
To appreciate the functional complexity in retinogeniculate processing, we first describe the underlying synaptic structure. The architecture of the retinogeniculate synapse is conserved across species. Glutamate is the excitatory neurotransmitter packed into numerous round vesicles contained in large synaptic terminals along the axon (Montero & Wenthold, 1989). A single retinal axon terminal can span ~1–4 microns in diameter and contain multiple spatially distinct neurotransmitter release sites (cat and mouse studies: Famiglietti & Peters, 1972; Rafols & Valverde, 1973; Sur & Sherman, 1982; Hamos et al., 1987; Robson, 1993; Bickford et al., 2010; Budisantoso et al., 2012; Morgan et al., 2016). RGC boutons contact a TC neuron near its cell body, synapsing directly onto the dendritic shaft or dendritic appendages that protrude from the proximal shaft or primary dendritic branch points (Rafols & Valverde, 1973; Robson & Mason, 1979; Wilson et al., 1984; Hamos et al., 1987; Bickford et al., 2010; Morgan et al., 2016). Retinal inputs account for only 5–10% of a TC neuron’s synaptic input, whereas cortical feedback projections from Layer 6 occupy the distal dendrites, providing as much as 50% of synaptic input (Wilson et al., 1984; Montero, 1991; Van Horn et al., 2000). Nonetheless, the proximal position of retinogeniculate synapses along the dendrite, their synaptic structure with multiple release sites, and their large number of synaptic contacts drives powerful and reliable transmission that has earned the retinal input the moniker of “driver” to all other inputs’ “modulator” (Guillery & Sherman, 2002).
The fine details of retinogeniculate connectivity reveal potential heterogeneity of circuit organization and, thereby, of function. A RGC axon contacts a TC neuron with as many as 59 terminals in cat or mouse (Fig. 1A–1C; Hamos et al., 1987; Robson, 1993; Morgan et al., 2016). The morphology of these numerous synaptic contacts between a single RGC axon and its target TC neuron can range from the simple to the complex (Jones & Powell, 1969; Famiglietti & Peters, 1972; Hammer et al., 2015; for finer categorization, see Lund & Cunningham, 1972; Robson & Mason, 1979; Morgan et al., 2016). Simple retinogeniculate contacts consist of one small or large crenulated bouton contacting a dendrite or dendritic appendage. Complex contacts comprise a glomerular structure containing multiple boutons from retinal, inhibitory, and neuromodulatory inputs (Robson & Mason, 1979; Koch, 1985; Sherman & Guillery, 1996; Sherman, 2004). Although it is not known whether differences in bouton morphology correlate with specializations for retinogeniculate information transfer, work on cerebellar parallel fibers shows that bouton size can predict sensitivity to neuromodulation, and induction of LTP or LTD can induce plasticity in the size of hippocampal presynaptic boutons (Toni et al., 1999; Becker et al., 2008; Zhang & Linden, 2009). Furthermore, the morphology of synaptic contacts is optimized to the requirements of sensory transmission in the retina, inner ear, and central auditory synapses (Taschenberger et al., 2002; Matthews & Fuchs, 2010; Freche et al., 2011; Graydon et al., 2014). It is therefore likely that the diversity of retinogeniculate contact morphologies reflects differences in their contribution to transmission. Indeed, different TC neuron types in cats exhibit biases in presynaptic morphology. Cat RGCs and TC neurons are distinguished into three categories (X, Y, W) based on the properties of their responses to visual stimuli, including the size of the receptive field and the degree of linearity of spatial summation, as well as morphological markers. The X and Y classifications refer to relatively homogeneous populations (and are often compared to primate M and P pathways), whereas the W (compared to K in primates) encompasses a more diverse set of cells with rarely-encountered physiological responses (Wilson et al., 1976; Fukuda et al., 1984; Felch & Van Hooser, 2012). Simple contacts dominate retinal input onto cat Y cells, whereas X cell dendritic appendages preferentially participate in complex synaptic structures (Robson & Mason, 1979; Hamos et al., 1985; Koch, 1985; Sherman & Guillery, 1996; Sherman, 2004). Similar distinctions among TC neurons have been observed in the mice by morphological analysis, but have not been associated with distinct patterns of synaptic structures nor delineated by physiology (Krahe et al., 2011; El-Danaf et al., 2015; Sriram et al., 2016).
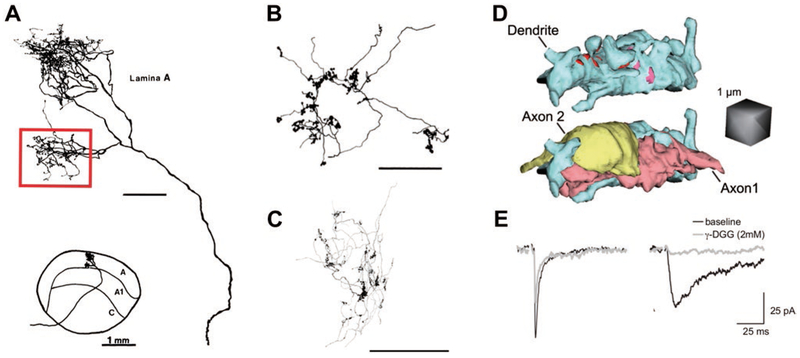
Fig. 1.
Synaptic structure shapes retinogeniculate transmission. (A) Tracing of an HRP-filled X-RGC arbor in the cat dLGN shows the location and morphology of a single branch (red box) of the X-RGC arbor used for EM reconstruction. This branch of the axon contacts 4 TC neurons out of 40 available neurons in the territory of the arbor. The remainder of the axon was not reconstructed, and likely contacts several other TC neurons. Bottom inset shows the location of the axonal arbor in the context of the cat LGN. Figure modified from Hamos et al. (1987). Unmarked scale bar = 100 μm. (B, C) Reconstructed arbors of single RGC axons showing distribution of presynaptic boutons into dense clusters in the LGN of (B) an adult cat and (C) a p20 mouse. Note the clustering of boutons along the arbor. Image in B is modified from Robson et al. (1993), showing a segment of a RGC axon; Image in C is from Hong et al. (2014), showing a BD-RGC axon. Scales bars are 100 μm. (D) A 3D reconstruction of a TC neuron dendrite and sites of contact between two neighboring RGC boutons from Budisantoso et al. (2012). In the top image, the dendrite and its appendages are depicted in blue, whereas pink and red sites label the postsynaptic densities of the two axons. In the bottom image, the structure of the terminals of two axons has been added. Spillover can occur between these two nearby terminals. (E) Evidence of spillover-mediated responses to the stimulation of a single RGC axon before eye opening. Two different synaptic responses were observed in response to single retinal fiber stimulation. Shown are recordings from TC neurons in whole cell voltage clamp at −70 mV in a dLGN slice in the presence of the NMDAR blocker, 20 μM CPP. On the left is an example of a retinogeniculate AMPAR EPSC with characteristic rapid rise time and decay kinetics (black trace). On the right is an atypical AMPAR EPSC response notable for significantly slower rise time and decay kinetics (black trace). The two types of EPSCs differ in their sensitivity to the low-affinity AMPAR antagonist, γ−DGG. Low affinity antagonists can be used to assess the relative concentration of glutamate in the synaptic cleft (Clements et al., 1992; Diamond & Jahr, 1997). As γ−DGG competes with glutamate for binding to AMPAR, its efficacy of inhibition decreases with increasing glutamate concentration. γ−DGG has only a small effect on the amplitude of the fast EPSC, but dramatically reduces the amplitude of the slow EPSC (overlaid gray traces), consistent with lower peak glutamate concentration in the synaptic cleft of the slow EPSC. Because the EPSCs are evoked by minimal stimulation, the rapid EPSC represents a direct input from a single RGC axon that forms a direct synapse onto the voltage-clamped relay neuron, whereas the slow EPSC corresponds to the activation of a RGC axon that does not directly synapse onto the voltage-clamped neuron. Modified from Hauser et al. (2014). All figures reprinted with permission.
In addition to potential functional differences reflected in the morphology of presynaptic geniculate boutons, the close proximity of clustered multisynaptic boutons in the mature synapse makes possible novel interactions between glutamate transients originating in separate RGC inputs. Large simple boutons in the rat contain an average of 27 independent release sites, whereas each of the boutons in a glomerulus has approximately 6 (Hamos et al., 1987; Budisantoso et al., 2012; Hammer et al., 2015). Notably, multiple RGCs may contribute to the same cluster or glomerulus of boutons (Hammer et al., 2015; Morgan et al., 2016). At other CNS synapses, glia ensheath individual boutons and interdigitate into the synaptic cleft, preventing glutamate from diffusing, or “spilling over,” to neighboring boutons, in part through the action of glutamate transporters, which clear glutamate from the extracellular space (Diamond & Jahr, 1997; Danbolt, 2001; Tzingounis & Wadiche, 2007; Hauser et al., 2013; Rimmele & Rosenberg, 2016). In contrast, glia do not interdigitate into the cleft of single RGC boutons nor within geniculate glomeruli, making the retinogeniculate connection conducive to glutamate spillover within and between individual boutons (Famiglietti & Peters, 1972; Robson & Mason, 1979; Winfield et al., 1980; Mason, 1982; Bickford et al., 2010). Although interbouton spillover has not been experimentally assessed in the mature dLGN, a simulation that demonstrated the likelihood of spillover between distant synapses within the same bouton also implies the possibility of spillover of glutamate between closely spaced boutons. Fig. 1D shows an example of two such close retinogeniculate boutons along one TC neuron dendrite, highlighting that glutamate released from one bouton can diffuse to postsynaptic release sites of the neighboring bouton (Budisantoso et al., 2012). At a developmental phase when boutons are less clustered (Sur et al., 1984; Hong et al., 2014) and glomeruli have not yet formed, retinogeniculate transmission exhibits extensive gluta-mate spillover between neighboring boutons. In fact, glutamate from the bouton of one RGC axon can spill over to the synaptic cleft of a neighboring RGC axon before eye opening (Hauser et al., 2014). Glutamate spillover can be distinguished from direct retinogeniculate synaptic activation by the slower kinetics of the α−amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated Excitatory Postsynaptic Current (EPSC) and increased sensitivity to γ−DGG (gray traces), a low affinity AMPAR antagonist (Fig. 1E). These characteristics indicate that the receptors mediating the spillover current are exposed to a lower glutamate concentration than those mediating the direct EPSC. Glutamate spillover can therefore diminish synaptic specificity, but also result in complex and graded integration of information transmitted from different RGC inputs. This mechanism of transmission may be particularly relevant for high-frequency presynaptic activity (RGCs can reach up to 500 Hz, Nirenberg & Meister, 1997), which would promote the pooling and spillover of glutamate. Therefore, the intricate morphology of retinogeniculate contacts presents the possibility for a diversity of modes of retinogeniculate information transfer, which could vary as a function of preceding sensory or modulatory activity.
Short-term plasticity
The mature retinogeniculate synapse boasts multiple bouton contacts, each with many release sites, leading to a high probability of release (Yeow & Peterson, 1991; Chen & Regehr, 2000; Budisantoso et al., 2012). Combined with the proximity of retinal contacts to the cell body that minimizes potential dendritic filtering of the synaptic signal, these structural features give rise to an EPSC characterized by rapid kinetics (time constant of decay ~2 ms) and large amplitudes in vitro (Chen & Regehr, 2000; example recording in Fig. 2A, p26–32). Studies in slices have identified several pre and post-synaptic mechanisms of short-term plasticity that modulate these EPSCs to further shape transmission based on the activity of the RGC input itself as well as other retinal and nonretinal inputs to that neuron. In shaping retinogeniculate transmission, these short-term plasticity mechanisms provide the means to dynamically modify the information transmitted from RGC to TC neuron output.
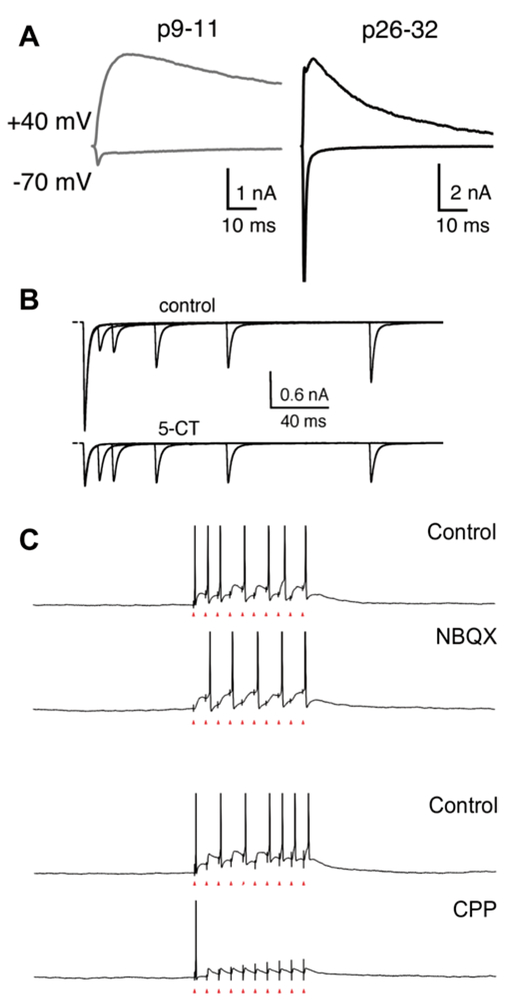
Fig. 2.
Contributions of retinogeniculate short-term plasticity. (A) Representative traces of AMPAR and NMDAR mediated currents recorded before eye opening (left) and in a mature mouse (right) in response to the stimulation of the optic tract. Whole-cell voltage clamp recordings were performed with bicuculline to block GABAA−receptor mediated currents. At −70 mV holding potential, AMPARs mediate the fast activating and decaying current. AMPAR and NMDAR currents both contribute to the EPSCs recorded at +40 mV with AMPARs contributing to the rapid rise and the NMDAR currents contributing to the slow decay of the EPSC. The average amplitude of AMPAR currents increases over development. (B) 5-CT-mediated activation of serotonin receptors alters retinogeniculate short-term plasticity. Experiments were performed in retinogeniculate slices from mature mice. Top and bottom traces overlay pairs of retinogeniculate EPSCs evoked with varying ISI before (top) and after (bottom) the application of 5-CT to active 5HT-1 receptors expressed in presynaptic retinogeniculate boutons. Application of 5-CT reduces the amplitude of the first EPSC and relieves short-term depression, increasing the amplitude of the second EPSC preferentially at short interstimulus interval. (C) Physiologically relevant stimulation frequencies preferentially diminish the contribution of AMPARs to relay neuron firing. Current clamp recordings of action potential firing in response to trains of optic tract stimulation in the presence of AMPAR (NBQX) or NMDAR (CPP) antagonists. Holding potential −50 mV. Blockade of AMPARs alters the latency to first spike but only minimally reduces the overall number of spikes. In contrast, blockade of NMDARs abolished EPSC summation toward action potential firing; only the first stimulus evokes an action potential, reflecting the contribution of AMPARs that rapidly desensitize after the first pulse. Therefore, NMDAR currents can sustain action potential generation without AMPAR contribution. Adapted from (A) Chen and Regehr (2000), B) Liu and Chen (2008) and (C) Augustinaite and Heggelund (2007). All figures reprinted with permission.
A prominent feature of retinogeniculate transmission studied in vitro is short-term depression: the second of two impulses separated by a short interval generates a weaker response than the first. Both presynaptic mechanisms involving vesicle depletion and postsynaptic mechanisms including AMPAR desensitization and N−methyl-D-aspartate receptor (NMDAR) saturation contribute to this plasticity (Chen et al., 2002; Blitz et al., 2004; Budisantoso et al., 2012). In contrast to the short-term depression observed in vitro in mice, extracellular recordings of TC neuron activity in vivo in cats and primates consistently show paired-stimulus enhancement, such that the second of two retinal impulses separated by a brief (<30 ms) interval is more effective at driving a postsynaptic action potential (Mastronarde, 1987; Usrey et al., 1998; Levine & Cleland, 2001; Rowe & Fischer, 2001; Carandini et al., 2007; Rathbun et al., 2007; Sincich et al., 2007, 2009). In part, this contradiction is due to the dependence of short-term depression on the recent history of activity at the synapse. Activation of the retinogeniculate synapse in slice usually follows a period of quiescence, whereas baseline spontaneous activity maintains synaptic transmission in a chronically depressed state in vivo (Levick & Williams, 1964; Stoelzel et al., 2015). A train of spikes preceding optic tract stimulation in vitro attenuates the degree of synaptic depression (Seeburg et al., 2004; Augustinaite & Heggelund, 2007; Liu & Chen, 2008). Further, neurotransmitter inputs from the brainstem and inhibitory neurons can modulate retinogeniculate transmission in a context-dependent manner in vivo. To understand the relationship between in vitro and in vivo manifestations of short-term plasticity, we review the prominent sources of modulation of retinogeniculate transmission.
Presynaptic modulation
Retinogeniculate transmission is known to be modulated presynaptically by a number of neurotransmitter receptors, including GABAB, serotonin 5HT1B (Chen & Regehr, 2003; Seeburg et al., 2004), as well as adenosine A1 (Yang et al., 2014) and metabotropic glutamate receptors (Hauser et al., 2013; Lam & Sherman, 2013). Activation of the GABAB or 5-HT1B receptors strongly depresses neurotransmitter release and relieves short-term depression by decreasing the entry of calcium into the presynaptic terminal (see Fig. 2B; Chen & Regehr, 2003; Seeburg et al., 2004). While these modulators decrease the strength of the retinogeniculate EPSC, they also alter the pattern of action potentials transmitted from the pre-to post-synaptic neuron. For example, activation of pre-synaptic 5-HT1B receptors leads to preferential transmission of high-frequency over lowfrequency activity, essentially acting as a high-pass filter (Seeburg et al., 2004). In some cases, presynaptic modulation can be additive. The combined activation of 5HT1B and adenosine A1 receptors, can convert presynaptic depression into facilitation (Yang et al., 2014). Therefore, the activity of neuromodulatory inputs in vivo can dynamically shape retinogeniculate information transfer by modulating the degree of short-term plasticity. It is not known whether the expression of presynaptic receptors differs between RGC types or RGC bouton morphologies. However, any such differences would add an additional layer of modularity to retinogeniculate transmission.
Postsynaptic modulation
In addition to presynaptic depression, postsynaptic glutamate receptor properties also contribute to short-term depression and shape the efficacy of retinogeniculate transmission. Postsynaptic AMPA and NMDA receptors both exhibit short-term depression, and perform complementary functions in retinogeniculate transmission.
AMPAR channel gating properties and the high density of their expression contribute to the large, rapid activation, and decay kinetics of the retinogeniculate EPSC (Tarusawa et al., 2009). Because AMPARs readily conduct at negative potentials, they are effective at initiating postsynaptic spiking, even from a relatively hyperpolarized membrane potential (Blitz & Regehr, 2003; Augustinaite & Heggelund, 2007; Liu & Chen, 2008). However, AMPARs desensitize upon exposure to glutamate and recover with a time constant of ~100 ms (Chen et al., 2002; Kielland & Heggelund, 2002). These properties lead to short-term depression of the AMPAR EPSC. Therefore, AMPARs contribute to the onset of an action potential train transmitted from a RGC, initiating robust short-latency spikes during low frequency activity, but cannot sustain the robust transmission of high-frequency retinogeniculate activity (Blitz & Regehr, 2003). Fig. 2C demonstrates that pharmacological blockade of AMPAR with NBQX reduces the initial spikes in response to a stimulus train (Turner et al., 1994; Augustinaite & Heggelund, 2007).
NMDA receptor-mediated currents have distinct kinetics and voltage dependence from AMPAR (Fig. 3A and 3B). At the retinogeniculate synapse, NMDARs exhibit short-term depression due to their high affinity to glutamate and receptor saturation (Traynelis et al., 2010). Recovery from NMDAR saturation occurs more quickly than from AMPAR desensitization (Chen et al., 2002; Kielland & Heggelund, 2002). TC neuron NMDARs experience incomplete Mg2+ block at hyperpolarized potentials, and therefore conduct significant current at negative potentials (Liu & Chen, 2008). Fig. 3B and 3C illustrates the contribution of NMDAR to transmission over development. NMDARs conduct current for many tens of milliseconds during a prolonged decay, which permits the summation of closely timed EPSCs, especially within the range of interstimulus intervals (ISIs) that exhibit paired-pulse enhancement in vivo, and supports multiple TC neuron spikes even in “tonic” mode (Chen et al., 2002; Blitz & Regehr, 2003; Augustinaite & Heggelund, 2007; Liu & Chen, 2008; Budisantoso et al., 2012). Blockade of NMDAR dramatically reduces retinogeniculate transmission in vivo (Sillito et al., 1990; Kwon et al., 1991), and NMDAR current summation in vitro can even drive action potential firing in the presence of AMPAR blockers, though with less temporal precision (Fig. 2C; Chen et al., 2002; Blitz & Regehr, 2003; Augustinaite & Heggelund, 2007; Budisantoso et al., 2012). In fact, the NMDAR component of the first EPSC may sufficiently depolarize a TC neuron to spike threshold, such that a small or depressed AMPAR current can shorten the latency to first spike (Kielland & Heggelund, 2002; Augustinaite & Heggelund, 2007; Budisantoso et al., 2012). Therefore, summation of NMDAR currents enhances the probability of TC neuron spiking in response to the second and later RGC action potentials during a train. Together with modulation and postsynaptic integration (Carandini et al., 2007), the properties of NMDA and AMPA receptor currents can reconcile the robust short-term depression seen in vitro with paired-pulse enhancement observed in vivo.
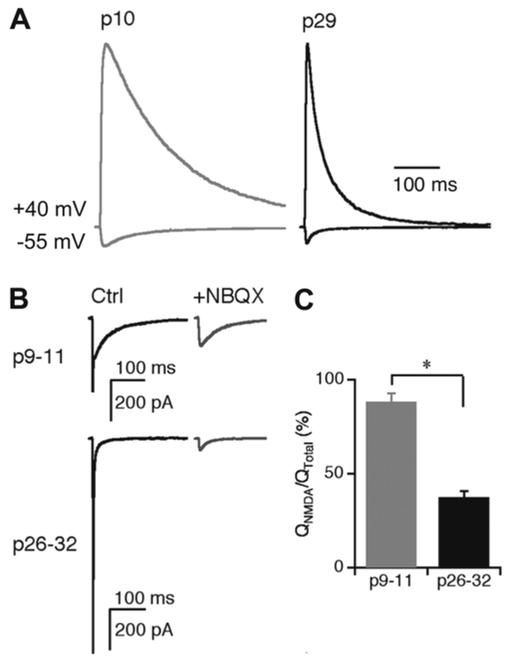
Fig. 3.
Contribution of NMDAR-currents to retinogeniculate transmission over development. (A) NMDAR EPSCs recorded in the presence of the AMPAR blocker, NBQX, at +40 and −55 mV holding potentials in a p10 (left) and a p29 (right) retinogeniculate slice. Normalized traces are shown. Note the acceleration in NMDAR current decay time over development. (B) Example EPSCs recorded in young (top) and mature (bottom) TC neuron in slice before (left) and during (right) the application of NBQX. Holding potential, −55 mV. (C) NMDAR currents contribute more to the total retinogeniculate charge transfer at p9–11 than p26–32; however, even at the mature synapse, NMDARs contribute nearly half of the total charge transfer. Figure adapted from Liu and Chen (2008). All figures reprinted with permission.
An additional factor that could contribute to differences in in vitro and in vivo short-term plasticity is the expression of calcium-permeable AMPARs. The retinogeniculate synapse differs from other synapses in that the expression of calcium-permeable AMPARs increases over development (see Fig. 4A and 4B; Budisantoso et al., 2012; Hauser et al., 2014; Louros et al., 2014; compare to Kumar et al., 2002; Soto et al., 2007). Calcium permeable AMPARs (those lacking the GluA2 subunit) exhibit stronger desensitization-mediated paired pulse depression (Budisantoso et al., 2012). However, depolarization-mediated reduction of polyamine block may partly rescue this effect (Rozov et al., 1998; Soto et al., 2007), increasing the contribution from AMPARs to transmission later in a train of high frequency activity.
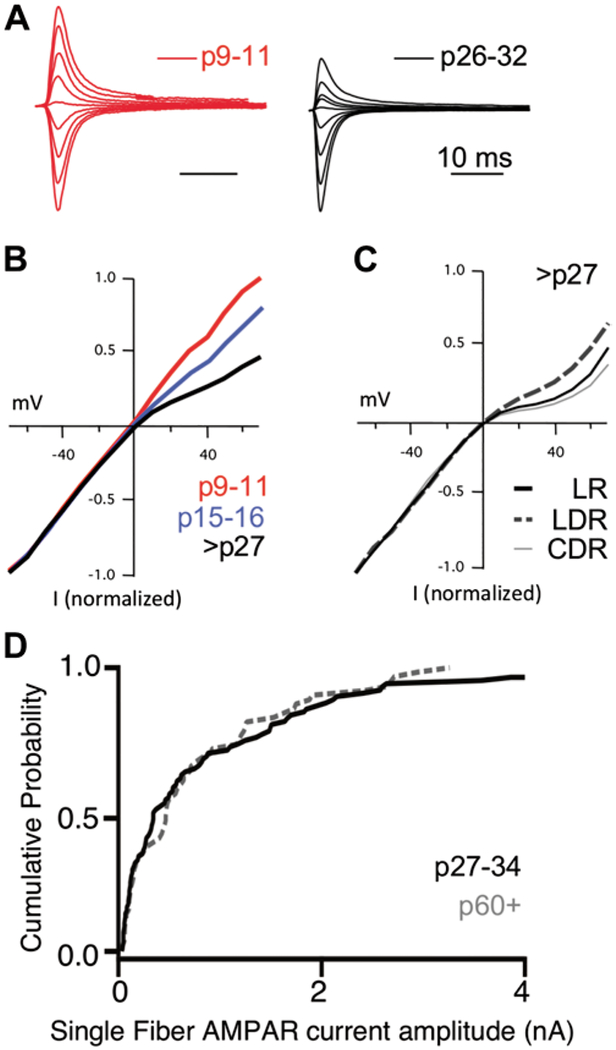
Fig. 4.
Substrates for retinogeniculate plasticity. (A) Overlaid AMPAR current traces recorded from different holding potentials to assess the current voltage (I–V) relationship. Currents in the presence of CPP to block NMDAR currents and with spermine in the internal solution to examine the degree of I–V rectification. Calcium-permeable AMPARs exhibit a rectifying I–V relationship. Traces were recorded at 20 mV increments from −60 to +60 mV holding potentials. Left-example obtained before eye opening; right, example from a mature slice. From Hauser et al. (2014). (B) Change in the average AMPAR EPSC I–V relationship over development. Rectification of I–V currents increases significantly from p9–11 to maturity, indicating a gradual increase in the contribution of CP-AMPARs to AMPAR-mediated currents. Modified from Hauser et al. (2014). Red: p9–11; blue: p15–16; black: p27–32. (C) Changes in AMPAR subunit composition in response to visual experience. The effect of visual deprivation from p20 (late-dark rear, LDR) or dark rearing from birth (chronic dark reared, CDR) on the AMPAR EPSC I–V relationship. Rectification of AMPAR currents is reduced in LDR but not in chronically dark reared (CDR) mice when compared to normally reared mice (light rear, LR) mice. P = 0.03. Recordings performed at p27–32. Modified from Louros et al. (2014). (D) Comparison of the distribution of amplitudes of single fiber RGC inputs in juvenile (p27–34) and adult (p60+) mice show the persistence of weak (small-amplitude) inputs with age. Modified from Hong et al. (2014). All figures reprinted with permission.
The in vitro retinogeniculate preparation has permitted the identification of mechanisms regulating retinogeniculate synaptic transmission at an unmatched resolution. The synaptic mechanisms discussed above are part of a larger array of factors that affect retinogeniculate information transfer, including circuit elements that influence postsynaptic integration in the TC neuron: local and extrageniculate GABAergic circuits, reciprocal connectivity with the cortex, and brainstem modulatory inputs (reviewed in Sherman & Guillery, 2002). Together, these synaptic and circuit mechanisms impart the dynamic features that regulate transmission of information at the retinogeniculate synapse. Interestingly, the paired-stimulus enhancement of retinogeniculate transmission in vivo acts not only to increase the signal-to-noise of retino-geniculo-cortical information transfer, but also encode emergent features in the spike code (Sincich et al., 2009; Rathbun et al., 2010; Wang et al., 2010). Thus these synaptic mechanisms may also impart novel functionality at the level of dLGN. Further, as these mechanisms can rapidly alter the contribution of a particular retinal input to postsynaptic spiking, they may regulate the contribution of strong versus weak RGC inputs to visual processing (discussed below).
Neurotransmission before eye-opening
The distinct features of the immature retinogeniculate synapse suggest that the immature dLGN carries out a different set of computations on incoming retinal information than the mature dLGN. Many of the modulatory circuits that shape transmission in the adult dLGN begin to innervate TC neurons shortly before eye-opening: corticogeniculate innervation is not complete in mice until p14 (Jacobs et al., 2007; Seabrook et al., 2013; Grant et al., 2016), and cholinergic innervation develops over several postnatal weeks in cats (Carden et al., 2000). GABAergic interneurons continue to be recruited into the dLGN at the end of the first postnatal week in mice, and GABAergic innervation in rodents and carnivores occurs gradually (Shatz and Kirkwood, 1984; Ramoa & McCormick, 1994a; Pirchio et al., 1997; Ziburkus et al., 2003; Golding et al., 2014). In addition, presynaptic ultrastructural morphology and TC dendritic arbor complexity are immature at eye-opening (Bickford et al., 2010). Therefore, retinogeniculate transmission before eye-opening occurs in a very different environment than after circuits have matured.
Whereas mature TC neurons receive and integrate information from one or several strong retinal inputs that can reach several nA in amplitude, numerous weak inputs, measuring on average ~40 pA in amplitude (peak AMPAR EPSC) innervate a TC neuron before eye opening (in vitro in mice; Hooks and Chen, 2006). Remarkably, retinogeniculate transmission to cortex does occur before input refinement: both the spontaneous patterns of activity (retinal waves) that prominently feature in the developing retina, and visually-evoked stimuli detectable through the closed eyelid influence the activity of the visual cortex (Katz & Shatz, 1996; Mooney et al., 1996; Feller, 1999; Akerman et al., 2002; Hanganu et al., 2006; Ackman et al., 2012). In slice, the synaptic charge transfer needed to drive TC neuron spiking before eye-opening is relatively small: an AMPAR EPSC with a peak amplitude of 120 pA is adequate (Liu & Chen, 2008). The coincident activation of a subset of the dozen or more converging RGC inputs, perhaps relying on synchronous activity that dominates retinal activity during this period of development, can achieve this amplitude (Wong et al., 1993; Wong, 1999; Butts & Rokhsar, 2001; Feller, 2009).
Multiple mechanisms contribute to the efficacy of neurotrans-mission at the immature weak retinogeniculate synapse. A study using the slow calcium chelator, EGTA-AM, suggested that the distance between the presynaptic release machinery and calcium channels at retinal terminals is greater at immature presynaptic specializations, resulting in delayed or asynchronous release (Borst & Sakmann, 1996; Hauser et al., 2014). Additionally, the immature nervous system produces a slower action potential waveform (Taschenberger & von Gersdorff, 2000; Murphy & du Lac, 2001) and a lower density of glutamate transporters in surrounding astrocytes (Thomas et al., 2011). These properties of the immature synapse lead to the prolonged exposure of postsynaptic receptors to glutamate, promoting the integration of retinal EPSCs over a longer time scale than after maturation.
Several postsynaptic mechanisms also improve the integration of weak RGC inputs at young ages. The temporal window for postsynaptic summation is much greater before eye opening (Liu & Chen, 2008). This is due in part to the higher input resistance in immature neurons (Ramoa & McCormick, 1994b; Macleod et al., 1997; Pirchio et al., 1997), as well as enhanced conduction through NMDA receptors at negative potentials because of a greater contribution of both NR2B as well as NR2C/D subunits at young synapses (Ramoa & McCormick, 1994a; Liu & Chen, 2008). These receptors exhibit slower decay kinetics and a lower sensitivity to magnesium block (compare between ages in Fig. 3), and their contribution declines over development in an activity-regulated manner (Ramoa & Prusky, 1997; Chen & Regehr, 2000; Liu & Chen, 2008).
AMPAR current amplitudes at the immature retinogeniculate synapse are much smaller than later in life (Figs. 2A and 3B). In fact, a substantial fraction (22%) of immature RGC inputs are “silent” (lacking detectable AMPAR currents; Isaac et al., 1995; Liao et al., 1995; Chen & Regehr, 2000). Transmission before eye opening therefore seems to rely almost entirely on NMDAR transmission, with AMPARs influencing the latency to spike (Liu & Chen, 2008). Finally, immature neurons also exhibit calcium plateau potentials (Jaubert-Miazza et al., 2005; Lo et al., 2013), and more depolarized resting membrane potentials that are closer to firing threshold (Ramoa & McCormick, 1994b; Macleod et al., 1997; Pirchio et al., 1997), increasing the efficacy of individual inputs.
In summary, the developing retinogeniculate synapse exhibits numerous adaptations that permit it to integrate and transfer visual signals to cortex even while it undergoes dramatic synaptic rearrangement. As these signals arise from the summation of multiple weak convergent RGC inputs, the computations that the immature dLGN performs, and therefore its role in visual processing, is substantially different from that of the mature dLGN. In addition to a role in conveying visual information, retinogeniculate transmission is important for cortical map formation (Huberman et al., 2008b; Cang & Feldheim, 2013; Owens et al., 2015), though its precise computational role is not yet understood.
Retinogeniculate connectivity
Developmental refinement of retinogeniculate connectivity
Retinogeniculate refinement is thought to lead to the maturation of receptive field properties in the dLGN. Before eye opening, RGC axons from the two eyes segregate into eye-specific layers: segments of the axon arbor that occupy the inappropriate layer are pruned, while the appropriately positioned portion of the arbor becomes more elaborate in a number of species (Robson, 1981; Mason, 1982; Sretavan & Shatz, 1984, 1986; Campbell & Shatz, 1992; Garraghty & Sur, 1993; Dhande et al., 2011; Hong et al., 2014). In vitro studies of the dLGN in rodents show that each TC neuron receives weak inputs from more than a dozen RGCs. Some of these inputs are subsequently pruned while others strengthen to become dominant drivers of postsynaptic activity (reviewed in Guido, 2008; Huberman et al., 2008a; Hong & Chen, 2011; Thompson et al., 2017). This refinement occurs over several weeks following eye opening in mice. The earliest phases depend on spontaneous input from the retina, while visual experience maintains the mature configuration and modifies connectivity via feedback from the cortex during a critical period (Hooks & Chen, 2006; Thompson et al., 2016).
The robust refinement of retinogeniculate connectivity demonstrated in vitro in mice corresponds temporally to the developmental transformation of initially broad, irregularly-shaped or temporally imprecise receptive fields to smaller, sharper, or temporally precise ones that closely match the receptive field of the dominant retinal inputs in vivo (cat: Wiesel & Hubel, 1963; Daniels et al., 1978; Tootle & Friedlander, 1989; Gary-Bobo et al., 1995; Cai et al., 1997; Ferret: Tavazoie & Reid, 2000, Akerman et al., 2002, Davis et al., 2015; primate: Blakemore & Vital-durand, 1985; mouse in vitro: Chen & Regehr, 2000; Jaubert-Miazza et al., 2005). Surprisingly, there is a disconnect between structure and function—studies in cats, mice, and primates fail to show large-scale pruning of the axon arbor during this later window of development (Sur et al., 1984, 1987; Lachica & Casagrande, 1988; Hong et al., 2014). A recent study that examined individually reconstructed axon arbors of a subtype of mouse RGCs, the BD-RGC (ON–OFF direction-selective RGC, Kim et al., 2010), found that their size and branching complexity remain stable in the 2–3 weeks following eye opening. Instead, during the period of robust functional refinement, changes occur in bouton size and distribution along the arbor structure (Hong et al., 2014). Before eye-opening, boutons are distributed broadly along the terminal arbor in mice, but gradually form tight clusters over the later window of development (for examples of mouse and cat RGC axon bouton clustering, see Fig. 1B and 1C). This development suggests that an immature axon makes transient contacts with a large number of potential postsynaptic targets, but redistributes its inputs onto a few targets during the period of activity-dependent refinement. Final pruning of the arbor skeleton, however, does not occur until well after the end of the geniculate and cortical critical periods (Hong et al., 2014). These findings are consistent with observations in cat and primate studies of the complexity of mature RGC axon morphology (Fig. 1A), with multiple segments that can branch off the primary axon within the optic tract (Sur & Sherman, 1982; Hamos et al., 1987; Sur et al., 1987; Garraghty et al., 1988; Dhande et al., 2011; Hong et al., 2014), although the arbors may be more restricted in the primate dLGN (Glees & Le Gros Clark, 1941; Lachica & Casagrande, 1988; Michael, 1988; Conley & Fitzpatrick, 1989). In cat, one RGC axon arbor spans the territory of far more postsynaptic neurons than it contacts (Hamos et al., 1987). Reduction of the X-RGC axon arbor occurs between 4 and 12 weeks postnatal, after the peak of ocular dominance plasticity (Hubel & Wiesel, 1970; Sur et al., 1984).
Therefore, while retinogeniculate development yields a circuit with appreciable functional specificity, the anatomical correlates of this process suggests latent complexity in the mature system. The breadth of the RGC axon arbor, which may impart the potential to synapse onto new TC partners even in the adult, together with short-term plasticity mechanisms that modulate the efficacy of existing contacts, provide the scaffold for dynamic computation beyond the relay of retinal firing patterns to the cortex (Alonso et al., 2006; Martinez et al., 2014; Usrey & Alitto, 2015).
Convergence at the retinogeniculate synapse
Retinogeniculate convergence (and divergence) add complexity to visual processing in the dLGN. The simplest circuit, where 1 RGC contacts 1 TC neuron, is most consistent with the concept of a thalamic “relay” (Glees & Le Gros Clark, 1941; Sherman & Guillery, 1996). More complex circuits with converging RGC inputs and/or diverging single RGC axons onto multiple target TC neurons increase the likelihood of the emergence of novel visual features or receptive field properties (Dan et al., 1998; Alonso et al., 2006; Koepsell et al., 2009; Usrey & Alitto, 2015; Sherman, 2016; Weyand, 2016). For these reasons, studies quantifying connectivity, and in particular, the degree of retinogeniculate convergence, is an active area of research.
A tour-de-force serial electron microscopy (EM) reconstruction of the synaptic contacts of one branch of an X-type retinal axon in the cat dLGN demonstrated that a RGC axon makes connections selectively rather than randomly. The reconstructed portion of the axon (reproduced in Fig. 1A) innervated three X-cells and one Y-cell, and its inputs accounted for as much as 33, 49, and 100% of total innervation to the X-cells, and as little as <6% to the Y-cell. This study concluded simultaneously that a TC neuron can receive inputs from multiple RGCs (convergence), and that some of those inputs can also contact other TC neurons (divergence/multiplexing; Hamos et al., 1987). However, the single X-cell that received all of its inputs from the labeled axon remains the best-recognized result, serving as exemplary anatomical evidence for low retinogeniculate convergence. In contrast, Robson (1993) estimated that cat Y-cells receive upwards of 10 inputs per cell, suggesting that convergence varies depending on cell type.
Recent studies using new anatomical methods in p30 mice, however, came to a conclusion that counters the general view of low convergence. Morgan and colleagues used an approach that combines serial section EM with circuit tracing, to identify the presynaptic RGC axons that connect to reconstructed postsynaptic TC neurons in the dLGN. They observed at least 40 RGC axon segments contacting one of these TC neurons (Morgan et al., 2016). Many axons also promiscuously diverged to innervate numerous other TC neurons. Hammer and colleagues reached a number closer to 10 inputs per cell from observations of bouton clustering of multi-color fluorescently labeled RGC axons (Brainbow labeling) in the mouse LGN (Hammer et al., 2015). However, these studies were not able to trace the axon segments to the primary axon, raising the possibility that they were overestimating the number of inputs to a given TC neuron. Overcoming this limitation, and despite low efficiency of rabies tracing, Rompani and colleagues showed that 1–36 RGCs innervate monocular neurons, and up to 91 RGCs from both eyes converge onto binocularly innervated neurons in the mouse dLGN. The three anatomical studies made no distinctions between X- and Y-cells, but the rabies tracing identified three different patterns of convergence in the binocular dLGN region (Rompani et al., 2017). Importantly, these studies cannot determine whether all the identified contacts are functional; many convergent inputs could be nonfunctional remnants of refinement, as final pruning of the axon arbor occurs between p30 and p60 (Hong et al., 2014). Nonetheless, these independent studies using disparate anatomical methods demonstrate that tens of RGCs may converge onto mature mouse TC neurons.
To date, most functional studies have yielded a more conservative estimate of convergence than the ultrastructural literature. In the rodent slice prep, estimates of the number of afferent inputs obtained by varying the intensity of optic tract stimulation yield numbers ranging from 1 to 5 (Chen & Regehr, 2000; Jaubert-Miazza et al., 2005; Ziburkus & Guido, 2006; Hooks & Chen, 2007; Chung et al., 2013; Lee et al., 2014; Dilger et al., 2015). However, this approach likely underestimates convergence due to the severing of axons in slice. It also averages across the population of TC neurons accessible with this method, with no distinction between TC neuron sub-types with different input convergence that may exist in the mouse dLGN (Krahe et al., 2011).
Estimates of retinogeniculate connectivity from carnivores and nonhuman primates have largely utilized recordings that assess the correlation of spiking activity of RGC-TC neuron pairs in vivo (Cleland et al., 1971; Levick et al., 1972; Kaplan & Shapley, 1984; Mastronarde, 1987, 1992; Usrey et al., 1998; Rowe & Fischer, 2001; Carandini et al., 2007; Sincich et al., 2007; Rathbun et al., 2010). Many of these experiments show that a geniculate X-cell (cat) or M or P cell (primate) receives at least one dominant input that reliably drives EPSCs preceding all or most of a TC neuron’s spikes (Cleland et al., 1971; Cleland & Lee, 1985; Soodak et al., 1987; Sincich et al., 2007). Others, however (especially those focusing on Y cells in cats), show that the contribution from individual RGCs exhibits greater variability, and the activity of a single retinal input rarely accounts for the entirety of the activity of its TC neuron partner (Hubel & Wiesel, 1961; Cleland & Levick, 1971; Cleland et al., 1971; Levick et al., 1972; Mastronarde, 1992). Interestingly, one study using paired recordings across both X- and Y-cells yielded examples of RGCs that drove as few as ~1% to as many as 82% of a TC neuron’s action potentials (Usrey et al., 1999); similar results later emerged in the Y pathway (Yeh et al., 2009; Rathbun et al., 2016; considered in detail in; Weyand, 2016). Furthermore, several studies corroborate anatomical observations of divergence, such that neurons with most closely matching receptive fields exhibit the greatest correlation among their firing patterns (Alonso et al., 1996; Usrey et al., 1998).
The variability in the contribution of a single RGC to postsynaptic spiking in cat dLGN is consistent with anatomical studies, if the number of contacts between a single RGC axon and TC cell relates to the functional strength of the individual input (Hamos et al., 1987). The findings in cat are also consistent with in vitro functional data from mice. Even in adult mice (p60), the distribution of single RGC input amplitudes ranges from a tens of pA to several nA in strength (Fig. 4D; Hooks & Chen, 2008; Hong et al., 2014; Thompson et al., 2016). The fact that weak convergent inputs persist into adulthood, in both cats and mice, suggest that they have relevance for retinogeniculate function (Alonso et al., 1996; Dan et al., 1998; Usrey et al., 1999).
Taken together, the retinogeniculate circuit exhibits organization that is set up to actively tune, select, or elaborate information that is being conveyed from the retina to the cortex, suggesting that the dLGN participates in complex processing of visual information (Sherman, 2016). While similarities across species support this view, insight into the function of dLGN should come from further elucidation of the differences between mice, cat and primates.
Retinogeniculate plasticity
In addition to short-term plasticity, the retinogeniculate circuit exhibits long-term plasticity of synaptic weights. The finding that both weak and strong inputs innervate mature TC neurons and contribute to their spiking activity highlights the possibility that experience-dependent plasticity of RGC input strength and number relies on the balancing of synaptic weights in the adult circuit (Thompson et al., 2016). The retinogeniculate connectivity map remodels to experience during development, and may also do so in mature animals. Depriving juvenile mice of visual experience for a week starting at p20 (late dark rearing) disrupts retinogeniculate connectivity, decreasing the amplitude of the average retinal input and increasing the overall number of RGC inputs onto TC neurons (Hooks & Chen, 2006, 2008; Narushima et al., 2016). This manipulation also reduces the clustering of RGC axon boutons without significantly altering the size of the arbor or the number of boutons (Hong et al., 2014). Together, these observations suggest that while connectivity between axons and targets is selective, the large size of the arbor builds flexibility into the system: dramatic change in visual experience, such as late dark rearing can resculpt connectivity by rearranging boutons and adjusting input strength (Louros et al., 2014) without investing into remodeling the entire axonal arbor. Because excess branches of RGC arbors do not prune down until at least p60 in mice, retinogeniculate connectivity may exhibit substantial plasticity until at least this age (Hong et al., 2014).
A well-established mechanism for altering synaptic strength in response to activity or experience is through changes in AMPAR content of the post-synaptic density (Huganir & Nicoll, 2013). Several studies link the regulation of AMPAR trafficking and function to modulation of the strength of juvenile retinogeniculate synapses. The retinogeniculate synapse is one among several synapses that recruit GluA1-containing AMPARs in response to sensory stimulation (Clem & Barth, 2006; Kielland et al., 2009; Louros et al., 2014), suggesting that GluA1-dependent AMPAR-driven forms of transmission play an important role in the development and plasticity at this synapse (Fig. 4A–4C; activity-dependent changes in AMPAR subunit composition reviewed in Cull-Candy et al., 2006; Liu & Zukin, 2007; Lee et al., 2014). AMPAR subunit content is sensitive to visual experience: mice subjected to late dark rearing exhibited a decrease in AMPAR current rectification, a measure of the fraction of calcium-permeable to calcium-impermeable AMPARs in the postsynaptic density. In contrast, mice that never had any visual experience (chronically dark reared from birth) exhibited normal rectification (Fig. 4C; Louros et al., 2014). Experience-dependent changes in AMPAR content and function at the retinogeniculate synapse rely in part on stargazin, a transmembrane AMPA regulatory protein that modifies the trafficking and channel kinetics of AMPARs (Straub & Tomita, 2012). Indeed, late dark rearing increases the expression and phosphorylation of star-gazin, which can in turn regulate the composition of postsynaptic AMPARs in both a Hebbian (Tomita et al., 2005) or homeostatic manner (Louros et al., 2014). Finally, changes in AMPAR expression also mediate the role of MHC class I molecule H2-Db in retinogenicu-late developmental refinement (Lee et al., 2014). H2-Db is one of a series of immune-related molecules that shape retinogeniculate development (Shatz, 2009; Schafer & Stevens, 2010). Mice lacking H2-Db expression exhibit an increase in calcium-permeable AMPARs at retinogeniculate synapses, corresponding to a deficit in LTD. Together, these studies bespeak a critical role of AMPAR regulation in retinogeniculate synaptic plasticity, which may persist into adulthood.
Both Hebbian and homeostatic mechanisms of synaptic plasticity have been shown to alter retinogeniculate synaptic strength during development (Mooney et al., 1993; Butts et al., 2007; Ziburkus et al., 2009; Krahe & Guido, 2011; Lin et al., 2014; Louros et al., 2014). High frequency stimulation of the optic tract, or low frequency stimulation coincident with postsynaptic depolarization in slices from immature ferret dLGN result in long-term enhancement of the EPSC, with contribution from NMDAR activation (Mooney et al., 1993). However, in rat, the same high-frequency stimulus results in long-term depression in dLGN explants before eye-opening, but long-term potentiation later in development (Ziburkus et al., 2009). Finally, plasticity rules based on burst timing have been identified before eye-opening (Butts et al., 2007 in rat), but these plasticity rules have not been examined in more mature slices. On the other hand, the contribution of homeostatic plasticity in retinogeniculate plasticity has been suggested through studies involving monocular deprivation, chronic dark rearing, manipulation of stargazin, and deletion of Mecp2 (a transcription factor necessary for homeostatic scaling up in the visual cortex; Blackman et al., 2012; Noutel et al., 2011; Krahe & Guido, 2011; Lin et al., 2014; Louros et al., 2014). Similar paradigms likely also drive synaptic plasticity at the fully mature retinogeniculate synapse.
Recently described instances of rapid plasticity across species could also engage Hebbian or homeostatic mechanisms at the retinogeniculate synapse and cause a shift in the strength of individual retinogeniculate inputs (Moore et al., 2011; Aguila et al., 2017). In fact, dLGN neurons readily adapt to changes in visual input. For example, pharmacologic blockade of On-center RGC activity in adult cats rapidly uncovers Off-center responses in dLGN neurons previously exhibiting On-center responses, instead of silencing them (Moore et al., 2011). Further, acute suppression of cortical feedback in awake monkeys shifted the receptive field position of a subset of TC neurons (Aguila et al., 2017). Finally, rabbit genicu-late neurons exhibit bidirectional sensory adaptation that improves signal detection (Stoelzel et al., 2015). While modulatory mechanisms could shape the response of the TC neuron to rapid changes in upstream inputs, the specificity that TC neuron responses exhibit in these studies indicates instead a role for rapid shifts in the synaptic efficacy of retinogeniculate connections. These changes occur too rapidly to rely on structural rearrangements of retinogeniculate connections, their timecourse is consistent with possible unsilencing or strengthening of functionally silent or weak sub-threshold inputs via the insertion of postsynaptic receptors at dormant synaptic sites. Of course, local inhibitory circuits may also contribute to rapid shifts in RGC input efficacy (Fisher et al., 2017). Changes that occur over days rather than hours may also recruit the redistribution of presynaptic boutons along the broad RGC axon arbor. The capacity of the diverse types of retinogenicu-late synaptic contacts for functional plasticity remains unexplored.
The expression of a variety of mechanisms for modification of synaptic weights is layered on top of a potentially densely interconnected network that is evident in considerable retinogeniculate divergence and convergence. Combined with the observation that both weak and strong inputs innervate mature TC neurons, these plasticity mechanism may endow the dLGN with a role in visual learning on multiple time scales (Ramos et al., 1976; Albrecht et al., 1990).
Conclusion
Recent work is revealing new complexities of retinogeniculate transmission and circuit organization that further expand the potential role of the dLGN in visual processing beyond its classic attributes (Steriade et al., 1997; Sherman & Guillery, 2001). The morphological diversity of synaptic motifs and complex connectivity patterns, combined with short- and long-term plasticity mechanisms of the retinogeniculate circuit demonstrate that the retinogeniculate synapse makes substantial and dynamic contributions to the processing of visual information. Weak or non-dominant retinogeniculate inputs in the mature dLGN, which have repeatedly been dismissed as insignificant, as errors of development or leftovers from developmental plasticity with no functional relevance (Sur et al., 1984; Garraghty et al., 1985; Hamos et al., 1987), likely enhance extraction of visual features in the geniculate and visual cortex, and serve as strategic reserves of plasticity. Moreover, the convergence of potentially heterogeneous RGC inputs onto single geniculate neurons could give rise to new receptive field features such as orientation selectivity in mouse dLGN, as recently proposed (Stafford & Huberman, 2017). Finally, the interplay between the strength and short-term plasticity properties of RGC inputs in the context of convergence and divergence adds to the richness of the dLGN circuitry.
Much is still left to understand about the extent and underlying basis of plasticity in the dLGN. However, the idea that the mature geniculate system can utilize activity-dependent plasticity mechanisms to fine-tune the contribution of its individual inputs in response to novel visual challenges, experiences, changes in modulatory state, or retinal degeneration appears to be rapidly gaining experimental support. Future studies are needed to clarify the differences in the number of inputs that converge onto TC neurons between species, because the results may correlate with the degree by which new receptive field features emerge at the level of dLGN. Elucidation of whether and how weak inputs contribute to visual processing in different species will also uncover the richness of thalamic function.
The continually expanding toolbox for interrogating diversity in neuronal circuits is already uncovering nuances in the contribution of different RGC types to TC neuron function (Storchi et al., 2015; Denman et al., 2016). Advances in methods for labeling, activating, and measuring the activity of different neuronal populations that have been deployed extensively in mice may also reveal underappreciated subtleties of retinogeniculate transmission in other species (Scholl et al., 2013; Zaltsman et al., 2015; Zeater et al., 2015; Suresh et al., 2016). Shifting models of the organization of the visual system that take into account the important nuances of retinogeniculate functional organization and plasticity are certain to provide new models of visual system development and function.
Acknowledgment
The authors thank C.-E. Stephany, G. Rankin and A.D. Thompson for their critical reading and helpful discussions on the manuscript. This work was supported by the NIH R01EY013613 and NIH U54 HD090255 to CC and the Edward R. and Anne G. Lefler Center Predoctoral Fellowship to EYL.
References
- Ackman JB, Burbridge TJ & Crair MC (2012). Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguila J, Cudeiro FJ & Rivadulla C (2017). Suppression of V1 feedback produces a shift in the topographic representation of receptive fields of LGN cells by unmasking latent retinal drives. Cerebral Cortex 27, 3331–3345. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Smyth D & Thompson ID (2002). Visual experience before eye-opening and the development of the retinogeniculate pathway. Neuron 36, 869–879. [DOI] [PubMed] [Google Scholar]
- Albrecht D, Davidowa H & Gabriel H (1990). Conditioning-related changes of unit activity in the dorsal lateral geniculate nucleus of urethane-anaesthetized rats. Brain Research Bulletin 25, 55–63. [DOI] [PubMed] [Google Scholar]
- Alitto HJ, Moore BD, Rathbun DL & Martin Usrey W (2011). A comparison of visual responses in the lateral geniculate nucleus of alert and anaesthetized macaque monkeys. The Journal of Physiology 589, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Usrey WM & Reid RC (1996). Precisely correlated firing in cells of the lateral geniculate nucleus. Nature 383, 815–819. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Yeh CI, Weng C & Stoelzel CR (2006). Retinogeniculate connections: A balancing act between connection specificity and receptive field diversity. Progress in Brain Research 154, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinaite S & Heggelund P (2007). Changes in firing pattern of lateral geniculate neurons caused by membrane potential dependent modulation of retinal input through NMDA receptors. The Journal of Physiology 582, 297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T & Nägerl UV (2008). LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron 60, 590–597. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, He Z & Goldberg JL (2017). Reaching the brain: Advances in optic nerve regeneration. Experimental Neurology 287, 365–373. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Slusarczyk A, Dilger EK, Krahe TE, Kucuk C & Guido W (2010). Synaptic development of the mouse dorsal lateral geniculate nucleus. Journal of Comparative Neurology 518, 622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P, Burke W & Davis R (1959). Activation of single lateral genicu-late cells by stimulation of either optic nerve. Science 130, 506–507. [DOI] [PubMed] [Google Scholar]
- Blackman MP, Djukic B, Nelson SB & Turrigiano GG (2012). A critical and cell-autonomous role for MeCP2 in synaptic scaling up. The Journal of Neuroscience 32, 13529–13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C & Vital-durand F (1985). Organization and post-natal development of the monkey’s lateral geniculate nucleus. Journal of Physiology 380, 453–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Foster KA & Regehr WG (2004). Short-term synaptic plasticity: A comparison of two synapses. Nature Reviews Neuroscience 5, 630–640. [DOI] [PubMed] [Google Scholar]
- Blitz DM & Regehr WG (2003). Retinogeniculate synaptic properties controlling spike number and timing in relay neurons. Journal of Neurophysiology 90, 2438–2450. [DOI] [PubMed] [Google Scholar]
- Borst JG & Sakmann B (1996). Calcium influx and transmitter release in a fast CNS synapse. Nature 383, 431–434. [DOI] [PubMed] [Google Scholar]
- Budisantoso T, Matsui K, Kamasawa N, Fukazawa Y & Shigemoto R (2012). Mechanisms underlying signal filtering at a multisynapse contact. The Journal of Neuroscience 32, 2357–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Kanold PO & Shatz CJ (2007). A burst-based ‘Hebbian’ learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biology 5, e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA & Rokhsar DS (2001). The information content of spontaneous retinal waves. Journal of Neuroscience 21, 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, DeAngelis GC & Freeman RD (1997). Spatiotemporal receptive field organization in the lateral geniculate nucleus of cats and kittens. Journal of Neurophysiology 78, 1045–1061. [DOI] [PubMed] [Google Scholar]
- Campbell G & Shatz CJ (1992). Synapses formed by identified retinogeniculate axons during the segregation of eye input. The Journal of Neuroscience 12, 1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J & Feldheim DA (2013). Developmental mechanisms of topo-graphic map formation and alignment. Annual Review of Neuroscience 36, 51–77. [DOI] [PubMed] [Google Scholar]
- Carandini M, Horton JC & Sincich LC (2007). Thalamic filtering of retinal spike trains by postsynaptic summation. Journal of Vision 7, 20.1–20.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden WB, Datskovskaia A, Guido W, Godwin DW & Bickford ME (2000). Development of the cholinergic, nitrergic, and GABAergic innervation of the cat dorsal lateral geniculate nucleus. The Journal of Comparative Neurology 418, 65–80. [DOI] [PubMed] [Google Scholar]
- Chen C, Blitz DM & Regehr WG (2002). Contributions of receptor desensitization and saturation to plasticity at the retinogeniculate synapse. Neuron 33, 779–788. [DOI] [PubMed] [Google Scholar]
- Chen C & Regehr WG (2000). Developmental remodeling of the retinogeniculate synapse. Neuron 28, 955–966. [DOI] [PubMed] [Google Scholar]
- Chen C & Regehr WG (2003). Presynaptic modulation of the retinogeniculate synapse. The Journal of Neuroscience 23, 3130–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong SKK, Tailby C, Solomon SG & Martin PR (2013). Cortical-like receptive fields in the lateral geniculate nucleus of marmoset monkeys. Journal of Neuroscience 33, 6864–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W-S, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ & Barres BA (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW & Levick WR (1971). Sustained and transient neurones in the cat’s retina and laterla geniculate nucleus. Journal of Physiology 217, 473–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG & Lee BB (1985). A comparison of visual responses of cat lateral geniculate nucleus neurones with those of ganglion cells afferent to them. Journal of Physiology 369, 249–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG & Levick WR (1971). Simultaneous recording of input and output of lateral geniculate neurones. Nature 231, 191–192. [DOI] [PubMed] [Google Scholar]
- Clem RL & Barth A (2006). Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron 49, 663–670. [DOI] [PubMed] [Google Scholar]
- Clements J, Lester R, Tong G, Jahr C & Westbrook G (1992). The time course of glutamate in the synaptic cleft. Science 258, 1498–1501. [DOI] [PubMed] [Google Scholar]
- Conley M & Fitzpatrick D (1989). Morphology of retinogeniculate axons in the macaque. Visual Neuroscience 2, 287–296. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Kelly L & Farrant M (2006). Regulation of Ca2+−permeable AMPA receptors: Synaptic plasticity and beyond. Current Opinion in Neurobiology 16, 288–297. [DOI] [PubMed] [Google Scholar]
- Dan Y, Alonso JM, Usrey WM & Reid RC (1998). Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nature Neuroscience 1, 501–507. [DOI] [PubMed] [Google Scholar]
- Danbolt NC (2001). Glutamate uptake. Progress in Neurobiology 65, 1–105. [DOI] [PubMed] [Google Scholar]
- Daniels JD, Norman JL & Pettigrew JD (1977). Biases for oriented moving bars in lateral geniculate nucleus neurons of normal and stripe-reared cats. Experimental Brain Research 29, 155–72. [DOI] [PubMed] [Google Scholar]
- Daniels JD, Pettigrew JD & Norman JL (1978). Development of single-neuron responses in kitten’s lateral geniculate nucleus. Journal of Neurophysiology 41, 1373–1393. [DOI] [PubMed] [Google Scholar]
- Davis ZW, Chapman B & Cheng JL (2015). Increasing spontaneous retinal activity before eye opening accelerates the development of geniculate receptive fields. Journal of Neuroscience 35, 14612–14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman D, Siegle JH, Koch C, Reid RC & Blanche TJ (2016). Spatial organization of chromatic pathways in the mouse dorsal lateral geniculate nucleus. Journal of Neuroscience 37, 1102–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Hua EW, Guh E, Yeh J, Bhatt S, Zhang Y, Ruthazer ES, Feller MB & Crair MC (2011). Development of single retinofugal axon arbors in normal and β2 knock-out mice. The Journal of Neuroscience 31, 3384–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS & Jahr CE (1997). Transporters buffer synaptically released glutamate on a submillisecond time scale. The Journal of Neuroscience 17, 4672–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger EK, Krahe TE, Morhardt DR, Seabrook TA, Shin H-S & Guido W (2015). Absence of plateau potentials in dLGN cells leads to a breakdown in retinogeniculate refinement. The Journal of Neuroscience 35, 3652–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Danaf RN, Krahe TE, Dilger EK, Bickford ME, Fox M & Guido W (2015). Developmental remodeling of relay cells in the dorsal lateral geniculate nucleus in the absence of retinal input. Neural Development 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV & Peters A (1972). The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. The Journal of Comparative Neurology 144, 285–333. [DOI] [PubMed] [Google Scholar]
- Felch DL & Van Hooser SD (2012). Molecular compartmentalization of lateral geniculate nucleus in the gray squirrel (Sciurus carolinensis). Frontiers in Neuroanatomy 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB (1999). Spontaneous Correlated Activity in Developing Neural Circuits. Neuron 22, 653–656. [DOI] [PubMed] [Google Scholar]
- Feller MB (2009). Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Development 4, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TG, Alitto HJ & Usrey WM (2017). Retinal and non-retinal contributions to extraclassical surround suppression in the lateral genicu-late nucleus. Journal of Neuroscience 37, 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freche D, Pannasch U, Rouach N & Holcman D (2011). Synapse geometry and receptor dynamics modulate synaptic strength. PLoS ONE 6, e25122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Hsiao CF, Watanabe M & Ito H (1984). Morphological correlates of physiologically identified Y-, X-, and W-cells in cat retina. Journal of Neurophysiology 52, 999–1013. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Salinger WL & Macavoy MG (1985). The development of cell size in the dorsal lateral geniculate nucleus of monocularly paralyzed cats. Brain Research 353, 99–106. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Shatz CJ, Sretavan DW & Sur M (1988). Axon arbors of X and Y retinal ganglion cells are differentially affected by prenatal disruption of binocular inputs. Proceedings of the National Academy of Sciences 85, 7361–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty PE & Sur M (1993). Competitive interactions influencing the development of retinal axonal arbors in cat lateral geniculate nucleus. Physiological Reviews 73, 529–545. [DOI] [PubMed] [Google Scholar]
- Gary-Bobo E, Przybyslawski J & Saillour P (1995). Experience-dependent maturation of the spatial and temporal characteristics of the cell receptive fields in the kitten visual cortex. Neuroscience Letters 189, 147–150. [DOI] [PubMed] [Google Scholar]
- Glees P & Le Gros Clark WE (1941). The termination of optic fibres in the lateral geniculate body of the monkey. The Journal of Anatomy 75, 295–307. [PMC free article] [PubMed] [Google Scholar]
- Golding B, Pouchelon G, Bellone C, Murthy S, Di Nardo AA, Govindan S, Ogawa M, Shimogori T, Lüscher C, Dayer A & Jabaudon D (2014). Retinal input directs the recruitment of inhibitory interneurons into thalamic visual circuits. Neuron 81, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Grant E, Hoerder-Suabedissen A & Molnar Z (2016). The regulation of corticofugal fiber targeting by retinal inputs. Cerebral Cortex 26, 1336–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graydon CW, Cho S, Diamond JS, Kachar B, von Gersdorff H & Grimes WN (2014). Specialized postsynaptic morphology enhances neurotransmitter dilution and high-frequency signaling at an auditory synapse. The Journal of Neuroscience 34, 8358–8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W (2008). Refinement of the retinogeniculate pathway. The Journal of Physiology 586, 4357–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R & Sherman SM (2002). Thalamic relay functions and their role in corticocortical communication: Generalizations from the visual system. Neuron 33, 163–175. [DOI] [PubMed] [Google Scholar]
- Hammer S, Monavarfeshani A, Lemon T, Su J & Fox MA (2015). Multiple retinal axons converge onto relay cells in the adult mouse thalamus. Cell Reports 12, 1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D & Sherman SM (1987). Synaptic circuits involving an individual retinogeniculate axon in the cat. The Journal of Comparative Neurology 259, 165–192. [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Uhlrich DJ & Sherman SM (1985). Synaptic connectivity of a local circuit neurone in lateral geniculate nucleus of the cat. Nature 317, 618–621. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y & Khazipov RR (2006). Retinal waves trigger spindle bursts in the neonatal rat visual cortex. The Journal of Neuroscience 26, 6728–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser JL, Edson EB, Hooks BM & Chen C (2013). Metabotropic glutamate receptors and glutamate transporters shape transmission at the developing retinogeniculate synapse. Journal of Neurophysiology 109, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser JL, Liu X, Litvina EY & Chen C (2014). Prolonged synaptic currents increase relay neuron firing at the developing retinogenicu-late synapse. Journal of Neurophysiology 112, 1714–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei X, Stoelzel CR, Zhuang J, Bereshpolova Y, Huff JM, Alonso JM & Swadlow HA (2014). Directional selective neurons in the awake LGN: Response properties and modulation by brain state. Journal of Neurophysiology 112, 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK & Chen C (2011). Wiring and rewiring of the retinogeniculate synapse. Current Opinion in Neurobiology 21, 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Park S, Litvina EY, Morales J, Sanes JR & Chen C (2014). Refinement of the retinogeniculate synapse by bouton clustering. Neuron 84, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM & Chen C (2006). Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52, 281–291. [DOI] [PubMed] [Google Scholar]
- Hooks BM & Chen C (2007). Critical periods in the visual system: Changing views for a model of experience-dependent plasticity. Neuron 56, 312–326. [DOI] [PubMed] [Google Scholar]
- Hooks BM & Chen C (2008). Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. The Journal of Neuroscience 28, 4807–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth M, Walmsley L & Brown TM (2014). Report binocular integration in the mouse lateral geniculate nuclei. Current Biology 24, 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (1961). Integrative action in the cat’s lateral geniculate body. The Journal of Physiology 155, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (1970). The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of Physiology 206, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB & Chapman B (2008a). Mechanisms underlying development of visual maps and receptive fields. Annual Review of Neuroscience 31, 479–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA & Barres BA (2008b). Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron 59, 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL & Nicoll RA (2013). AMPARs and synaptic plasticity: The last 25 years. Neuron 80, 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JTR, Nicoll RA & Malenka RC (1995). Evidence for silent synapses: Implications for the expression of LTP. Neuron 15, 427–434. [DOI] [PubMed] [Google Scholar]
- Jacobs EC, Campagnoni C, Kampf K, Reyes SD, Kalra V, Handley V, Xie Y-Y, Hong-Hu Y, Spreur V, Fisher RS & Campagnoni AT (2007). Visualization of corticofugal projections during early cortical development in a τ-GFP-transgenic mouse. European Journal of Neuroscience 25, 17–30. [DOI] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo F-S, Bui K, Mills J & Guido W (2005). Structural and functional composition of the developing retinogeniculate pathway in the mouse. Visual Neuroscience 22, 661–676. [DOI] [PubMed] [Google Scholar]
- Jones EG & Powell TPS (1969). Electron microscopy of synaptic glomruli in the thalamic relay nuclei in cat. Proceedings of the Royal Society 172, 153–171. [DOI] [PubMed] [Google Scholar]
- Kano M & Hashimoto K (2009). Synapse elimination in the central nervous system. Current Opinion in Neurobiology 19, 154–161. [DOI] [PubMed] [Google Scholar]
- Kaplan E (2014). The M, P and K pathways of the primate visual system revisited Ehud Kaplan In The New Visual Neuroscience, pp. 215–226. MIT Press. [Google Scholar]
- Kaplan E & Shapley R (1984). The origin of the S (slow) potential in the mammalian lateral geniculate nucleus. Experimental Brain Research 55, 111–116. [DOI] [PubMed] [Google Scholar]
- Kastner S, Schneider KA & Wunderlich K (2006). Beyond a relay nucleus: neuroimaging views on the human LGN. Progress in Brain Research 155, 125–143. [DOI] [PubMed] [Google Scholar]
- Katz LC & Shatz CJ (1996). Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138. [DOI] [PubMed] [Google Scholar]
- Kielland A, Bochorishvili G, Corson J, Zhang L, Rosin DL, Heggelund P & Zhu JJ (2009). Activity patterns govern synapse-specific AMPA receptor trafficking between deliverable and synaptic pools. Neuron 62, 84–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielland A & Heggelund P (2002). AMPA and NMDA currents show different short-term depression in the dorsal lateral geniculate nucleus of the rat. The Journal of Physiology 542, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I-J, Zhang Y, Meister M & Sanes JR (2010). Laminar restriction of retinal ganglion cell dendrites and axons: Subtype-specific developmental patterns revealed with transgenic markers. The Journal of Neuroscience 30, 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C (1985). Understanding the intrinsic circuitry of the cat’s lateral geniculate nucleus: Electrical properties of the spine-triad arrangement. Proceedings of the Royal Society of London Series B 225, 365–390. [DOI] [PubMed] [Google Scholar]
- Koepsell K, Wang X, Vaingankar V, Wei Y, Wang Q, Rathbun DL, Usrey WM, Hirsch JA & Sommer FT (2009). Retinal oscillations carry visual information to cortex. Frontiers in Systems Neuroscience 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe TE, El-Danaf RN, Dilger EK, Henderson SC & Guido W (2011). Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. Journal of Neuroscience 31, 17437–17448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe TE & Guido W (2011). Homeostatic plasticity in the visual thalamus by monocular deprivation. The Journal of Neuroscience 31, 6842–6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V & Huguenard JR (2002). A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. The Journal of Neuroscience 22, 3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Esguerra M & Sur M (1991). NMDA and non-NMDA receptors mediate visual responses of neurons in the cat’s lateral geniculate nucleus. Journal of Neurophysiology 66, 414–428. [DOI] [PubMed] [Google Scholar]
- Lachica EA & Casagrande VA (1988). Development of primate retinogeniculate axons. Visual Neuroscience 1, 103–123. [DOI] [PubMed] [Google Scholar]
- Lam Y-W & Sherman SM (2013). Activation of both group I and group II metabotropic glutamatergic receptors suppress retinogeniculate transmission. Neuroscience 242, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Creutzfeldt OD & Elepfandt A (1979). The responses of magno- and parvocellular cells of the monkey’s lateral geniculate body to moving stimuli. Experimental Brain Research 35, 547–557. [DOI] [PubMed] [Google Scholar]
- Lee H, Brott BK, Kirkby LA, Adelson JD, Cheng S, Feller MB, Datwani A & Shatz CJ (2014). Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature 509, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick AWR, Oyster CW & Takahashi E (2010). Rabbit lateral geniculate nucleus: Sharpener of directional information rabbit lateral geniculate nucleus: Sharpener of directional information. Science 165, 712–714. [DOI] [PubMed] [Google Scholar]
- Levick WR, Cleland BG & Dubin MW (1972). Lateral geniculate neurons of cat: Retinal inputs and physiology. Investigative Ophthalmology 11, 302–311. [PubMed] [Google Scholar]
- Levick WR & Thibos LNLN (1980). Orientation bias of cat retinal ganglion cell. Nature 286, 389–390. [DOI] [PubMed] [Google Scholar]
- Levick WR & Williams WO (1964). Maintained activity of lateral geniculate neurones in darkness. The Journal of Physiology 170, 582–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MW & Cleland BG (2001). An analysis of the effect of retinal ganglion cell impulses upon the firing probability of neurons in the dorsal lateral geniculate nucleus of the cat. Brain Research 902, 244–254. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA & Malinow R (1995). Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375, 400–404. [DOI] [PubMed] [Google Scholar]
- Lin DJ-P, Kang E & Chen C (2014). Changes in input strength and number are driven by distinct mechanisms at the retinogeniculate synapse. Journal of Neurophysiology 112, 942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ & Zukin RS (2007). Ca2+−permeable AMPA receptors in synaptic plasticity and neuronal death. Trends in Neurosciences 30, 126–134. [DOI] [PubMed] [Google Scholar]
- Liu X & Chen C (2008). Different roles for AMPA and NMDA receptors in transmission at the immature retinogeniculate synapse. Journal of Neurophysiology 99, 629–643. [DOI] [PubMed] [Google Scholar]
- Lo F, Ziburkus J & Guido W (2013). Synaptic mechanisms regulating the activation of a Ca2+−mediated plateau potential in developing relay cells of the LGN. Journal of Neurophysiology 87, 1175–1185. [DOI] [PubMed] [Google Scholar]
- Louros SR, Hooks BM, Litvina L, Carvalho AL & Chen C (2014). A role for stargazin in experience-dependent plasticity. Cell Reports 7, 1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD & Cunningham TJ (1972). Aspects of synaptic and laminar organization of the mammalian lateral geniculate body. Investigative ophthalmology 11, 291–302. [PubMed] [Google Scholar]
- Luo L & O’Leary DDM (2005). Axon retraction and degeneration in development and disease. Annual Review of Neuroscience 28, 127–156. [DOI] [PubMed] [Google Scholar]
- Macleod N, Turner C & Edgar J (1997). Properties of developing lateral geniculate neurones in the mouse. International Journal of Developmental Neuroscience 15, 205–224. [DOI] [PubMed] [Google Scholar]
- Marshel JH, Kaye AP, Nauhaus I & Callaway EM (2012). Anterior–posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron 76, 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LM, Molano-Mazón M, Wang X, Sommer FT & Hirsch JA (2014). Statistical wiring of thalamic receptive fields optimizes spatial sampling of the retinal image. Neuron 81, 943–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CA (1982). Development of terminal arbors of retino-geniculate axons in the kitten–I. Light microscopical observations. Neuroscience 7, 541–559. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN (1987). Two classes of single-input X-cells in cat lateral geniculate nucleus. II. Retinal inputs and the generation of receptive-field properties. Journal of Neurophysiology 57, 381–413. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN (1992). Nonlagged relay cells and interneurons in the cat lateral geniculate nucleus: Receptive-field properties and retinal inputs. Visual Neuroscience 8, 407–441. [DOI] [PubMed] [Google Scholar]
- Matthews G & Fuchs P (2010). The diverse roles of ribbon synapses in sensory neurotransmission. Nature Reviews Neuroscience 11, 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA & Bal T (1994). Sensory gating mechanisms of the thalamus. Current Opinion in Neurobiology 4, 550–555. [DOI] [PubMed] [Google Scholar]
- Michael CR (1988). Retinal afferent arborization patterns, dendritic field orientations, and the segregation of function in the lateral geniculate nucleus of the monkey. Proceedings of the National Academy of Sciences 85, 4914–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero VM (1991). A quantitative study of synpatic contacts on inter-neurons and relay cells of the cat lateral geniculate nucleus. Experimental Brain Research 86, 2570270. [DOI] [PubMed] [Google Scholar]
- Montero VM & Wenthold RJ (1989). Quantitative immunogold analysis reveals high glutamate levels in retinal and cortical synaptic terminals in the lateral geniculate nucleus of the macaque. Neuroscience 31, 639–647. [DOI] [PubMed] [Google Scholar]
- Mooney R, Madison DV & Shatz CJ (1993). Enhancement of transmission at the developing retinogeniculate synapse. Neuron 10, 815–825. [DOI] [PubMed] [Google Scholar]
- Mooney R, Penn AA, Gallego R & Shatz CJ (1996). Thalamic relay of spontaneous retinal activity prior to vision. Neuron 17, 863–874. [DOI] [PubMed] [Google Scholar]
- Moore BD, Kiley CW, Sun C & Usrey WM (2011). Rapid plasticity of visual responses in the adult lateral geniculate nucleus. Neuron 71, 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Berger DR, Wetzel AW & Lichtman JW (2016). The fuzzy logic of network connectivity in mouse visual thalamus. Cell 165, 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ & du Lac S (2001). Postnatal development of spike generation in rat medial vestibular nucleus neurons. Journal of Neurophysiology 85, 1899–1906. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Yagasaki Y, Harada T, Nagumo Y, Uesaka N, Hashimoto K, Aiba A, Watanabe M, Miyata M & Kano M (2016). The metabotropic glutamate receptor subtype 1 mediates experience-dependent maintenance of mature synaptic connectivity in the visual thalamus. Neuron 91, 1097–1109. [DOI] [PubMed] [Google Scholar]
- Nirenberg S & Meister M (1997). The light response of retinal ganglion cells is truncated by a displaced amacrine circuit. Neuron 18, 637–650. [DOI] [PubMed] [Google Scholar]
- Noutel J, Hong YK, Leu B, Kang E & Chen C (2011). Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice. Neuron 70, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MT, Feldheim DA, Stryker MP & Triplett JW (2015). Stochastic interaction between neural activity and molecular cues in the formation of topographic maps. Neuron 87, 1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirchio M, Turner JP, Williams SR, Asprodini E & Crunelli V (1997). Postnatal development of membrane properties and delta oscillations in thalamocortical neurons of the cat dorsal lateral geniculate nucleus. Journal of Neuroscience 17, 5428–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD & Niell C (2013). Diverse visual features encoded in mouse lateral geniculate nucleus. Journal of Neuroscience 33, 4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafols JA & Valverde F (1973). The structure of the dorsal lateral geniculate nucleus in the mouse. A Golgi and electron microscopic study. The Journal of Comparative Neurology 150, 303–331. [DOI] [PubMed] [Google Scholar]
- Ramoa AS & McCormick DA (1994a). Enhanced activation of NMDA receptor responses at the immature retinogeniculate synapse. The Journal of Neuroscience 14, 2098–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa AS & McCormick DA (1994b). Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. The Journal of Neuroscience 14, 2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa AS & Prusky G (1997). Retinal activity regulates developmental switches in functional properties and ifenprodil sensitivity of NMDA receptors in the lateral geniculate nucleus. Developmental Brain Research 101, 165–175. [DOI] [PubMed] [Google Scholar]
- Ramos A, Schwartz EL & Roy E (1976). Stable and plastic unit discharge patterns during behavioral generalization. Science 192, 393–396. [DOI] [PubMed] [Google Scholar]
- Rathbun DL, Alitto HJ, Warland DK & Usrey WM (2016). Stimulus contrast and retinogeniculate signal processing. Frontiers in Neural Circuits 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun DL, Alitto HJ & Weyand TTG (2007). Interspike interval analysis of retinal ganglion cell receptive fields. Journal of Neurophysiology 98, 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun DL, Warland DK & Usrey WM (2010). Spike timing and information transmission at retinogeniculate synapses. The Journal of Neuroscience 30, 13558–13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele TS & Rosenberg PA (2016). Neurochemistry international GLT-1: The elusive presynaptic glutamate transporter. Neurochemistry International 98, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson JA (1981). Abnormal axonal growth in the dorsal lateral geniculate nucleus of the cat. The Journal of Comparative Neurology 195, 453–476. [DOI] [PubMed] [Google Scholar]
- Robson JA (1993). Qualitative and quantitative analyses of the patterns of retinal input to neurons in the dorsal lateral geniculate nucleus of the cat. The Journal of Comparative Neurology 334, 324–336. [DOI] [PubMed] [Google Scholar]
- Robson JA & Mason CA (1979). The synaptic organization of terminals traced from individual labeled retino-geniculate axons in the cat. Neuroscience 4, 99–111. [DOI] [PubMed] [Google Scholar]
- Rompani SB, Mullner FE, Wanner A, Roth CN, Yonehara K, Zhang C & Roska B (2017). Different modes of visual integration in the lateral geniculate nucleus revealed by single-cell-initiated trans-synaptic tracing. Neuron 93, 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MH & Fischer Q (2001). Dynamic properties of retino-geniculate synapses in the cat. Visual Neuroscience 18, 219–231. [DOI] [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP & Burnashev N (1998). Facilitation of currents through rat Ca2+−permeable AMPA receptor channels by activity-dependent relief from polyamine block. Journal of Physiology 511, 361–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson KJ, Bishop P & Darian-Smith I (1971). The properties of the binocular receptive fields of lateral geniculate neurons. Experimental Brain Research 13, 178–207. [DOI] [PubMed] [Google Scholar]
- Schafer DP & Stevens B (2010). Synapse elimination during development and disease: Immune molecules take centre stage. Biochemical Society Transactions 38, 476–481. [DOI] [PubMed] [Google Scholar]
- Scholl B, Tan AYY, Corey J & Priebe NJ (2013). Emergence of orientation selectivity in the Mammalian visual pathway. The Journal of Neuroscience 33, 10616–10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, Eleftheriou CG, Krahe TE, Fox MA & Guido W (2013). Retinal input regulates the timing of corticogeniculate innervation. The Journal of Neuroscience 33, 10085–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg DP, Liu X & Chen C (2004). Frequency-dependent modulation of retinogeniculate transmission by serotonin. The Journal of Neuroscience 24, 10950–10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengpiel F & Kind PC (2002). The role of activity in development of the visual system. Current Biology 12, 818–826. [DOI] [PubMed] [Google Scholar]
- Shatz CJ (2009). MHC class I: An unexpected role in neuronal plasticity. Neuron 64, 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ & Kirkwood PA (1984). Prenatal development of functional connections in the cat’s retinogeniculate pathway. The Journal of Neuroscience 4, 1378–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (2004). Interneurons and triadic circuitry of the thalamus. Trends in Neurosciences 27, 670–675. [DOI] [PubMed] [Google Scholar]
- Sherman SM (2005). Thalamic relays and cortical functioning. Progress in Brain Research 149, 107–126. [DOI] [PubMed] [Google Scholar]
- Sherman SM (2007). The thalamus is more than just a relay. Current Opinion in Neurobiology 17, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (2016). Thalamus plays a central role in ongoing cortical functioning. Nature Neuroscience 16, 533–541. [DOI] [PubMed] [Google Scholar]
- Sherman SM & Guillery R (1996). Functional organization of thalamocortical relays. Journal of Neurophysiology 76, 1367–1395. [DOI] [PubMed] [Google Scholar]
- Sherman SM & Guillery R (2002). The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society of London Series B. Biological Sciences 357, 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM & Guillery RW (2001). Exploring the Thalamus. Elsevier. [Google Scholar]
- Shou TD & Leventhal AG (1989). Organized arrangement of orientation-sensitive relay cells in the cat’s dorsal lateral geniculate nucleus. Journal of Neuroscience 9, 4287–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Murphy PC, Salt TE & Moody CI (1990). Dependence of retinogeniculate transmission in cat on NMDA receptors. Journal of Neurophysiology 63, 347–355. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Adams DL, Economides JR & Horton JC (2007). Transmission of spike trains at the retinogeniculate synapse. The Journal of Neuroscience 27, 2683–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Horton JC & Sharpee T (2009). Preserving information in neural transmission. Journal of Neuroscience 29, 6207–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Chino Y, Ridder WH, Kitagawa K & Langston A (1990). Orientation bias of neurons in the lateral geniculate nucleus of macaque monkeys. Visual Neuroscience 5, 525–545. [DOI] [PubMed] [Google Scholar]
- Soodak RE, Shapley RM & Kaplan E (1987). Linear mechanism of orientation tuning in the retina and lateral geniculate nucleus of the cat. Journal of Neurophysiology 58, 267–275. [DOI] [PubMed] [Google Scholar]
- Soto D, Coombs ID, Kelly L, Farrant M & Cull-Candy SG (2007). Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nature Neuroscience 10, 1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan DW & Shatz CJ (1984). Prenatal development of individual retinogeniculate axons during the period of segregation. Nature 308, 845–848. [DOI] [PubMed] [Google Scholar]
- Sretavan DW & Shatz CJ (1986). Prenatal development of retinal ganglion cell axons: Segregation into eye-specific layers within the cat’s lateral geniculate nucleus. The Journal of Neuroscience 6, 234–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram B, Meier PM & Reinagel P (2016). Temporal and spatial tuning of dorsal lateral geniculate nucleus neurons in unanesthetized rats. Journal of Neurophysiology 115, 2658–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford BK & Huberman AD (2017). Signal integration in thalamus: Labeled lines go cross-eyed and blurry. Neuron 93, 717–720. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA & Stevens B (2012). The complement system: An unexpected role in synaptic pruning during development and disease. Annual Review of Neuroscience 35, 369–389. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG & McCormick DA (1997). Thalamus. Oxford: Elsevier. [Google Scholar]
- Stoelzel CR, Huff JM, Bereshpolova Y, Alonso JM, Swadlow HA, Zhuang J, Hei X, Alonso JM & Swadlow HA (2015). Hour-long adaptation in the awake early visual system. Journal of Neurophysiology 114, 1172–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchi R, Milosavljevic N, Eleftheriou CG, Martial FP, Orlowska-Feuer P, Bedford RA, Brown TM, Montemurro MA, Petersen RS & Lucas RJ (2015). Melanopsin-driven increases in maintained activity enhance thalamic visual response reliability across a simulated dawn. Proceedings of the National Academy of Sciences 112, E5734–E5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C & Tomita S (2012). The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Current Opinion in Neurobiology 22, 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Esguerra M, Garraghty PE, Kritzer MF & Sherman SM (1987). Morphology of physiologically identified retinogeniculate X- and Y-axons in the cat. Journal of Neurophysiology 58, 1–32. [DOI] [PubMed] [Google Scholar]
- Sur M & Sherman SM (1982). Retinogeniculate terminations in cats: Morphological differences between X and Y cell axons. Science 218, 389. [DOI] [PubMed] [Google Scholar]
- Sur M, Weller RE & Sherman SM (1984). Development of X and Y-cell retinogeniculate terminations in kittens. Nature 310, 246–249. [DOI] [PubMed] [Google Scholar]
- Suresh V, Ciftcio lu UM, Wang X, Lala BM, Ding KR, Smith WA, Sommer FT & Hirsch JA (2016). Synaptic contributions to receptive field structure and response properties in the rodent lateral geniculate nucleus of the thalamus. Journal of Neuroscience 36, 10949–10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa E, Matsui K, Budisantoso T, Molnár E, Watanabe M, Matsui M, Fukazawa Y & Shigemoto R (2009). Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. The Journal of Neuroscience 29, 12896–12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H & von Gersdorff H (2000). Fine-tuning an auditory synapse for speed and fidelity: Developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. The Journal of Neuroscience 20, 9162–9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Leão RM, Rowland KC, Spirou GA & von Gersdorff H (2002). Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron 36, 1127–1143. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF & Reid RC (2000). Diverse receptive fields in the lateral geniculate nucleus during thalamocortical development. Nature Neuroscience 3, 608–616. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Tian H & Diamond JS (2011). The relative roles of diffusion and uptake in clearing synaptically released glutamate change during early postnatal development. The Journal of Neuroscience 31, 4743–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Gribizis A, Chen C & Crair MC (2017). Activity-dependent development of visual receptive fields. Current Opinion in Neurobiology 42, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AD, Picard N, Min L, Fagiolini M & Chen C (2016). Cortical feedback regulates feedforward retinogeniculate refinement. Neuron 91, 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Zhou Y & Leventhal AG (1994). Direction-sensitive X and Y cells within the a laminae of the cat’s LGNd. Visual Neuroscience 11, 927–938. [DOI] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA & Bredt DS (2005). Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron 45, 269–277. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR & Muller D (1999). LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425. [DOI] [PubMed] [Google Scholar]
- Tootle JS & Friedlander MJ (1989). Postnatal development of the spatial contrast sensitivity X- and Y-cells in the kitten retinogeniculate pathway. The Journal of Neuroscience 9, 1324–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ & Dingledine R (2010). Glutamate receptor ion channels: Structure, regulation, and function. Pharmacological Reviews 62, 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JP, Leresche N, Guyon A, Soltesz I & Crunelli V (1994). Sensory input and burst firing output of rat and cat thalamocortical cells: The role of NMDA and non-NMDA receptors. The Journal of Physiology 480, 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV & Wadiche JI (2007). Glutamate transporters: Confining runaway excitation by shaping synaptic transmission. Nature Reviews Neuroscience 8, 935–947. [DOI] [PubMed] [Google Scholar]
- Usrey WM & Alitto HJ (2015). Visual functions of the thalamus. Annual Review of Vision Science 1, 351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB & Reid RC (1998). Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature 395, 384–387. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB & Reid RC (1999). Specificity and strength of retinogeniculate connections. Journal of Neurophysiology 82, 3527–3540. [DOI] [PubMed] [Google Scholar]
- Van Horn SC, Erişir A & Sherman SM (2000). Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. Journal of Comparative Neurology 416, 509–520. [PubMed] [Google Scholar]
- Vidyasagar TR & Urbas JV (1982). Orientation sensitivity of cat LGN neurones with and without inputs from visual cortical areas 17 and 18. Experimental Brain Research 46, 157–169. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Bal T & McCormick D (1993). Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261, 361–364. [DOI] [PubMed] [Google Scholar]
- Wang X, Hirsch JA & Sommer FT (2010). Recoding of sensory information across the retinothalamic synapse. The Journal of Neuroscience 30, 13567–13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wei Y, Vaingankar V, Wang Q, Koepsell K, Sommer FT & Hirsch JA (2007). Feedforward excitation and inhibition evoke dual modes of firing in the cat’s visual thalamus during naturalistic viewing. Neuron 55, 465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand TG (2007). Retinogeniculate transmission in wakefulness. Journal of Neurophysiology 98, 769–785. [DOI] [PubMed] [Google Scholar]
- Weyand TG (2016). The multifunctional lateral geniculate nucleus. Reviews in the Neurosciences 27, 135–157. [DOI] [PubMed] [Google Scholar]
- Wiesel TN & Hubel DH (1963). Effects of visual deprivation on morphology and physiology of cells in the cat’s lateral geniculate body. Journal of Neurophysiology 26, 978–993. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Friedlander JM & Sherman SM (1984). Fine structural of identified X- and Y-cells morphology in the cat’s lateral geniculate nucleus. Proceedings of the Royal Society of London Series B 221, 411–436. [DOI] [PubMed] [Google Scholar]
- Wilson PD, Rowe MH & Stone J (1976). Properties of relay cells in cat’s lateral geniculate nucleus: A comparison of W-cells with X- and Y-cells. Journal of Neurophysiology 39, 1193–1209. [DOI] [PubMed] [Google Scholar]
- Winfield DA, Hiorns RW & Powell TPS (1980). A quantitative electron-microscopical study of the postnatal development of the lateral geniculate nucleus in normal kittens and in kittens with eyelid suture. Proceedings of the Royal Society 210, 211–234. [DOI] [PubMed] [Google Scholar]
- Wong RO (1999). Retinal waves and visual system development. Annual Review of Neuroscience 22, 29–47. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M & Shatz CJ (1993). Transient period of correlated bursting activity during development of the mammalian retina. Neuron 11, 923–938. [DOI] [PubMed] [Google Scholar]
- Yang YC, Hu CC, Huang CS & Chou PY (2014). Thalamic synaptic transmission of sensory information modulated by synergistic interaction of adenosine and serotonin. Journal of Neurochemistry 128, 852–863. [DOI] [PubMed] [Google Scholar]
- Yeh C-I, Stoelzel CR, Weng C & Alonso JM (2009). Functional consequences of neuronal divergence within the retinogeniculate pathway. Journal of Neurophysiology 101, 2166–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeow MBL & Peterson EH (1991). Active zone organization and vesicle content scale with bouton size at a vertebrate central synapse. Journal of Comparative Neurology 307, 475–486. [DOI] [PubMed] [Google Scholar]
- Zaltsman JB, Heimel JA & Van Hooser SD (2015). Weak orientation and direction selectivity in lateral geniculate nucleus representing central vision in the gray squirrel Sciurus carolinensis. Journal of Neurophysiology 113, 2987–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeater N, Cheong SKK, Solomon SGG, Dreher B & Martin PR (2015). Binocular visual responses in the primate lateral geniculate nucleus. Current Biology 25, 1–6. [DOI] [PubMed] [Google Scholar]
- Zhang W & Linden DJ (2009). Neuromodulation at single presynaptic boutons of cerebellar parallel fibers is determined by bouton size and basal action potential-evoked Ca transient amplitude. Journal of Neuroscience 29, 15586–15594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chen H, Liu X & Cang J (2013). Orientation-selective responses in the mouse lateral geniculate nucleus. The Journal of Neuroscience 33, 12751–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Dilger EK, Lo F-S & Guido W (2009). LTD and LTP at the developing retinogeniculate synapse. Journal of Neurophysiology 102, 3082–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J & Guido W (2006). Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. Journal of Neurophysiology 96, 2775–2784. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Lo F-S & Guido W (2003). Nature of inhibitory postsynaptic activity in developing relay cells of the lateral geniculate nucleus. Journal of Neurophysiology 90, 1063–1070. [DOI] [PubMed] [Google Scholar]