Abstract
Acute otitis media (AOM) represents a significant disease burden in the pediatric population. Besides vaccinations, there are no robust measures of reducing incidence of AOM in this age-group. This is a randomized controlled clinical trial evaluating the efficacy of a non-invasive middle ear aeration device, the EarPopper device (EP). We aim to investigate the reduction of episodes AOM in children with recurrent otitis media. The control arm will be observational. The intervention arm will have the EP used. The primary endpoint is incidence of AOM. The secondary endpoints are hazard ratio of time to AOM, proportion without AOM and antibiotics use, quality of life (OMO-22 Form), and adherence to treatment. Sample size is a minimum of 150 patients. The inclusion criteria is ages 4–11, with history of recurrent Acute Otitis Media (AOM).
Keywords: Otitis media, Acute otitis media
1. Introduction
Otitis media (OM) is an inflammatory disease of the middle ear predominantly observed in the pediatric population, accounting for 10–15% of all childhood doctor visits [1]. The diagnosis of Acute OM (AOM) confers a significant incremental health-care utilization burden on both patients and the health care system. With its high prevalence across the United States, pediatric AOM accounts for approximately $2.88 billion in added health care expense annually and is a significant health-care utilization concern [2]. Left untreated, OM can result in serious complications such as hearing loss, perforation of the eardrum, or infectious spread to the inner ear and the brain. While interventions include prophylactic antibiotics, tympanostomy tubes, and adenoidectomy, the mainstay initial treatment has been antibiotics [1]. However, there is only weak evidence that routine antibiotic treatment improves the course or prevents subsequent infections, indicating the need for an alternative solution to protect children from OM recurrence and complications [1,3].
Recent studies suggest a new device, the EarPopper, as a non-invasive treatment for middle ear effusion [[4], [5], [6]]. The EarPopper is indicated for the treatment of negative middle ear pressure. Negative middle ear pressure can lead to fluid accumulation in the middle ear, impaired hearing and hearing loss. The EarPopper provides a method of ventilating the middle ear by momentarily increasing the air pressure in the nose and the eustachian tube. Equalizing the pressure can prevent the accumulation of fluid and prevent hearing loss. The EP device is 510(K) regulated (510(K) Number K073401) as a non-surgical, non-drug related treatment for middle ear pressure problems such as: Middle ear fluid (Otitis Media with Effusion), Eustachian Tube Dysfunction, Temporary hearing loss, Ear pain and pressure caused by air travel, Ear fullness caused by colds, allergies and sinusitis. The device is based on the Politzer Maneuver, and works by opening the Eustachian tube by delivering a safe, constant stream of air into the nasal cavity [7,8]. In clinical studies funded by the National Institutes of Health (Grant#: 5R44DC003613-03), the EarPopper has proven to be effective in reducing chronic middle ear effusions [[4], [5], [6]].
We hypothesize that the EarPopper device will be an effective prophylactic measure to reduce incidence of AOM in children with recurrent OM. Here, we present the protocol for our study, which has been approved by the Institutional Review Board (IRB) of the Northwell Health System (IRB Approval Number: 18-0388).
2. Overall design
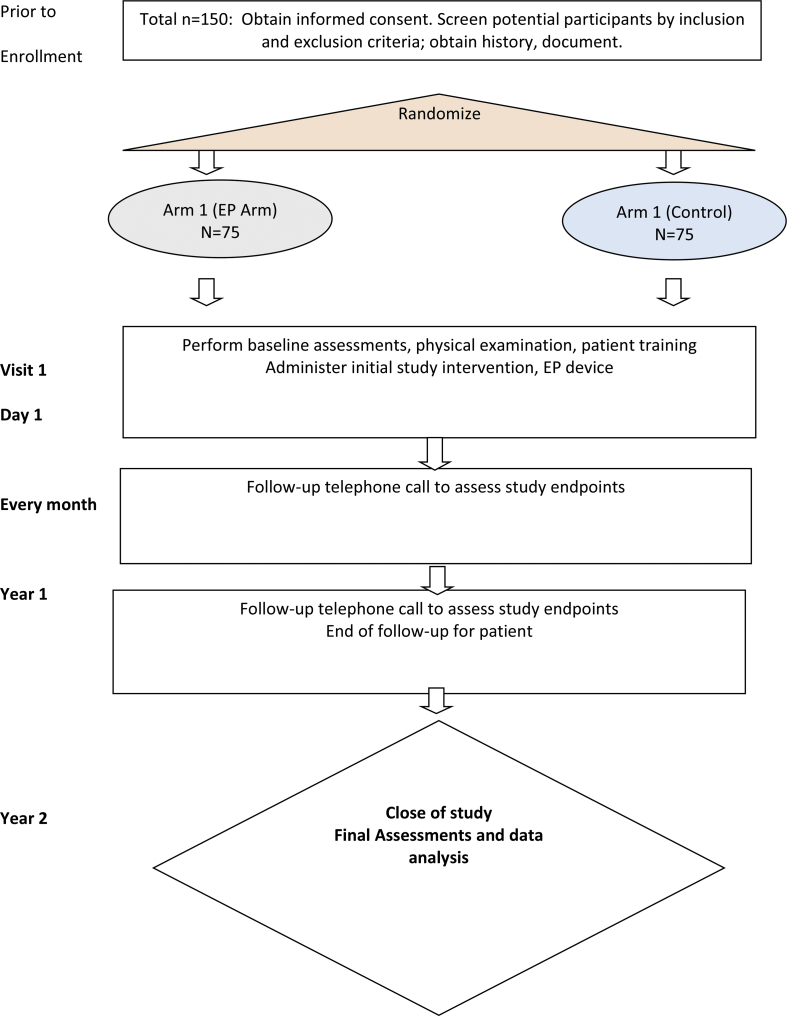
The hypothesis of this randomized controlled trial is that the EP device will be able to prophylactically decrease incidence of AOM in children with recurrent AOM. The secondary hypothesis is that the EP device will be able to decrease morbidity of AOM and severity of AOM in children with recurrent AOM (by measuring quality of life via the OMO-22 form and associated endpoints, Table 1). This is a randomized, controlled, blinded study. This is a randomized controlled clinical trial evaluating the efficacy of the EarPopper device (EP) in the reduction of episodes of acute otitis media (AOM) in children with recurrent otitis media. The control arm will be observational. The intervention arm will have the EP used. Copy of schema presented in Fig. 1.
Table 1.
Objectives and endpoints.
| Objective | Endpoint | Justification |
|---|---|---|
| Primary | ||
| To assess the prophylactic efficacy of the EP in preventing AOM |
% Incidence of AOM
|
Our hypothesis is that prophylactic use of the EP device will decrease episodes of AOM. Therefore, we are able to assess this if we compare the % incidence of AOM in the intervention group versus the control group. |
| Secondary | ||
| To assess the efficacy of the EP in reducing severity and morbidity of AOM |
Hazard Ratio (HR) of time to AOM, 95% CI
|
These easily obtainable data and will enable us to investigate if the EP has ancillary benefits, independent of the primary endpoint. |
| To assess adherence to using the device | Adherence to treatment | This endpoint acts as a control for the above endpoints, to reduce bias of the result |
| To assess quality of life | Quality of life, measured by the OMO-22 Form | This feedback will be used to see if there is any improvement in the quality of life between groups of patients across time |
Fig. 1.
Flow diagram of Randomized Controlled Trial.
2.1. Scientific rationale for study design
The rationale of a randomized controlled trial is to test the hypothesis of reduction of incidence of AOM in children with recurrent AOM. In order to increase the robustness of the data, comparator control arm was designed in the study to compare the incidence of AOM. Control arm in this study is not a placebo control. This is because patients will be acutely aware if they are using a dummy device or not, as they will be able to notice lack of air pressure delivered by the dummy device. The baseline rate of otitis media for children age 4 and up is higher than 40%, which would therefore provide a control arm with high baseline event rate [9].
2.2. Justification for dose
The dose is twice-daily, once in the morning and once in the evening. The dose justification is based on previous randomized controlled trials featuring the EP device, which had an excellent safety profile with no longterm sequelae or side-effects [5,6]. This is the dose delivered in 3 previously published randomized controlled trials [4,5,8]. Those previous publications did not mention issues with treatment adherence, so we envision a sufficient level of adherence in our population as well. We would like to stay as close to the previously proven efficacious dose to reduce the chance of delivering a suboptimal dose. Since this is not a Phase I trial, the dose-finding aspect is beyond the scope of this study.
2.3. End of study definition
A participant is considered to have completed the study if he or she has completed all phases of the study including the last visit or the last scheduled procedure. The end of the study is defined as completion of the last visit or procedure in the trial globally.
2.4. Inclusion criteria
In order to be eligible to participate in this study, an individual must meet all of the following criteria:
Provision of signed and dated informed consent form from parent, plus assent form (if age appropriate); Stated willingness to comply with all study procedures and availability for the duration of the study; Male or female, aged 4–11; Diagnosed with recurrent AOM, defined as: at least 2 episodes of AOM within the preceding year of date of screening; Must be able to follow directions to use EarPopper, or have a caregiver able to administer the device; Patient must be currently free of middle ear effusion or current acute OM. This will be determined on physical examination during screening visit.
2.5. Exclusion criteria
An individual who meets any of the following criteria will be excluded from participation in this study: Patient with chronic middle ear effusion; Patients with potential complications or confounding conditions: asthma, chronic sinusitis, immunodeficiency, diabetes mellitus; Patient with cleft palate.
2.6. Study intervention description
The EarPopper is indicated for the treatment of negative middle ear pressure. Negative middle ear pressure can lead to fluid accumulation in the middle ear, impaired hearing and hearing loss. The EarPopper provides a method of ventilating the middle ear by momentarily increasing the air pressure in the nose and the eustachian tube. Equalizing the pressure can prevent the accumulation of fluid and prevent hearing loss. The device is based on the Politzer Maneuver, and works by opening the Eustachian tube by delivering a safe, constant stream of air into the nasal cavity [7,8]. By regularly aerating the middle ear, we hypothesize that the EarPopper device will be an effective prophylactic measure to reduce incidence of AOM in children with recurrent OM. The device delivers a jet of air pressure from the nozzle at 5.2PSI, at a volume velocity of 1,524 mL/min [5].
2.7. Dosing and administration
Dose: Dosing of the EP device will be twice per day, once in the morning and once before bedtime. This is consistent with previous dosing which showed no adverse events and an excellent safety profile [5,6].
Administration: Step 1. Hold nosepiece firmly against nostril opening creating a good, tight seal is crucial. Plug the other nostril closed. Step 2. Push button to start the airflow and swallow while the device is running. Step 3. Repeat on other nostril. After 5 min, repeat steps 1–3. This will complete one treatment.
2.8. Measures to minimize bias: randomization and blinding
Blinding: Since there will be no dummy devices in this trial, we will be unable to blind the patients and physicians to whom is using the EP device. The assessors/data collectors will be blinded for patient follow-up, and the statistician will be blinded.
Randomization and Consent: Subjects will be randomized after the consent process. Randomization and consent will be carried out in the following sequence. Every eligible patient will be approached and told that the ENT service is conducting a research study of how the EP device will be able to reduce incidence of AOM in children with recurrent AOM. All eligible patients will be told that they will receive the EP device either (1) at the start of the clinical trial, or (2) at the end of 1 year of follow-up. In this case, the group that receives the EP device at the start will be the intervention arm, and the group that receives the device later is the control arm. The rationale for giving the devices to the control arm is to ensure that patients have an incentive for continued participation for follow-up in the clinical trial.
All patients will be asked to sign a consent document After obtaining written patient consent, the consenting physician will step out of the room, go to the nurse's station or to any computer with internet capabilities. The physician will log on to the Biostatistics Randomization Management System (BRMS) and randomize the subject to either EP or control.
If assigned to control, the patient will be informed that they were randomized to receive the device after one year and will be followed up normally, receive telephone check-ins from the research team, and the EP device will be given to them, free of charge, at the end of 1 year of follow-up.
If EP arm, then the physician returns to the subject's room and informs the subject that he/she has been selected to use the EP device. The device will be explained to the subject. All subjects will be randomized in a 1:1 ratio using permuted blocks. Subjects will be stratified by site (Lenox Hill) prior to randomization.
Rationale for using the design: Under a standard randomized design (i.e., consent to be randomized followed by randomization), it is likely that a significant number of subjects will drop out of the study after being randomized to the control group because they were hoping to be assigned to the EP group. This is common in randomized studies where a standard method is being compared to an attractive “high-tech” method. By providing all patients with the EP device whether or not which arm they are in, this removes the likelihood of patients in the control arm dropping out of the study.
Ethical Considerations: First, the randomization does not add any risk to subjects. All subjects will receive EP device, and those who receive EP initially do not incur any greater risk. Furthermore, EP subjects are free to reject the EP device. Finally, there is no deception in this design. All subjects are told the truth about what they are consenting to.
3. Study assessments and procedures
3.1. Screening visit/enrollment/baseline visit
The screening and enrollment visit may be conducted on the same day. This visit will include recording of demographic information, physical examination, history, and training of the use of the EP device. Consenting Investigator will take the consent, demographic information, physical examination, history, and training of the use of the EP device. Non-consenting investigator may be able to perform all of the above routines, except consent, under the direct supervision of a consenting investigator.
Establishing validity of endpoint measurement (OMO-22): Validity of our telephone questionnaire will be tested. An expanded version of Rosenfeld's previously validated otitis media quality-of-life survey, the Otitis Media–6 (OM-6), was used to assess disease-specific quality of life [10]. The questionnaire was expanded into individual variables to allow for analysis of each specific variable. It also included demographic data at each administration. The questionnaire we use is the Otitis Media Outcome–22 (OMO-22) based on the study by Richards et al. [11] (Supplementary materials) It is a 22-item questionnaire based on a 7-point Likert scale with associated demographic questions. The questionnaire can be divided into a physical, emotional, hearing loss, speech, and social symptoms subsets.
Validation of instrument: The validity of this expanded version of Rosenfeld's OM-6 questionnaire, the OMO-22, will be evaluated. Since we are following up the patients via telephone call, the investigator be will delivering the questionnaire verbally. Test consistency was evaluated in a subset of patients for both test-retest reliability and internal consistency. A subset of 10 patients will answer the questionnaire at two separate timepoints prior to finishing their baseline visit. The same investigator will deliver these questionnaires at all timepoints to ensure consistency. A paired t-test will be used to investigate statistical differences between the mean OMO-22 scores. The overall internal consistency will be measured with Cronbach α coefficient. The ability of the questionnaire to adequately measure ear-related symptoms and quality of life will also be evaluated. The discriminant validity was assessed by comparing the total ear scores of children with recurrent AOM with a cohort of 10 children without a significant history of ear problems. The mean score between groups will be compared with a t-test.
3.2. Primary endpoint (% incidence of AOM)
In each telephone interview, the parent will be asked whether the subject had any unscheduled visits to a health care provider since previous contact, and if so, for what reasons; a list of possible reasons for such visits, including ‘ear infection’ among other common pediatric illnesses, will be read to the parent to ensure that all visits will be reported. Additionally, patients are given a symptom sheet with common presenting symptoms, as well as instructions on what to do when they experience those symptoms. (Supplementary materials) Patients will provide researchers with contact details of PCP. HIPAA release forms will be signed by the patient and sent to the PCP. PCP records will be obtained by investigators/research coordinators of the study. Medical records from each subject's primary care physician and from any other health care provider whom the parent identified as having treated the subject during the study period will be obtained and reviewed by the principal investigator (Tristan Tham, MD) in a blinded fashion. Non-consenting investigator may review these records only under the direct supervision of consenting investigator. Endpoint definition: For the primary outcome of clinical diagnoses of AOM, the medical record from PCP will be considered the gold standard. For those cases in which the medical record was not available, the parent's report of AOM diagnoses will be used. Previous research in AOM through telephone surveys has demonstrated that parents' recall of recent episodes of AOM is highly accurate [12]. This study design relying on the validity of telephone interviews as the primary outcome is based data validations of Vernacchio et al. [12,13] Data point for occurrence of AOM ‘yes’/’no’ will be made by the PI, which will be a binary data point. The incidence difference in two groups will be calculated with a 2-tailed t-test. . We are calculating the incidence of ‘at least one episode of AOM’ per patient. Therefore if: Patient A, Arm 1 gets 2 episodes; Patient B, Arm 1 gets 0 episodes; Patient C, Arm 2 gets 3 episodes; Patient D, Arm 2 gets 4 episodes.
Then: Arm 1 = 1 patient with at least 1 episode of AOM; Incidence = 1/2 = 50%; Arm 2 = 2 patients with at least 1 episode of AOM; Incidence = 2/2 = 100%. If patient discontinuous the device earlier, this will be accounted for in the subgroup stratification via adherence in section 4.9.
3.3. Secondary endpoint: hazard ratio of AOM
In addition to the above primary endpoint (incidence of AOM), we will also compare time to first clinically diagnosed AOM episode after randomization in the 2 study groups using the Cox Proportional Hazards Model (CPH). Data point for occurrence of AOM ‘yes’/’no’ will be determined by the PI from review of clinic notes, which will be a binary data point. Data point for time to AOM will be taken from the clinic note. This will be verified by the PI reviewing the clinic not. Using these above two data points, CPH and Kaplan Meier curves can be constructed.
3.4. Secondary endpoint: proportion without AOM and antibiotics use
Proportion of subjects in each group with no AOM episodes and no antibiotic use will be recorded. They will be compared across groups using the Fisher Exact Test. Data point for occurrence of AOM ‘yes’/’no’ will be determined by the PI from review of clinic notes, which will be a binary data point. Data point for use of antibiotics ‘yes’/’no’ will be determined by the PI from review of clinic notes, which will be a binary data point. Proportion of subjects in each group with no AOM episodes and no antibiotic use will be recorded. They will be compared across groups using the Fisher Exact Test.
3.5. Secondary endpoint: quality of life
Standardized questionnaire based on the OMO-22 will be delivered via telephone call to the patient or parent. This will be conducted monthly until end of study period for each participant. Significance testing between times was performed using paired t-test statistics for parametric data with equal distributions and the Mann-Whitney test for nonparametric data. Consenting investigator will deliver the modified OMO-22 form. Non-consenting investigator may do this only under direct supervision of consenting investigator. See attached structured survey (OMO-22) for more details. Data point for Overall OMO-22 score are calculated from the total score of the OMO-22 form. Likert data points will be recorded from the OMO-22 form and compared using the Mann Whitney U test.
3.6. Secondary endpoint: adherence to treatment
Adherence will be assessed during every monthly interview, by asking the parent how often the subject used the EP device since the last interview: “All or nearly all of the prescribed doses”; “More than half of the doses”; “Less than half”; “None or nearly none”. Consenting investigator will deliver this question. Non-consenting investigator may do this only under direct supervision of consenting investigator. This data point is included in the modified OMO-22 form attached to this protocol. The above responses will be recorded as ordinal variables. Subgroup analysis may be performed according to adherence to treatment. We will additionally investigate if adherence to treatment may affect any of the prior endpoints.
4. Statistical considerations
4.1. Null hypotheses
Null hypothesis of primary endpoint: The incidence of AOM is the same between two arms of the study.
Null hypothesis of secondary endpoints: Hazard ratios between groups for time to AOM event is the same; Proportion of patients without AOM and antibiotics use is the same between groups;
OMO-22 outcomes are the same between the two arms of the study.
4.2. Sample size determination
Primary Endpoint was Used for Sample Size Justification: Assuming that 10% of patients in experimental arm and 30% of patients in control arm have acute otitis media (AOM), then a chi-square test with a 0.05 two-sided significance level will have 80% power to detect the difference between these proportions when the sample size is 72 subjects per group (144 total). The values of 10–30% for the baseline incidence were derived from one of the only epidemiological studies of incidence of AOM in an adjacent geographical area by Teele et al. [9].
4.3. General approach to analysis
We are using Intention-to-Treat (ITT) Analysis on all randomized patients. For descriptive statistics, categorical and continuous data will be presented as percentages, means with standard deviations, median, range, where appropriate. Incidence of AOM will be presented as %. All inferential tests will be two tailed with an alpha of 0.05. Checks of normality will be performed, and if distribution is not normal, non-parametric tests will be used instead.
4.4. Interim analysis
Planned interim analysis will be carried out when the study reaches 100 patients. P value penalties will be applied via the Pocock rule. We plan to terminate this clinical trial if a statistically significant effect is shown in the primary endpoint when 100 patients have finished 1 year of follow-up each. (ie, null hypothesis rejected in the primary endpoint).
4.5. Sub-group analysis/controlling for confounders
The primary endpoint will be analyzed based on age, sex, race/ethnicity, household smoking, history of episodes of AOM. The above parameters will be input into the multiple logistic regression model, or multiple cox proportional hazards model (when investigating time to AOM), to control for confounding factors. Adherence analysis will also be performed. Adherence will be assessed during every monthly interview, by asking the parent how often the subject used the EP device since the last interview: “All or nearly all of the prescribed doses”; “More than half of the doses”; “Less than half”; “None or nearly none”. Consenting investigator will deliver this question. Non-consenting investigator may do this only under direct supervision of consenting investigator. This data point is included in the modified OMO-22 form attached to this protocol. The above responses will be recorded as ordinal variables. Subgroup analysis may be performed according to adherence to treatment. We will additionally investigate if adherence to treatment may affect any of the prior endpoints. This will be conducted through multiple logistic regression analysis, or in multiple cox proportional hazards regression.
A clinical practice guideline issued jointly by the American Academy of Pediatrics (AAP) and American Academy of Family Physicians (AAFP) recognizes the benefit of pneumococcal conjugate vaccine (PCV) for preventing AOM [14]. PCV vaccination at 2, 4, 6, and 12–15 months of age is part of the routine childhood immunization series recommended by the Advisory Committee on Immunization Practices [15]. Since our cohort is comprised of patients treated at clinics where this vaccine is routinely scheduled as the standard-of-care, there is no plan to stratify the patients based on PCV vaccination status.
5. Discussion
This is the first randomized controlled trial (RCT) to investigate the use of the EP device as a prophylactic measure to reduce incidence of AOM in children. AOM constitutes a significant burden of disease in the USA, with up to 15% of doctors visits attributed to AOM, with almost $3 billion spent annually in this country [1,2]. Indeed, the subsequent sequelae and complications of OM are hearing loss, perforation of the eardrum, or infectious spread to the inner ear and the brain. Influenza vaccinations have been proven interventions in reducing the incidence of AOM in children, especially during influenza season. However, there is only weak evidence that routine antibiotic treatment improves the course or prevents subsequent infections, indicating the need for an alternative solution to protect children from OM recurrence and complications [1,3]. The EP device has been shown in previous studies to be a safe and effective treatment for middle ear effusion. In this study, we hope to see if the EP device will additionally have some prophylactic activity to reduce episodes of AOM in children with recurrent AOM.
Conflicts of interest
No conflicts of interest to declare. Earpopper devices were provided without charge by Innovia Medical (Centennial, CO, USA). None of the authors are reimbursed by Innovia Medical.
Funding source
Earpopper devices were provided without charge by Innovia Medical (Centennial, CO, USA). No other funding was provided.
Acknowledgements
We would like to thank Dr Peter Costantino for departmental support, and Ahn Seung Jun for statistical advice. We would also like to thank Yonatan Bardash and Dr Sushma Teegala for assistance in drafting early versions of this protocol.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2018.09.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Worrall G. Acute otitis media. Can. Fam. Physician. 2007;53(12):2147. [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed S., Shapiro N.L., Bhattacharyya N. Incremental health care utilization and costs for acute otitis media in children. Laryngoscope. 2014;124(1):301–305. doi: 10.1002/lary.24190. [DOI] [PubMed] [Google Scholar]
- 3.Granath A. Recurrent acute otitis media: what are the options for treatment and prevention? Curr. Otorhinolaryngol. Rep. 2017;5(2):93–100. doi: 10.1007/s40136-017-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banigo A., Hunt A., Rourke T., Whiteside O., Aldren C. Does the EarPopper® device improve hearing outcomes in children with persistent otitis media with effusion? A randomised single‐blinded controlled trial. Clin. Otolaryngol. 2016;41(1):59–65. doi: 10.1111/coa.12480. [DOI] [PubMed] [Google Scholar]
- 5.Arick D.S., Silman S. Nonsurgical home treatment of middle ear effusion and associated hearing loss in children. Part I: clinical trial. Ear Nose Throat J. 2005;84(9):567. [PubMed] [Google Scholar]
- 6.Silman S., Arick D.S., Emmer M.B. Nonsurgical home treatment of middle ear effusion and associated hearing loss in children. Part II: validation study. Ear Nose Throat J. 2005;84(10):646. [PubMed] [Google Scholar]
- 7.Casale M., Rinaldi V., Setola R., Salvinelli F. The old‐fashioned politzer maneuver: a video clip demonstration. Laryngoscope. 2007;117(11):2002. doi: 10.1097/MLG.0b013e31812eeded. [DOI] [PubMed] [Google Scholar]
- 8.Silman S., Arick D. Efficacy of a modified politzer apparatus in management of eustachian tube dysfunction in adults. J. Am. Acad. Audiol. 1999;10:496–501. [PubMed] [Google Scholar]
- 9.Teele D.W., Klein J.O., Rosner B., Group G.B.O.M.S. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J. Infect. Dis. 1989;160(1):83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld R.M., Goldsmith A.J., Tetlus L., Balzano A. Quality of life for children with otitis media. Arch. Otolaryngol. Head Neck Surg. 1997;123(10):1049–1056. doi: 10.1001/archotol.1997.01900100019002. [DOI] [PubMed] [Google Scholar]
- 11.Richards M., Giannoni C. Quality-of-life outcomes after surgical intervention for otitis media. Arch. Otolaryngol. Head Neck Surg. 2002;128(7):776–782. doi: 10.1001/archotol.128.7.776. [DOI] [PubMed] [Google Scholar]
- 12.Vernacchio L., Vezina R.M., Ozonoff A., Mitchell A.A. Validity of parental reporting of recent episodes of acute otitis media: a Slone Center Office-Based Research (SCOR) Network study. J. Am. Board Fam. Med. 2007;20(2):160–163. doi: 10.3122/jabfm.2007.02.060125. [DOI] [PubMed] [Google Scholar]
- 13.Vernacchio L., Corwin M.J., Vezina R.M. Xylitol syrup for the prevention of acute otitis media. Pediatrics. 2014;133(2):289–295. doi: 10.1542/peds.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AAoPSoMoAO Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 15.Kroger A., Sumaya C., Pickering L., Atkinson W. The national center for immunization and respiratory diseases. General recommendations on immunization—recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2011;60:1–64. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.