Abstract
Introduction
The clinical significance of enterococci is mostly related to its antibiotic resistance which contributes to colonization and infection, in particular amongst the hospitalized patients. The present review has examined the literature to provide a comprehensive data on enterococci antibiotic resistance during the last 20 years in Iran.
Methods
Search engines such as Google Scholar and PubMed were used to identify all Persian and English-language articles investigating enterococci in Iran from 1996 to 2017. The search terms were “enterococci”, “enterococcal”, “enterococcus”, “Iran”, “bacterial resistance”, “antibiotic resistance” and “resistance”.
Results
Decrease in the resistance trend against ampicillin, gentamycin and ciprofloxacin was observed over a period of 15 years (2001 to 2016) in Iran. During a 10 years period from 2001 to 2015, the rate of resistance among Enterococcus faecalis species was less than Enterococcus faecium. The resistancerate, however, was considerably increased for both species during this period. The mean resistance rates for vancomycin, gentamicin, ciprofloxacin, erythromycin, nitrofurantoin, chloramphenicol, trimethoprim-sulfametoxazol, imipenem and teicoplanin were higher among complicated cases (patients with underlying debilitating disorders) compared to general cases (hospitalized or outpatients with no specific underlying disorder).
Conclusions
E. faecalis and E. faecium showed a rise in the mean resistance against all the antibiotics during a 10-year period from 2010 to 2015. With the exception of penicillin and ampicillin, resistance to all antibiotics was higher amongst complicated cases compared to general patients.
Keywords: Antimicrobial resistance, clinical isolates, enterococci, Iran, review, resistance trend
Introduction
Enterococci are natural parts of the intestinal flora in humans and animals which are ubiquitously present in nature, soil, plants, vegetables and treated or untreated water. They can cause severe opportunistic infections, including endocarditis as well as urinary tract (UTIs), intra-abdominal and wound infections [1]. Enterococci gain entry into foodstuffs through water supplies, food processes or unsanitary conditions related to food handling [2]. Drug resistance is the main reason for the dramatic emergence of these organisms as a cause of healthcare-associated infections throughout the world, including Iran [3].
The treatment of enterococcal infection has been complicated by the emergence of strains possessing a high level resistance to almost all of the antibiotics used in clinical settings, especially aminoglycosides, β-lactams and glycopeptides. Alternative therapeutic options are consequently being evaluated to manage multidrug-resistant strains [4]. In Iran, enterococci have gained resistance to almost the entire antimicrobial spectrum used against this organism. This includes resistance to vancomycin, the most important antimicrobial agent for the treatment of enterococcal infections; resistance has been reported to be as high as 9.4% in Iran [5]. The high rate of antibiotic resistance among enterococci isolates has greatly limited the therapeutic options to treat infections caused by this organism in Iran.
Enterococcal clinical and epidemiologic data in each country are needed in order to make informed decisions on ways to control enterococcal infections. Such information could help scientists, healthcare workers and policy makers to understand the emergence of multidrug-resistant strains of enterococci and their association with underlying diseases at both regional and global levels.
We performed a review to evaluate the published literature over the last 20 years regarding the evolution of enterococcal infections and antibiotic resistance in Iran.
Methods
Search strategy and selection criteria
A detailed search was conducted of the Google Scholar, Scopus, PubMed, ISI, Iranmedex, Magiran, SID and ISC databases with articles published from 1996 to 2017 to identify all of the Persian- and English-language articles which have investigated enterococci in Iran. The search terms were ‘enterococci,’ ‘enterococcus,’ ‘enterococcal,’ ‘Iran,’ ‘bacterial resistance’ and ‘antibiotic resistance.’
All of the articles were carefully examined for the reports of antibiotic susceptibility patterns among general and complicated cases. General cases were defined as hospitalized patients or outpatients, with no specific underlying disorder, who had transient general illnesses such as diarrhoea, UTIs and blood infections. Complicated cases included patients with underlying debilitating disorders such as cardiovascular diseases, diabetic foot, gallbladder stones, lymphoblastic leukaemia, and renal or hepatic failure, as well as patients undergoing haematopoietic stem-cell transplantation, patients receiving chronic haemodialysis and patients hospitalized in the intensive care unit.
Articles which had no examination of the antibiotic susceptibility pattern and those investigating nonclinical isolates of enterococci, as well as duplicate articles and studies presented only in abstract form, were excluded from analysis.
Data extraction
The following data were extracted from each article: corresponding author's name, year of data collection, country, number of isolates, resistance rate (as a percentage) for all the antibiotics investigated in the article and the specific disorder of the patients, if present.
Statistical analysis
Spearman correlation was used to analyse the significance of resistance trends over time. The independent t test method was used to compare the changes in antibiotic resistance over a period of 10 years (2005–2015) either in Enterococcus faecium or Enterococcus faecalis spp. and to evaluate the significance of any possible difference in antibiotic resistance between general and complicated cases. The means ± standard deviation of different variables are indicated in the tables and figures, and p ≤ 0.05 was considered statistically significant.
Results
During the initial database search, a total of 150 articles were found. After exclusion of the articles according to our criteria, a total of 44 articles were included in the analysis [3], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]. Among these 44 articles, 24 were associated with general patients (3071 isolates), 12 studies assessed their enterococcal populations on the basis of species (3906 isolates) and eight investigated colonization of enterococci among complicated patients with specific underlying conditions (589 isolates). The most common methods used to assess the antimicrobial susceptibility pattern in these studies included disc diffusion and broth microdilution.
In total, 38 antibiotics were assessed within the 44 articles (Table 1). The most commonly examined antibiotics in these studies were vancomycin (n = 32 studies), gentamycin (n = 21), ciprofloxacin (n = 20), ampicillin (n = 18), penicillin (n = 19) and erythromycin (n = 17), whereas amoxicillin, tobramycin, kanamycin, fusidic acid, nafcillin, tazobactam/piperacillin and ofloxacin were only investigated by one study each (Table 1).
Table 1.
Number of total studies and mean resistance rates associated with different antibiotics among enterococci in Iran during 2001–2016
| Antibiotic | Total studies (n) | Total isolates (n) | Mean resistance (%) |
|||
|---|---|---|---|---|---|---|
| General cases | Complicated cases | Enterococcus faecalis | Enterococcus faecium | |||
| Vancomycin | 32 | 6703 | 15.6 | 21.8 | 12.5 | 47.0 |
| Gentamicin | 21 | 4155 | 51 | 76.0 | 65.1 | 74.9 |
| Ciprofloxacin | 20 | 3885 | 35.4 | 61.0 | 51.0 | 77.4 |
| Penicillin | 19 | 3107 | 74.1 | 57.5 | 45.1 | 70.7 |
| Ampicillin | 18 | 5141 | 43.5 | 34.3 | 17.1 | 69.0 |
| Erythromycin | 17 | 4656 | 58.3 | 42.7 | 67.0 | 76.3 |
| Nitrofurantoin | 15 | 1962 | 19 | 35.3 | 3.5 | 16.2 |
| Chloramphenicol | 14 | 2503 | 17.2 | 26.4 | 34.4 | 28.8 |
| Trimethoprim/sulfamethoxazole | 14 | 2346 | 48.6 | 100.0 | 53.8 | 80.5 |
| Tetracycline | 12 | 2592 | 56 | — | 65.3 | |
| Imipenem | 10 | 1978 | 21.5 | 41.2 | 26.2 | 81.7 |
| Linezolid | 10 | 1588 | 1.4 | 0.5 | — | 0.0 |
| Teicoplanin | 10 | 1599 | 23.2 | 39.4 | 9.2 | 62.8 |
| Amikacin | 8 | 708 | 43.0 | — | — | |
| Clindamycin | 8 | 1436 | 68.0 | 100.0 | — | — |
| Oxacillin | 7 | 1202 | 56.6 | 49.0 | — | |
| Synercid | 5 | 986 | — | 23.5 | — | 24.3 |
| Rifampin | 5 | 1413 | 55.4 | 70.1 | — | 86.5 |
| Streptomycin | 5 | 393 | 33.0 | 85.3 | — | 90.0 |
| cephalothin | 4 | 924 | 86.7 | — | — | |
| Nalidixic acid | 3 | 247 | 65.3 | — | — | |
| Cephalexin | 3 | 199 | 33.3 | — | — | |
| Ceftizoxime | 3 | 140 | 57.4 | — | — | |
| Cefotaxime | 3 | 1189 | 51.7 | — | — | |
| Ceftriaxone | 3 | 1009 | 41.5 | — | — | |
| Norfloxacin | 3 | 276 | — | 85.3 | — | 84.0 |
| Meropenem | 3 | 121 | 96.7 | — | — | — |
| Levofloxacin | 2 | 149 | 25.6 | — | — | — |
| Co-amoxiclava | 2 | 137 | 12 | — | — | — |
| Cefazolin | 2 | 102 | 94.0 | — | — | |
| Cefotetan | 2 | 772 | 76.0 | 85.3 | — | — |
| Nafcillin | 1 | 738 | 74.6 | — | — | — |
| Amoxicillin | 1 | 606 | 16.7 | 33.3 | — | — |
| Tobramycin | 1 | 138 | 11.4 | — | — | — |
| Kanamycin | 1 | 138 | 80.2 | — | — | — |
| Fusidic acid | 1 | 220 | 89.5 | — | — | — |
| Tazobactam/piperacillina | 1 | 180 | 42 | — | — | — |
| Ofloxacin | 1 | 89 | 5.0 | — | — | — |
Amoxicillin/clavulanic acid.
Enterococci isolated from general patients showed the highest resistance to meropenem (97%), cefazolin (94%), fusidic acid (90%), cephalothin (87%), kanamycin (80%), cefotetan (76%), nafcillin (75%), penicillin (74%), clindamycin (68%), nalidixic acid (65%) and erythromycin (58%). However, the isolates were highly sensitive to the following antibiotics: co-amoxiclav (0 resistance), linezolid (1%), ofloxacin (5%) and tobramycin (11%) (Table 1).
E. faecalis had a high resistance rate against erythromycin (67% resistance), gentamicin (65%), trimethoprim/sulfamethoxazole (54%), ciprofloxacin (51%) and oxacillin (49%), whereas nitrofurantoin (4% resistance) and teicoplanin (9%) were the most active agents against this species.
E. faecium isolates were mostly resistant against erythromycin (78%), norfloxacin (84% resistance), imipenem (82%) and trimethoprim/sulfamethoxazole (81%), whereas linezolid with no resistance and nitrofurantoin (16%) were the most effective antibiotics.
Enterococci isolated from complicated cases were fully resistant (100% resistance) against trimethoprim/sulfamethoxazole and clindamycin. Linezolid with 0.5% resistance was the most effective antibiotic against these isolates (Table 1).
Trend of antibiotic resistance during 2001–2016
The antibiotic resistance profiles of ampicillin, gentamicin, ciprofloxacin, vancomycin and erythromycin were found to be reported by more than 90% of the studies during 2001–2016 (Fig. 1, Fig. 2). A lack of data on the remaining 32 antibiotics did not permit us to estimate the resistance trend over time for these antibiotics.
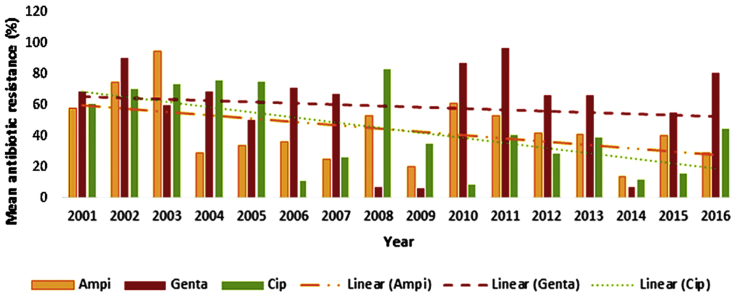
Fig. 1.
Trend of mean resistance to ampicillin, gentamycin and ciprofloxacin among enterococci during 2001–2016 in Iran.
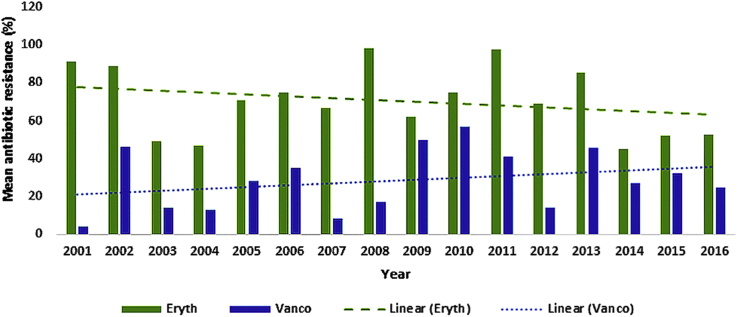
Fig. 2.
Trend of mean resistance to erythromycin and vancomycin among enterococci during 2001–2016 in Iran.
An insignificant decrease in the resistance trend for ampicillin (p 0.149) and erythromycin (p 0.356), and an insignificant increase trend was observed for vancomycin (p 0.292) during the 15 years of study. The decrease was significant for ciprofloxacin (p 0.053) and somewhat consistent for gentamycin (p 0.760) during this time period.
The highest and lowest rates of enterococci resistance, with reduced resistance over time, occurred during 2003 (95%) and 2014 (14%) for ampicillin, 2008 (83%) and 2010 (8%) for ciprofloxacin, 2001 (11%) and 2008 (98%) for erythromycin and 2009 (6%) and 2011 (97%) for gentamycin (Fig. 1, Fig. 2). However, the highest and lowest rates of enterococci resistance, with increased resistance over time, occurred in 2003 (0.1%) and 2010 (57%) for vancomycin (Fig. 2). Increases and decreases in the resistance rates are, however, observed for all antibiotics during 2001–2016 (Fig. 1, Fig. 2).
Antibiotic resistance among E. faecalis and E. faecium spp.
The total number of studies associated with enterococci resistance against the four most frequently investigated antibiotics—erythromycin, gentamycin, vancomycin and ampicillin—during 2004–2006 were 20 for E. faecalis and 18 for E. faecium spp. The studies were 11 for E. faecalis and eight for E. faecium spp. for the same antibiotics during 2014–2016 (Table 2). The mean resistance rates against the above-mentioned antibiotics for both species are indicated in Table 2.
Table 2.
Mean resistance rates associated with different antibiotics among Enterococcus faecalis and Enterococcus faecium during 2005–2015
| Species | Antibiotic | Mean resistance (%), 2005 | Mean resistance (%), 2015 | Total studies during 2005 (n) | Total studies during 2015 (n) |
|---|---|---|---|---|---|
| E. faecalis | Erythromycin | 37 | 70 | 4 | 2 |
| Gentamicin | 49 | 80 | 4 | 3 | |
| Vancomycin | 14 | 28 | 6 | 3 | |
| Ampicillin | 6 | 26 | 6 | 3 | |
| E. faecium | Erythromycin | 57 | 80 | 4 | 2 |
| Gentamicin | 64 | 64 | 4 | 2 | |
| Vancomycin | 53 | 60 | 5 | 2 | |
| Ampicillin | 62 | 65 | 5 | 2 |
The mean resistance rates of E. faecalis and E. faecium spp. against four commonly investigated antibiotics in 10-year period time (2005 and 2015) were compared (Fig. 3). Using the independent t test, the following p values were attained by comparing resistance between the two species during 2005: erythromycin (p 0.14), gentamicin (p 0.041), vancomycin (p 0.032) and ampicillin (p 0.007). The same comparison during 2015 was made, and the results showed the following p values: erythromycin (p 0.216), gentamicin (p 0.08), vancomycin (p 0.048) and ampicillin (p 0.029).
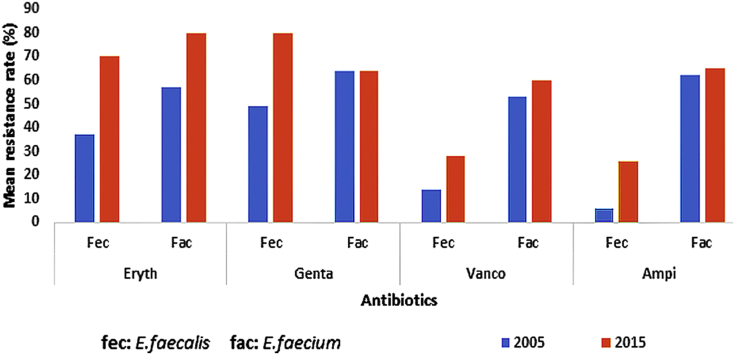
Fig. 3.
Comparison between mean resistance rates against most frequently used antibiotics in 2005 and 2015 among Enterococcus faecalis and Enterococcus faecium species.
The mean rate of resistance was generally higher among E. faecalis species compared to E. faecium for all antibiotics either in 2005 or 2015; except for gentamycin during 2015, the resistance to which was higher among E. faecium species.
The mean antibiotic resistance rate was considerably increased for both E. faecium and E. faecalis during this 10-year time period (2005–2015) (Fig. 3). Statistical analysis revealed that the mean resistance rates during this time period were as follows for E. faecalis and E. faecium, respectively: erythromycin (p 0.018, p 0.038), gentamicin (p 0.071, p 0.08), vancomycin (p 0.159, p 0.163) and ampicillin (p 0.014, p 0.162).
Antibiotic resistance among general and complicated cases
Table 3 shows a resistance assessment among enterococci in more than ten investigations conducted during 2001–2016 of 12 antibiotics. The lowest and highest antibiotic resistance rates belonged to vancomycin (14%) and penicillin (75%) for general cases and to vancomycin (26%) and trimethoprim/sulfamethoxazole (100%) for complicated cases, respectively. Overall, the analysis of the data suggested that the mean resistance rates for all of the examined antibiotics were higher among complicated cases compared to general cases, except for penicillin and ampicillin (Table 3).
Table 3.
Mean resistance rates of enterococci against 12 most studied antibiotics among general and complicated cases during 2001–2016 in Iran
| Antibiotic | Total studies (n) | Total isolates (n) | Total mean resistance (%) |
Significance of difference between general and complicated cases (p) | |
|---|---|---|---|---|---|
| General cases | Complicated cases | ||||
| Vancomycin | 32 | 6703 | 15.6 | 26 | 0.597 |
| Gentamicin | 21 | 4155 | 51 | 76 | 0.440 |
| Ciprofloxacin | 20 | 3885 | 35 | 61 | 0.155 |
| Penicillin | 19 | 3885 | 74 | 58 | 0.560 |
| Ampicillin | 18 | 5141 | 43.5 | 39 | 0.676 |
| Erythromycin | 17 | 4656 | 58.3 | 85 | 0.425 |
| Nitrofurantoin | 15 | 1962 | 19 | 35 | 0.235 |
| Chlorophenol | 14 | 2503 | 17 | 27 | 0.604 |
| Trimethoprim/sulfamethoxazole | 14 | 2346 | 49 | 100 | 0.141 |
| Tetracycline | 12 | 2592 | 56 | — | — |
| Imipenem | 10 | 1978 | 22 | 41 | 0.065 |
| Teicoplanin | 10 | 1599 | 23.2 | 39 | 0.289 |
Discussion
As normal residents of the gastrointestinal tract, vagina and male urethra, enterococci can occasionally cause infections in humans. Resistance is the main reason for the dramatic emergence of these organisms as a cause of healthcare-associated infections throughout the world. The current review is to our knowledge the first to inclusively report the prevalence and trend of antibiotic resistance of enterococci over 20 years in Iran. Data analysis showed noticeable increases and decreases in the trends of resistance to ampicillin, gentamicin, ciprofloxacin, vancomycin and erythromycin during 2001–2016. A decreased resistance trend for ampicillin and erythromycin and an insignificant increased trend was observed for vancomycin. However, the trend was significantly decreased for ciprofloxacin. The level of gentamicin resistance was consistent during this time period.
The reasons for the observed increases and decreases in the level of the antibiotic resistance of enterococci could be sample size, geographic location and methodology. For example, in a study carried out in the east of Iran (Azerbaijan) in 2003, only eight enterococcal isolates were isolated from 676 samples from cases of UTI [14]. All eight isolates were resistant to ampicillin. Another study which was carried out in the same year in Tehran reported 90% ampicillin resistance among enterococcal isolates recovered from cases of UTI. The small sample size of these two articles, and hence during 2003, might have caused a bias (overestimation) in the rate of resistance to ampicillin during this year. However, no variations were observed in different provinces where the same methodology was used.
During 2014, the mean rate of ampicillin resistance from two distinct studies, one in the south of Iran (Bandar Abbas, n = 54) and another in its south-central region (Shiraz, n = 24), was determined as 14% [49], [50]. Moreover, no significant difference was seen where different methodologies were used. For example, a low mean rate of resistance was deduced for gentamycin during 2008 (7%), 2009 (6%) and 2014 (7%) [3], [35], [41], [45].
Ciprofloxacin, as an occasional empirical prescription for UTIs, showed a significant decreased resistance trend among enterococcal infections in Iran. A possible reason for this decrease over time might be the scarcity of studies in regions where ciprofloxacin is frequently used. However, ciprofloxacin is typically an antibiotic of choice for outpatients, whereas the majority of studies reported and examined were of hospitalized patients.
Since 2001, resistance to erythromycin has been high among enterococcal isolates. High emergence of resistance has precluded its empirical use in clinical settings. Macrolides are not generally used to treat enterococcal infections; however, incidental exposure of enterococci to these antibiotics might happen during the course of therapy for other bacterial infections. The most frequent type of macrolide resistance is the production of a methylase enzyme, which specifically methylates an adenine residue in the 23S rRNA of the 50S ribosomal subunit. This reduces the binding affinity of macrolides for the ribosome and hence renders macrolides ineffective [51].
The trend of resistance to vancomycin was insignificantly increased during 2001–2016. The year 2002 seems to be the start of a sudden rise in vancomycin resistance in Iran. A study carried out in Tehran during 2002 revealed that 102 (8%) of 1231 urine bacterial isolates were identified as enterococci, among which 46% were found to be vancomycin resistant.
The apparent upsurge and downsurge in vancomycin resistance seems to have occurred every 2 to 3 years during the last 15 years in Iran. During 2007, 8.5% vancomycin-resistant enterococci (VRE) was reported among clinical enterococci recovered from two hospitals in Tehran [44]. In 2016, 4% VRE were isolated from patients in southeast Iran (Zabol province), which included the first reported case of vancomycin-resistant Enterococcus mundtii in Iran [18]. In the same year, another study in the south of Iran (Shiraz) reported 45% VRE [52]. The difference in the percentages reported within the same year from difference provinces could be due to the lack of proper medical reporting in some provinces, different methodology and in particular different vancomycin discs used, or geographic variations.
Fig. 3 shows a clear indication that both E. faecalis and E. faecium have had an increase in the mean resistance against all the antibiotics during a 10-year period. This increase in resistance was only significant in the case of erythromycin and ampicillin for E. faecalis and erythromycin in the case of E. faecium species. Erythromycin, as an empirical therapy, might have increased during this time period, resulting in constant exposure with enterococcal species and eventual evolving of resistance mechanisms by the microorganism.
The mean resistance rates against gentamicin, vancomycin and ampicillin were significantly higher among E. faecium species compared to E. faecalis during 2005. In 2015, however, gentamicin resistance among E. faecium species fell below that of E. faecalis. This decrease, however, was statistically insignificant.
Higher rates of antibiotic resistance among E. faecium compared to E. faecalis has been well documented. The presence of resistance genes and mechanisms such as the aminoglycoside modifying enzymes Aph(2″)-Ib and Ant(3″)-Ia, responsible for high-level gentamicin and streptomycin resistance or altered cell wall structures due to L,D-transpeptidase causing resistance to β-lactams, have been extensively reported in E. faecium [53]. Such alterations have resulted in MICs for β-lactam to be typically about 2 to 8 mg/mL for E. faecalis and 8 to 16 mg/mL for E. faecium. Additionally, because of the species genomic structure, vancomycin resistance by vanA is relatively uncommon in clinical isolates of E. faecalis compared to E. faecium.
Our analysis showed that with the exception of penicillin and ampicillin, resistance to all antibiotics was higher (although not statistically significant) among complicated cases compared to general patients. Complicated patients are more exposed to antibiotics through hospitalization. The balance in the microbiota becomes altered by the overuse of antibiotics such as cephalosporins and metronidazole, which are often used empirically in critically ill patients. These antibiotics are able to exterminate many Gram-negative and anaerobic bacteria, leaving more resistant enterococci intact. Moreover, some cephalosporins (such as ceftriaxone) are concentrated in the gut through gut secretions, thus emphasizing its effect against gut microbiota and facilitating the expansion of resistant enterococci from the gut to other sites within the body [53].
Conclusion
A notable increase and decrease in the trends of enterococcal resistance to ampicillin, gentamicin, ciprofloxacin, vancomycin and erythromycin were found during 2001–2016. The mean resistance against all the antibiotics has continued to rise in E. faecalis and E. faecium during a 10-year period from 2010 to 2015. With the exception of penicillin and ampicillin, resistance to all antibiotics was higher among complicated patients compared to general patients. Although more data are needed to confirm our results, it seems that the use of newer antibiotics such as linezolid, and in case of E. faecium infections Synercid, appears to be a potentially reasonable therapeutic choice in Iran.
Acknowledgements
We thank the Faculty of Medicine and the vice chancellor of research, as well as the technology department of Iran University of Medical Sciences for their support.
Conflict of interest
None declared.
References
- 1.Mansouri S., Shakibaei M., Mahboub S. Antibiotic susceptibility in enterococci isolated from patients in Kerman, Southeastern Iran. Iran J Med Sci. 2005;30:68–72. [Google Scholar]
- 2.Lukášová J., Šustáčková A. Enterococci and antibiotic resistance. Acta Veterinaria Brno. 2003;72:315–323. [Google Scholar]
- 3.Tabatabaei S.R., Karimi A., Navidinia M., Fallah F., Fard A.T., Rahbar M. A study on prevalence of vancomycin-resistant enterococci carriers admitted in a children’s hospital in Iran. Ann Biol Res. 2012;3:5441–5445. [Google Scholar]
- 4.van Harten R.M., Willems R.J., Martin N.I., Hendrickx A.P. Multidrug-resistant enterococcal infections: new compounds, novel antimicrobial therapies? Trends Microbiol. 2017;25:467–479. doi: 10.1016/j.tim.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Emaneini M., Hosseinkhani F., Jabalameli F., Nasiri M., Dadashi M., Pouriran R. Prevalence of vancomycin-resistant Enterococcus in Iran: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2016;35:1387–1392. doi: 10.1007/s10096-016-2702-0. [DOI] [PubMed] [Google Scholar]
- 6.Khani M., Fatollahzade M., Pajavand H., Bakhtiari S., Abiri R. Increasing prevalence of aminoglycoside-resistant Enterococcus faecalis isolates due to the aac(6′)-aph(2″) gene: a therapeutic problem in Kermanshah, Iran. Jundishapur J Microbiol. 2016;9 doi: 10.5812/jjm.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talebi M., Pourshafie M., Katouli M., Möllby R. Molecular structure and transferability of Tn1546-like elements in Enterococcus faecium isolates from clinical, sewage, and surface water samples in Iran. Appl Environ Microbiol. 2008;74:1350–1356. doi: 10.1128/AEM.02254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pourshafie M.R., Talebi M., Saifi M., Katouli M., Eshraghi S., Kühn I. Clonal heterogeneity of clinical isolates of vancomycin-resistant Enterococcus faecium with unique vanS. Trop Med Int Health. 2008;13:722–727. doi: 10.1111/j.1365-3156.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- 9.Karimaei S., Sadeghi J., Asadian M., Esghaei M., Pourshafie M.R., Talebi M. Antibacterial potential and genetic profile of Enterococcus faecium strains isolated from human normal flora. Microb Pathog. 2016;96:67–71. doi: 10.1016/j.micpath.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Behrooozi A., Rahbar M., Jalil V. A survey on epidemiology of urinary tract infections and resistance pattern of uropathogens in an Iranian 1000-bed tertiary care hospital. Afr J Microbiol Res. 2010;4:753–756. [Google Scholar]
- 11.Rahbar M., Hajia M., Farzanehkhah M. Activity of nitrofurantoin against urinary tract infection (UTI) isolates of vancomycin-resistant enterococci (VRE): a three-year survey in an Iranian hospital. Iran J Pathol. 2007;2:171–174. [Google Scholar]
- 12.Saffar M., Enayti A., Abdolla I., Razai M., Saffar H. Antibacterial susceptibility of uropathogens in 3 hospitals, Sari, Islamic Republic of Iran, 2002–2003. East Mediterr Health J. 2008;14:556–563. [PubMed] [Google Scholar]
- 13.Saifi M., Pourshafie M.R., Eshraghian M.R., Soltan Dallal M.M. Anti-microbial resistance of enterococci isolated from urinary tract infections in Iran. Iran Biomed J. 2008;12:185–190. [PubMed] [Google Scholar]
- 14.Farajnia S., Alikhani M.Y., Ghotaslou R., Naghili B., Nakhlband A. Causative agents and antimicrobial susceptibilities of urinary tract infections in the northwest of Iran. Int J Infect Dis. 2009;13:140–144. doi: 10.1016/j.ijid.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Mirzaei B., Babaei R., Asiabar A.P.D., Bameri Z. Detection of both vanA and vanB genes in vanA phenotypes of enterococci by TaqMan RT-PCR. Braz J Microbiol. 2015;46:161–165. doi: 10.1590/S1517-838246120131234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haghi-Ashteiani M., Sadeghifard N., Abedini M., Taheri-Kalani S.S.M. Etiology and antibacterial resistance of bacterial urinary tract infections in Children’s Medical Center, Tehran, Iran. Acta Med Iran. 2007;45:153–157. [Google Scholar]
- 17.Jabalameli F., Emaneini M., Shahsavan S., Sedaghat H., Abdolmaliki Z., Aligholi M. Evaluation of antimicrobial susceptibility patterns of enterococci isolated from patients in Tehran University of Medical Sciences teaching hospitals. Acta Med Iran. 2009;47:325–328. [Google Scholar]
- 18.Sharifi-Rad M., Shadanpour S., van Belkum A., Soltani A., Sharifi-Rad J. First case of vanA-positive Enterococcus mundtii in human urinary tract infection in Iran. New Microbes New Infect. 2016;11:68–70. doi: 10.1016/j.nmni.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shokoohizadeh L., Mobarez A.M., Zali M.R., Ranjbar R., Alebouyeh M., Sakinc T. High frequency distribution of heterogeneous vancomycin resistant Enterococcous faecium (VRE fm) in Iranian hospitals. Diagn Pathol. 2013;8:163. doi: 10.1186/1746-1596-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dadfarma N., Fooladi A.A.I., Oskoui M., Hosseini H.M. High level of gentamicin resistance (HLGR) among enterococcus strains isolated from clinical specimens. J Infect Public Health. 2013;6:202–208. doi: 10.1016/j.jiph.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Feizabadi M.M., Sayadi S., Shokrzadeh L., Parvin M., Yadegarynia D. Increase in prevalence of vancomycin resistant isolates of Enterococcus faecium at Labbafinejad Hospital. Iran J Clin Infect Dis. 2008;3:73–77. [Google Scholar]
- 22.Aligholi M., Emaneini M., Taherikalani M., Shahsavan S., Jabalameli F., Asadollahi P. Time-kill study and synergistic activity of cell-wall inhibitor antibiotics in combination with gentamicin against Enterococcus faecalis and Enterococcus faecium. Acta Microbiol Immunol Hung. 2011;58:219–226. doi: 10.1556/AMicr.58.2011.3.5. [DOI] [PubMed] [Google Scholar]
- 23.Sharifi Y., Hasani A., Ghotaslou R., Naghili B., Aghazadeh M., Milani M. Virulence and antimicrobial resistance in enterococci isolated from urinary tract infections. Adv Pharm Bull. 2013;3:197–201. doi: 10.5681/apb.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eslami G., Nowruzi J., Fllah F., Goudarzi H., Hakemivala M., Jahangiri S. Detection of bacteria responsible for gallbladder inflammation and gallstones. Iran J Clin Infect Dis. 2007;2:139–141. [Google Scholar]
- 25.Anvarinejad M., Pouladfar G., Japoni A., Bolandparvaz S., Satiary Z., Mardaneh J. Diabetic foot infections: antibiotic susceptibility patterns and determination of antibiotic cross-resistance in clinical isolates of Enterococcus species during 2012–2014 in Shiraz, Iran. Arch Pediatr Infect Dis. 2016;5 [Google Scholar]
- 26.Kaveh M., Bazargani A., Ramzi M., Ebrahim-Saraie H.S., Heidari H. Colonization rate and risk factors of vancomycin-resistant enterococci among patients received hematopoietic stem cell transplantation in Shiraz, Southern Iran. Int J Organ Transplant Med. 2016;7:197–205. [PMC free article] [PubMed] [Google Scholar]
- 27.Hasibi M., Rezaii J., Iravani B.M., Moslemi S.B., Haji-Abadi M.R., Taghavi S.M. Hospital-acquired vancomycin-resistant enterococci: a report of 2-year experience. Acta Med Iran. 2009;47:469–472. [Google Scholar]
- 28.Nateghian A., Robinson J., Arjmandi K., Vosough P., Karimi A., Behzad A. Epidemiology of vancomycin-resistant enterococci in children with acute lymphoblastic leukemia at two referral centers in Tehran, Iran: a descriptive study. Int J Infect Dis. 2011;15:e332–e335. doi: 10.1016/j.ijid.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Haghighat M., Dehghani S.M., Alborzi A., Imanieh M.H., Pourabbas B., Kalani M. Organisms causing spontaneous bacterial peritonitis in children with liver disease and ascites in Southern Iran. World J Gatroenterol. 2006;12:5890–5892. doi: 10.3748/wjg.v12.i36.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javadi A., Ataei B., Khorvash F., Toghyani S., Mobasherzadeh S., Soghrati M. Prevalence of vancomycin resistant enterococci colonization in gastrointestinal tract of hospitalized patients. Arch Clin Infect Dis. 2008;3 http://journals.sbmu.ac.ir/infectiousinvisible/article/view/207 Available at: [Google Scholar]
- 31.Hassanzadeh P., Motamedifar M., Hadi N. Prevalent bacterial infections in intensive care units of Shiraz University of medical sciences teaching hospitals, Shiraz, Iran. Jpn J Infect Dis. 2009;62:249–253. [PubMed] [Google Scholar]
- 32.Askarian M., Afkhamzadeh R., Monabbati A., Daxboeck F., Assadian O. Risk factors for rectal colonization with vancomycin-resistant enterococci in Shiraz, Iran. Int J Infect Dis. 2008;12:171–175. doi: 10.1016/j.ijid.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Zarinfar N., Cyrus A., Sharafkhah M., Akbari M. Antibiotic resistance profile of Gram-positive bacteria isolated from inpatients of an academic and a private hospital in Arak, Central Iran, 2013–2014. J Iran Clin Res. 2016;2:168–173. [Google Scholar]
- 34.Aligholi M., Emaneini M., Jabalameli F., Shahsavan S., Abdolmaleki Z., Sedaghat H. Antibiotic susceptibility pattern of Gram-positive cocci cultured from patients in three university hospitals in Tehran, Iran during 2001–2005. Acta Med Iran. 2009;47:329–334. [Google Scholar]
- 35.Asadian M., Sadeghi J., Lari A.R., Razavi S., Bibalan M.H., Talebi M. Antimicrobial resistance pattern and genetic correlation in Enterococcus faecium isolated from healthy volunteers. Microb Pathog. 2016;92:54–59. doi: 10.1016/j.micpath.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Khalili H., Dashti-Khavidaki S., Karimzadeh I., Jafari S., Abdollahi A., Shahidi M.R. Changes in 4-year antimicrobial resistance pattern of Gram-positive bacteria at the main referral teaching hospital, Tehran, Iran. Acta Med Iran. 2012;50:493–504. [PubMed] [Google Scholar]
- 37.Talebi M., Eshraghi S., Pourshafie M., Pourmand M., Eshraghian M. Characterization of vancomycin resistant Enterococcus faecium. Iran J Public Health. 2007;36:20–25. [Google Scholar]
- 38.Arabestani M.R., Nasaj M., Mousavi S.M. Correlation between infective factors and antibiotic resistance in enterococci clinical isolates in west of Iran. Chonnam Med J. 2017;53:56–63. doi: 10.4068/cmj.2017.53.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emaneini M., Hashemi F.B., Aligholi M., Fatholahzadeh B., Kazemi B., Sadeghi F. Detection of vanB genotype enterococci in Iran. Int J Antimicrob Agents. 2005;26:98–99. doi: 10.1016/j.ijantimicag.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Haeili M., Ghodousi A., Nomanpour B., Omrani M., Feizabadi M.M. Drug resistance patterns of bacteria isolated from patients with nosocomial pneumonia at Tehran hospitals during 2009–2011. J Infect Dev Ctries. 2013;7:312–317. doi: 10.3855/jidc.2604. [DOI] [PubMed] [Google Scholar]
- 41.Mohammadi F., Ghafourian S., Mohebi R., Taherikalani M., Pakzad I., Valadbeigi H. Enterococcus faecalis as multidrug resistance strains in clinical isolates in Imam Reza Hospital in Kermanshah, Iran. Br J Biomed Sci. 2015;72:182–184. doi: 10.1080/09674845.2015.11665750. [DOI] [PubMed] [Google Scholar]
- 42.Mamishi S., Pourakbari B., Ashtiani M.H., Hashemi F.B. Frequency of isolation and antimicrobial susceptibility of bacteria isolated from bloodstream infections at Children’s Medical Center, Tehran, Iran, 1996–2000. Int J Antimicrob Agents. 2005;26:373–379. doi: 10.1016/j.ijantimicag.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Rezvani J., Nasr R., Shamsabadi F.T., Eidgahi M.R.A. Frequency of vanA, vanB and vanH variants amongst vancomycin-resistant enterococci isolated from patients in central region of Iran. Gastroenterol Hepatol Bed Bench. 2016;9:308–315. [PMC free article] [PubMed] [Google Scholar]
- 44.Yasliani S., Mobarez A.M., Doust R.H., Satari M., Teymornejad O. Linezolid vancomycin resistant Enterococcus isolated from clinical samples in Tehran hospitals. Indian J Med Sci. 2009;63:297–302. [PubMed] [Google Scholar]
- 45.Ghasemi E., Mansouri S., Shahabinejad N. Vaginal colonization and susceptibility to antibiotics of enterococci during late pregnancy in Kerman City, Iran. Arch Clin Infect Dis. 2016;11 [Google Scholar]
- 46.Akhi M., Farzaneh F., Oskouei M. Study of enterococcal susceptibility patterns isolated from clinical specimens in Tabriz, Iran. Pak J Med Sci. 2009;25:211–216. [Google Scholar]
- 47.Kafil H.S., Asgharzadeh M. Vancomycin-resistant Enterococcus faecium and Enterococcus faecalis isolated from education hospital of Iran. Maedica (Buchar) 2014;9:323–327. [PMC free article] [PubMed] [Google Scholar]
- 48.Sharifi Y., Hasani A., Ghotaslou R., Varshochi M., Hasani A., Soroush M.H. Vancomycin-resistant enterococci among clinical isolates from north-west Iran: identification of therapeutic surrogates. J Med Microbiol. 2012;61:600–602. doi: 10.1099/jmm.0.036780-0. [DOI] [PubMed] [Google Scholar]
- 49.Ghalandarzadeh Daryaii Z., Javadpour S., Kargar M. Frequency of vancomycin-resistant enterococci isolated from clinical samples of Shahid Mohammadi Hospital through the E-test method. Bimonth J Hormoz Univ Med Sci. 2015;19:211–217. [Google Scholar]
- 50.Karimzadeh I., Mirzaee M., Sadeghimanesh N., Sagheb M.M. Antimicrobial resistance pattern of Gram-positive bacteria during three consecutive years at the nephrology ward of a tertiary referral hospital in Shiraz, Southwest Iran. J Res Pharm Pract. 2016;5:238–247. doi: 10.4103/2279-042X.192460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kristich C.J., Rice L.B., Arias C.A. Enterococcal infection—treatment and antibiotic resistance. In: Gilmore M.S., Clewell D.B., Ike Y., Shankar N., editors. Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary; Boston, MA: 2014. [PubMed] [Google Scholar]
- 52.Heidari H., Hasanpour S., Ebrahim-Saraie H.S., Motamedifar M. High incidence of virulence factors among clinical Enterococcus faecalis isolates in Southwestern Iran. Infect Chemother. 2017;49:51–56. doi: 10.3947/ic.2017.49.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollenbeck B.L., Rice L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence. 2012;3:421–569. doi: 10.4161/viru.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]