Abstract
Schizophrenia is the eighth leading cause of disability worldwide in people aged 15–44 years. Before antidopaminergic antipsychotics were introduced in the 1950s, no effective medications existed for the treatment of schizophrenia. This review summarizes key meta-analytic findings regarding antipsychotic efficacy in the acute treatment of schizophrenia, including clozapine in treatment-resistant patients. In the most comprehensive meta-analysis of randomized controlled trials conducted in multi-episode schizophrenia, antipsychotics outperformed placebo regarding total symptoms, positive symptoms, negative symptoms, depressive symptoms, quality of life and social functioning. Amongst these outcomes, the standardized mean difference for overall symptoms was largest, that is, 0.47 (95% credible interval = 0.42–0.51), approaching a medium effect size, being reduced to 0.38 when publication bias and small-trial effects were accounted for. A comparison of two meta-analyses indicated that first-episode patients, compared with multi-episode patients, were more likely to have at least minimal treatment response [⩾20% Positive and Negative Syndrome Scale (PANSS)/Brief Psychiatric Rating Scale (BPRS) score reduction: 81% versus 51%] and good response (⩾50% PANSS/BPRS score reduction: 52% versus 23%). In multi-episode schizophrenia, no response or worsening after 2 weeks of a therapeutic antipsychotic dose was highly predictive of not achieving a good response at endpoint (median treatment = 6 weeks: specificity = 86%; positive predictive value = 90%), suggesting a change in treatment should be considered in such cases. In first-episode psychosis, adequately dosed antipsychotic treatment trials for more than 2 weeks are recommended before using no response or worsening as a decision point for aborting a given antipsychotic. In clearly defined treatment-resistant schizophrenia, clozapine generally outperformed other antipsychotics, especially when dosed appropriately (target = 3–6 months’ duration; trough clozapine level ⩾350–400 μg/L) with a response rate (⩾20% PANSS/BPRS) of 33% by 3 months of treatment. High antipsychotic doses and psychotropic combinations are unlikely to be superior to standard doses of antipsychotic monotherapy. Acute antipsychotic efficacy in schizophrenia depends on the targeted symptom domain (greater efficacy: total and positive symptoms, lesser efficacy: negative symptoms, depressive symptoms, social functioning and quality of life). Greater antipsychotic efficacy is associated with higher total baseline symptom severity, treatment-naïveté/first-episode status, shorter illness duration, and trials that are nonindustry sponsored and that have a lower placebo effect. The heterogeneity of antipsychotic response across individuals and key symptom domains, the considerable degree of nonresponse/treatment resistance in multi-episode patients, and the adverse effect potential of antipsychotics are major limitations, underscoring the need to develop new medications for the treatment of schizophrenia. Drug development should include matching patient subgroups, which are identified by means of clinical and biomarker variables, to mechanisms of action of novel medications, targeting specific symptom domains, and investigating mechanisms of action other than dopaminergic blockade.
Keywords: antipsychotic, clozapine, efficacy, meta-analysis, randomized controlled trial, response, schizophrenia
Introduction
Schizophrenia is a common and serious psychiatric illness. The lifetime risk is approximately 1%.1 The morbidity, mortality and impact on quality of life are considerable and the illness has high economic costs for society. The World Health Organization (WHO) ranked schizophrenia as the eighth leading cause of disability worldwide in the age group 15–44 years.2 Data for 2013 estimated the total economic costs of the illness in the United States at $155.7 billion.3 The largest components were unemployment (38%), productivity loss associated with caregiving (34%), and direct health care costs (24%).
The longitudinal course following a first psychotic episode is highly variable, as shown by the ÆSOP-10 study, a multicentre 10-year follow up of a cohort of 557 people with first-episode psychosis in the UK.4 The two extremes in course were represented by 23% of participants who had continuous symptoms over the 10-year follow-up period and 13% of participants whose symptoms remitted within 6 months of first contact and who remained symptom free throughout follow up. Between these two extremes were the majority of individuals who had a relapsing course, that is, they experienced at least one remission but one or more relapses.
Chlorpromazine was the world’s first antipsychotic drug. It was synthesized in 1950 by the French pharmaceutical company Rhône-Poulenc and introduced into clinical practice in France in 1952.5 Since that time, new antipsychotics have regularly been introduced. To date, all share the ability to block D2 receptors and reduce postsynaptic dopamine transmission. With the exception of clozapine, which is more effective in treatment-resistant schizophrenia, differences in efficacy between individual antipsychotic drugs are relatively small, at least at group levels.6 In contrast, there are major differences between antipsychotics drugs in risk of individual side effects.6
This paper reviews the efficacy of antipsychotics in acute schizophrenia. The reviewed data reflect the authors’ knowledge of the literature, with the emphasis being on data from recently published, high-quality meta-analyses. By acute efficacy we refer to the benefits of antipsychotics as demonstrated in short-term clinical trials, typically 6–12 weeks in duration. Acute effectiveness in real world clinical practice is influenced by many additional factors, including therapeutic alliance, adherence and substance misuse, which are outside the remit of this paper. Antipsychotics are also effective in reducing the risk of relapse in schizophrenia,7 an area that is due to be reviewed in a forthcoming paper in the journal.8 Efficacy, acute or long term, needs to be balanced against the adverse effects of medication, an area outside our remit. The adverse effect profiles of antipsychotics differ markedly and there is high-quality meta-analytic evidence to assist patients and clinicians select antipsychotics according to the likelihood of specific adverse effects occurring.6,9 We start by reviewing some of the early placebo-controlled trials of antipsychotics conducted in the 1960s before moving on to consider recent meta-analyses of antipsychotic treatment in first-episode and multi-episode patients. We then consider the efficacy of pharmacological strategies when there has been limited response to antipsychotics before considering clozapine in treatment-resistant schizophrenia, as well as opportunities for future research and drug development.
Early trials of antipsychotics in schizophrenia
Chlorpromazine entered clinical practice in 1952 without any supporting clinical trials having been conducted. Contemporary accounts from the 1950s indicate that clinicians were impressed by its benefits, first recognizing the tranquilizing and later the antipsychotic effects, and it was felt that a new era of treatment was starting.10 Other antipsychotics followed, including haloperidol, synthesized in 1958. During the 1950s, case reports and noncontrolled studies supporting the effectiveness of various antipsychotics were published.10,11 However, it was not until the early 1960s that the results of sufficiently large, double-blind, placebo-controlled, randomized trials of antipsychotic treatment were published. These were highly supportive.12–14
In 1960, a Veteran Administration study was published showing that chlorpromazine was superior in reducing overall symptoms versus placebo, phenobarbital (an active control) and promazine over a 12-week period in the treatment of male inpatients with chronic schizophrenia.12 The average patient in the study had been ill for 10 years and hospitalized for over 7 years. Soon thereafter, a further study was published comparing four phenothiazine antipsychotics to both an inert and an active placebo in male and female inpatients over 8 months of treatment.13 All patients had been hospitalized for 2–10 years. All four phenothiazines were superior to the two placebos in reducing overall symptoms. The phenothiazine-treated group was also superior to the placebo-treated group in clinicians’ ratings of the proportion of patients who had improved (48% for antipsychotics; 6% for the combined placebo group) and who were deemed suitable for discharge when the study ended and blinding was broken (36% antipsychotics versus 5% placebo).
In 1964, the National Institute of Mental Health (NIMH) Psychopharmacology Service Center Collaborative Study Group showed the benefits of antipsychotics in inpatients with acute schizophrenia.14 The study recruited 463 individuals, newly admitted to one of nine hospitals in the United States, half of whom were treated for their first episode. They were randomized to placebo or one of three antipsychotics (chlorpromazine, thioridazine or fluphenazine). After 6 weeks, the proportion of patients who showed a marked to moderate degree of improvement was 75% for those who received antipsychotic treatment and 23% for those who received placebo. There were no significant differences in efficacy between the three antipsychotics assessed. The striking benefit of antipsychotic treatment in this study partly reflects the high proportion of patients included with first-episode psychosis, a group now known to be more responsive to antipsychotics than those with multi-episode psychosis.15 The NIMH study showed that antipsychotics were effective in treating a wide range of symptoms of schizophrenia, including auditory hallucinations, ideas of persecution, hebephrenic symptoms, incoherent speech, irritability and hostility; that is, their benefit was not confined to treating overactivity and behavioural disturbances.
Acute antipsychotic efficacy in first-episode schizophrenia
A systematic review by Zhu and colleagues assessed response rates of first-episode patients with schizophrenia in randomized trials of antipsychotics.16 Blinded and open-label randomized studies were included. In total, 17 studies were included (n = 3156 participants). A weakness of the evidence base was that the systematic review did not identify any placebo-controlled trials. This absence is a major omission in the literature. However, conducting a placebo-controlled trial in first episode psychosis presents significant ethical issues; the relatively short duration of illness would mean that associated risks (e.g. self harm, aggression, self neglect) would often be less clear than in a person who had a chronic illness with well documented previous episodes of psychosis. Furthermore, delaying effective treatment (i.e. extending the duration of untreated psychosis) has been associated with poorer symptomatic and social outcomes and would go against the ethos of early intervention services. The primary outcome for the meta-analysis was the change in the total score on the Positive and Negative Syndrome Scale (PANSS), or if this was not available, the change in the score on the Brief Psychiatric Rating Scale (BPRS), or if neither scale was used, then the score or another rating scale for symptoms of schizophrenia. Two definitions of response were used, namely those who achieved a reduction of at least 20% on the PANSS/BPRS from baseline to endpoint and those who showed a reduction of at least 50% on the PANSS/BPRS. This decision is supported by earlier work that showed that a 20% PANSS/BPRS reduction approximates to minimal improvement on the Clinical Global Impressions (CGI) scale, and a 50% PANSS/BPRS reduction approximates to much improved on the CGI.17–19
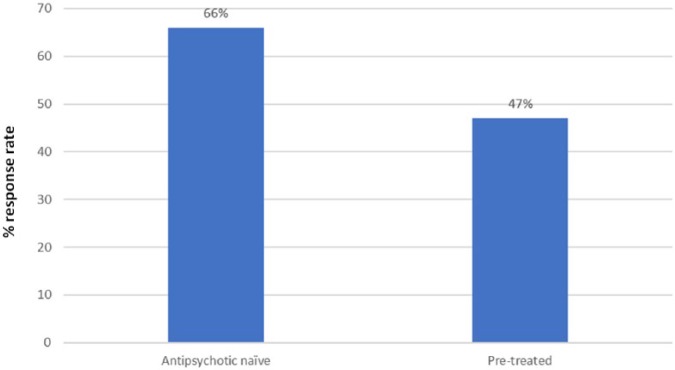
On average, 81% of the first-episode patients showed at least a 20% PANSS or BPRS reduction from their baseline score and when the more rigorous 50% reduction criterion was applied this reduced to 52%.16 These rates are considerably higher than the comparative rates seen in patients with chronic schizophrenia with 51% achieving at least a 20% PANSS/BPRS reduction and 23% achieving at least a 20% PANSS/BPRS reduction from baseline.9 The researchers used meta-regression to investigate the effect of potential moderators on response in first-episode patients. Being female, antipsychotic naïve (Figure 1), more severely ill and having a shorter duration of illness at baseline were each significantly associated with a higher response rate.16 Response rate was also higher in open versus blinded studies, but this just missed statistical significance (57% versus 48%; p = 0.06). A better response in female patients20 and more severely ill patients21 (see below) has been reported in other analyses. The higher response rate in antipsychotic-naïve patients may be because prior antipsychotic treatment has reduced baseline symptom severity, thereby reducing the scope for further improvement with the antipsychotic being assessed. The better outcome in those with a shorter illness duration may reflect illness duration acting as a marker for duration of untreated psychosis (DUP) (i.e. the time between the onset of the first psychotic episode and the start of first adequate treatment). However, this is speculative, as DUP per se could not be analysed in this meta-analysis since the individual studies rarely reported it. Nevertheless, many studies have shown that a longer DUP is significantly correlated with poorer symptomatic as well as functional outcomes in the short and long term, although the relationship is strongest for short-term outcomes.22–25
Figure 1.
Antipsychotic response rate in studies in first-episode psychosis: studies in antipsychotic naïve patients versus studies that allowed pretreatment (p = 0.004) (data from Zhu et al.16).
A related systematic review investigated the efficacy and tolerability of 12 individual antipsychotics in the first episode of schizophrenia.26 In this network meta-analysis, haloperidol was inferior to several second-generation drugs in terms of overall symptom reduction and all-cause discontinuation. There was little difference between second-generation antipsychotics in terms of efficacy. The quality of evidence was generally low. The authors recommended that medication should be selected primarily by the different side effect profiles of antipsychotics. The relative risk of a range of antipsychotic side effects has been established in an earlier and larger network meta-analysis in patients with chronic schizophrenia.6
Efficacy of antipsychotics versus placebo in multi-episode patients
In this section, we focus on a systematic review by Leucht and colleagues, which includes the most comprehensive meta-analysis of double-blind, randomized, placebo-controlled oral antipsychotic drug trials in acute schizophrenia.9 The review was restricted to trials in adults with an acute exacerbation of schizophrenia or related disorders; that is, schizoaffective disorder, schizophreniform disorder and delusional disorder. It is worth noting that this entry criterion would encompass chronically ill patients who experience an exacerbation of psychosis as well as patients with an acute relapse following various degrees of remission. It is unclear to what degree these groups differ in terms of treatment response. Similarly, the pooling of patients with schizophrenia and related disorders does not allow investigation of whether diagnosis alters treatment response. However, it is common practice for meta-analyses, and individual clinical trials, of treatment in ‘schizophrenia’ to also include people with schizoaffective disorder, schizophreniform disorder and delusional disorder. The minimum trial duration was 3 weeks. All antipsychotics licensed in at least one country were considered with the exception of clozapine. Trials of antipsychotic long-acting injections and short-acting intramuscular antipsychotics were also excluded. As with the meta-analysis in first-episode schizophrenia discussed in the previous section,16 outcome was response rate defined as either at least a 20% or at least a 50% reduction in the PANSS or BPRS total score from baseline. The authors referred to these two outcomes as representing a ‘minimal’ and ‘good’ response, respectively.
The analysis included 167 randomized controlled trials (RCTs) published between 1955 and 2016 (n = 28,102 participants).9 The mean age of participants was 38.7 years and the mean duration of illness was 13.4 years. The analysis did not include any studies that exclusively recruited patients with a first episode of psychosis (no such studies were identified in the systematic review). Therefore, the placebo-controlled findings relate to patients with multi-episode schizophrenia. The median duration of the studies was 6 weeks (range = 3–28 weeks). About half the studies, for which sponsorship could be identified, were industry sponsored.
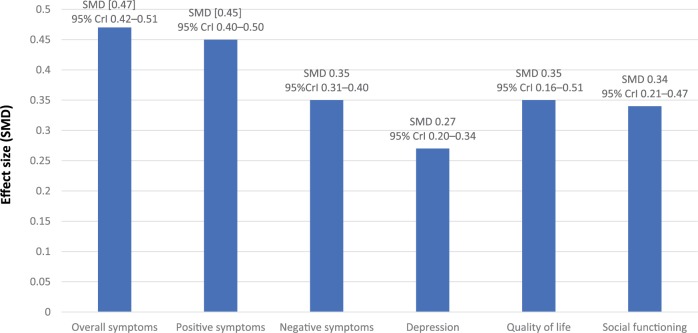
The standardized mean difference (SMD) size for overall symptoms was 0.47 (95% credible interval = 0.42–0.51), which approaches the conventional threshold for a medium effect and was equivalent to a reduction in the PANSS of 9.6 points. The SMD reduced to 0.38 when publication bias and small-trial effects were accounted for. The SMD for positive symptoms was comparable to that for total symptoms and greater than for depressive or negative symptoms (Figure 2). Antipsychotics also showed a benefit, similar in magnitude to negative symptoms, in improving quality of life data and social functioning (Figure 2), outcomes that are highly relevant to patients. A multivariate meta-regression investigated trial characteristics that could predict drug–placebo differences. This analysis showed that the only independent moderators of greater antipsychotic efficacy were nonindustry sponsorship and smaller placebo response, both of which were associated with greater efficacy. Importantly, the placebo effect was shown to have increased over time while antipsychotic response had remained unchanged over time (12.2-point versus 1.2-point increase over 45 years of study conduct).
Figure 2.
Effect size [expressed as standardized mean difference (SMD) between antipsychotic treatment versus placebo) for various outcomes as reported in a comprehensive meta-analysis of placebo-controlled antipsychotic drug trials in acute schizophrenia (data from Leucht et al.9).
95% CrI = 95% credible interval.
Table 1 shows the percentage of patients in the antipsychotic- and placebo-treated groups who were responders, according to two definitions, as well as those leaving the study for any reason or inefficacy. It is sobering that only a minority of those treated with antipsychotics achieved a good response [23% for antipsychotics versus 14% for placebo; number needed to treat (NNT) = 8]. Those treated with antipsychotics were significantly more likely to experience movement disorders, sedation, weight gain, prolactin increase and QTc prolongation than those treated with placebo.9
Table 1.
Response and dropout rates according to different criteria in the antipsychotic-treated and placebo-treated groups as reported in a comprehensive meta-analysis of placebo-controlled antipsychotic drug trials in acute schizophrenia (data from Leucht et al.9).
| Outcome | % of patients treated with antipsychotic | % of patients treated with placebo | No. of studies | Total sample | Number needed to treat to benefit (NNT) (credible interval) |
|---|---|---|---|---|---|
| Minimal response | 51 | 30 | 46 | 8918 | 5 (4–5) |
| Good response | 23 | 14 | 38 | 8403 | 8 (6–11) |
| Stopping treatment: any reason | 38 | 56 | 105 | 22,851 | 11 (9–14) |
| Stopping treatment: inefficacy | 13 | 26 | 94 | 23,017 | 7 (6–9) |
Effect of illness severity on antipsychotic efficacy
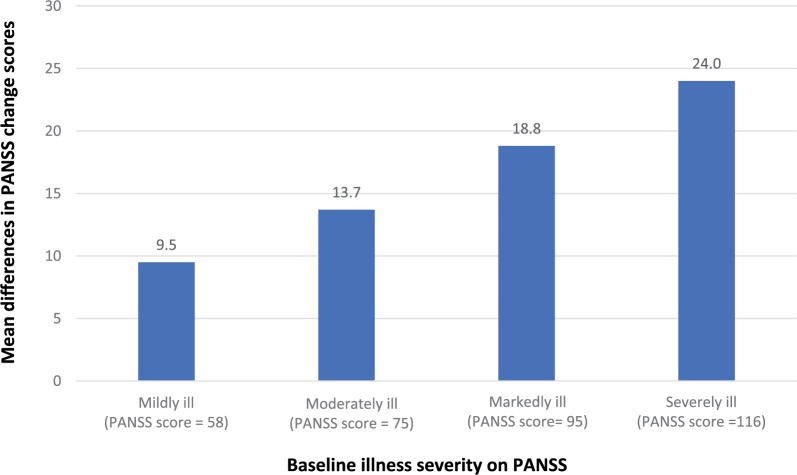
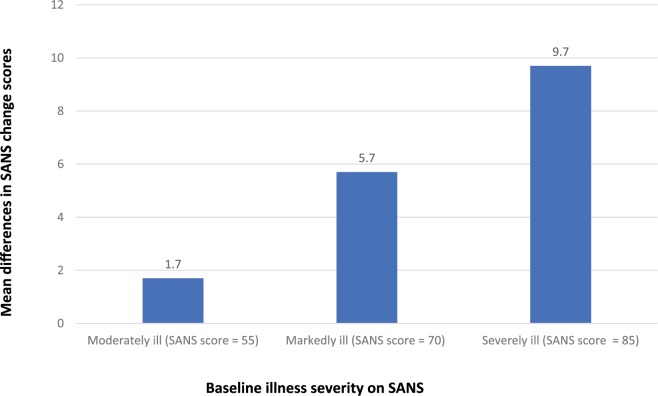
Furukawa and colleagues conducted a meta-analysis to examine the effect of baseline severity of both positive and negative symptoms on the efficacy of antipsychotic drugs.21 Individual-level data from three placebo-controlled trials in acute schizophrenia (n = 611) and three trials in patients with predominantly negative symptoms of schizophrenia (n = 475) were analysed. The main outcome was change on the PANSS and the Scale for the Assessment of Negative Symptoms (SANS) from baseline up to 6 weeks later. The relationship between the baseline score and change scores for the drug-treated and placebo-treated patients was examined using a series of mixed-effects models. The best-fitting models showed that greater baseline severity was significantly (p < 0.01) associated with a greater magnitude of differences between active treatment and placebo. This relationship was seen for both change in the PANSS (Figure 3) and the SANS in those with predominantly negative symptoms (Figure 4). A higher antipsychotic response rate in more severely ill patients has been reported in other meta-analyses, including in first-episode studies discussed earlier.16 In summary, antipsychotics can benefit patients throughout a spectrum of illness severity, but the benefits are greatest in the most severely ill. Nevertheless, symptom improvement does not mean that the patient is well. Since response is a relative measure, more severely ill patients may improve more, but may still not reach the absolute threshold remission,15 defined as no more than mild positive and negative symptoms maintained for at least 6 months.27 Thus, even in acute studies, cross-sectional remission should also be considered.
Figure 3.
Estimated mean differences in Positive and Negative Syndrome Scale (PANSS) change scores by baseline illness severity (based on best-fit model and data from a participant-level meta-analysis of placebo-controlled studies in acute schizophrenia conducted by Furukawa et al.21).
Estimated mean differences in Scale for the Assessment of Negative Symptoms (SANS) change scores by baseline illness severity in patients with predominantly negative symptoms (based on best-fit model and data from a participant-level meta-analysis of placebo-controlled studies in schizophrenia with predominantly negative symptoms conducted by Furukawa et al.21).
Time course of antipsychotic response
For decades it was believed that antipsychotics had a delayed onset of action. In 2003, a meta-analysis by Agid and colleagues,28 assessing response over 4 weeks of antipsychotic treatment, showed that this was incorrect and that the reduction in PANSS/BPRS scores seen during the first 2 weeks of treatment (21.9%) was significantly greater than the improvement seen in the third and fourth weeks (9.8%). This finding has been confirmed by many studies since, but inconsistencies in methodology, in particular in defining early and late response, have prevented this research from being translated into clear guidance for clinicians. Given this, it is not surprising that clinical guidelines produced by different organizations vary in their recommendations as to how long a trial of antipsychotic medication should continue before consideration is given to switching.
Trying to provide clarity in answering this question, Samara and colleagues conducted a diagnostic test meta-analysis using data from 34 antipsychotic trials in individuals experiencing an acute exacerbation of schizophrenia (n = 9460).29 In most cases, individual patient data were analysed. The investigators determined whether lack of improvement at week 2 predicted future nonresponse. Lack of improvement at 2 weeks was defined as less than 20% PANSS or BPRS improvement from baseline. This approximates to a rating of less than minimal improvement made by clinicians using the CGI scale; that is, no improvement or worsening. ‘Later nonresponse’ was defined as less than 50% reduction in total score on either PANSS or BPRS from baseline to endpoint. The corollary of this (i.e. ⩾50% improvement in PANSS/BPRS) approximates to a CGI rating of much improved; that is, a robust clinically meaningful change. If possible, a 6-week endpoint was used (range = 4–12 weeks). When data from fixed dose studies were used, only those which related to at least a minimal therapeutic dose were analysed.
The analyses confirmed that less than minimal improvement after 2 weeks predicted future nonresponse at endpoint (4–12 weeks), with a specificity of 86% and positive predictive value of 90%. In other words, of 100 patients who show no improvement at week 2 (<20% PANSS or BPRS score reduction), 90 patients will fail to show much improvement at endpoint (<50% PANSS or BPRS score reduction). Conversely, of 100 patients who show at least minimal improvement at week 2 (⩾20% PANSS or BPRS score reduction), 86 patients will also show much improvement at endpoint (⩾50% PANSS or BPRS score reduction). Three independent factors increased the specificity of the diagnostic test: keeping the duration of the treatment trial shorter, that is, 4 compared with 6 weeks or longer (p = 0.0005); greater illness severity at baseline (p = 0.0001); and a shorter duration of illness (p = 0.02). Patient age, sex, whether patients had first-episode or multi-episode psychosis, antipsychotic class (first versus second generation) and whether a fixed or flexible dosing schedule was employed were all nonsignificant in affecting the results.
The diagnostic test remained accurate when it was applied to data for individual drugs, namely amisulpride, haloperidol, olanzapine and risperidone. The consistency of the results, despite the pharmacological differences between these drugs, suggests that the results can be generalized to other antipsychotics.
Although all of the meta-analysed trials based their analyses on PANSS or BPRS score ratings, scales that are not used in clinical care, the approximations to the CGI make these results clinically relevant. Furthermore, one study conducted in adolescents with schizophrenia-spectrum disorders using the CGI, without rating any other psychopathology scale, had similar predictive results in patients showing less than minimal improvement for ultimate nonresponse, defined as not reaching much or very much improved status.30 Taken together, these results imply that a switch of antipsychotic should be considered if there has been no response that meets the clinical eye after 2 weeks of antipsychotic treatment at a therapeutic dose. This strategy would prevent patients from undergoing further treatment with an antipsychotic that is unlikely to benefit them, but that may lead to new or ongoing adverse effects. Earlier switching could also identify treatment resistance sooner and reduce the delay before patients are offered a trial of clozapine. However, there are three important caveats to adopting the 2-week test in clinical practice, two of which were highlighted by the authors of the meta-analysis.29 First, there were only six studies in first-episode patients and one study in treatment-resistant patients. Therefore, this diagnostic test is most appropriate for multi-episode patients and should not be applied to those who are in their first episode or are treatment resistant, in whom longer periods may be required to reliably predict ultimate nonresponse. Several individual studies suggest that the time course of response in first-episode31,32 and treatment-resistant patients33 differs to that seen in patients with chronic disease, with both populations possibly including relevant subgroups who show a later treatment response, arguing that longer treatment trials are needed until ‘early’ nonresponse triggers a change in treatment. Clearly, additional studies on the time course of antipsychotic nonresponse are needed in these subpopulations to guide clinical practice.
The second caveat when interpreting the 2-week nonresponse test is to ensure that the 2-week trial has allowed treatment with a sufficiently high dose of antipsychotic. Most of the trials included in the meta-analysis by Samara and colleagues29 adopted a rapid titration schedule enabling target doses to be reached within 3 days. In routine clinical practice, doses of antipsychotics are often titrated more slowly in an attempt to improve tolerability or because clinical pressures mean a patient’s medication is reviewed less frequently than is ideal, or because a patient is followed in an ambulatory setting. Samara and colleagues highlight that the 2-week trial should be interpreted as 2 weeks of treatment at target doses or ‘even near the upper limits of these ranges’. A Delphi international survey of international experts has generated recommended target dose ranges for most antipsychotic drugs that can be referred to (see Table 2 for examples).34
Table 2.
Examples of target dose ranges for oral antipsychotics based on recommendations in a Delphi survey of international experts (note: respondents were asked to consider doses for a moderately symptomatic adult man with schizophrenia with ⩾2 years of antipsychotic treatment and not considered treatment resistant; that is, doses do not relate to first episode). Data adapted from Gardner et al.34
| Antipsychotic | Target dose range (mg/day) |
|---|---|
| Amisulpride | 400–800 |
| Aripiprazole | 15–30 |
| Chlorpromazine | 300–600 |
| Haloperidol | 5–10 |
| Olanzapine | 10–20 |
| Paliperidone | 6–9 |
| Quetiapine | 400–800 |
| Risperidone | 4–6 |
| Trifluoperazine | 10–20 |
| Ziprasidone | 120–160 |
| Zuclopenthixol | 20–60 |
The third caveat is that nonadherence to antipsychotics is a common problem in schizophrenia35,36 and before making any assumptions about the efficacy of an antipsychotic trial, it is essential to ensure that there has been adequate adherence. In other words, one needs to differentiate between nonadherence and nonresponse.
Acute efficacy of pharmacological strategies following antipsychotic nonresponse
A key clinical question is not only how to determine when an antipsychotic trial has failed (see section above), but also what to do in this case. Limited space means we only review this area briefly. A first step is to assess adherence, the duration of previous treatment trials and to identify any reversible factors that may be driving the psychosis, for example substance misuse or psychosocial stressors that may be amenable to psychosocial intervention.37 Pharmacological strategies commonly adopted following an inadequate antipsychotic response include dose increase, switching and combination strategies.
There is no convincing evidence that doses of antipsychotic drugs higher than the maximum licensed dose are more effective than standard doses.38 Indeed, for many antipsychotics the near-maximal effective dose (i.e. the threshold dose necessary to produce all or almost all the clinical responses for each drug) is lower than the maximum licensed dose, at least in patients enrolled in clinical trials.39 Switching is widely used in clinical practice where it often appears effective. However, this impression could partly reflect expectation bias, or the natural fluctuation in the clinical course of schizophrenia. Switching has not been adequately investigated by RCTs. Of published studies, probably the most robust is a study by Kinon and colleagues,40 in which patients who had not responded to 2 weeks of treatment with risperidone were randomised to either continue risperidone or switch to olanzapine for a further 10 weeks. Those who responded to risperidone at 2 weeks continued risperidone. Among the early nonresponder group, switching from risperidone to olanzapine led to a small but significantly greater reduction in PANSS total score and also in depressive symptoms at end point. Efficacy in the switch arm was higher in those who were at least moderately ill at the time of the switch. The SWITCH41 and OPTiMiSE (Optimization of Treatment and Management of Schizophrenia in Europe)42 studies are two large-scale RCTs that have been undertaken to investigate switching strategies in patients who have not shown an early response to antipsychotic treatment. At the time of writing the results were not available.
Given the limited effectiveness of antipsychotics in schizophrenia, various psychotropic medications have been added to antipsychotics to try and enhance their effectiveness. This is referred to as combination or augmentation treatment. This approach is common in clinical practice both for antipsychotic cotreatment (i.e. combining two antipsychotics) and for combinations involving adding a nonantipsychotic medication to an antipsychotic. For example, the median prevalence of antipsychotic polypharmacy (i.e. the concomitant use of ≥2 antipsychotics) was 19.6% based on pooled data from 147 studies published between 1970 and 2009.43 A recent systematic review of reviews identified 29 meta-analyses with 381 individual trials and 19,833 participants that compared the efficacy of an antipsychotic combined with another antipsychotic or 41 other nonantipsychotic drugs versus placebo or antipsychotic monotherapy.44 Although 14 different combination strategies significantly improved ratings of overall symptoms compared with monotherapy with a non-clozapine antipsychotic and six strategies were superior for positive symptoms, and although effect sizes for superiority ranged from small (0.2) to large (⩽1.3), study quality was generally not high and the effect sizes were inversely correlated with study quality. Taking the results of the individual study quality and cohesiveness of the results into account, overall the authors concluded that no single combination strategy had sufficient high-quality evidence that it could be recommended.
Efficacy of clozapine in treatment-resistant schizophrenia
Clozapine was synthesized by the Swiss pharmaceutical company Wander in 1956 but did not become available clinically until 1972 when it was launched in Europe. The delay has been attributed to its lack of extrapyramidal side effects leading some to doubt it was an effective antipsychotic.45 This view reflected a common opinion at the time that extrapyramidal side effects and antipsychotic efficacy were closely linked, something now known to be incorrect. The delay is also likely to have reflected concerns about clozapine’s ability to cause hypotension and seizures. In 1975, a series of cases of agranulocytosis, some fatal, were reported in elderly patients treated with clozapine in Finland.46 This led the then manufacturer, Sandoz, to voluntarily withdraw clozapine from the market. Over 10 years later, prompted by clinicians who argued that clozapine had been particularly beneficial, a well designed RCT was undertaken to compare clozapine with chlorpromazine in patients known to be refractory to at least three antipsychotics and who in addition had failed a prospective trial of haloperidol. The results, published by Kane and colleagues in the Archives of General Psychiatry in 1988, showed a clear advantage for clozapine.47 The response rate was 30% for those treated with clozapine but only 4% for those treated with chlorpromazine. This landmark study paved the way for clozapine to be reintroduced for treatment-resistant schizophrenia in 1990 with mandatory monitoring of the white cell count to ensure early detection of any decrease in the white cell count enabling appropriate action to be taken, including if necessary stopping clozapine treatment.
In the already discussed network meta-analysis of acute antipsychotic efficacy in multi-episode patients with schizophrenia,6 clozapine demonstrated significantly greater efficacy for total psychopathology than all the other antipsychotics, having a large effect size (0.88). However, time effects cannot be excluded, as earlier trials showed greater effect sizes and in nonresistant patients the superiority of clozapine should be less evident. Two meta-analyses, both published in 2016, evaluated the efficacy of clozapine in treatment-resistant patients, with treatment resistance being defined with varying degrees of stringency.48,49
The first meta-analyses by Siskind and colleagues (21 RCTs; n = 2364 participants) found that clozapine was superior to other antipsychotics for positive symptoms in both the short and long term.48 Clozapine was only superior for total and negative symptoms in the short term. Better outcomes were seen in nonindustry-funded studies and in patients with higher baseline symptom scores. Clozapine was associated with significantly less insomnia and dry mouth [number needed to treat (NNT) = 13 and 7, respectively], and there were no significant differences versus non-clozapine antipsychotics regarding hypotension, headache and even weight gain. However, 8 of the 13 meta-analysed adverse effects were more prevalent with clozapine with the number needed to harm (NNH) ranging from 4 for sialorrhoea to 19 for fever and nausea/vomiting.
In the second meta-analysis by Samara and colleagues,49 no significant superiority for total symptoms was found for clozapine when pooling all trials. However, clozapine was significantly superior to first-generation antipsychotics (chlorpromazine, haloperidol), yet not versus second-generation antipsychotics (risperidone, olanzapine, ziprasidone). Methodological issues need to be considered when interpreting these results. Mean doses of clozapine in the studies that compared clozapine with a second-generation antipsychotic comparator were significantly lower than in the studies that used a first-generation antipsychotic comparator (392 versus 511 mg per day), which may have biased the results to nondifferential findings versus second-generation antipsychotics. In a prior subanalysis of studies that compared clozapine with risperidone, clozapine was only superior in studies in which the clozapine dose was greater than 400 mg/day.50 Moreover, it is possible that patients who were not treatment resistant were included in some of the second-generation antipsychotic comparator trials, which could have also increased the response to the non-clozapine treatments.51 Importantly, a real-world, large, national database study from Sweden found clozapine to be the most efficacious oral antipsychotic to prevent hospitalization and treatment failure, being superior to both first- and second-generation antipsychotics.52 A similar database study from Finland showed that clozapine was associated with a significantly lower mortality rate than other antipsychotics.53
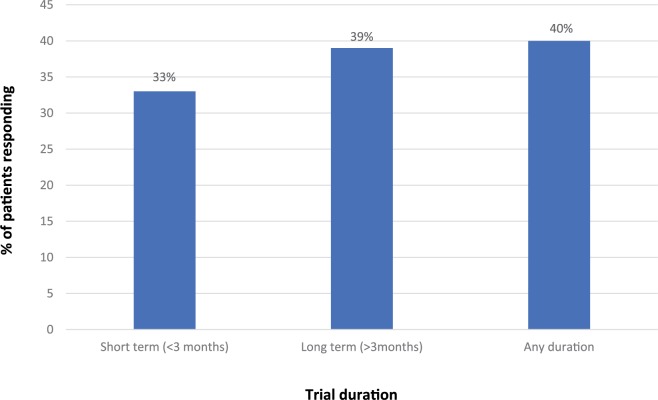
A further analysis using data from the clozapine systematic meta-analysis published by Siskind and colleagues,48 reviewed above, reported the proportion of people treated with clozapine who were responders as well as the absolute and percentage change in PANSS scores.54 Trials varied from 6 to 78 weeks in duration. Data were analysed for all time points (i.e. irrespective of trial duration), for short-term trials (⩽3 months’ duration) and for long-term trials (>3 months’ duration). Figure 5 summarizes the response rates by trial duration based on data from 11 trials. A weakness with the analysis is that different definitions of response were used in the individual RCTs, though all required a reduction of at least 20% BPRS/PANSS. Table 3 shows the mean reduction in PANSS and the percentage change scores in PANSS by trial duration. In summary, a substantial proportion of patients (33–40% depending on trial duration) who were nonresponders to non-clozapine antipsychotics will respond to clozapine. Although the average PANSS reduction (Table 3) that is achieved is equivalent to minimally improved on the CGI scale, one has to consider that this mean score is driven downwards by up to two-thirds of patients who are nonresponders even to clozapine treatment.
Figure 5.
Response rates (various criteria) in meta-analysis of randomized controlled trials of clozapine treatment in schizophrenia by trial duration (data from Siskind et al.54).
Table 3.
Mean absolute reduction and percentage change in PANSS in meta-analysis of randomized controlled trials (RCTs) of clozapine in treatment-resistant schizophrenia according to trial duration (table adapted from Siskind et al.54).
| Trial duration |
|||
|---|---|---|---|
| Short-term trials (<3 months) |
Long-term trials (>3 months) |
Any duration | |
| Mean absolute reduction in PANSS (95% CI) | 19.4 (19.0–20.9) | 24.0 (19.4–22.8) | 22.0 (20.9–13.1) |
| Mean % change in PANSS (95% CI) | 24.3 (23.1–25.5) | 20.9 (19.1–22.7) | 25.8 (24.7–26.9) |
CI, confidence interval; PANSS, Positive and Negative Syndrome Scale.
In the UK, the National Institute for Health and Care Excellence recommends that clozapine is offered to patients who have not responded sufficiently to sequential use of adequate doses of at least two different antipsychotic drugs, at least one of which should be a non-clozapine second-generation antipsychotic.55 In practice, there are usually substantial delays before eligible patients receive a trial of clozapine and rates of use vary markedly in different countries. For example, an audit conducted in a large London Mental Health Trust showed that the average delay before starting clozapine was 4 years.56 These issues partly reflect clinicians’ attitudes and knowledge about clozapine and in some countries service factors that influence the ability to initiate and monitor clozapine.
Recommendations as to the optimal duration of a trial of clozapine vary, including 2 months,57 12–24 weeks,58 3–6 months59 and 6 months.60,61 Irrespective of the duration adopted, it should relate to treatment with a clozapine trough plasma level of at least 350–400 μg/L.62 In practice, the duration of a clozapine trial will also need to consider the side effects of clozapine and be a joint decision made by the clinician and the patient. Nevertheless, many side effects associated with clozapine are manageable.63 Given that there is no high-quality evidence to support any pharmacological strategy after a failed trial of clozapine monotherapy,44,64 and some studies which indicate a delayed response to clozapine in a proportion of patients,33 we recommend continuing clozapine for 3–6 months before judging its effectiveness. This guidance is consistent with the recommendation in the British Association for Psychopharmacology Schizophrenia guideline.59
Meta-analyses do not allow any augmentation strategy to be recommended in those who have shown only a partial response to clozapine.44,64 Correll and colleagues44 found that none of the five meta-analysed combination strategies with clozapine were superior to clozapine monotherapy with regards to total psychopathology; and out of four, one was superior for positive symptoms. However, in the latter case the evidence was insufficient for it to be recommended. An earlier meta-analysis by Sommer and colleagues64 of 29 studies that assessed 15 different augmentations, including adding another antipsychotic, an antidepressant, an anticonvulsant or a glutamatergic drug to clozapine, showed either negative results or positive findings that reflected an outlying or a single study. If a trial of clozapine augmentation is adopted it should be undertaken as an individual trial in that patient and if there is no evidence of improvement after an appropriate period the augmentation agent should be withdrawn. Failure to do so puts the patient at risk of additional side effects for no benefit.
Summary and conclusions
Antipsychotics revolutionized the management of schizophrenia following their introduction in the 1950s. For the first time, medications were available that effectively treated a wide range of symptoms of the illness. In addition, continuing antipsychotic treatment after effective acute treatment significantly reduces the risk of future relapse.7 However, on average the acute efficacy of antipsychotics is modest. In a recent meta-analysis in patients with chronic schizophrenia, the effect size above and beyond that of placebo for total symptom reduction was medium, according to standard conventions.9 Nevertheless, since clinicians see the entire effect, including that of placebo, clinical effects are large. Furthermore, even when the threshold for response was defined in terms of ‘minimal improvement’ (⩾20% PANSS/BPRS reduction from baseline to endpoint), 51% of those treated with antipsychotics compared with 30% treated with placebo were responders, resulting in a favourable NNT of five.9 Good response (⩾50% PANSS/BPRS reduction from baseline to endpoint) was lower; that is, 23% versus 14% with placebo (NNT = 8).9 Higher cumulative response rates may be achieved in real-world practice by switching antipsychotic treatment to improve tolerability and efficacy, although the effectiveness of switching has not been adequately investigated in RCTs. Reponses rates are significantly higher in first-episode patients.16
In the meta-analysis of multi-episode schizophrenia by Leucht and colleagues, data on quality of life were only available from 6 trials (n = 1900) and data on social functioning from 10 trials (n = 3077).9 These are outcomes that are highly relevant to patients and more attention should be paid to them in future research. Nevertheless, the pooled sample sizes for both outcomes were sufficiently large, so that one can be confident that antipsychotics were beneficial in improving these outcomes even in the short term, albeit at only a small to medium effect size in the acute phase, but also without formal psychosocial interventions added.
The benefits of antipsychotics in terms of symptom reduction are greatest in those with the most severe symptoms, as they have more room for improvement.21 However, more research is needed regarding what predicts remission of more than mild psychotic symptoms. Nevertheless, assuming the same dose is used, the burden of side effects from medication will be the same irrespective of illness severity. This means the tradeoff between benefit and side effects for antipsychotics is most favourable in the most severely ill and least favourable in the most mildly ill, at least when starting from an antipsychotic-free baseline. However, prevention of progression of the illness and maintenance effects also need to be considered, and multiple datasets have indicated that antipsychotic use is associated with lower mortality than no antipsychotic use in people with schizophrenia.53,65,66
There is a pressing need to develop antipsychotic medications with improved efficacy and better side-effect profiles.67 Developing medications that have mechanisms of action other than dopamine antagonism is one important direction, but to date such research programmes have proved disappointing.68–70 The unsatisfactory results may partly reflect weaknesses in trial design,71 including studies recruiting too broad a range of patients. Schizophrenia almost certainly involves subgroups of people with different neurotransmitter abnormalities. Thus, ideally, trials (and later clinical practice) would match medications to different underlying disease mechanisms and appropriate patient subgroups. However, this approach of stratification requires the identification of reliable genetic, neuroimaging, electrophysiological, neurochemical or other biomarkers that would predict response to specific medications. However, despite technological advances, results from such research have not yet yielded results that are applicable to drug discovery or clinical care. A related approach is to develop medications that target specific symptom domains in schizophrenia, such as cognitive dysfunction and primary negative symptoms, but such medications would need to be investigated in samples enriched for the symptom domain in question. At present, there are no approved medications to treat either cognitive impairment or negative symptoms in patients with schizophrenia.
The management of schizophrenia always requires a comprehensive individualized treatment plan that includes appropriate psychosocial support and interventions, as well as pharmacological treatment.55,72–76 While there is a major need to develop medications with improved and expanded efficacy and less side-effects, antidopaminergic antipsychotics have remained the cornerstone of treatment of people with schizophrenia since their discovery and introduction more than six decades ago.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: In the last 3 years, Dr Haddad has received honoraria for lecturing and consultancy work from Allergan, Galen, Janssen, Lundbeck, NewBridge Pharmaceuticals, Otsuka, Sunovion and Teva, plus conference support from Janssen, Lundbeck, NewBridge Pharmaceuticals and Sunovion.
Dr Correll has been a consultant or advisor to or has received honoraria from Alkermes, Allergan, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer, ROVI, Sunovion, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Pfizer, Roche and ROVI. He received royalties from UpToDate and grant support from Janssen, Neurocrine and Takeda. He is also a shareholder of LB Pharma.
Contributor Information
Peter M. Haddad, Department of Psychiatry, Hamad Medical Corporation, Doha, Qatar. Neuroscience and Psychiatry Unit, University of Manchester, Stopford Building, Oxford Road, Manchester, UK.
Christoph U. Correll, The Zucker Hillside Hospital, Psychiatry Research, Glen Oaks, NY, USA Department of Psychiatry and Molecular Medicine, Hofstra Northwell School of Medicine, Hempstead, NY, USA Department of Child and Adolescent Psychiatry, Charité Universitätsmedizin, Berlin, Germany
References
- 1. Peräla J, Suvisaari J, Samuli I, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry 2007; 64: 19–28. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organisation. The WHO World Health Report 2001 - Mental Health: New Understanding, New Hope. Geneva: World Health Organization, 2001. [Google Scholar]
- 3. Cloutier M, Aigbogun MS, Guerin A, et al. The Economic Burden of Schizophrenia in the United States in 2013. J Clin Psychiatry 2016; 77: 764–771. [DOI] [PubMed] [Google Scholar]
- 4. Revier CJ, Reininghaus U, Dutta R, et al. Ten-Year Outcomes of First-Episode Psychoses in the MRC ÆSOP-10 Study. J Nerv Ment Dis 2015; 203: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. López-Muñoz F, Alamo C, Cuenca E, et al. History of the discovery and clinical introduction of chlorpromazine. Ann Clin Psychiatry 2005; 17: 113–135. [DOI] [PubMed] [Google Scholar]
- 6. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382: 951–962. [DOI] [PubMed] [Google Scholar]
- 7. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 2012; 379: 2063–2071. [DOI] [PubMed] [Google Scholar]
- 8. A full list of the papers available in the Special Collection on Antipsychotics: Advances, Limitations, and Alternatives in Therapeutic Advances in Psychopharmacology is available at: http://journals.sagepub.com/page/tpp/special-collection/antipsychotics
- 9. Leucht S, Leucht C, Huhn M, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry 2017; 174: 927–942. [DOI] [PubMed] [Google Scholar]
- 10. Swazey JP. Chlorpromazine in psychiatry: a study of therapeutic innovation. Cambridge: MIT Press, 1974. [Google Scholar]
- 11. Lehmann HE, Hanrahan GE. Chlorpromazine, new inhibiting agent for psychomotor excitement and manic states. Arch Neurol Psychiatry 1954; 71: 227–237. [PubMed] [Google Scholar]
- 12. Casey JF, Bennett IF, Lindley CJ, et al. Drug therapy in schizophrenia. A controlled study of the relative effectiveness of chlorpromazine, promazine, phenobarbital, and placebo. Arch Gen Psychiatry 1960; 2: 210–220. [DOI] [PubMed] [Google Scholar]
- 13. Adelson D, Epstein LJ. A study of phenothiazines with male and female chronically ill schizophrenic patients. J Nerv Ment Dis 1962; 134: 543–554. [DOI] [PubMed] [Google Scholar]
- 14. National Institute of Mental Health Psychopharmacology Service Center Collaborative Study Group. Phenothiazine treatment of acute schizophrenia: Effectiveness. Arch Gen Psychiatry 1964; 10: 246–261. [PubMed] [Google Scholar]
- 15. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci 2014; 16: 505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu Y, Li C, Huhn M, et al. How well do patients with a first episode of schizophrenia respond to antipsychotics: a systematic review and meta-analysis. Eur Neuropsychopharmacol 2017; 27: 835–844. [DOI] [PubMed] [Google Scholar]
- 17. Schennach-Wolff R, Obermeier M, Seemüller F, et al. Does clinical judgment of baseline severity and changes in psychopathology depend on the patient population? Results of a CGI and PANSS linking analysis in a naturalistic study. J Clin Psychopharmacol 2010; 30: 726–731. [DOI] [PubMed] [Google Scholar]
- 18. Leucht S, Engel RR, Davis JM, et al. Equipercentile linking of the Brief Psychiatric Rating Scale and the Clinical Global Impression Scale in a catchment area. Eur Neuropsychopharmacol 2012; 22: 501–505. [DOI] [PubMed] [Google Scholar]
- 19. Leucht S, Kane JM, Etschel E, et al. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology 2006; 31: 2318–2325. [DOI] [PubMed] [Google Scholar]
- 20. Rabinowitz J, Werbeloff N, Caers I, et al. Determinants of antipsychotic response in schizophrenia: implications for practice and future clinical trials. J Clin Psychiatry 2014; 75: e308–e316. [DOI] [PubMed] [Google Scholar]
- 21. Furukawa TA, Levine SZ, Tanaka S, et al. Initial severity of schizophrenia and efficacy of antipsychotics: participant-level meta-analysis of 6 placebo-controlled studies. JAMA Psychiatry 2015; 72: 14–21. [DOI] [PubMed] [Google Scholar]
- 22. Marshall M, Lewis S, Lockwood A, et al. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry 2005; 62: 975–983. [DOI] [PubMed] [Google Scholar]
- 23. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry 2005; 162: 1785–1804. [DOI] [PubMed] [Google Scholar]
- 24. Velden WT, Ten Hegelstad W, Larsen TKT, et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am J Psychiatry 2012; 169: 374–380. [DOI] [PubMed] [Google Scholar]
- 25. Drake RJ, Haley CJ, Akhtar S, et al. Causes and consequences of duration of untreated psychosis in schizophrenia. Br J Psychiatry 2000; 177: 511–515. [DOI] [PubMed] [Google Scholar]
- 26. Zhu Y, Krause M, Huhn M, et al. Antipsychotic drugs for the acute treatment of patients with a first episode of schizophrenia: a systematic review with pairwise and network meta-analyses. Lancet Psychiatry 2017; 4: 694–705. [DOI] [PubMed] [Google Scholar]
- 27. Andreasen NC, Carpenter WT, Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005; 162: 441–449. [DOI] [PubMed] [Google Scholar]
- 28. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 2003; 60: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 29. Samara MT, Leucht C, Leeflang MM, et al. Early improvement as a predictor of later response to antipsychotics in schizophrenia: a diagnostic test review. Am J Psychiatry 2015; 172: 617–629. [DOI] [PubMed] [Google Scholar]
- 30. Stentebjerg-Olesen M, Jeppesen P, Pagsberg AK, et al. Early nonresponse determined by the clinical global impressions scale predicts poorer outcomes in youth with schizophrenia spectrum disorders naturalistically treated with second-generation antipsychotics. J Child Adolesc Psychopharmacol 2013; 23: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry 2011; 72: 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first episode schizophrenia. Am J Psychiatry 2006; 163: 743–745. [DOI] [PubMed] [Google Scholar]
- 33. Lieberman JA, Safferman AZ, Pollack S, et al. Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry 1994; 151: 1744–1752. [DOI] [PubMed] [Google Scholar]
- 34. Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry 2010; 167: 686–693. [DOI] [PubMed] [Google Scholar]
- 35. Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014; 5: 43–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kane JM, Kishimoto T, Correll CU. Factors contributing to non-adherence in patients with psychotic disorders. World Psychiatry 2013; 12: 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Howes OD, McCutcheon R, Agid O, et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry 2017; 174: 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Royal College of Psychiatrists. CR190 Consensus statement on High-dose Antipsychotic medication. London, 2014. [Google Scholar]
- 39. Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004; 24: 192–208. [DOI] [PubMed] [Google Scholar]
- 40. Kinon BJ, Chen L, Ascher-Svanum H, et al. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology 2010; 35: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ClinicalTrials.gov. The Switch Study—Efficacy of an Early Antipsychotic Switch in Case of Poor Initial Response to the Treatment of Schizophrenia, https://clinicaltrials.gov/show/NCT01029769 (last accessed 11 June 2018).
- 42. Leucht S, Winter-van Rossum I, Heres S, et al. The Optimization of Treatment and Management of Schizophrenia in Europe (OPTiMiSE) Trial: Rationale for its Methodology and a Review of the Effectiveness of Switching Antipsychotics. Schizophr Bull 2015; 41(3): 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res 2012; 138: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Correll CU, Rubio JM, Inczedy-Farkas G, et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 2017; 74: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ackenheil M, Hippius H. Clozapine. In: Usdin E, Forrest IS. (eds) Psychotherapeutic drugs: part II. New York: Marcel Dekker; 1977, pp.923–956. [Google Scholar]
- 46. Idanpaan-Heikkila J, Alhava E, Olkimora M, et al. Clozapine and agranulocytosis. Lancet 1975: 611. [DOI] [PubMed] [Google Scholar]
- 47. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45: 789–796. [DOI] [PubMed] [Google Scholar]
- 48. Siskind D McCartney L Goldschlager R et al.Clozapine v.. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2016; 209: 385–392. [DOI] [PubMed] [Google Scholar]
- 49. Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry 2016; 73: 199–210. [DOI] [PubMed] [Google Scholar]
- 50. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 2009; 166: 152–163. [DOI] [PubMed] [Google Scholar]
- 51. Kane JM, Correll CU. What is the role of clozapine in schizophrenia? JAMA Psychiatry 2016; 73: 187–188. [DOI] [PubMed] [Google Scholar]
- 52. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry 2017; 74: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 2009; 374: 620–627. [DOI] [PubMed] [Google Scholar]
- 54. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry 2017; 62: 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. National Institute for Health and Care Excellence (NICE). Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178], February 2014. [Google Scholar]
- 56. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry 2012; 201: 481–485. [DOI] [PubMed] [Google Scholar]
- 57. Conley RR, Carpenter WT, Jr, Tamminga CA. Time to clozapine response in a standardized trial. Am J Psychiatry 1997; 154: 1243–1247. [DOI] [PubMed] [Google Scholar]
- 58. Lieberman JA, Safferman AZ, Pollack S, et al. Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry 1994; 151: 1744–1752. [DOI] [PubMed] [Google Scholar]
- 59. Barnes TR. and the Schizophrenia Consensus Group of the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2011; 25: 567–620. [DOI] [PubMed] [Google Scholar]
- 60. Kerwin RW, Bolona A. Management of clozapine-resistant schizophrenia. Adv Psychiatr Treat 2005; 11: 101–106. [Google Scholar]
- 61. Meltzer HY. Treatment of the neuroleptic-nonresponsive schizophrenic patient. Schizophr Bull 1992; 18: 515–542. [DOI] [PubMed] [Google Scholar]
- 62. Schulte P. What is an adequate trial with clozapine? Therapeutic drug monitoring and time to response in treatment-refractory schizophrenia. Clin Pharmacokinet 2003; 42: 607–618. [DOI] [PubMed] [Google Scholar]
- 63. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry 2013; 74: 603–613. [DOI] [PubMed] [Google Scholar]
- 64. Sommer IE, Begemann MJ, Temmerman A, et al. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. Schizophr Bull 2012; 38: 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull 2015; 41: 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vermeulen J, van Rooijen G, Doedens P, et al. Antipsychotic medication and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis. Psychol Med 2017; 47: 2217–2228. [DOI] [PubMed] [Google Scholar]
- 67. Correll CU. What are we looking for in new antipsychotics? J Clin Psychiatry 2011; 72(Suppl. 1): 9–13. [DOI] [PubMed] [Google Scholar]
- 68. Köster LS, Carbon M, Correll CU. Emerging drugs for schizophrenia: an update. Expert Opin Emerg Drugs 2014; 19: 511–531. [DOI] [PubMed] [Google Scholar]
- 69. Garay RP, Citrome L, Samalin L, et al. Therapeutic improvements expected in the near future for schizophrenia and schizoaffective disorder: an appraisal of phase III clinical trials of schizophrenia-targeted therapies as found in US and EU clinical trial registries. Expert Opin Pharmacother 2016; 17: 921–936. [DOI] [PubMed] [Google Scholar]
- 70. Albert C. Yang and Shih-Jen Tsai. New Targets for Schizophrenia Treatment beyond the Dopamine Hypothesis. Int J Mol Sci 2017; 18: 1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Correll CU, Kishimoto T, Kane JM. Randomized controlled trials in schizophrenia: opportunities, limitations, and trial design alternatives. Dialogues Clin Neurosci 2011; 13: 155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhao S, Sampson S, Xia J, et al. Psychoeducation (brief) for people with serious mental illness. Cochrane Database Syst Rev 2015; (4): CD010823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dieterich M, Irving CB, Bergman H, et al. Intensive case management for severe mental illness. Cochrane Database Syst Rev 2017; (1): CD007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers 2015; 1: 15067. [DOI] [PubMed] [Google Scholar]
- 75. Jauhar S, McKenna PJ, Radua J, et al. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry 2014; 204: 20–29. [DOI] [PubMed] [Google Scholar]
- 76. Carmona VR, Gómez-Benito J, Huedo-Medina TB, et al. Employment outcomes for people with schizophrenia spectrum disorder: a meta-analysis of randomized controlled trials. Int J Occup Med Environ Health 2017; 30: 345–366. [DOI] [PubMed] [Google Scholar]