Abstract
Background:
Reconstruction for complex urethral strictures may necessitate grafting. Buccal mucosal graft (BMG) harvest involves additional morbidity, making ‘off-the-shelf’ options attractive. Multiple extracellular matrices (ECMs) have been used with varying degrees of success. We reviewed our experience with MatriStem (ACell, Inc., Columbia, MD, USA) to assess safety and clinical/histologic outcomes.
Methods:
All patients undergoing acellular matrix-based reconstruction were included. Data regarding indications for surgery, patient demographics, subsequent procedures, clinical outcomes, and histologic analysis, when present, were collected.
Results:
Eight patients undergoing urethral reconstruction with ECM were identified. All repairs were performed as staged procedures. Grafting was performed with either MatriStem alone or MatriStem and concomitant BMG. Seven patients (88%) underwent prior endoscopic intervention and five patients (71%) had failed to respond to one or multiple prior urethroplasties. Length of involved segments ranged from 2.5 to 15 cm. ECM graft placement was feasible and demonstrated excellent graft take. Among patients undergoing second-stage repairs (four of eight, 50%), 50% remained patent without the need for subsequent dilation.
Conclusions:
Use of acellular matrix grafts in urethral reconstruction appears safe and feasible. Acellular matrix performs similarly to BMG with respect to graft take and contraction following staged repair. Further study is warranted.
Keywords: acellular matrix, off-the-shelf, reconstruction, urethral stricture disease, urethroplasty
Introduction
Urethral stricture disease threatens quality of life for numerous men, accounting for greater than 5000 inpatient and 1.5 million office visits annually. The diagnosis increases health care expenditures by more than $6000 per person each year.1 Bothersome lower urinary tract symptoms, frank urinary retention, and recurrent urinary tract infections can ultimately lead to identification and consideration of definitive repair by a reconstructive urologist. Short, bulbar strictures are generally treated with excision and primary anastomosis, while longer or more distal strictures are generally treated with substitution urethroplasty.
Buccal mucosal graft (BMG) has become the standard graft material for substitution urethroplasty due to numerous advantages over other materials. BMG is readily accessible, easy to handle, relatively resistant to infection, and compatible with a moist environment.2,3 Reported success rates for BMG substitution urethroplasty are 85–96%. However, harvesting oral mucosa has been associated with donor site morbidity. Possible complications include hemorrhage, infection, damage to the parotid duct, pain, parasthesias, change in salivary flow, and oral tightness.3–6 Although most of these complications are generally transient, parasthesia and oral tightness may persist. Harvesting oral mucosa also adds time, cost, and technical difficulty to the procedure. Additionally, some patients may have already undergone previous BMG harvest for prior surgery, or the stricture length may be in excess of the amount of oral mucosa available. Thus, a graft material with ‘off-the-shelf’ availability that performs comparably to BMG would be advantageous to both patient and surgeon.
To date, applications of tissue-engineered grafts as a substitute for donor-harvested tissue have met with varied success. Proposed substitutes range from acellular cadaveric and porcine matrices to those seeded with autologous patient cells obtained from biopsy or washings.7 Although seeded matrices are not dependent upon the ingrowth of mucosal cells, they require increased time and expense as cells must be harvested or grown in cell culture for many weeks prior to implantation.
MatriStem Surgical Matrix (ACell, Inc., Columbia, MD, USA) is a commercially available extracellular matrix (ECM) graft material derived from porcine urinary bladders. The goal of the present study is to assess safety and feasibility of substitution urethroplasty using MatriStem graft.
Materials and methods
As part of an Institutional Review Board (IRB)-approved protocol, a prospectively maintained single-surgeon database of urethral reconstruction was retrospectively reviewed. Cases involving ECM were identified and reviewed. Informed consent was documented in all cases.
At the time of surgery, the urethra was opened throughout the entire length of the stricture, and the urethral plate was evaluated for suitability for single-stage repair based on surgeon judgment. The urethral plate was preserved when deemed nonobliterative. Grafting was performed with either ECM alone or in combination with BMG. 5-0 polydioxanone (PDS) (Ethicon, Inc., Somerville, New Jersey), 5-0 poliglecaprone 25 (Monocryl) (Ethicon, Inc., Somerville, New Jersey), or polyglactin 910 (Vicryl) (Ethicon, Inc., Somerville, New Jersey) suture was used to approximate graft materials to the urethral plate, to other graft material, or to the skin, as appropriate. Patients were assessed postoperatively for wound healing and graft take at each follow-up visit.
Results
Eight patients undergoing urethral reconstruction with ECM were identified (mean age 52 years; range 33–72 years). All repairs were performed as staged procedures. Grafting was performed with either MatriStem alone (two patients) or MatriStem and concomitant BMG (six patients). Mean body mass index was 28 kg/m2. Current and former tobacco use was noted in two and three patients, respectively. Diabetes was noted in two patients and one patient had a history of pelvic radiation. Seven patients (88%) underwent prior endoscopic intervention in the form of urethral dilation or direct vision internal urethrotomy. Five patients (63%) had failed to respond to one or multiple prior urethroplasties.
Length of involved segments in this patient population ranged from 2.5 to 15 cm. Location was pendulous in five, bulbar in two, and a combination of pendulous and bulbar in one patient. Strictures were due to various etiologies, including trauma, prior hypospadias repairs, pelvic radiation, lichen sclerosus (LS), or iatrogenic.
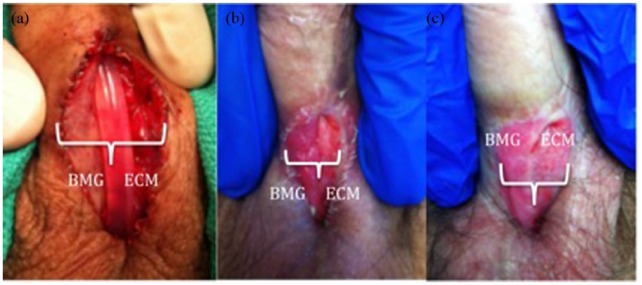
ECM graft placement was feasible in all cases. Mean follow up from time of first stage urethroplasty to last clinical follow up was 35 months (range 4–74 months). ECM graft take was subjectively excellent in seven of eight patients, with suboptimal appearance noted in one patient in conjunction with extremely poor personal hygiene. Patients having concomitant ECM and BMG placement had calculation of graft contraction based on measured width of graft segments at placement and at follow up, with no significant difference noted between materials (Figure 1). Among patients completing second-stage repairs (four of eight, 50%), 50% remained patent without need for dilation, with one, however, requiring excision of a urethrocutaneous fistula. Among the remainder, one required subsequent dilation and another progressed to definitive perineal urethrostomy for recurrent disease. For patients content with a first-stage procedure alone, 75% required no further interventions. One, however, underwent subsequent urinary diversion due to outlet obliteration and bladder dysfunction secondary to radiation for prostate cancer.
Figure 1A–C.
Patient with a BMG (patient’s right) and Acell MatriStem graft (patient’s left) placed concomitantly at the time of first-stage urethroplasty. Graft contraction rates were similar between the BMG and Acell. (a) At time of surgery (BMG 50%/ECM 50%), (b) 1 month postoperatively (BMG 53%/ECM 47%), (c) 4 months postoperatively (BMG 50%/ECM 50%). BMG, buccal mucosal graft; ECM, extracellular matrix.
Biopsies of matured ECM and BMG graft sites were taken at the time of second-stage urethroplasty in one patient with extensive LS. Biopsy of both the MatriStem site and penile skin showed spongiotic dermatitis consistent with contact dermatitis. Biopsy of both the native urethral plate and BMG site showed lichenoid inflammation consistent with LS.
Discussion
Complex urethral reconstruction requires identification of appropriate materials to replace diseased tissue. Currently, autologous BMG remains the gold standard for substitution urethroplasty; however, this represents a limited resource whose harvest adds time and morbidity to the operation. Additionally, in complex repairs such as those performed on patients with multiple prior surgeries, hypospadias cripples, or extensive LS, there may be insufficient BMG available for harvest. If a safe, readily accessible, cost-effective substitution material were available ‘off the shelf’, it would represent a welcome addition to the reconstructive urologist’s armamentarium.
Resorbable ECM grafts have numerous characteristics that make them well suited for urethroplasty, such as immunocompatibility, tissue strength, and promotion of cellular ingrowth.8 Several groups have investigated alternative substitutes for genitourinary tissue replacement, including cadaveric decellularized bone matrix, cadaveric bladder submucosa, small intestinal or colonic submucosa.9–12 Additionally, groups have studied the use of bladder acellular matrix grafts as well as grafts seeded with autologous cells (i.e. oral keratinocytes) in animal models with urethral stricture disease.13
El-Kassaby and colleagues investigated the use of bone matrix derived grafts from cadavers in patients with complex anterior strictures.9 Thirty patients with urethral strictures (including bulbar, penile, and bulbopendulous strictures) ranging from 2 to 18 cm were treated with either BMG or demineralized bone matrix grafts via onlay. In patients with healthy urethral plates, the success rate was nearly universal, showing similar results between BMG and bone matrix grafts. In patients with unhealthy urethral plates, successful repair was achieved in 100% (five of five) with BMG substitution, but only 33% (two of six) in patients who underwent replacement with bone-derived matrix grafts. Regardless of the graft material used, both groups showed patency and improvement in postoperative uroflowmetry. Biopsies revealed normal urethral tissue in all participants.
Atala and colleagues used donor cadaveric bladder submucosa to perform repairs in four patients with hypospadias who had failed to respond to prior surgical repair.14 Of these patients, one developed a subcoronal fistula and underwent subsequent uncomplicated fistula repair. All patients showed urethroscopic patency and biopsies demonstrated normal urethral stratified epithelium. El-Kassaby and colleagues subsequently expanded on this work, using cadaveric bladder submucosa to perform repairs in 28 patients with anterior urethral strictures of varying etiologies ranging from 1.5 to 16 cm in length.15 Of this cohort, four developed narrowing at anastomotic sites, which were incised and required no further treatment. As in the previous study, all patients showed improved uroflowmetry, cystoscopic patency, and normal urethral tissue on biopsy.
Palminteri and colleagues retrospectively reported on 25 men with bulbar urethral strictures who underwent substitution urethroplasty using small intestinal submucosa (SIS) with a 76% success rate at 71-month follow up.11 For strictures under 4 cm, the success rate was 86%, however for strictures over 4 cm, the success rate was 0%. Due to poor results in another study using SIS (unsuccessful repair in four of five patients), SIS was discontinued for open urethral stricture repair.16 Xu and colleagues used colonic mucosal grafts for 36 consecutive patients with complex urethral strictures averaging 15.1 cm in length with a success rate of 85.7% at a mean follow up of 53.6 months, with success being defined as normal voiding without the need for further procedures.12
Furthermore, porcine acellular collagen matrix (Pelvichol Implant; Bard, Covington, GA, USA) has been shown to be safe and useful as a ‘splint’ (additional layer of coverage) at the time of urethrocutaneous fistula repair, and at the time of second-stage urethroplasty in patients with inadequate tissue coverage.17
Our study builds on an expanding knowledge base of graft materials in genitourinary reconstruction. ACell MatriStem, derived from porcine bladder epithelial basement membrane and tunica propria (termed ‘urinary bladder matrix’), has previously shown positive results when used as a graft material in complex pilonidal wound management, closure of radiation wounds of the thigh, abdomen, and sacrum, failed flaps in facial reconstruction, and esophageal repair in animal models.18–22 As MatriStem provides a genitourinary-derived graft material with minimal necessary preoperative preparation, it appears well suited for urethral replacement in stricture disease.
Our cohort was heterogeneous in terms of etiology and stricture location. Although this can be considered a limitation of the presented analysis, this finding is common among series of patients undergoing complex substitution urethroplasty. It seems noteworthy that ECM was associated with excellent graft take and visibly demonstrated contraction rates similar to BMG. In regard to the histologic analysis of tissue taken from graft sites at the time of second-stage repair, the possibility is suggested, albeit in only a single case, that ECM could be more resistant to LS changes than BMG. The clinical implication of dermatitis within the ECM and penile skin at the time of biopsy is unclear and it is unknown whether or not these changes resolve during the healing process. Further investigation in a larger cohort and additional biopsies performed after a longer interval of time would be required to determine if these findings are valid and if they persist.
This study is limited by several factors, including small sample size and retrospective design. As mentioned earlier, heterogeneity of the population complicates generalizability of the findings presented. However, it seems worth emphasizing that ECM may represent a viable option for some patients with complex disease. Extended follow up and additional studies are needed to either reinforce or refute the experience noted in our series.
Conclusion
Our findings suggest that commercially available ECM can be successfully used for substitution urethroplasty. In staged repairs, ECM appears to perform similarly to BMG, but patients with complex disease remain at risk of subsequent recurrence. Further study and longer follow up are warranted before we can state that ECM is an acceptable replacement for BMG.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Amy Marcia Pearlman, Urology, Wake Forest Baptist Medical Center, Medical Center Boulevard, Winston-Salem, NC 27157-0001, USA.
Vaidehi Mujumdar, Wake Forest Baptist Medical Center, Winston-Salem, NC, USA.
Kara Elizabeth McAbee, Wake Forest Baptist Medical Center, Winston-Salem, NC, USA.
Ryan Patrick Terlecki, Wake Forest Baptist Medical Center, Winston-Salem, NC, USA.
References
- 1. Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol 2007; 177: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 2. Mangera A, Chapple C. Management of anterior urethral stricture: an evidence-based approach. Curr Opin Urol 2010; 20: 453–458. [DOI] [PubMed] [Google Scholar]
- 3. Lumen N, Oosterlinck W, Hoebeke P. Urethral reconstruction using buccal mucosa or penile skin grafts: systematic review and meta-analysis. Urol Int 2012; 89: 387–394. [DOI] [PubMed] [Google Scholar]
- 4. Jang TL, Erickson B, Medendorp A, et al. Comparison of donor site intraoral morbidity after mucosal graft harvesting for urethral reconstruction. Urol 2005; 66: 716–720. [DOI] [PubMed] [Google Scholar]
- 5. Dublin N, Stewart LH. Oral complications after buccal mucosal graft harvest for urethroplasty. BJU Int 2004; 94: 867–869. [DOI] [PubMed] [Google Scholar]
- 6. Rourke K, McKinny S, St Martin B. Effect of wound closure on buccal mucosal graft harvest site morbidity: results of a randomized prospective trial. Urol 2012; 79: 443–447. [DOI] [PubMed] [Google Scholar]
- 7. Mangera A, Chapple CR. Tissue engineering in urethral reconstruction-an update. Asian J Androl 2013; 15: 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santucci RA, Barber TD. Resorbable extracellular matrix grafts in urologic reconstruction. Int Braz J Urol 2005; 31: 192–203. [DOI] [PubMed] [Google Scholar]
- 9. El-Kassaby A, AbouShwareb T, Atala A. Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol 2008; 179: 1432–1436. [DOI] [PubMed] [Google Scholar]
- 10. Hodde J. Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Eng 2004; 8: 295–308. [DOI] [PubMed] [Google Scholar]
- 11. Palminteri E, Berdondini E, Fusco F, et al. Long-term results of small intestinal submucosa graft in bulbar urethral reconstruction. Urology 2012; 79: 695–701. [DOI] [PubMed] [Google Scholar]
- 12. Xu YM, Qiao Y, Sa YL, et al. Urethral reconstruction using colonic mucosa graft for complex strictures. J Urol 2009; 182: 1040–1043. [DOI] [PubMed] [Google Scholar]
- 13. Li C, Xu YM, Song LJ, et al. Urethral reconstruction using oral keratinocyte seeded bladder acellular matrix grafts. J Urol 2008; 180: 1538–1542. [DOI] [PubMed] [Google Scholar]
- 14. Atala A, Guzman L, Retik A. A novel inert collagen matrix for hypospadias repair. J Urol 1999; 162: 1148–1152. [DOI] [PubMed] [Google Scholar]
- 15. El-Kassaby, Retik AB, Yoo JJ, et al. Urethral stricture repair with an off-the-shelf collagen matrix. J Urol 2003; 169: 170–173. [DOI] [PubMed] [Google Scholar]
- 16. Hauser S, Bastian PJ, Fechner G, et al. Small intestine submucosa in urethral stricture repair in a consecutive series. Urology 2006; 68: 263–266. [DOI] [PubMed] [Google Scholar]
- 17. Springer A, Subramaniam R. Preliminary experience with the use of acellular collagen matrix in redo surgery for urethrocutaneous fistula. Urology 2012; 80: 1156–1160. [DOI] [PubMed] [Google Scholar]
- 18. Sasse KC, Brandt J, Lim DC, et al. Accelerated healing of complex open pilonidal wounds using MatriStem extracellular matrix xenograft: nine cases. J Surg Case Rep 2013; 2013: rjt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rommer EA, Peric M, Wong A. Urinary bladder matrix for the treatment of recalcitrant nonhealing radiation wounds. Adv Skin Wound Care 2013; 26: 450–455. [DOI] [PubMed] [Google Scholar]
- 20. Kruper G, Vandegriend ZP, Lin HS, et al. Salvage of failed local and regional flaps with porcine urinary bladder extracellular matrix aided tissue regeneration. Case Rep Otolaryngol 2013; 2013: 917183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nieponice A, Gilbert TW, Badylak SF. Reinforcement of esophageal anatsomoses with an extracellular matrix scaffold in a canine model. Ann Thorac Surg 2006; 82: 2050–2058. [DOI] [PubMed] [Google Scholar]
- 22. Badylak SF, Vorp DA, Spievack AR. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res 2005; 128: 87–97. [DOI] [PubMed] [Google Scholar]