Short abstract
Pulmonary arterial hypertension (PAH) in humans manifests as a chronic process. However, PAH induced by high-dose monocrotaline (MCT) in animals occurs as a subacute process. To establish a chronic PAH model, rats were randomly divided into three groups, control (ctrl), single injection (SI), and twice injection (TI) groups. Rats in the SI group received a single intraperitoneal injection of 40 mg/kg MCT on day 0. Rats in the TI group received twice injections of 20 mg/kg MCT on days 0 and 7. Survival percentage, characteristic changes of pulmonary arterial variables, and right ventricular features were evaluated. Thirty-five days after the first MCT injection, survival percentage in TI group was higher than that in the SI group. The mean pulmonary arterial pressure (mPAP), right ventricular hypertrophy index (RVHI), pulmonary vascular remodeling, serum tumor necrosis factor α (TNFα), and interleukin-6 (IL-6) were higher either in SI or in TI 28 and 35 days after the first MCT injection. The rats in the SI and TI groups exhibited higher right ventricle end diastolic diameter (RVEDD) and lower adjusted pulmonary artery acceleration time (PAAT/HR), tricuspid annular plane systolic excursion (TAPSE), cardiac output (CO) and right ventricle fractional shortening (RVFS) when compared with controls. However, mPAP, RVHI, TAPSE, PAAT/HR, CO, TNFα, and IL-6 were lower and RVEDD were higher in the TI group than in the SI group. Pulmonary macrophage infiltration and right ventricle (RV) fibrosis were lower in TI than SI groups. The cardiomyocyte cross-sectional area and the beta myosin heavy chain (MYH7) mRNA level of RV were lower in TI than SI, whereas alpha myosin heavy chain (MYH6) was increased. These results show that two intraperitoneal injections of 20 mg/kg MCT with seven days interval could induce a model similar to chronic PAH with increased survival percentage in rats.
Impact statement
We demonstrated previously that a single intraperitoneal injection of 40 mg/kg MCT produced a subacute, not chronic, PAH model in rats, and the short survival periods of these rats did not represent adequately the chronic PAH seen in humans. To overcome this limitation, in this study, the single dose of 40 mg/kg MCT was divided into twice injections of 20 mg/kg with an interval of seven days. This modified administration of MCT produced an animal model much more similar to chronic PAH with prolonged survival and characteristic changes of structures and function in pulmonary arteries and right ventricles.
Keywords: Pulmonary arterial hypertension, monocrotaline, pulmonary arterial pressure, survival percentage, pulmonary vascular remodeling
Introduction
Pulmonary arterial hypertension (PAH) is characterized by increased pulmonary arterial pressure and pulmonary vascular remodeling, leading to right heart failure and death.1,2 In most cases, PAH in humans manifests as a chronic process. Conventionally, PAH animal models were set up by using a single subcutaneous injection of monocrotaline (MCT), ranging from 30 to 60 mg/kg and PAH would be evident by structure remodeling.3–6 Previously, our group modified successfully setting up a PAH model by intraperitoneal injection.7–15 However, these models have significant limitations and shortcomings. For example, most of the animal models induced by MCT create subacute conditions with the short survival period, usually less than one month. Pathological changes of these animal models were far from of chronic PAH in humans.16 As a result, due to the short survival period, these models of PAH were not appropriate for cell treatment in which a longer period of time was required.
The aim of this study was to establish a rat PAH model much closer to a chronic progress in humans using twice intraperitoneal injections of MCT at dose of 20 mg/kg with an interval of seven days. The results were compared with single intraperitoneal injection strategy with a focus on the number of surviving rats, pulmonary arterial pressure, vascular remodeling, and cardiac structure and function.
Materials and methods
Animals
Adult male Sprague-Dawley rats (200–250 g) were purchased from Shanghai SLACCAS Laboratory Animal Co., Ltd (Shanghai, China; Certificate No. SCXK 2012–0002). The animals were provided with water and food ad libitum.17 All the animal procedures were carried out in strict accordance with recommendations from the “Guide for the Care and Use of Laboratory Animals.” The study was approved by the Laboratory Animal Welfare and Ethics Committee of Fujian Medical University (Approval No. 2017–070, Fuzhou, China). All surgeries were performed with sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.18
The experimental protocol
A total of 154 male SD rats were sacrificed in the present study. The experimental protocol was designed as shown in Figure 1(a). Ninety-six rats were randomly divided into three groups, including normal control (ctrl, n = 32), single injection (SI, n = 32), and twice injections (n = 32) groups with each group containing subgroups of eight sacrificed on days 7, 14, 21, and 28 after the first MCT injection). For the survival analysis, rats in ctrl (n = 14), SI (n = 22), and TI (n = 22) groups were monitored for 35 days following the first MCT injection. In the SI group, rats received a single intraperitoneal injection of 40 mg/kg MCT (Sigma-Aldrich, CA, USA) on day 0 and normal saline on day 7. The rats in the TI group received twice injections of 20 mg/kg MCT on day 0 and day 7, respectively, while the rats in the ctrl group received an identical volume of normal saline on the same days. All the procedures were performed as described in our previous studies.7,9,11,15
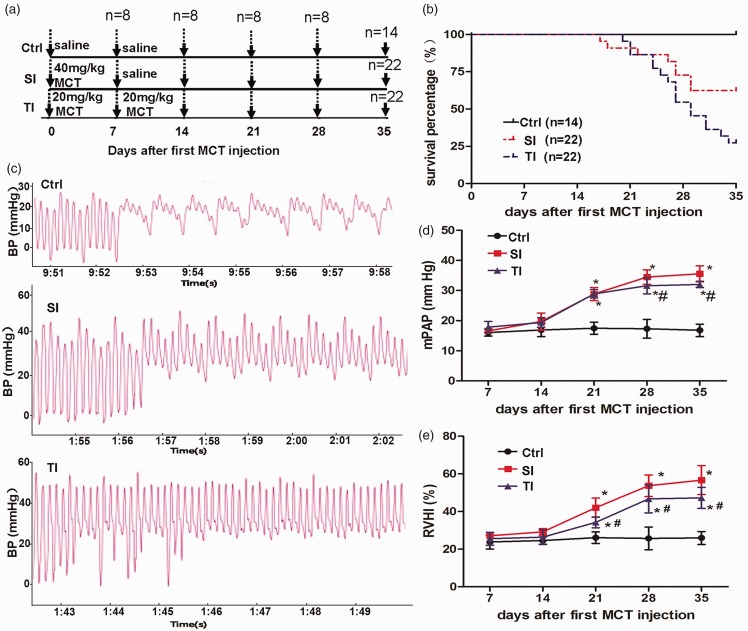
Figure 1.
Survival percentage, pulmonary arterial pressure, and right ventricular hypertrophy index (RVHI) in rats during 35 days after first monocrotaline (MCT) injection. (a) Experimental protocol: rats received a single intraperitoneal injection of 40 mg/kg MCT (SI group), twice injections of 20 mg/kg MCT (TI group), or normal saline (ctrl). (b) Kaplan-Meier survival curves showed that rats had a significantly higher survival percentage in the TI group than in the SI group (log-rank test, P < 0.01). (c) Representative waveforms of pulmonary arterial pressure in rats, showing continued movement of catheter when inserted into the pulmonary artery through right ventricle. Hemodynamic data were obtained by right cardiac catheterization. (d) Changes of mean pulmonary arterial pressure (mPAP) after injection of MCT. (e) Changes of RVHI during the progression of pulmonary arterial pressure on days 7, 14, 21, 28, and 35 after the first MCT injection. *P < 0.05 vs. ctrl of the corresponding day; #P < 0.05 vs. the SI group.
Evaluation of cardiac function
Transthoracic echocardiography was performed on day 35 after the first MCT injection as described previously.19 The rats were anesthetized with 2% sodium pentobarbital (50 mg/kg, IP) and were laid in the supine position. Echocardiography was performed using a GE Vivid-E9 ultrasound device (General Electric Co., Fairfield, CT, USA) with a 12.0 MHz linear array transducer. During the ultrasonic examination, the electrocardiogram was recorded and the heart rate (HR) was monitored. Pulmonary artery acceleration time (PAAT), right ventricular end systolic diameter (RVESD), right ventricular end diastolic diameter (RVEED), tricuspid annular plane systolic excursion (TAPSE), cardiac output (CO), right ventricular, and left ventricular fractional shortening (RVFS, LVFS) were determined. Since PAAT is HR-dependent, we corrected the PAAT using the revised formula PAAT/HR. All the results were monitored by a professional in the field.
Hemodynamic measurements
Hemodynamic measurements were described previously10,15 and were carried out on days 7, 14, 21, 28, and 35 after the first injection of MCT. Rats were anesthetized with 2% sodium pentobarbital at a dose of 50 mg/kg body weight. During the procedure, the animals were placed on a heating pad to maintain body temperature in the physiological range. Local disinfection was performed and a custom-made polyethylene catheter (Chinese Peking Union Medical Physiology, Beijing, China) was catharized via physiological pressure transducer from the right jugular vein through the right ventricle to the pulmonary artery. The mean pulmonary arterial pressure (mPAP) was measured, and the data were processed via a Powerlab-ML221 data acquisition and analysis system (AD Instruments Pvt Ltd, Bella Vista, New South Wales, Australia).
Measurement of serum inflammatory factors
After the hemodynamic measurements, blood samples were collected from the abdominal aortae and the samples were centrifuged at 3000g for 10 min. The supernatants were collected and stored at –80°C. Serum tumor necrosis factor-α (TNF-α) and interleukin -6 (IL-6) were evaluated 14, 21, 28, and 35 days after MCT injection. The assays were carried out using colorimetric enzyme-linked immunosorbent assay (ELISA) kits (BOSTER Biological Technology Co., Ltd, Wuhan, China) with a lower detection limit of 10 pg/mL, according to the manufacturer’s instructions.
RVHI
After blood sampling, the rats were euthanized. The right ventricle (RV) and left ventricle plus the septum (LV+S) weights were measured. RVHI was calculated based on the ratio RV/(LV+S), which is considered as the level of right ventricular hypertrophy.19
Morphometric analysis
Parts of the lung and heart were harvested from the rats, fixed with 4% paraformaldehyde and embedded in paraffin for morphological analysis. Sections (5 μm) were stained with hematoxylin and eosin (HE). Pulmonary arterioles with an external diameter (ED) approximately 100 μm were chosen for morphological analysis. Wall thickness, ED, total vascular area (TA), and lumen area (LA) of the pulmonary arterioles were determined by IPP 6.0 image analysis software quantitatively.19 The percentage of total wall thickness to external diameters of pulmonary arterioles diameter (WT%, WT%=2×WT/ED × 100%) and the percentage of wall area (WA) to the total area of vessels (WA%, WA%=(TA–LA)/TA × 100%) were calculated.
The size of cardiomyocyte in the RV was expressed as the average cross-sectional area (CSA) of transversely cut myocardium stained with HE. Picrosirius red staining was carried out for identifying collagen volume, which was used for analysis of cardiac fibrosis. LV and RV fibrosis were expressed as the percentage of tissue area positively stained for collagen. All the parameters were measured minimally for five randomly chosen areas per ventricle. Immunohistochemical staining was performed for analysis of the cardiac and lung inflammation. Briefly, after dewaxing and rehydration, cardiac and lung paraffin-embedded sections were blocked with 5% bovine serum albumin and incubated for 60 min with primary anti-mouse CD68 (1:100, Santa Cruz), followed by goat anti-mouse secondary antibody staining and counterstained with hematoxylin. The sections were observed under light microscope Nikon 80i Microscope (Nikon, Japan). Microphage infiltration was expressed as the number of positive CD68 cells per section area, measured for at least five randomly chosen sections.
Total RNA extraction and reverse transcription quantitative polymerase chain reaction
Total RNA extraction and real-time PCR for the RV were described previously.20 Briefly, the total RNA was extracted from 50 mg rat RV tissues using 1 mL Trizol reagent (Life Technology, USA) according to the manufacturer’s instructions. The RNA concentration and purity were determined at A260 nm and A280 nm wavelengths using Thermo Scientific™ NanoDrop™ instruments, and RNA samples with an A260 nm/A280 nm ratio in the range of 1.8 to 2.0 were used for reverse transcription. Reverse transcription system from Life Technology was used for cDNA synthesis. The cDNA samples were stored at −20°C for future analysis. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed in accordance with the instructions included with the FastStart Essential DNA Green Master kit (Roche), and the primer synthesis was performed by Sangon Biotechnology Co., Ltd (Shanghai, China). Primer sequences for the RT-PCR are listed as follows: forward primer of beta myosin heavy chain (MYH6): 5′-GTCAGCAGAACAGTAAAATAGAGG-3′, reverse primer of MYH6: 5′-GGCT TCCTCTAGCCTCTCAC-3′, forward primer of alpha myosin heavy chain (MYH7): 5′-CA GAGCAGATCGCCCTCAAG-3′, reverse primer of MYH7: 5′-CTGCAGTCGCAGTAGGT TCT-3′, forward primer of GAPDH: 5′-CTCATGACCACAGTC CATGCCA-3′, and reverse primer of GAPDH: 5′-GCCTTGGCAGCACCAGTGGATG-3′. The relative expression of MYH6 and MYH7 mRNA was calculated based on the 2−ΔΔCT method. GADPH was used as the internal reference gene.
Statistical analysis
The data were expressed as the mean ± standard deviation. One way and two-way analysis of variance (ANOVA) were used to test the differences among groups, followed by SNK-q test for pairwise comparisons. Kaplan-Meier method was used to derive survival curves, and a log-rank test was used to compare them. The Statistical Package for Social Sciences (SPSS, version 13.0, Chicago, IL, USA) was used for all the statistical analysis. P < 0.05 was considered significant.
Results
Survival and hemodynamic analysis
On day 14, loss of appetite, decreased weight and activity, slowed breathing rate, and obscure hair were observed in both SI and TI groups. However, the symptoms observed in rats of TI group were much less severe. From day 18, bleeding from the gums and lips, shortness of breath and other symptoms were observed in some of rats in the SI and TI groups. As shown in Figure 1(b), the Kaplan-Meier survival curves demonstrated that rats received twice injections of 20 mg/kg MCT had a significantly higher survival percentage than those that received a single injection of 40 mg/kg MCT (log-rank test, P<0.01). The survival percentage in the TI group was higher than that in the SI group (72.73 vs. 54.55%; n = 22) on day 28 after the first MCT injection, and this difference was more obvious on day 35 (62.34 vs. 27.27%; n = 22). All the rats in the control group were alive.
Hemodynamic data were obtained by right cardiac catheterization on days 7, 14, 21, 28, and 35 after the first MCT administration, respectively. The representative waveforms of pulmonary arterial pressure in rats are presented in Figure 1(c). No significant change in mPAP was observed among the three groups on day 7. The mPAP was slightly but not significantly elevated in the SI group in comparison with ctrl (19.72 ± 2.81 mmHg vs. 16.88 ± 2.20 mmHg) on day 14. However, on day 21, mPAP significantly increased both in MCT injected rats when compared with control rats (SI 28.85 ± 2.14 mmHg, TI 28.89 ± 1.36 mmHg vs. ctrl 17.50 ± 2.01 mmHg, P < 0.05) and was elevated further on day 28 (SI 34.48 ± 2.37 mmHg, TI 31.58 ± 2.69 mmHg vs. ctrl 17.30 ± 3.10 mmHg, P < 0.05). Compared with the SI group, the mPAP of the TI group was lower on day 28. On day 35, the mPAP in all groups remained similar to that of day 28 (Figure 1(d)).
Pulmonary vascular remodeling
The control rats showed an intact pulmonary arterial wall and intimal structure, without interstitial infiltration of inflammatory cells. However, infiltration of inflammatory cells in the interstitium was observed on day 7 in both of the SI and TI groups. These changes were aggravated further and were observed alongside damaged alveoli and hyperplastic lung interstitium from day 14 (Figure 2(a)).
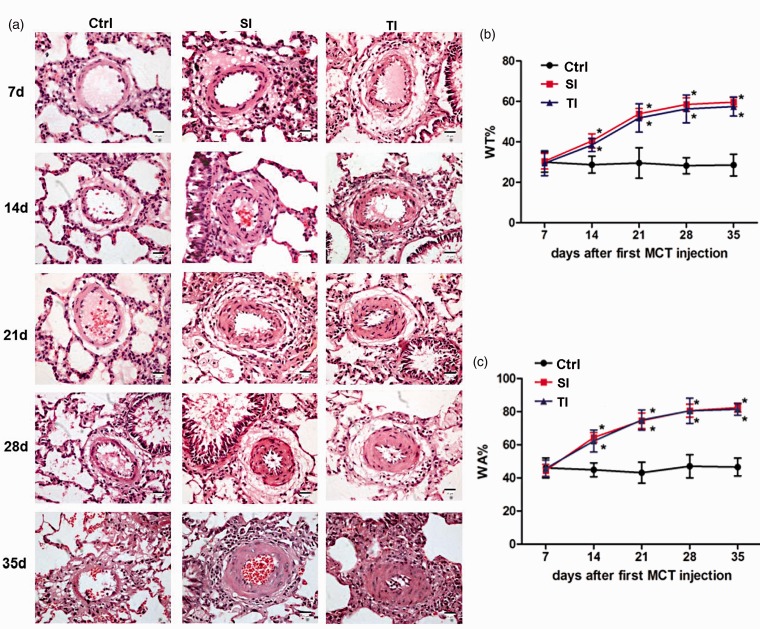
Figure 2.
Monocrotaline (MCT)-induced structural changes and pulmonary vascular remodeling in lungs from SD rats. (a) Representative histological pictures correspond to SD rats that received normal saline (ctrl), single injection of 40 mg/kg MCT (SI group), and twice injections of 20 mg/kg MCT (TI group) on days 7, 14, 21, 28, and 35. Structure of pulmonary arteries with HE staining showed an intact pulmonary arterial wall and structure, without interstitial inflammatory cells in the control group. In contrast, inflammatory cells were observed in the interstitium on day 7 in both the SI and TI groups. These changes were aggravated further, along with impaired alveoli, and hyperplastic lung interstitium on days 14 through 35. (b, c) No changes in the percentage of total wall thickness to external diameters of pulmonary arterioles diameter (WT%) and the percentage of wall area to the total area of vessels (WA%) were observed on day 7 for all three groups. However, WA% and WT% of the pulmonary arteries were significantly increased in both the SI and TI groups vs. control on days 14, 21, and 28; whereas, no significant difference between the two injection groups was observed. On day 35, WA% and WT% in all groups remained similar to day 28. Scale bar = 25 μm. *P < 0.05 vs. ctrl of the corresponding day; #P < 0.05 vs. SI group.
Pulmonary arterial remodeling was observed either in SI or in TI group in which WA% and WT% were increased from day 14 and peaked on day 28 (WA%, day 28: SI 80.57 ± 3.92, TI 80.49 ± 7.63 vs. ctrl 47.03 ± 6.95; P < 0.05; WT%, day 28: SI 58.50 ± 3.27, TI 56.33 ± 6.87 vs. ctrl 28.21 ± 3.94; P < 0.05) as compared with control rats. There was no significant difference both in the SI and TI groups (Figure 2(b) and (c)). On day 35, WA% and WT% in all the groups remained similar to day 28.
RV hypertrophy and function
The RVHI was significantly higher in both of the SI and TI groups on days 21, 28, and 35 as compared with control rats. In accordance with the trends of the mPAP, the RVHI in the TI group was significantly higher than control rats, although mildly when compared with the SI group on days 21, 28 and 35 (P < 0.05) (Figure 1(e)).
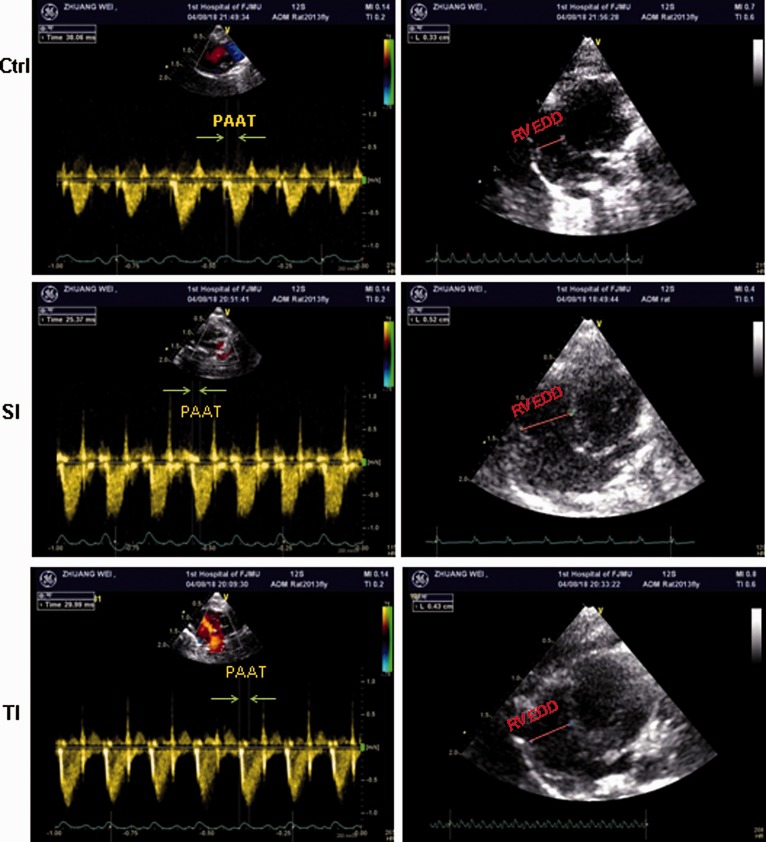
Pulmonary blood flow spectra of rats at 35 days after the first MCT injection are shown in Figure 3 (left panel). In the control group, the blood flow through pulmonary arteries showed a classical “rounded” shape. However, in the SI and TI groups, the velocity profiles of the pulmonary arteries were changed to “dirk” morphology with increased PAAT. Compared with the SI group, PAAT/HR was significantly higher than in the TI group. Furthermore, the hemodynamic, structural, and functional parameters for pulmonary arteries and hearts, including TAPSE, CO, and RVFS in the TI group were significantly higher than in the SI group, but lower than ctrl (Table 1). RVEDD (Figure 3, right panel; Table 1) and RVESD in the DI group were significantly lower than in the SI group. The data indicated that RV structure and function were less impaired in TI group. There were no statistically significant differences in PA diameter and LVFS among these three groups.
Figure 3.
Representative pulmonary blood flow spectra and a four-chamber view of heart at end-diastolic phase in pulmonary hypertensive rats induced by MCT. SD rats received normal saline (ctrl), single injection of 40 mg/kg monocrotaline (MCT) (SI group) and twice injections of 20 mg/kg (TI group). Pulmonary artery acceleration time (PAAT) and right ventricular end diastolic diameter (RVEED) are presented in the left and right panels, respectively.
Table 1.
Echocardiographic parameters in pulmonary hypertensive rats induced by monocrotaline (MCT).
| Ctrl | SI | TI | |
|---|---|---|---|
| TAPSE (mm) | 3.15±0.21 | 1.75±0.12* | 2.83±0.23*# |
| RVEDD (mm) | 3.45±0.17 | 5.61±0.26* | 4.32±0.20*# |
| RVESD (mm) | 2.22±0.28 | 4.23±0.28* | 2.75±0.14*# |
| PAAT/HR | 0.25±0.03 | 0.15±0.01* | 0.18±0.01*# |
| CO (mL/min) | 125±16 | 86±5* | 100±20*# |
| RVFS (%) | 32.15±5.27 | 18.18±2.78* | 24.34±2.64*# |
| LVFS (%) | 41.37±4.28 | 39.12±3.26 | 40.17±3.23 |
Note: All the data were expressed as mean±SD (standard deviation) in ctrl, SI and TI groups.
TAPSE: tricuspid annular plane systolic excursion; RVEDD: right ventricular end diastolic diameter; RVESD: right ventricular end systolic diameter; PAAT: pulmonary artery acceleration time; HR: heart rate; CO: cardiac output; RVFS: right ventricular fractional shortening; LVFS: left ventricular fractional shortening. SI group: intraperitoneally injected with a single dose of 40 mg/kg MCT; TI group: twice injections of 20 mg/kg MCT; ctrl: injected with normal saline. *P < 0.05 vs. ctrl; #P < 0.05 vs. TI group.
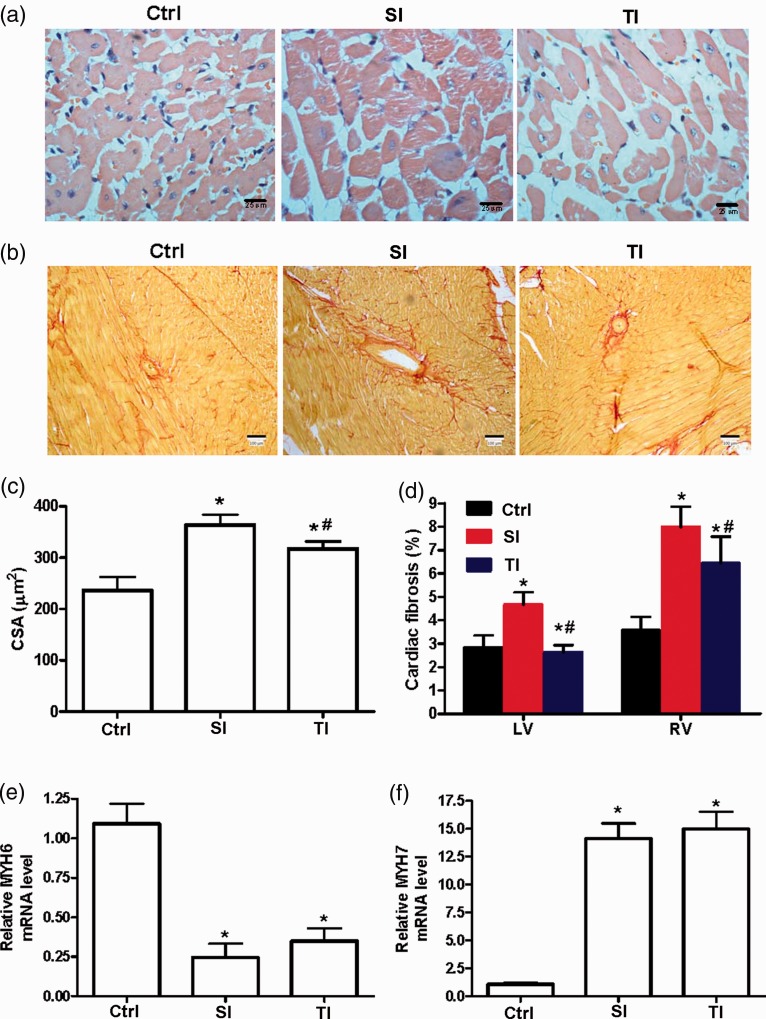
The size of cardiomyocyte in the RV in the SI and TI groups was increased in comparison with ctrl on day 35 (Figure 4(a)). The CSA of the RV was lower in the TI group when compared with the SI group (Figure 4(c)) and similar results were observed in RV (Figure 4(b)) and LV fibrosis (Figure 4(d)). The mRNA levels of cardiac hypertrophy marker MYH7 was lower in the RVs of TI than the SI group, whereas MYH6 mRNA was higher (Figure 4(e) and (f)).
Figure 4.
Cardiac hypertrophy and fibrosis of rats. (a) Representative images of right ventricle (RV) sections from male rats stained with HE in ctrl, SI, and TI groups. (b) Representative images of RV fibrosis stained with picrosirius red staining. (c, d) Quantification of cardiomyocyte cross-sectional area (CSA, c), left ventricle and RV fibrosis (d) in different groups. (e, f) RV hypertrophy markers alpha myosin heavy chain (MYH6) and beta myosin heavy chain (MYH7) were measured by RT-qPCR. SI group: intraperitoneally injected with a single dose of 40 mg/kg monocrotaline (MCT); TI group: twice injections of 20 mg/kg MCT with an interval of seven days; ctrl: injected with normal saline. Scale bar = 100 μm. *P < 0.05 vs. ctrl of the corresponding day; #P < 0.05 vs. SI group.
Inflammation
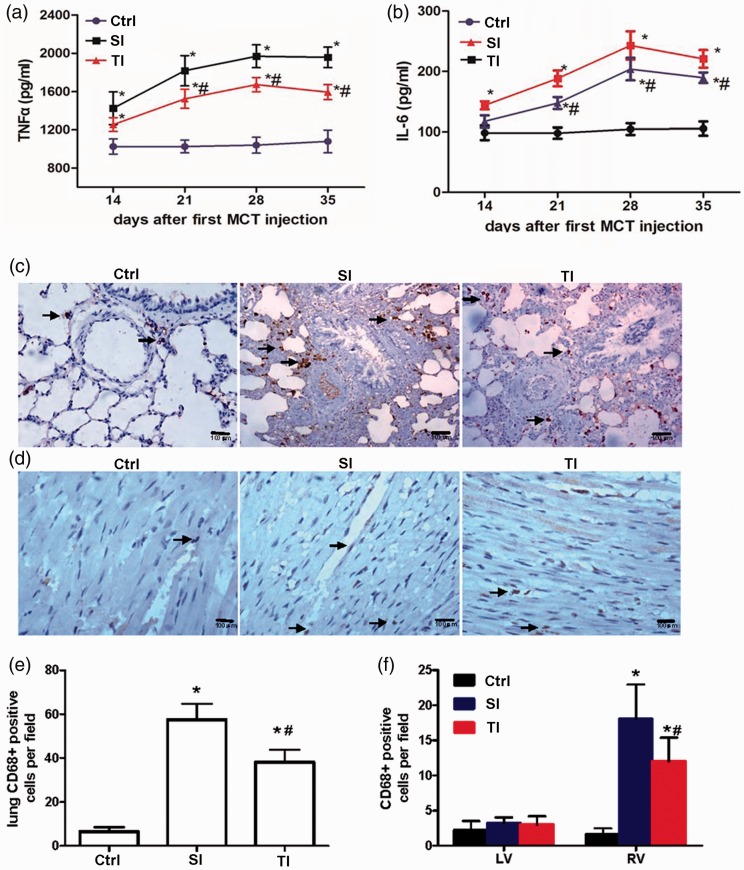
Fourteen days after the first injection of MCT, TNFα was significantly increased and peaked on day 28 (SI 1969.379 ± 118.97 pg/mL, TI 1672.67 ± 74.31 pg/mL vs. ctrl 1039.8 ± 83.60 pg/mL, P < 0.05), and decreased slightly on day 35 for both MCT injection groups. However, when compared with the SI group, the TNFα in the TI group was significantly lower (Figure 5(a)). Similarly, serum IL-6 in the TI group was also lower than in the SI group on days 21, 28, and 35 (Figure 5(b)).
Figure 5.
Changes in inflammation during the progression of pulmonary arterial hypertension. (a, b) Serum tumor necrosis factor α (TNFα) and interleukin-6 (IL-6) were measured 7, 14, 21, 28, and 35 days after the first monocrotaline (MCT) injection. (c, d) Typical examples of lungs and right ventricle (RV) macrophage infiltration (CD68 positive cells, Arrow) in ctrl, SI, and TI groups. (e, f) Quantification of lung (e) and cardiac inflammation (f) in different groups. SI group: intraperitoneally injected with a single dose of 40 mg/kg monocrotaline (MCT); TI group: twice injections of 20 mg/kg MCT with an interval of seven days; ctrl: normal saline. Scale bar = 100 μm. *P < 0.05 vs. ctrl of the corresponding day; #P < 0.05 vs. SI group.
In addition, pulmonary and RV macrophage infiltrations (CD68 positive cells) were lower in the TI group when compared with the SI group (Figure 5(c) to (f)). However, there was no significant difference of CD68-positive cells in the LV myocardium (Figure 5(f)).
Discussion
In the present study, a rat PAH model was set up by twice intraperitoneal injections of 20 mg/kg MCT with an interval of seven days. Compared with a single injection of 40 mg/kg MCT, this novel strategy produced higher survival percentage of rats with less impaired cardiac function, reduced inflammation, and less fibrosis during the experimental period.
In 1961, Lalich and Merkow firstly reported an experimental PAH model induced by MCT in which pulmonary vasculature remodeling, along with RV hypertrophy and failure, recapitulated certain characteristics of human PAH.21 Thereafter, this model was widely used for the study of PAH. MCT is not a directly active form. It needs to be transformed into active form, dehydrogenated MCT, by P450 mono oxidase in liver cells. Then, the dehydrogenated MCT plays a role in non-competitive inhibition of the respiratory chain complex I, destruction of mitochondrial membrane potential, consumption of intracellular adenosine triphosphate, and interference with various routes of glucose metabolism, inhibiting cell energy metabolism.17 MCT selectively impairs the endothelial cells in the pulmonary artery. Consequently, endothelial-derived nitric oxide synthesis and secretion are significantly reduced. On the contrary, production of endothelin 1, platelet-derived growth factor, and other vasoconstrictors are increased, leading to pulmonary artery contraction, stenosis, or occlusion.22,23 Our previous and current data show that a subacute rat PAH model can be established by a single intraperitoneal injection of 40 mg/kg MCT, based on evidence of significantly elevated mPAP, RVHI, and other pulmonary artery remodeling indices, such as WA% and WT%. However, survival percentage of rats in this model was low after four weeks. To establish a rat PAH model with higher survival percentage and longer duration, in this report, SD rats were administrated with twice intraperitoneal injections of 20 mg/kg MCT with an interval of seven days.
Our results showed that mPAP was significantly elevated in this model to an average of over 30 mmHg after 28 days. Additionally, although a mild significant decrease was noted in RVHI, however, the RVHI was still much higher than control rats after 35 days of injection. Importantly, to our expectation, the number of surviving rats was significantly higher in TI group when compared with the single injection group. Based on the above data, it was believed that a novel MCT-induced PAH model, much closer to chronic process, was successfully set up.
MCT is a known toxicant that contributes to pulmonary vascular injury, inflammatory response, smooth muscle hypertrophy, and vascular remodeling.10,24 Moreover, the size and extend of the lesion caused by MCT directly depend on the dosage; high doses of MCT lead to pulmonary edema or even death, while low doses induce pulmonary arterial remodeling, endothelial apoptosis, and perivascular inflammation.25 In a previous study, we determined that MCT-induced pulmonary arteriolar remodeling occurred prior to the increase of pulmonary arterial pressure in rats.15 The present study show that, after day 14, pulmonary arterial remodeling indices, WA% and WT%, were significantly increased in both MCT-induced PAH rats compared with control rats. However, there was no significant difference was observed between the two MCT-administration groups before 14 days. Moreover, at this time point, neither MCT-induced PAH group had a significant increase in mPAP or RVHI.
The present results showed that PAH was successfully induced by both a single dose of 40 mg/kg and the novel twice injections of 20 mg/kg MCT in rats. The novel strategy was more appropriate to mimic human chronic PAH, as evident by the moderate increase in mPAP and chronic progression, which may be associated with slow injury caused by longer duration of response to elevated concentration of MCT and prolonged interaction time. In contrast, the single injection method might not be appropriate to establish rat PAH model to investigate the effect of drug treatments, especially for cell treatment, since it was associated with high mortality rate, suggesting a subacute progression and more severe inflammation response based on our current and previous data. Therefore, the novel method to establish rat PAH could be more suitable for testing the effects of cell treatment.
Importantly, a higher survival percentage was also observed in the TI group in the present study. Three weeks after the first MCT injection, death of rats occurred, concomitant with elevated mPAP, and severe RV remodeling. However, at days 28 and 35, numbers of surviving rats in the TI group was maintained at relatively high level while numbers in the SI group sharply decreased. Increased survival percentage in the TI group can be explained by the following: First, after intraperitoneal injection, MCT is metabolized by cytochrome P450 in hepatocytes to form biodegradable MCT which can injure pulmonary vascular endothelial cells, lead to underneath collagen exposure, local pulmonary microvascular thrombosis, and resulting in increase in pulmonary artery pressure. Second, the area of drug reaction of intraperitoneal cavity is larger than a subcutaneous injection and other routes of administration.26 Therefore, the plasma concentration is increased after MCT absorption within a short time, and the toxic effect and inflammation may be more severe. Our data support this explanation. In this study, concentrations of serum TNFα, IL-6, and RV inflammation were significantly lower in the TI group than in the SI group during PAH. Third, compared with the SI group, the elevation in time and amplitude of pulmonary artery pressure, as well as RVHI, was delayed in the SI group. Last, RV function was better in the TI group than in the SI group. The rats that received two injections of 20 mg/kg MCT showed slight inflammation in lung and lower pulmonary artery pressure. It is likely that plasma concentration of MCT in rats caused by this process stimulates pulmonary vascular smooth muscle hyperplasia and pulmonary hypertension but avoids severe damage to the vessels. Therefore, PAH induced by the single injection method manifested a more serious inflammatory response, pulmonary artery endothelial damage, and pulmonary interstitial hyperplasia. A weaker inflammation response may lead to better survival in the novel PAH model using two low-dose MCT injections.
In conclusion, two injections of 20 mg/kg MCT, 7 days apart, could produce a chronic PAH in rats with prolonged survival and characteristic changes of structures in pulmonary arteries and right ventricles. Thus, rats receiving an MCT dosage divided into two injections afforded an improved PAH model compared with rats receiving a single high-dose MCT injection.
Acknowledgments
The authors thank Li Liu for her technical and secretarial assistance. The authors would like to thank Enago (www.enago.cn) for the English language review.
Author contributions
LX, WZ, and GL designed the study. WZ, BH, AD, GX, JG, CX, and WH performed the experiments. WZ and GL conducted data analysis. WZ prepared the manuscript. All authors read the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by National Natural Science Foundation of China (81570446/and 81270111).
References
- 1.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122:164–72 [DOI] [PubMed] [Google Scholar]
- 2.Murakami K, Mathew R, Huang J, Farahani R, Peng H, Olson SC, Etlinger JD. Smurf1 ubiquitin ligase causes downregulation of BMP receptors and is induced in monocrotaline and hypoxia models of pulmonary arterial hypertension. Exp Biol Med (Maywood) 2010; 235:805–13 [DOI] [PubMed] [Google Scholar]
- 3.Akagi S, Nakamura K, Miura D, Saito Y, Matsubara H, Ogawa A, Matoba T, Egashira K, Ito H. Delivery of imatinib-incorporated nanoparticles into lungs suppresses the development of monocrotaline-induced pulmonary arterial hypertension. Int Heart J 2015; 56:354–9 [DOI] [PubMed] [Google Scholar]
- 4.Sirmagul B, Ilgin S, Atli O, Usanmaz SE, Demirel-Yilmaz E. Assessment of the endothelial functions in monocrotaline-induced pulmonary hypertension. Clin Exp Hypertens 2013; 35:220–7 [DOI] [PubMed] [Google Scholar]
- 5.Yin X, Wang L, Qin G, Luo H, Liu X, Zhang F, Ye Z, Zhang J, Wang E. Rats with chronic, stable pulmonary hypertension tolerate low dose sevoflurane inhalation as well as normal rats do. PloS One 2016; 11:e0154154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dias-Neto M, Luisa-Neves A, Pinho S, Goncalves N, Mendes M, Eloy C, Lopes JM, Goncalves D, Ferreira-Pinto M, Leite-Moreira AF, Henriques-Coelho T. Pathophysiology of infantile pulmonary arterial hypertension induced by monocrotaline. Pediatr Cardiol 2015; 36:1000–13 [DOI] [PubMed] [Google Scholar]
- 7.Liang M, Li H, Zheng S, Ning J, Xu C, Wang H, Xie L. Comparison of early and delayed transplantation of adipose tissue-derived mesenchymal stem cells on pulmonary arterial function in monocrotaline-induced pulmonary arterial hypertensive rats. Eur Heart J Suppl 2015; 17:F4–F12 [Google Scholar]
- 8.Xiao T, Xie L, Huang M, Shen J. Differential expression of microRNA in the lungs of rats with pulmonary arterial hypertension. Mol Med Rep 2017; 15:591–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin T, Gu J, Huang C, Zheng S, Lin X, Xie L, Lin D. ( 1)H NMR-based analysis of serum metabolites in monocrotaline-induced pulmonary arterial hypertensive rats. Dis Markers 2016; 2016:5803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li HH, Xie LJ, Xiao TT, Huang M, Shen J. Mibefradil suppresses the proliferation of pulmonary artery smooth muscle cells. J Investig Med 2016; 64:45–9 [DOI] [PubMed] [Google Scholar]
- 11.Luo L, Lin T, Zheng S, Xie Z, Chen M, Lian G, Xu C, Wang H, Xie L. Adipose-derived stem cells attenuate pulmonary arterial hypertension and ameliorate pulmonary arterial remodeling in monocrotaline-induced pulmonary hypertensive rats. Clin Exp Hypertens 2015; 37:241–8 [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Su L, Li Y, Guo N, Xie L, Zhang D, Zhang X, Li H, Zhang G, Wang Y, Liu C. The synergistic therapeutic effect of hepatocyte growth factor and granulocyte colony-stimulating factor on pulmonary hypertension in rats. Heart Vessels 2014; 29:520–31 [DOI] [PubMed] [Google Scholar]
- 13.Yin Y, Wu X, Yang Z, Zhao J, Wang X, Zhang Q, Yuan M, Xie L, Liu H, He Q. The potential efficacy of R8-modified paclitaxel-loaded liposomes on pulmonary arterial hypertension. Pharm Res 2013; 30:2050–62 [DOI] [PubMed] [Google Scholar]
- 14.Gao G, Wang X, Qin X, Jiang X, Xiang D, Xie L, Hu J, Gao J. Effects of trimethoxystilbene on proliferation and apoptosis of pulmonary artery smooth muscle cells. Cell Biochem Biophys 2012; 64:101–6 [DOI] [PubMed] [Google Scholar]
- 15.Xie L, Lin P, Xie H, Xu C. Effects of atorvastatin and losartan on monocrotaline-induced pulmonary artery remodeling in rats. Clin Exp Hypertens 2010; 32:547–54 [DOI] [PubMed] [Google Scholar]
- 16.Maarman G, Lecour S, Butrous G, Thienemann F, Sliwa K. A comprehensive review: the evolution of animal models in pulmonary hypertension research; are we there yet? Pulm Circ 2013; 3:739–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva-Neto J, Barreto R, Pitanga B, Souza C, Silva V, Silva A, Velozo EdS Cunha S, Batatinha M, Tardy M. Genotoxicity and morphological changes induced by the alkaloid monocrotaline, extracted from Crotalaria retusa, in a model of glial cells. Toxicon 2010; 55:105–17 [DOI] [PubMed] [Google Scholar]
- 18.Sekinishi A, Suzuki J, Aoyama N, Ogawa M, Watanabe R, Kobayashi N, Hanatani T, Ashigaki N, Hirata Y, Nagai R, Izumi Y, Isobe M. Periodontal pathogen Aggregatibacter actinomycetemcomitans deteriorates pressure overload-induced myocardial hypertrophy in mice. Int Heart J 2012; 53:324–30 [DOI] [PubMed] [Google Scholar]
- 19.Luo L, Zheng W, Lian G, Chen H, Li L, Xu C, Xie L. Combination treatment of adipose-derived stem cells and adiponectin attenuates pulmonary arterial hypertension in rats by inhibiting pulmonary arterial smooth muscle cell proliferation and regulating the AMPK/BMP/Smad pathway. Int J Mol Med 2018; 41:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong H, Wang T, Lian G, Xu C, Wang H, Xie L. TRPM7 regulates angiotensin II-induced sinoatrial node fibrosis in sick sinus syndrome rats by mediating Smad signaling. Heart and Vessels. Epub ahead of print 6 March 2018. DOI: 10.1007/s00380-018-1146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalich JJ, Merkow L. Pulmonary arteritis produced in rat by feeding Crotalaria spectabilis. Lab Invest 1961; 10:744–50 [PubMed] [Google Scholar]
- 22.Lame MW, Jones AD, Wilson DW, Segall HJ. Monocrotaline pyrrole targets proteins with and without cysteine residues in the cytosol and membranes of human pulmonary artery endothelial cells. Proteomics 2005; 5:4398–413 [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Hwangbo C, Hu X, Kang Y, Papangeli I, Mehrotra D, Park H, McLean DL, Ju H, Comhair SA. Restoration of impaired endothelial MEF2 function rescues pulmonary arterial hypertension. Circulation 2015; 131:190–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong M, Yao J-P, Wu Z-K, Liao B, Liang Y-J, Zhang X, Wang Z-P. Fibrosis of pulmonary vascular remodeling in carotid artery-jugular vein shunt pulmonary artery hypertension model of rats. Eur J Cardiothorac Surg 2012; 41:162–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol 2012; 302:L363–9 [DOI] [PubMed] [Google Scholar]
- 26.Flessner MF, Lofthouse J. Improving contact area between the peritoneum and intraperitoneal therapeutic solutions. J Am Soc Nephrol 2001; 12:807–13 [DOI] [PubMed] [Google Scholar]