Short abstract
Stress-associated premature senescence plays a major role in retinal diseases. In this study, we investigated the relationship between endothelial dysfunction, endoplasmic reticulum (ER) stress, and cellular senescence in the development of retinal dysfunction. We tested the hypothesis that constant endothelial activation by transmembrane tumor necrosis factor-α (tmTNF-α) exacerbates age-induced visual deficits via senescence-mediated ER stress in this model. To address this, we employed a mouse model of chronic vascular activation using endothelial-specific TNF-α-expressing (tie2-TNF) mice at 5 and 10 months of age. Visual deficits were exhibited by tie2-TNF mice at both 5 months and 10 months of age, with the older mice showing statistically significant loss of visual acuity compared with tie2-TNF mice at age 5 months. The neural defects, as measured by electroretinogram (ERG), also followed a similar trend in an age-dependent fashion, with 10-month-old tie2-TNF mice showing the greatest decrease in “b” wave amplitude at 25 cd.s.m2 compared with age-matched wildtype (WT) mice and five-month-old tie2-TNF mice. While gene and protein expression from the whole retinal extracts demonstrated increased inflammatory (Icam1, Ccl2), stress-associated premature senescence (p16, p21, p53), and ER stress (Grp78, p-Ire1α, Chop) markers in five-month-old tie2-TNF mice compared with five-month-old WT mice, a further increase was seen in 10-month-old tie2-TNF mice. Our data demonstrate that tie2-TNF mice exhibit age-associated increases in visual deficits, and these data suggest that inflammatory endothelial activation is at least partly at play. Given the correlation of increased premature senescence and ER stress in an age-dependent fashion, with the loss of visual functions and increased endothelial activation, our data suggest a possible self-enhanced loop of unfolded protein response pathways and senescence in propagating neurovascular defects in this model.
Impact statement
Vision loss in most retinal diseases affects the quality of life of working age adults. Using a novel animal model that displays constant endothelial activation by tmTNF-α, our results demonstrate exacerbated age-induced visual deficits via premature senescence-mediated ER stress. We have compared mice of 5 and 10 months of age, with highly relevant human equivalencies of approximately 35- and 50-year-old patients, representing mature adult and middle-aged subjects, respectively. Our studies suggest a possible role for a self-enhanced loop of ER stress pathways and senescence in the propagation of retinal neurovascular defects, under conditions of constant endothelial activation induced by tmTNF-α signaling.
Keywords: p53, Icam1, UPR, GRP78, aging, tm-TNF
Introduction
Tumor necrosis factor-alpha (TNF-α), a pro-inflammatory cytokine, has been implicated as a contributing factor in the development of retinal diseases that are often associated with chronic retinal inflammation and endothelial activation.1,2 Interestingly, low-grade inflammation and endothelial dysfunction are related to cognitive decline and dementia in humans, in a complex interplay with vascular factors and aging.3 Moreover, previous studies have suggested that circulating levels of TNF-α are elevated in an age-associated fashion.4 In animal models, age-related upregulation of TNF-α in rat coronary arteries has been shown to induce endothelial apoptotic cell death and impaired endothelial function.5 While the effects of TNF-α are well known to affect growth rate and life span of endothelial cells in vitro,6 much less is known about the bystander effects of endothelial dysfunction on neuronal function, specifically the visual response.
We and others have also shown the direct role of TNF-α in mediating stress-associated premature senescence in endothelial cells in vitro.7–9 Studies on endothelial cell senescence reported changes such as decreased endothelial nitric oxide synthase (eNOS) activity and consequent decreased NO production,10 reduction in mitochondrial membrane potential,11 and increase in pro-inflammatory proteins including Icam1, IL-6, and IL-8 levels.9,10 This directly correlates senescence with endothelial dysfunction. Although TNF-α is known to affect these pathways in vitro, the question of whether TNF-α can directly induce senescence has not been answered conclusively in vivo. Moreover, the causes and consequences of these senescent endothelial cells in retinal pathologies have not been clearly defined.
Accumulating data suggest that the endoplasmic reticulum (ER) and senescence are cross regulated.12 During aging, increased oxidative stress associated with cellular senescence may be a consequence of accumulation of damaged biomolecules in the ER.13,14 ER stress is characteristically induced by the accumulation of misfolded proteins in the ER arising from several factors that affect folding within the ER.15 Inflammation and the accompanying cytokines have been shown to induce the unfolded protein response (UPR), through the damaging effects of oxidative stress on protein folding in the ER16 and may be propagated through signaling molecules, such as c-Jun N-terminal kinase (JNK)17 and nuclear factor-kappaB (NF-κB).18 Although many lines of evidence support a strong connection between senescence and the UPR, the effects of persistent/unresolved ER stress compounded by endothelial activation on age-associated senescence are not known.
TNF-α exists in two forms. The soluble TNF-α is generated by cleavage from its precursor transmembrane TNF-α (tmTNF-α) by TNF-α converting enzyme (TACE). While the soluble TNF-α binds preferentially to TNFR1 and induces apoptosis and inflammation, tmTNF-α is known to act predominantly through TNFR2 and plays a role in cell survival, although it also binds to TNFR1 to induce chronic inflammation, as observed in an arthritis model.19 Previously, using an endothelial-specific tmTNF-α (tie2-TNF) transgenic model, we have demonstrated spontaneous development of nephropathy features in mice and correlated those with increased pro-inflammatory markers and changes in gene expression involved in endothelial dysfunction.20 In addition, we developed diabetes in these mice and reported retinal inflammation and ER stress accompanied by visual deficits.21 In tie2-TNF transgenic animals, the endothelial promoter tie2-driven overexpression of transmembrane TNF, through a mutation of its TACE cleavage site, remains bound to endothelium,22 leading to constant endothelial activation and chronic inflammation.23,24 In this study, we investigated the relationship between endothelial dysfunction, ER stress, and cellular senescence in the development of retinal dysfunction. We tested the hypothesis that constant endothelial activation by tmTNF-α exacerbates age-induced neural (visual) deficits via ER stress in this model.
Materials and methods
tie2-TNF transgenic animals
Animal studies were approved by the Institutional Animal Care and Use Committee at UTHSC, Memphis, following guidelines per the Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Ophthalmic and Vision Research and the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. Transgenic animals used in this study were a gracious gift from Matthias Clauss, PhD (Indiana University School of Medicine, Indianapolis, IN). The generation of tie2-TNF transgenic animals was made possible by introducing the transgene uncleavable murine tmTNF-α mutant {mTNF-α 1–9, K(11)E}] between the endothelial-specific tie2 promoter and the tie2 first intron to confine TNF-α specific to the endothelium.22 The expression of tmTNF-α is considered to be global and not specific to retinal vascular endothelium. Since tie2 may also be expressed during embryogenesis in all cells of the yolk sac,25 including endothelial and immune cell precursors,26 it is possible that the expression of tmTNF-α in this model may also be expressed in other cell types. Mice used for this study had been backcrossed for more than eight generations in C57BL/6 animals and generated from founder line Tg 5382.22 The inflammatory phenotype in the transgenic line was shown to be dose dependent, suggesting that the effects are not due to random transgene integration effects.22 Animals were housed under an alternating 12-h light and dark cycle with free access to food and water. Mice of 5 and 10 months old tie2-TNF were used in the studies with age-matched littermate (C57BL/6) animals serving as controls.
Optokinetic reflex measurement
Awake mice were placed on a platform inside the OptoMotry virtual reality optokinetic reflex nystagmus (OKN) system to quantify the visual acuity and contrast sensitivity thresholds (OptoMotry, CerebralMechanics, Lethbride, Alberta, Canada) as described previously.27 According to published methodologies,28 a stepwise paradigm defined by OptoMotry software was used with the screens of contrasting bars of light, not visible to the investigator, and the investigator was blinded to the groups. Acuity testing was performed at 100% contrast with varying spatial frequency threshold (i.e. white versus black stripes), while contrast sensitivity testing was performed at fixed spatial frequency threshold (0.042 c/d) in the study groups.
Electroretinogram measurement
Scotopic threshold ERG recordings were obtained using the Espion E2 ERG system (Diagnosys LLC, Lowell, MA). Animals, dark adapted overnight, were anesthetized with ketamine/dexmedetomidine hydrochloride (0.5 mg/kg and 0.025 mg/kg respectively). Body temperatures of the mice were regulated with a heating pad at 37°C during recordings. Tropicamide (0.3%) was used to dilate eyes and 2.5% sterile hypromellose ophthalmic demulcent solution (AKORN Inc, Lake Forest, IL) was applied. Electrodes were placed on the cornea of both eyes; the reference electrode was positioned in between the eyes and a ground electrode in the tail. Mice were presented with different flashes of increasing intensity (0.0025, 0.025, 0.25, 2.5, 25 cd.s.m2), each repeated five times, with an inter-stimulus interval ranging from 20 s for dim flashes to 1 min for the brightest flashes. Three to five ERG traces at each flash luminance were averaged to measure b-wave amplitude.
Gene expression analysis
Whole mouse retinal tissue was used to isolate RNA using NucleoSpin® RNA Plus kit (Macherey-Nagel GmbH, Takara Bio, USA) followed by cDNA using SuperScript III first-strand synthesis supermix (Thermo Fisher Scientific, Waltham, MA). Real-time qPCR was performed with Quantstudio3 (Applied Biosystems, Foster City, CA) using cDNA as a template with TaqMan probes (Table 1). The expression levels of gene transcripts were determined using 2−DDCt and normalized to 18s ribosomal RNA.
Table 1.
TaqMan Gene Expression Assay Primers and Probes.
| Genes | Assay ID | Reference sequence | Amplicon length |
|---|---|---|---|
| 18S ribosomal RNA (18s rRNA) | Mm04277571 | NR_003278 | 115 |
| Glucose regulated protein78 (Grp78) | Mm00517691 | NM_001163434 | 75 |
| eukaryotic translation initiation factor 2 alpha kinase 3 (Perk) | Mm00438700 | NM_010121 | 62 |
| ER to nucleus signaling 1 (Ire1α) | Mm00470233 | NM_023913 | 95 |
| Activating transcription factor 6 (Atf6) | Mm01295319 | NM_001081304 | 74 |
| Spliced X-box binding protein 1 (sXbp1) | Mm00457357 | NM_001271730 | 56 |
| DNA-damage inducible transcript 3 (Chop) | Mm01135937 | NM_001290183 | 92 |
| Intercellular adhesion molecule 1 (Icam1) | Mm01175876 | NM_010493.2 | 94 |
| Vascular cell adhesion molecule 1 (Vcam1) | Mm01320973 | NM_011693.3 | 126 |
| Chemokine (C-C motif) ligand 2 (Ccl2) | Mm00441242 | NM_011333.3 | 74 |
| Endothelin 2 (Edn2) | Mm00432983 | NM_007902.2 | 74 |
| Tissue inhibitor of metalloproteinase 1 (Timp1) | Mm01341361 | NM_001044384.1 | 100 |
| Cholesterol 25-hydroxylase (Ch25h) | Mm00515486 | NM_009890.1 | 126 |
| Cyclin-dependent kinase inhibitor 2A (p16/Cdkn2a) | Mm00494449 | NM_009877.2 | 55 |
| cyclin-dependent kinase inhibitor 1A (p21/Cdkn1a) | Mm04205640 | NM_007669.4 | 80 |
| tumor protein 53 (p53) | Mm01731290 | NM_011640.3 | 119 |
Protein expression analysis
Whole mouse retinal tissues were lysed using RIPA buffer, sonicated and incubated for 1 h on ice and centrifuged at 16,000g for 5 min at 4°C. The clear supernatant was collected and quantified for total protein by the Bradford method. About 50 μg of protein sample was resolved using NuPAGE Bis-Tris pre-cast gels (Thermo Fisher Scientific) and transferred to a nitrocellulose membrane. After blocking the membrane for 1 h in blocking buffer, membranes were probed with primary antibodies (Table 2). This was followed by incubation with HRP-conjugated secondary antibodies. Detection was performed using an enhanced chemiluminescence kit (GE Healthcare, Chicago, IL). Targeted proteins were probed in separate blots. Mean densitometry values from independent experiments were calculated using Image-J (NIH, Bethesda, MD) software and represented as the ratio of target protein to β-tubulin or total protein of interest, where applicable.
Table 2.
Antibodies used in the study.
| Protein ID | Manufacturer | Catalog# |
|---|---|---|
| Grp78 | Thermo Fisher Scientific | PA1-014A |
| Ire1α | Thermo Fisher Scientific | PA5-20189 |
| p-Ire1α | Thermo Fisher Scientific | PA1-16927 |
| Chop | Thermo Fisher Scientific | MA1-250 |
| β-tubulin | Thermo Fisher Scientific | MA5-16308 |
| p21 | Santacruz Antibody (F-5) | sc-6246 |
| p16 | Santacruz Antibody (C-3) | sc-166760 |
Statistical analysis
Results are expressed as mean ± SEM. For all quantitative experiments, statistical analyses were performed with a two-way ANOVA with Newmann-Keuls post hoc test for multiple group comparisons (Prism 6 software; GraphPad Software, La Jolla, CA, USA) and a p-value < 0.05 was considered statistically significant.
Results
Accelerated visual deficits in tie2-TNF transgenic mice
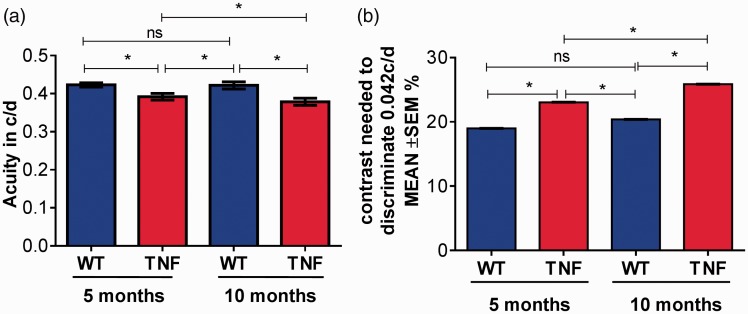
Visual deficits were assessed in live mice of 5 and 10 months of age with optokinetic measurements. As shown in Figure 1, visual acuity in five-month tie2-TNF mice was significantly decreased when compared with age-matched WT mice (five-month TNF, 0.39 ± 0.008 c/d; five-month WT, 0.42 ± 0.006 c/d, p < 0.05). As expected, 10-month tie2-TNF mice demonstrated a further decrease when compared with both the 10-month WT (10-month TNF, 0.37 ± 0.007 c/d; 10-month WT, 0.42 ± 0.009 c/d, p < 0.05) as well as the five-month tie2-TNF mice group (p < 0.05). Interestingly, five-month tie2-TNF mice showed significantly decreased acuity compared with 10-month WT mice. In corroboration with a decrease in visual acuity, contrast sensitivity was significantly increased in five-month tie2-TNF mice compared with five-month WT mice (five-month TNF, 23.0 ± 0.018%; five-month WT, 18.9 ± 0.010%, p < 0.05). Again as expected, 10-month tie2-TNF mice demonstrated a further increase when compared with both the 10-month WT (10-month TNF, 25.8 ± 0.02%; 10-month WT, 20.3 ± 0.01%, p < 0.05) as well as the five-month tie2-TNF mice group (p < 0.05). Consistent with visual acuity defects, contrast sensitivity also increased in the five-month tie2-TNF mice compared with 10-month WT mice (p < 0.05). There were no differences in visual acuity or contrast sensitivities between 5- and 10-month WT mice.
Figure 1.
Deterioration in visual acuity and contrast sensitivity in tie2-TNF mice. (a) Visual acuity expressed in c/d was measured by presenting black and white bars of varying spatial frequencies at 100% contrast, and the contrast sensitivity was measured by changing the gradient that generates tracking at a fixed spatial frequency of 0.042 c/d (b). Contrast sensitivity in mice expressed as percentage. Data represent Mean ± SEM from n = 5 animals. *p < 0.05 as indicated. (A color version of this figure is available in the online journal.)
TNF: tumor necrosis factor.
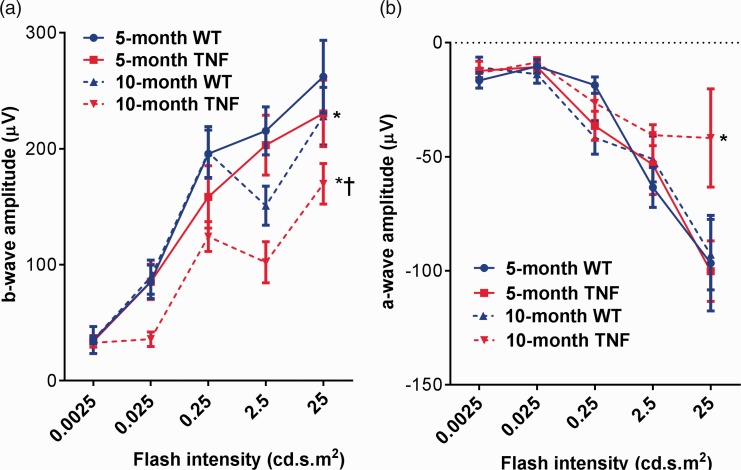
Next, we examined the electrophysiological changes generated by neuronal and non-neuronal cells in the mouse retina from all groups. Dark-adapted scotopic ERG responses were recorded from both eyes of WT and tie2-TNF transgenic mice (Figure 2(a)). When compared with age-matched WT mice, five-month tie2-TNF mice demonstrated a substantial decrease in the b-wave amplitude in the scotopic range with the data being significant at 25 cd.s.m2 flash intensity (five-month TNF, 230 ± 28 μV; five-month WT, 262 ± 31 μV, p < 0.05). This decrease was further exacerbated in 10-month tie2-TNF mice when compared with 10-month WT group (10-month TNF, 169 ± 17.4 μV; 10-month WT, 228 ± 24.7 μV, p < 0.05), also as compared with the five-month tie2-TNF mice group (p < 0.05). However, five-month tie2-TNF mice did not show a statistically significant decrease in b-wave response compared with 10-month WT mice, despite the reduction in b-wave amplitudes. Finally, no significant differences were observed in b-wave amplitudes between 5- and 10-month WT mice.
Figure 2.
Decrease in ERG amplitudes in tie2-TNF mice (a). b-wave amplitude measurement in mice expressed as μV. (b) a-wave amplitude measurement in mice expressed as μV. Data represent Mean ± SEM from n = 5 animals. *p < 0.05 compared with five-month WT mice; † p < 0.05 compared with five-month tie2-TNF mice. (A color version of this figure is available in the online journal.)
TNF: tumor necrosis factor.
Outer retinal defects are reflected in a-wave changes in ERG. When compared with age-matched WT mice, 10-month old tie2-TNF mice demonstrated a substantial decrease in the a-wave amplitude (10-month WT, –92.9682 ± 15.5 μV; 10-month TNF, –41.8247 ± 21.5 μV, p < 0.05). However, both five-month WT and five-month tie2-TNF mice did not show any alterations in a-wave response (five-month WT, –96 ± 20.9 μV; 5-month TNF, –100 ± 13.2 μV, p > 0.05) (Figure 2(b)). Finally, no significant differences were observed in a-wave amplitudes between 5- and 10-month WT mice.
tie2-TNF transgenic mice exhibit retinal premature senescence
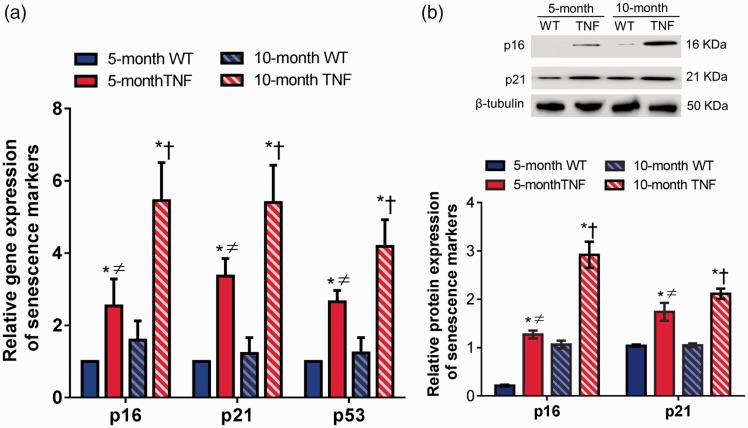
Retinal tissues from both 5-month and 10-month tie2-TNF mice and their age-matched WT mice were harvested for senescence-associated gene transcripts by quantitative real-time qPCR. The expressions of p16, p21, and p53 genes were significantly elevated (>2 fold, p < 0.05) in five-month tie2-TNF mice when compared with age-matched WT mice (Figure 3(a)). Subsequently, 10-month tie2-TNF mice group demonstrated a further increase in all three gene transcripts when compared with both the age-matched WT group and five-month tie2-TNF mice. Interestingly, five-month tie2-TNF mice demonstrated significant elevations of senescence markers when compared with 10-month WT mice group (>1.5 fold, p < 0.05). There were no significant differences in expressions of senescence markers between 5- and 10-month WT mice.
Figure 3.
Increased senescence in tie2-TNF mice. (a) Whole retinal tissue extract analyzed for gene expression of senescence markers by Taqman qPCR expressed as fold change normalized to internal control in the study groups. (b) Representative immunoblots of senescence markers in the whole retinal tissue extract analyzed for protein senescence markers from the study groups (top). Densitometric analysis of target protein expressed as fold change normalized to internal control in the study groups (bottom). Data represent Mean ± SEM from n = 5 animals. *p < 0.05 compared with five-month WT mice; † p < 0.05 compared with five-month tie2-TNF mice; ≠ p < 0.05 compared with 10-month WT mice. (A color version of this figure is available in the online journal.)
TNF: tumor necrosis factor.
Consistent with the gene transcript data, protein expression of senescence markers p16 and p21 was also increased in five-month tie2-TNF mice compared with WT mice (>2 fold, p < 0.05) (Figure 3(b)). Interestingly, the expression of these proteins was further increased significantly in 10-month tie2-TNF mice compared with both 10-month WT mice and five-month tie2-TNF mice (p < 0.05). Finally, five-month tie2-TNF mice demonstrated significant elevation of senescence markers when compared with the 10-month WT mice (>2 fold, p < 0.05).
tie2-TNF transgenic mice exhibit markers of retinal inflammation
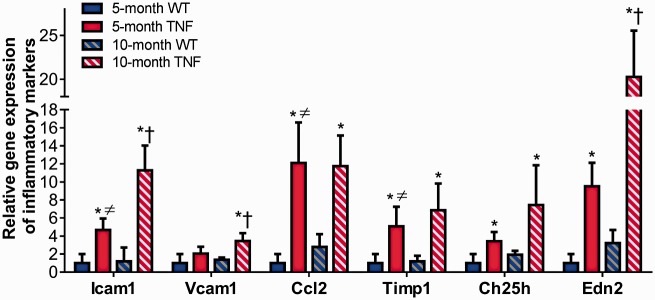
We next assessed retinal inflammatory gene transcripts by quantitative real-time qPCR assay in the five-month and 10-month tie2-TNF mice and their age-matched WT mice. A significant increase in adhesion molecules including Icam1 and pro-inflammatory cytokines and biomarker panel genes implicated in diabetic retinopathy research,29,30 including chemokine (C-C motif) ligand 2 (Ccl2), Timp1, Ch25h, and Edn2, were significantly upregulated (>2 fold, p < 0.05) in five-month tie2-TNF mice compared with age-matched WT mice (Figure 4). Interestingly, vascular cell adhesion molecule-1 levels (Vcam1), Timp1, Ch25h, and Edn2 were further increased in 10-month tie2-TNF mice (>2 fold, p < 0.05) compared with both age-matched WT group and five-month tie2-TNF mice. Also, five-month tie2-TNF mice demonstrated significant elevation of Icam1, Vcam1, and Edn2 gene transcripts when compared with 10-month WT mice group (>2 fold, p < 0.05). There were no significant differences in inflammatory markers between 5- and 10-month WT mice.
Figure 4.
Increased inflammation in tie2-TNF mice. Whole retinal tissue extract analyzed for gene expression of inflammatory markers by Taqman qPCR expressed as fold change normalized to internal control in the study groups. Data represent Mean ± SEM from n = 5 animals. *p < 0.05 compared with five-month WT mice; † p < 0.05 compared with five-month tie2-TNF mice; ≠ p < 0.05 compared with 10-month WT mice. (A color version of this figure is available in the online journal.)
TNF: tumor necrosis factor.
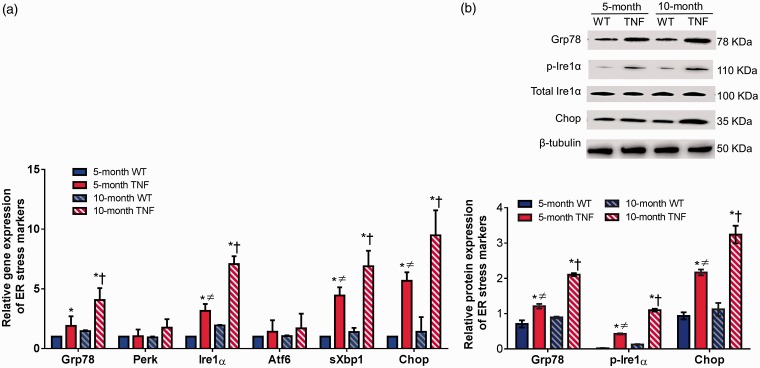
tie2-TNF transgenic mice exhibit markers of ER stress
We next investigated whether ER stress is involved in senescent cell-cycle arrest pathways in five-month and 10-month tie2-TNF mice and their age-matched WT mice. Using a quantitative real-time qPCR assay, we measured mRNA levels of ER stress markers including glucose-regulated protein-78 (Grp78), double-stranded RNA-activated protein kinase-like ER kinase (Perk), inositol requiring enzyme 1 (Ire1α), activating transcription factor 6 (Atf6), spliced X box-binding protein (sXbp1), and C/EBP homologous protein (Chop) in retinal tissues (Figure 5(a)). While the mRNA expression of Grp78, Ire1α, sXbp1, and Chop were significantly elevated in retinal tissues of five-month tie2-TNF mice compared with age-matched WT mice (>2 fold, p < 0.05), Perk and Atf6 both increased but without achieving statistical significance. Interestingly, the expression of these genes further increased significantly in 10-month tie2-TNF mice compared with both 10-month WT mice and five-month tie2-TNF mice (p < 0.05). Finally, five-month tie2-TNF mice demonstrated significant elevation of ER stress gene transcripts (Ire1α, sXbp1, and Chop) when compared with 10-month WT mice group (>2 fold, p < 0.05). There were no significant differences in ER stress markers between 5- and 10-month WT mice.
Figure 5.
Activation of ER stress markers in tie2-TNF mice. (a) Whole retinal tissue extract analyzed for gene expression of ER stress markers by Taqman qPCR expressed as fold change normalized to internal control in the study groups. (b) Representative immunoblots of senescence markers in the whole retinal tissue extract analyzed for protein ER stress markers from the study groups (top). Densitometric analysis of target protein expressed as fold change normalized to internal control in the study groups (bottom). Data represent Mean ± SEM from n = 5 animals. *p < 0.05 compared with five-month WT mice; † p < 0.05 compared with five-month tie2-TNF mice; ≠ p < 0.05 compared with 10-month WT mice. (A color version of this figure is available in the online journal.)
TNF: tumor necrosis factor.
Consistent with the gene transcript data, protein expression of other ER stress markers, Grp78, p-Ire1α, and Chop, were also increased in five-month tie2-TNF mice compared with WT mice (>2 fold, p < 0.05) (Figure 5(b)). The expression of these proteins was further increased significantly in 10-month tie2-TNF mice compared with both 10-month WT mice and five-month tie2-TNF mice (p < 0.05). Finally, five-month tie2-TNF mice demonstrated significant elevation of ER stress activation when compared with the 10-month WT mice (>2 fold, p < 0.05).
Discussion
Using a mouse model of chronic vascular activation through endothelial-specific TNF-α -expressing mice, in this study, we demonstrate accelerated age-associated visual deficits between mice of 5 and 10 months of age. Interestingly, these defects correlated with premature senescence (p16, p21, p53) in the retina accompanied by ER stress (Grp78, Ire1α, Chop). Our data demonstrate that age intensifies endothelial inflammation leading to visual deficits. Vision loss in most retinal diseases—like diabetic retinopathy, diabetic macular edema, and some inherited retinal disorders—affects the quality of life of working age adults.31 Our study using mice of 5 and 10 months of age is highly relevant for this demographic, with human equivalencies of approximately 35 and 50 years of age, representing mature and middle age adults, respectively.32
The role of tmTNF-α signaling in retinal diseases is emerging. We recently demonstrated that tie2-TNF-expressing diabetic mice display retinal inflammation and ER stress accompanied by visual deficits.21 Although the exact cellular signaling mechanisms are unclear, our studies show that constant endothelial activation induced by tmTNF-α, and further exacerbated by hyperglycemia, results in the activation of ER stress and chronic pro-inflammation in a feed forward loop, ultimately resulting in neurovascular alterations that lead to visual deficits in the retina.21 Germane to our results, tmTNF-α is known to participate in both forward and reverse signaling in certain type cells and causes activation or suppression of NFkB cell survival pathways, depending upon whether it acts like a ligand or receptor.33 Consequently, a variety of roles for tmTNF-α signaling have been established including: atheroprotection34; spontaneous nephropathy20; protection from con-A induced acute hepatitis22; resistance to apoptosis and drug resistance in breast cancer35; inducement of chronic inflammatory arthritis19; proliferation of endothelial colony-forming cells36; angiogenesis23; liver injury37; insulin sensitization38; neuroprotection39; inducement of colitis40; and VEGF-induced vascular permeability.41 It remains to be determined how tmTNF-α signaling in retina contributes to observed retinal degeneration, and whether targeting tmTNF-α signaling may be a viable therapeutic strategy in retinal diseases.
Significant increases in senescence markers in the TNF mice were observed at five months of age, with a further increase at 10 months, compared with their age-matched wildtype controls. The connection between inflammation and cellular senescence is evident from past studies, which have demonstrated that senescence is associated with gene expression patterns similar to those observed in inflammatory responses. Recent evidence in experiments with endothelial cells,42 fibroblasts,12 and epithelial cells43 have demonstrated that cellular senescence is accompanied by a prominent increase in the secretion of several inflammatory factors that participate in intercellular signaling, possibly playing a variety of roles including the clearance of senescent cells.44,45 In accordance with this, our studies demonstrated retinal inflammation at five months of age, with most inflammatory genes demonstrating a further increase at 10 months. Senescence is usually accompanied by the induction of expression and/or activity of p16INK4A and p5346,47; p53 in turn induces the expression of its transcriptional target p21WAF1. Together with p16INK4A, p21WAF1 inhibits the activity of cyclin-dependent kinases that phosphorylate retinoblastoma protein at the G1/S transition, thereby resulting in cell cycle arrest and other phenotypes of senescence. Accordingly, we show a significant increase in both p16INK4A and p53 in TNF mice, both at five months with further exacerbated levels at 10 months of age. Previously, we have shown that mature but not highly proliferative endothelial cells, expressed p16INK4A under the influence of TNF.7 Taken together, these studies establish the role for TNF in premature senescence in the retinal tissue, although further work is still necessary to elucidate the exact mechanisms by which p16INK4A induces senescence in this model.
Multiple factors including inflammation and senescence can result in a buildup of unfolded or misfolded proteins in the ER lumen, a condition known as ER stress. The UPR is evoked in response to ER stress to manifest cellular homeostasis by activation of three ER membrane-bound transducers; Ire1α, Atf6, and Perk. There are isolated observations demonstrating the implications of ER stress-mediated premature senescence through the Atf4/p16 pathway in epithelial cells,43 p21 signaling,48 or an increase of the Xbp1 spliced form which have been reported in studies involving adriamycin-induced senescence in lymphomas cells.49 A recent study has shown inflammation-induced upregulation of ER stress markers (eIF2α, Grp78, and Atf4) in the aged mouse brain.50 In accordance with these studies, our results show significant upregulation of Grp78, Ire1α, and Chop with increasing age, with 10-month tie2-TNF mice showing the greatest amount of change, possibly mediated by underlying endothelial inflammation. This is in agreement with a study that demonstrated that inhibition of Ire1α-mediated UPR initiates the p38/SKN-1(Nrf2) antioxidant response,51 thereby postponing age-related health decline. Future investigations, beyond the scope of the current study, need to establish more mechanistic insight, so as to delineate which arm of UPR is involved in the downstream effects of ER stress-mediated senescence in this model.
It is interesting to note that visual deficits seen in TNF mice increased in an age-dependent fashion, with animals 10 months old showing more defects when compared with those five months of age. Both ERG and OKN defects observed in the model are consistent with other rodent models that display inflammation,52 ER stress,53 neurotransmitter defects,54 or retinal microcirculation,55,56 all of which are correlated with premature cellular senescence and/or ER stress pathways. Specifically, inflammation and oxidative stress have been linked to outer retinal defects, with photoreceptors having the highest susceptibility to oxidative damage.57 It is possible that the expression of TNF in the inner retina may cause excessive inflammatory and/or oxidative stress in the outer retina, as reflected by decreases in a-wave amplitudes in our model. Since tie2 may also be expressed during embryogenesis25 and within immune cell precursors,26 it is possible that the expression of tmTNF- α in this model may also be expressed in other cell types of the retina, thereby influencing the observed inflammatory phenotype. In future studies, we plan to use other specific methods, including the use of pharmacological agonists or antagonists, to tease out the exact cellular origins of these defects in our model.
In conclusion, our data demonstrate that tie2-TNF mice demonstrate age-associated increases in visual deficits. Given the correlation of increased ER stress with the loss of visual function, and correlation of increased endothelial activation with premature senescence, our study suggests a self-perpetuating loop of UPR pathways and senescence in propagating age-associated neurovascular defects in this model.
Acknowledgments
The authors wish to acknowledge Matthias Clauss, Indiana University School of Medicine, Indianapolis, for helpful discussions.
Authors’ Contributions
Conceived, designed the experiments, and wrote the manuscript: RL, RG. Performed the experiments: RL, PGN, JG. Analyzed the data: RL, PGN, JG, RG. Reviewed and approved the manuscript: All authors.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Eye Institute (EY023427), gifts from the Hamilton Eye Institute, and an unrestricted grant from Research to Prevent Blindness. RL is a recipient of postdoc fellowship awards from the Neuroscience Institute, UTHSC, and the International Retinal Research Foundation. The funders played no role in the conduct of the study, collection of data, management of the study, analysis of data, interpretation of data, or preparation of the manuscript.
References
- 1.Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol 2008; 172:1411–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci 2011; 52:1336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heringa SM, van den Berg E, Reijmer YD, Nijpels G, Stehouwer CD, Schalkwijk CG, Teerlink T, Scheffer PG, van den Hurk K, Kappelle LJ, Dekker JM, Biessels GJ. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population – the Hoorn Study. Psychoneuroendocrinology 2014; 40:108–18 [DOI] [PubMed] [Google Scholar]
- 4.Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol 2000; 121:255–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 2004; 17:21–30 [DOI] [PubMed] [Google Scholar]
- 6.Shimada Y, Ito H, Kaji K, Fukuda M. Tumor necrosis factor reduces lifespan of human endothelial cells in vitro. Mech Ageing Dev 1990; 55:245–54 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Herbert BS, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB J 2009; 23:1358–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong PF, Jamal J, Tong KL, Khor ES, Yeap CE, Jong HL, Lee ST, Mustafa MR, Abubakar S. Deregulation of hsa-miR-20b expression in TNF-alpha-induced premature senescence of human pulmonary microvascular endothelial cells. Microvasc Res 2017; 114:26–33 [DOI] [PubMed] [Google Scholar]
- 9.Khan SY, Awad EM, Oszwald A, Mayr M, Yin X, Waltenberger B, Stuppner H, Lipovac M, Uhrin P, Breuss JM. Premature senescence of endothelial cells upon chronic exposure to TNFalpha can be prevented by N-acetyl cysteine and plumericin. Sci Rep 2017; 7:39501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 2002; 105:1541–4 [DOI] [PubMed] [Google Scholar]
- 11.Unterluggauer H, Hutter E, Voglauer R, Grillari J, Voth M, Bereiter-Hahn J, Jansen-Durr P, Jendrach M. Identification of cultivation-independent markers of human endothelial cell senescence in vitro. Biogerontology 2007; 8:383–97 [DOI] [PubMed] [Google Scholar]
- 12.Matos L, Gouveia AM, Almeida H. ER Stress response in human cellular models of senescence. J Gerontol A Biol Sci Med Sci 2015; 70:924–35 [DOI] [PubMed] [Google Scholar]
- 13.Basaiawmoit RV, Rattan SI. Cellular stress and protein misfolding during aging. Methods Mol Biol 2010; 648:107–17 [DOI] [PubMed] [Google Scholar]
- 14.Lamoke F, Shaw S, Yuan J, Ananth S, Duncan M, Martin P, Bartoli M. Increased oxidative and nitrative stress accelerates aging of the retinal vasculature in the diabetic retina. PLoS One 2015; 10:e0139664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 2005; 74:739–89 [DOI] [PubMed] [Google Scholar]
- 16.Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem 2005; 280:33917–25 [DOI] [PubMed] [Google Scholar]
- 17.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000; 287:664–6 [DOI] [PubMed] [Google Scholar]
- 18.Balakumar M, Raji L, Prabhu D, Sathishkumar C, Prabu P, Mohan V, Balasubramanyam M. High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol Cell Biochem 2016; 423:93–104 [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulou L, Pasparakis M, Kollias G. A murine transmembrane tumor necrosis factor (TNF) transgene induces arthritis by cooperative p55/p75 TNF receptor signaling. Eur J Immunol 1997; 27:2588–92 [DOI] [PubMed] [Google Scholar]
- 20.Rajashekhar G, Gupta A, Marin A, Friedrich J, Willuweit A, Berg DT, Cramer MS, Sandusky GE, Sutton TA, Basile DP, Grinnell BW, Clauss M. Soluble thrombomodulin reduces inflammation and prevents microalbuminuria induced by chronic endothelial activation in transgenic mice. Am J Physiol Renal Physiol 2012; 302:F703–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenin R, Nagy P, Alli S, Rao VR, Clauss M, Kompella UB, Gangaraju R. Critical role of endoplasmic reticulum stress in chronic endothelial activation induced visual deficits in tie2-tumor necrosis factor mice. J Cell Biochem 2018; Jul 27. doi: 10.1002/jcb.27072 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 22.Willuweit A, Sass G, Schoneberg A, Eisel U, Tiegs G, Clauss M. Chronic inflammation and protection from acute hepatitis in transgenic mice expressing TNF in endothelial cells. J Immunol 2001; 167:3944–52 [DOI] [PubMed] [Google Scholar]
- 23.Rajashekhar G, Willuweit A, Patterson CE, Sun P, Hilbig A, Breier G, Helisch A, Clauss M. Continuous endothelial cell activation increases angiogenesis: evidence for the direct role of endothelium linking angiogenesis and inflammation. J Vasc Res 2006; 43:193–204 [DOI] [PubMed] [Google Scholar]
- 24.Rajashekhar G, Grow M, Willuweit A, Patterson CE, Clauss M. Divergent and convergent effects on gene expression and function in acute versus chronic endothelial activation. Physiol Genomics 2007; 31:104–13 [DOI] [PubMed] [Google Scholar]
- 25.Li W, Johnson SA, Shelley WC, Ferkowicz M, Morrison P, Li Y, Yoder MC. Primary endothelial cells isolated from the yolk sac and para-aortic splanchnopleura support the expansion of adult marrow stem cells in vitro. Blood 2003; 102:4345–53 [DOI] [PubMed] [Google Scholar]
- 26.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004; 118:149–61 [DOI] [PubMed] [Google Scholar]
- 27.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci 2004; 45:4611–6 [DOI] [PubMed] [Google Scholar]
- 28.Umino Y, Everhart D, Solessio E, Cusato K, Pan JC, Nguyen TH, Brown ET, Hafler R, Frio BA, Knox BE, Engbretson GA, Haeri M, Cui L, Glenn AS, Charron MJ, Barlow RB. Hypoglycemia leads to age-related loss of vision. Proc Natl Acad Sci USA 2006; 103:19541–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman WM, Bixler GV, Brucklacher RM, Walsh E, Kimball SR, Jefferson LS, Bronson SK. Transcriptomic comparison of the retina in two mouse models of diabetes. J Ocul Biol Dis Inform 2009; 2:202–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman WM, Bixler GV, Brucklacher RM, Lin CM, Patel KM, VanGuilder HD, LaNoue KF, Kimball SR, Barber AJ, Antonetti DA, Gardner TW, Bronson SK. A multistep validation process of biomarkers for preclinical drug development. Pharmacogenomics J 2010; 10:385–95 [DOI] [PubMed] [Google Scholar]
- 31.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010; 376:124–36 [DOI] [PubMed] [Google Scholar]
- 32.Life span as a biomarker, The Jackson Laboratory, www.jax.org/research-and-faculty/research-labs/the-harrison-lab/gerontology/life-span-as-a-biomarker (accessed 12 March 2018)
- 33.Zhang H, Yan D, Shi X, Liang H, Pang Y, Qin N, Chen H, Wang J, Yin B, Jiang X, Feng W, Zhang W, Zhou M, Li Z. Transmembrane TNF-α mediates “forward” and “reverse” signaling, inducing cell death or survival via the NF-κB pathway in Raji Burkitt lymphoma cells. J Leukoc Biol 2008; 84:789–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canault M, Peiretti F, Mueller C, Kopp F, Morange P, Rihs S, Portugal H, Juhan V, I, Nalbone G. Exclusive expression of transmembrane TNF-alpha in mice reduces the inflammatory response in early lipid lesions of aortic sinus. Atherosclerosis 2004; 172:211–8 [DOI] [PubMed] [Google Scholar]
- 35.Yu M, Zhou X, Niu L, Lin G, Huang J, Zhou W, Gan H, Wang J, Jiang X, Yin B, Li Z. Targeting transmembrane TNF-alpha suppresses breast cancer growth. Cancer Res 2013; 73:4061–74 [DOI] [PubMed] [Google Scholar]
- 36.Green LA, Njoku V, Mund J, Case J, Yoder M, Murphy MP, Clauss M. Endogenous transmembrane TNF-alpha protects against premature senescence in endothelial colony forming cells. Circ Res 2016; 118:1512–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang P, Zhou W, Li C, Zhang M, Jiang Y, Jiang R, Ba H, Li C, Wang J, Yin B, Gong F, Li Z. Kupffer-cell-expressed transmembrane TNF-alpha is a major contributor to lipopolysaccharide and D-galactosamine-induced liver injury. Cell Tissue Res 2016; 363:371–83 [DOI] [PubMed] [Google Scholar]
- 38.Zhou W, Yang P, Liu L, Zheng S, Zeng Q, Liang H, Zhu Y, Zhang Z, Wang J, Yin B, Gong F, Wu Y, Li Z. Transmembrane tumor necrosis factor-alpha sensitizes adipocytes to insulin. Mol Cell Endocrinol 2015; 406:78–86 [DOI] [PubMed] [Google Scholar]
- 39.Novrup HG, Bracchi-Ricard V, Ellman DG, Ricard J, Jain A, Runko E, Lyck L, Yli-Karjanmaa M, Szymkowski DE, Pearse DD, Lambertsen KL, Bethea JR. Central but not systemic administration of XPro1595 is therapeutic following moderate spinal cord injury in mice. J Neuroinflammation 2014; 11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corazza N, Brunner T, Buri C, Rihs S, Imboden MA, Seibold I, Mueller C. Transmembrane tumor necrosis factor is a potent inducer of colitis even in the absence of its secreted form. Gastroenterology 2004; 127:816–25 [DOI] [PubMed] [Google Scholar]
- 41.Clauss M, Sunderkotter C, Sveinbjornsson B, Hippenstiel S, Willuweit A, Marino M, Haas E, Seljelid R, Scheurich P, Suttorp N, Grell M, Risau W. A permissive role for tumor necrosis factor in vascular endothelial growth factor-induced vascular permeability. Blood 2001; 97:1321–9 [DOI] [PubMed] [Google Scholar]
- 42.Eman MR, Regan-Klapisz E, Pinkse MW, Koop IM, Haverkamp J, Heck AJ, Verkleij AJ, Post JA. Protein expression dynamics during replicative senescence of endothelial cells studied by 2-D difference in-gel electrophoresis. Electrophoresis 2006; 27:1669–82 [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Yang JR, Chen XM, Cai GY, Lin LR, He YN. Impact of ER stress-regulated ATF4/p16 signaling on the premature senescence of renal tubular epithelial cells in diabetic nephropathy. Am J Physiol Cell Physiol 2015; 308:C621–30 [DOI] [PubMed] [Google Scholar]
- 44.Young AR, Narita M. SASP reflects senescence. EMBO Rep 2009; 10:228–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol Mech Dis 2010; 5:99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88:593–602 [DOI] [PubMed] [Google Scholar]
- 47.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. Embo J 2003; 22:4212–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Huang K, Cai GY, Chen XM, Yang JR, Lin LR, Yang J, Huo BG, Zhan J, He YN. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal 2014; 26:110–21 [DOI] [PubMed] [Google Scholar]
- 49.Dorr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Dabritz JH, Lisec J, Lenze D, Gerhardt A, Schleicher K, Kratzat S, Purfurst B, Walenta S, Mueller-Klieser W, Graler M, Hummel M, Keller U, Buck AK, Dorken B, Willmitzer L, Reimann M, Kempa S, Lee S, Schmitt CA. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 2013; 501:421–5 [DOI] [PubMed] [Google Scholar]
- 50.Palomera-Avalos V, Grinan-Ferre C, Izquierdo V, Camins A, Sanfeliu C, Canudas AM, Pallas M. Resveratrol modulates response against acute inflammatory stimuli in aged mouse brain. Exp Gerontol 2018; 102:3–11 [DOI] [PubMed] [Google Scholar]
- 51.Hourihan JM, Moronetti Mazzeo LE, Fernandez-Cardenas LP, Blackwell TK. Cysteine sulfenylation directs IRE-1 to activate the SKN-1/Nrf2 antioxidant response. Mol Cell 2016; 63:553–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeh P-T, Huang H-W, Yang C-M, Yang W-S, Yang C-H. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS One 2016; 11:e0146438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonnet Wersinger D, Benkafadar N, Jagodzinska J, Hamel C, Tanizawa Y, Lenaers G, Delettre C. Impairment of visual function and retinal ER stress activation in Wfs1-deficient mice. PLoS One 2014; 9:e97222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramsey DJ, Ripps H, Qian H. An electrophysiological study of retinal function in the diabetic female rat. Invest Ophthalmol Vis Sci 2006; 47:5116–24 [DOI] [PubMed] [Google Scholar]
- 55.Muir ER, Renteria RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci 2012; 53:6488–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Yadav AS, Leskova W, Harris NR. Inhibition of 20-HETE attenuates diabetes-induced decreases in retinal hemodynamics. Exp Eye Res 2011; 93:108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci USA 2013; 110:16586–91 [DOI] [PMC free article] [PubMed] [Google Scholar]