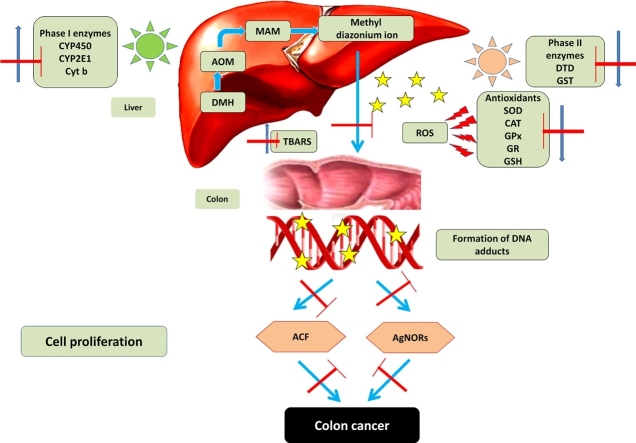
Graphical abstract
Keywords: Colorectal cancer, DMH, Orientin, Aberrant crypt foci, Cell proliferation
Highlights
-
•
DMH induced preneoplastic lesions in colonic mucosa.
-
•
Orientin treatment reduced DMH induction of cytochrome P450.
-
•
Orientin attenuates DMH induced aberrant crypt formation.
-
•
Orientin suppresses colonic tumor cell proliferation.
Abstract
Orientin, a c- glycosyl flavonoid found copiously in roobios tea and various medicinal plants is well known for its antioxidant, anti-inflammatory, and antitumor effects. The present study aims to investigate the anti-cancer efficacy of orientin on 1,2 dimethyl hydrazine induced colonic aberrant crypt foci (ACF) and cell proliferation in Wistar rats. Rats were randomly divided into six groups and fed with high fat diet. Group 1 left as untreated control. Group 2 administered with DMH (20 mg/kg body weight) for initial 4 weeks and left untreated. Group 3 received orientin (10 mg/kg body weight) alone for the entire period. Group 4 received orientin along with DMH for initial 4 weeks and left untreated; Group 5 administered DMH for initial 4 weeks and treated with orientin for remaining 12 weeks; Group 6 administered DMH and treated with orientin throughout the entire period. Our preclinical findings suggest that the administration of orientin decreases the occurrence of DMH induced colonic polyps and aberrant crypt foci, augments antioxidant defense and altered the activities of drug metabolizing phase I and phase II enzymes in colonic and hepatic tissues and thereby ensuring the detoxification of carcinogen. Furthermore, orientin attenuates the aberrant crypt foci formation and reinstates the DMH induced cell proliferation, as evident from the AgNORs staining of colonic tissues of experimental rats. Thus, our study emphasizes that orientin may prevent DMH induced precancerous lesions and proven to be a potent antioxidant and antiproliferative agent.
1. Introduction
Colorectal cancer is the third most commonly diagnosed cancer and fourth most causes of cancer related mortality worldwide [1]. The emerging countries also pose an alarming increase in the rate of incidence due to adaptation of the Westernized lifestyle (diet and reduced physical activity), rural populations and poor socioeconomic status [2,3]. The chemopreventive strategies using dietary supplements and pharmacologically active compounds serve as an effective therapy against colorectal cancer compared with other treatments [4,5]. The pro-carcinogenic 1, 2-dimethylhydrazine (DMH) is widely used to induce tumorigenesis in experimental rodent models [[6], [7], [8]]. It mimics human colon carcinoma in epithelial origin, colonic mucosa anatomy, morphology, histology and tumorigenic characteristics, thereby, serves as a perfect experimental model for chemoprevention studies [9,10]. DMH induction causes epithelial cells to undergo pathogenesis from preneoplastic lesion aberrant crypt foci (ACF) into adenomas and malignant adenocarcinomas. The macroscopic preneoplastic lesions appear after the exposure of DMH for two weeks, suggesting that ACF are antecedents of colorectal cancer [11,12]. Epidemiological studies suggest that rodents fed with high fat diet (HFD) leads to an increase in the number of carcinogen induced aberrant crypt foci [13]. High fat diet intake in DMH induced tumor model could promote the formation of colonic neoplasm through inflammation, metabolic dysfunctions and increased cell proliferation [14].
DMH itself is not a carcinogen, it is metabolically activated into DNA reactive metabolites by the phase I and phase II xenobiotic enzymes in order to exert lipid peroxidation and tumorigenic activity [15]. Primarily, the phase I enzymes cytochrome P450 dependant monooxygenases cause hydroxylation of procarcinogens into highly reactive methyldiazonium ion which alkylates DNA bases. This strong nucleophiles interacts with the DNA bases to form adducts including O6-methylguanine (O6-mG) and N7-methylguanine (N7-mG) ultimately resulting in mutagenesis and thereby tumorigenesis [16]. The phase II enzymes, including glutathione S-transferase (GST) and DT-diaphorase (DTD) detoxify the electrophilic intermediates [17,18]. The quantitative evaluation of argyrophilic nucleolar organizing regions (AgNORs) determines the rate of cell proliferation and their numbers can reflect the prognosis of colon cancer [19].
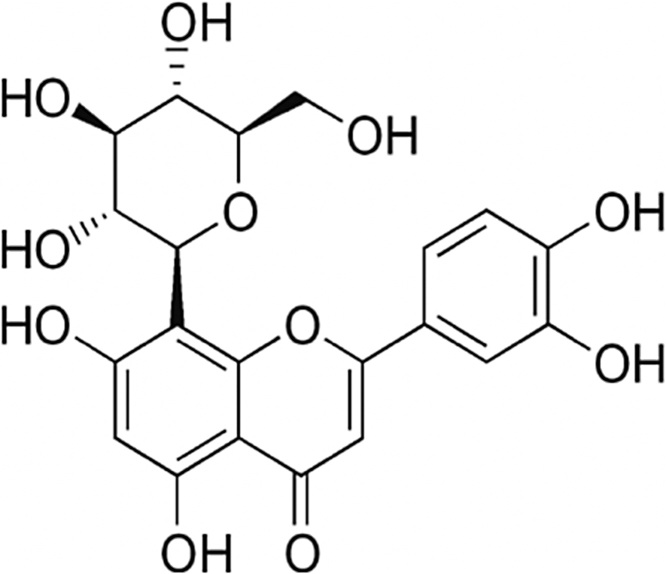
Orientin (Luteolin -8-glucoside) belonging to the subclass of flavones namely C-glycosyl flavonoid was copiously found in rooibos tea, passion fruit and isolated from various medicinal plants such as tulsi and bamboo Leaves [[20], [21], [22]]. It is a water-soluble flavonoid with a molecular formula of C21H20O11 and a molecular weight of 448.3769 g/mol; contains mostly phenol groups with two ether groups and a ketone group as shown in Fig. 1. It has been reported to possess a variety of pharmacological activities including antioxidant, anti-inflammatory, neuroprotective, cardioprotective and antitumor effects [23,24]. It also effectively attenuates chemically induced inflammatory bowel conditions in dextran sulphate sodium (DSS)-induced colitis in mice [25]. Orientin exhibits significant cytotoxicity in esophageal cancer EC109 cells [26] and MCF-7 breast cancer cells [27]. The present study aims to evaluate the protective efficacy of orientin against DMH-induced colorectal lesions in Wistar rats by analyzing the occurrence of colon lesions and changes in phase I & II metabolizing enzymes after DMH dosing with and without orientin treatment.
Fig. 1.
Structure of orientin.
2. Materials and methods
2.1. Chemicals
Orientin (ORI), 1, 2-dimethylhydrazine hydrochloride (DMH) and methylene blue was purchased from Sigma-Aldrich Chemical Company, Saint-Louis, MO, USA. All other chemicals and solvents employed were of analytical grade.
2.2. Colorectal tumor induction
DMH was dissolved in1mM EDTA just ahead of use and the pH adjusted to 6.5 with 1 mM NaOH to make sure the stability of carcinogen. Animals were given subcutaneous injections of DMH at a dose of 20 mg/kg body weight for 4 consecutive weeks of the total experimental period [28,29].
2.3. Orientin dissolution and administration
Orientin was dissolved in 0.01% of dimethyl sulfoxide (DMSO) just before use and administered intraperitoneally at a daily dose of 10 mg/kg body weight [30].
2.4. Animals
Male adult Wistar rats, aged 6 weeks of body weight 120–150 g were obtained from Sri Venkateshwara enterprises, Bangalore, India and were acclimatized under laboratory conditions for a week prior to experimental period. Animals were maintained as per the principles and guidelines of the Institutional Ethical Committee of Animal Care in accordance with the Indian National Law on animal care and use (Reg.No.:P.Col/08/2016/IAEC/VMCP). The animals were housed in solid bottomed polypropylene cages with a stainless steel grill on top and a hygienic rice husk bed in a specific-pathogen free environment. The animals were maintained under ambient conditions of 12 h light/dark cycle with relative humidity of 50 ± 10% and a temperature of 22 ± 2 °C throughout the experimental period. Animals were fed with a modified high fat diet prepared from commercial pellet diet of 4.2% fat obtained from Sri Venkateshwara Enterprises, Bangalore, India and mixed with 15.8% peanut oil, making a total of 20% fat in the diet (Table 1) and tap water ad libitum [10].
Table 1.
High fat diet composition.
| Ingredients | Modified High fat diet |
|---|---|
| Protein | 17.7 |
| Fat | 20% (4.2% + Pea nut oil 15.8%) |
| Carbohydrate | 50.5 |
| Fiber | 3.4 |
| Mineral | 6.7 |
| Vitamin | 1.7 |
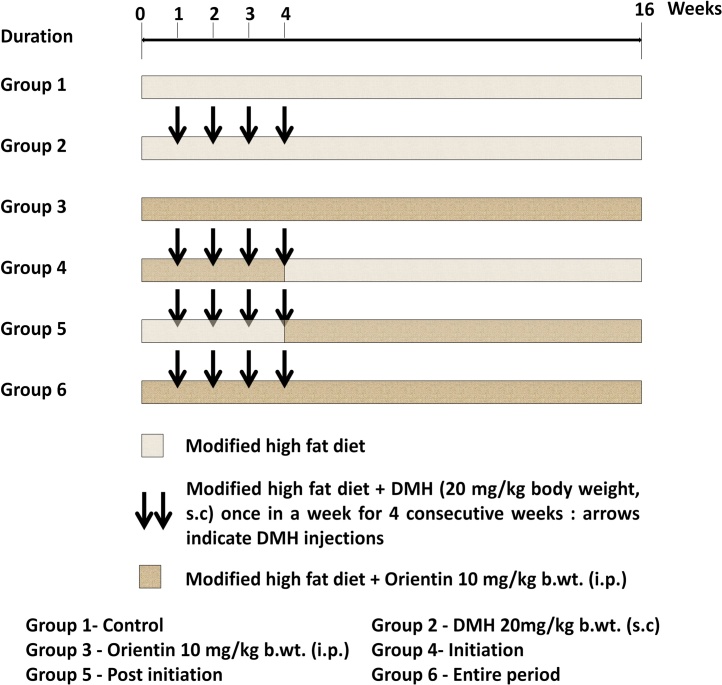
2.5. Experimental design and treatment schedule
Animals were randomly separated into six groups of six rats and fed with high fat pellet throughout the experimental period of 16 weeks. Group 1 rats left as untreated control. Group 2 rats were induced colorectal tumor via subcutaneous administration of 20 mg/kg body weight DMH once in a week for 4 consecutive weeks and left untreated for remaining 12 weeks. Group 3 rats received orientin alone intraperitoneally at a daily dose of 10 mg/kg body weight for the entire 16 weeks. Group 4 rats received orientin intraperitoneally at a daily dose of 10 mg/kg body weight along with weekly DMH exposure (20 mg/kg body weight) for the first 4 weeks and left untreated for remaining 12 weeks. Group 5 rats induced with DMH alone (20 mg/kg body weight, weekly) for first 4 weeks and received intraperitoneal administration of orientin (10 mg/kg body weight, daily) for the remaining 12 weeks. Group 6 rats induced with DMH (20 mg/kg body weight, weekly) for first 4 weeks and in addition received orientin intraperitoneally at a daily dose of 10 mg/kg body weight throughout the entire 16 weeks. In our study, Group 4 rats received orientin for first 4 weeks termed as ‘initiation’; Group 5 rats received orientin for the remaining 12 weeks after DMH induction termed as ‘post initiation’ and Group 6 rats received orientin for the entire 16 weeks termed as ‘entire period’. The detailed experimental protocol is shown in Fig. 2. At the end of 16 weeks, the rats were anesthetized by ketamine hydrochloride (30 mg/kg body weight, intramuscular) after an overnight fast and euthanized by cervical dislocation.
Fig. 2.
Schematic representation of treatment schedule.
2.6. Body weight and growth rate changes
Body weight and growth rate of the control and experimental rats were assessed throughout the experimental period of 16 weeks. All the rats were weighed before the experiment, and every week consecutively and finally before sacrifice.
2.7. Macroscopic assessment of polyp’s incidence
Rats were sacrificed and the entire colon was removed at the end of the experimental period, and flushed thoroughly with physiological saline. The colons were incised longitudinally without disturbing the polyps and vigilantly counted through macroscopic observation.
2.8. Hemolysate preparation
Blood was collected in 5.0 ml heparinized tubes, and the plasma was isolated upon centrifugation at 2000 g for 10 min. Further, the buffy coat was removed and the erythrocytes were washed repeatedly with cold physiological saline. The hemolysate was prepared by lysing a known volume of packed RBCs with hypotonic phosphate buffer and centrifuged at 3000 g for 10 min at 4 °C.
2.9. Cytosol and microsomal fractionation
Briefly, the liver and the intestinal mucosal scrapings were homogenized in ice-cold 0.01 M Tris–KCl buffer (pH 7.4) and centrifuged at 9000×g for 20 min. The resulting supernatant was transferred into pre-cooled ultracentrifugation tubes and centrifuged at 100,000 ×g for 60 min to yield cytosolic fraction for biochemical estimations [31]. The pellet was resuspended in ice-cold 0.15 M Tris–KCl buffer (pH 7.4) and re-centrifuged for 60 min at 100,000×g to obtain microsomal fraction for assaying cytochrome b5 and cytochrome P450 enzymes. The protein content was determined following the method of Lowry et al. [32] using bovine serum albumin as standard, at 660 nm.
2.10. Estimation of lipid peroxidation and antioxidant status
The level of thiobarbituric acid reactive substances (TBARS), the lipid peroxidation marker was quantified by the formation of pink chromogen complex on reaction with MDA, and the absorbance was read at 535 nm in tissues and plasma. SOD (EC.1.15.1.1) was measured based on 50% inhibition of the formation of NADH‑phenazine methosulfate‑NBT formazan complex at 560 nm. CAT (EC.1.11.16) was quantified by measuring the amount of chromium acetate formed while reducing dichromate in acetic acid. Reduced GSH content is measured by Ellman method based on the yellow colored formation when DTNB react with the compounds containing sulfhydryl groups. Glutathione peroxidase (GPx) (EC.1.11.1.9) was determined by incubating known quantity of the enzyme with H2O2 in the presence of GSH. Glutathione reductase (GR) (EC 1.8.1.7) was determined based on NADPH oxidation; the absorbance was read at 340 nm. Glutathione‑S‑transferase (GST) was measured by estimating the formation of GSH conjugate using CDNB and the absorbance was recorded at 340 nm. [30].
2.11. Assay of xenobiotic biotransformation enzymes (Phase I and Phase II)
Cytochrome P450 (CYP450, EC 1.14.14.1) and cytochrome b5 (CYB5, EC 1.6.2.4) content were assayed as described by Omura [33]. Cytochrome P4502E1 (CYP2E1, EC 1.14.13.n7) was measured by the method of Watt et al. [34]. The activity of glutathione S-transferase (GST, EC 2.5.1.18) was determined by Habig et al. [35] method. The activity of DT-diaphorase (DTD, EC 1.6.99.2) was assayed as described by Ernster [36].
2.12. Aberrant crypt foci examination
The screening of ACF was performed by the method described by Ochiai et al. [37]. The colon tissues were split open longitudinally and flushed with PBS (0.1 M, pH 7.2). Then the luminal surface of the colon was placed on strips of filter paper on either side. The colon was fixed in a 10% buffered formalin overnight. Each segment was placed in a petri dish and stained with 0.2% methylene blue solution for 2 min. The colonic sections were then transferred to another petri dish-containing PBS buffer to remove excess stains. The surface of the intestinal mucosa was visualized using a light microscope at low magnification to determine the total number of ACF and the number of crypts per focus [38].
2.13. Determination of colonic cell proliferation
Thin colon sections of 4 μm were cut, dewaxed and rehydrated through different grades of ethanol to water. The colloidal silver nitrate solution is freshly prepared by mixing a part of 2% gelatin in 1% formic acid with twice the part of the 50% aqueous silver nitrate solution for 30 min at room temperature and stained the slides. The sections were then washed with double distilled water. The sections were dehydrated through the increasing grades of alcohol. Finally the slides were washed with xylene and mounted using DPx [11]. More than 100 cells were selected randomly in each specimen in order to avoid conflicts and each AgNOR dots with a diameter of more than 1 mm were counted. The AgNOR counting was expressed as the number of AgNORs count/nucleus.
2.14. Statistical analysis
The values are given as the mean ± SD. The significant difference between the mean of the six groups was significantly analyzed by one way analysis of variance (ANOVA) and Duncan’s Multiple Range Test (DMRT). The results were considered statistically significant at P < 0.05. Statistical analysis was performed using SPSS 16.0 software package (SPSS, IBM product, Chicago, IL, USA).
3. Results
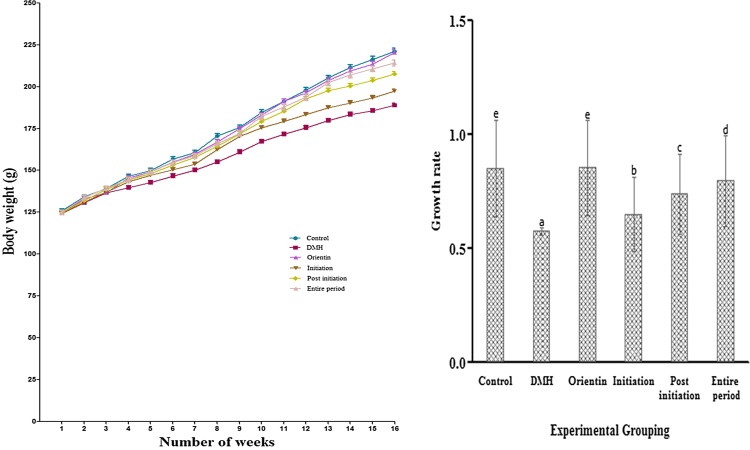
3.1. Effect of orientin on body weight and growth rate
Changes in the body weight and growth rate of control and treated groups were recorded throughout the experiment period (Fig. 3). DMH-alone-administered rats (group III) showed decrease in body weight as compared to the control and control. However, the loss of body mass was prevented significantly (P < 0.05) on administration with orientin (Group 3–6) to DMH alone exposed rats. The growth rate was decreased in DMH alone induced rats as compared to control and significantly increased in orientin treated rats (Group 3–6) as compared to the DMH-alone-exposed rats. Orientin at a dose of 10 mg/kg for entire period showed a gain in body weight and optimum growth rate. The above findings suggest that orientin offers chemo protective activity against DMH-induced colon carcinogenesis.
Fig. 3.
Body weight and growth rate profile of control and treated rats. DMH exposed rats showed decrease in body weight and growth rate when compared with control rats. Values are Mean ± SEM of six rats from each group.
3.2. Effect of orientin on polyp’s incidence
High fat/DMH-induced colon tumor development and crypt multiplicity are listed out in Table 2. Group 2 rats induced with DMH alone exhibit 100% tumor development throughout the colon, whereas orientin treatment in Group 3–6 resulted in the reduction in percentage of tumor incidence. Table 2 depicts the crypt multiplicity in Group 2 rats ranging from one, two, three, and four crypts after 16 weeks. The multiplicity of crypts was restricted and observed to be reduced in orientin-treated rats (Group 4–6) compared to DMH alone treated rats (Group 2). No significant difference was observed in control and orientin alone-treated group (Group 3).
Table 2.
Effect of Orientin on the incidence of colonic polyps in the colon of control and experimental rats.
| Groups | Number of rats bearing polyps | Total number of polyps | Average number of polyps-bearing rats | Percentage of polyps incidence* | Percentage of polyps inhibition | Crypt multiplicity# |
|---|---|---|---|---|---|---|
| Control | 0/6 | 0 | 0 | – | – | 0 |
| DMH | 6/6 | 16 | 2.7 | 100 | – | 0.88 ± 0.02 |
| Orientin | 0/6 | 0 | 0 | – | – | 0 |
| Initiation | 4/6 | 11 | 1.8 | 66.67 | 33.33 | 0.65 ± 0.02 |
| Post initiation | 3/6 | 8 | 1.3 | 50 | 50 | 0.32 ± 0.01 |
| Entire period | 1/6 | 4 | 0.67 | 16.67 | 83.33 | 0.12 ± 0.01 |
Group 1 - control, Group 2 - DMH, Group 3 - Orientin, Group 4 - Initiation, Group 5 - Post initiation, Group 6 - Entire period. *(Number of polyps-bearing rats/total number of rats in each group) x100. # Number of tumors/rat on colonic tumors.
3.3. Effect of orientin on lipid peroxidation and antioxidant status
The effect of orientin on circulatory and colonic lipid peroxidation and antioxidant status post treatment period is listed out in Table 3, Table 4. The circulatory TBARS were found to be increased on the exposure to DMH as compared with control rats, whereas on treatment with orientin the levels were found to be significantly decreased. In contrast, the colonic TBARS were observed to be decreased in DMH exposed rats (Group 2) when compared with control (Group 1). The reduced level of TBARS was significantly elevated when compared with that of carcinogen induced rats (Group 2) on treatment with orientin (Group 3–6). Both circulatory and colonic antioxidants were significantly decreased in DMH treated rats (Group 2) as compared to control rats (Group 1). Orientin administration (Group 3–6) significantly increased the level of antioxidants compared to that of DMH induced rats (Group 2).
Table 3.
Effect of orientin on circulatory and colonic level of TBARS, SOD and CAT in control and treated rats.
| Groups | TBARS |
SOD |
CAT |
|||
|---|---|---|---|---|---|---|
| Circulation& | Colon% | Circulation* | Colon^ | Circulation$ | Colon# | |
| Control | 1.76 ± 0.05a | 0.62 ± 0.04d | 4.12 ± 0.07e | 4.51 ± 0.06e | 30.22 ± 0.85e | 41.13 ± 1.07e |
| DMH | 4.21 ± 0.07e | 0.44 ± 0.02a | 2.94 ± 0.05a | 2.74 ± 0.07a | 16.5 ± 0.73a | 31.26 ± 1.32a |
| Orientin | 1.81 ± 0.05a | 0.73 ± 0.04e | 4.18 ± 0.04e | 4.67 ± 0.08f | 31.82 ± 1.17f | 42.83 ± 1.14f |
| Initiation | 3.69 ± 0.07d | 0.51 ± 0.02b | 3.07 ± 0.07b | 3.08 ± 0.07b | 21.10 ± 0.96b | 33.84 ± 1.18b |
| Post initiation | 3.17 ± 0.06c | 0.54 ± 0.01b | 3.22 ± 0.06c | 3.57 ± 0.06c | 24.11 ± 0.85c | 36.60 ± 1.03c |
| Entire period | 2.77 ± 0.05b | 0.59 ± 0.02c | 3.57 ± 0.08d | 4.26 ± 0.08d | 26.97 ± 0.73d | 38.11 ± 1.28d |
Group 1 - control, Group 2 - DMH, Group 3 - Orientin, Group 4 - Initiation, Group 5 - Post initiation, Group 6 - Entire period. TBARS – Thiobarbituric acid reactive substances. SOD - Superoxide dismutase, CAT – Catalase. The values are presented as the mean ± SD of six rats per each group. a–f P < 0.05; values not sharing a common superscript letter are significantly different from the DMHtreated groups (analysis of variance followed by DMRT). &mmoles/mg protein. %mmoles/mg protein. * Enzyme required for 50% inhibition of NBT reduction/min/mg of Hb. ^ Enzyme required for 50% inhibition of NBT reduction/min/mg protein. $ mmol H2O2 utilized/min/mg of Hb. # μmol H2O2 utilized/min/mg protein.
Table 4.
Effect of orientin on circulatory and colonic level of GSH, GPx and GR in control and treated rats.
| Groups | GSH |
GPx |
GR |
|||
|---|---|---|---|---|---|---|
| Circulation* | Colon^ | Circulation# | Colon& | Circulationα | Colon$ | |
| Control | 3.67 ± 0.05d | 12.49 ± 0.12e | 25.13 ± 0.72d | 19.34 ± 0.43e | 3.67 ± 0.05e | 12.49 ± 0.12e |
| DMH | 2.09 ± 0.06a | 9.74 ± 0.11a | 19.63 ± 0.39a | 15.37 ± 0.21a | 2.09 ± 0.06a | 9.74 ± 0.11a |
| Orientin | 3.73 ± 0.06d | 12.57 ± 0.14e | 25.87 ± 0.90d | 19.65 ± 0.19f | 3.73 ± 0.06e | 12.57 ± 0.14e |
| Initiation | 2.33 ± 0.05b | 10.21 ± 0.11b | 21.18 ± 0.68b | 16.60 ± 0.25b | 2.33 ± 0.05b | 10.21 ± 0.11b |
| Post initiation | 2.58 ± 0.05b | 10.54 ± 0.10c | 21.86 ± 0.83b | 17.03 ± 0.26c | 2.58 ± 0.05c | 10.54 ± 0.10c |
| Entire period | 2.88 ± 0.03c | 11.46 ± 0.12d | 23.5 ± 0.90c | 17.81 ± 0.39d | 2.88 ± 0.04d | 11.46 ± 0.12d |
Group 1 - control, Group 2 - DMH, Group 3 - Orientin, Group 4 - Initiation, Group 5 - Post initiation, Group 6 - Entire period. GSH – Reduced glutathione, GPx – Glutathione peroxidase, GR- Glutathione reductase. The values are presented as the mean ± SD of six rats per each group. a–f P < 0.05; values not sharing a common superscript letter are significantly different from the DMH-treated groups (analysis of variance followed by DMRT). * mg/dL. ^μg/dL. # mmoles of GSH utilized/min/mg of Hb. & μmoles of GSH utilized/min/mg protein.α mmoles of NADPH oxidized/min/mg of Hb. $ μmoles of NADPH oxidized/min/mg protein.
3.4. Effect of orientin on phase I biotransformation enzymes
The levels of phase I enzymes (CYP450, CYP2E1 and cytochrome b5) in the hepatic and colonic mucosa of control and experimental animals are shown in Table 5. The activities of phase I enzymes were significantly elevated in group 2 rats induced with DMH alone as compared to the control. Xenobiotic phase I enzyme activities were significantly decreased in all carcinogenic phases (group 4–6) as compared with the DMH alone administered rats. The effect of orientin was more pronounced in group 6 (entire period). However, the orientin alone treated rats (group 3) did not show any significant change as compared to control rats (group 1).
Table 5.
Effect of DMH and orientin on phase I xenobiotic metabolizing enzymes.
| Phase I enzymes | Groups |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | DMH | Orientin | Initiation | Post initiation | Entire period | ||||
| Cytochrome P450* | |||||||||
| Colonic mucosa | 3.20 ± 0.19a | 7.70 ± 0.41e | 3.49 ± 0.23ab | 6.64 ± 0.24d | 4.45 ± 0.18c | 3.62 ± 0.15b | |||
| Liver | 4.40 ± 0.18a | 8.49 ± 0.53e | 4.45 ± 0.21a | 6.27 ± 0.32d | 5.59 ± 0.26c | 4.94 ± 0.22b | |||
| Cytochrome P2E1# | |||||||||
| Colonic mucosa | 1.14 ± 0.14a | 2.24 ± 0.17e | 1.25 ± 0.10ab | 1.84 ± 0.13d | 1.60 ± 0.18c | 1.37 ± 0.15b | |||
| Liver | 5.17 ± 0.42a | 9.56 ± 0.63d | 5.41 ± 0.28a | 8.27 ± 0.59c | 7.10 ± 0.52b | 6.62 ± 0.30b | |||
| Cytochrome b5* | |||||||||
| Colonic mucosa | 0.92 ± 0.11a | 2.80 ± 0.15d | 1.17 ± 0.11bc | 1.32 ± 0.11c | 1.19 ± 0.12bc | 1.15 ± 0.10b | |||
| Liver | 2.26 ± 0.17a | 4.35 ± 0.26e | 2.36 ± 0.17ab | 3.39 ± 0.12d | 2.98 ± 0.13c | 2.52 ± 0.10b | |||
Group 1 - control, Group 2 - DMH, Group 3 - Orientin, Group 4 - Initiation, Group 5 - Post initiation, Group 6 - Entire period. *μmol/mg protein, #mmol of p-nitrocatechol liberated/min/mg protein. Data are presented as the mean ± SD of six rats in each group. Values not sharing a common superscript letter (a–e) differ significantly at P < 0.05 (DMRT).
3.5. Effect of orientin on phase II biotransformation enzymes
The effect of orientin and DMH on phase II biotransformation enzymes such as DT-diaphorase (DTD) and glutathione S-transferase (GST) in the hepatic and colonic mucosa are shown in Table 6. Group 2 (DMH) rats exhibited significant reduction in DTD and GST activities as compared to the control. On contrast, orientin administration to DMH treated rats produced a significant increase in DTD and GST activities in different stages of carcinogenesis namely group 4 (initiation), group 5 (post-initiation) and group 6 (entire period) rats. The effect of orientin on the activities of DTD and GST was more pronounced in group 6 (entire period) rats. There is no significant difference in orientin alone treated rats (group 3) compared to control rats. Thus, DMH-treated rats administered with orientin reverse back the adverse changes of phase I and phase II enzymes in the colonic and hepatic tissues.
Table 6.
Effect of DMH and orientin on phase II xenobiotic metabolizing enzymes.
| Phase II enzymes | Groups |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | DMH | Orientin | Initiation | Post initiation | Entire period | ||||
| DT-Diaphorase* | |||||||||
| Colonic mucosa | 1.42 ± 0.11cd | 0.48 ± 0.05a | 1.51 ± 0.10d | 1.06 ± 0.12b | 1.13 ± 0.10b | 1.38 ± 0.13c | |||
| Liver | 2.06 ± 0.13d | 0.90 ± 0.03a | 2.03 ± 0.12d | 1.28 ± 0.11b | 1.63 ± 0.12c | 1.94 ± 0.16d | |||
| GST# | |||||||||
| Colonic mucosa | 3.64 ± 0.18c | 2.42 ± 0.14a | 3.87 ± 0.16d | 3.35 ± 0.14b | 3.39 ± 0.15b | 3.52 ± 0.14bc | |||
| Liver | 2.40 ± 0.16d | 1.35 ± 0.08a | 2.35 ± 0.15d | 1.72 ± 0.10b | 1.92 ± 0.13c | 2.23 ± 0.17d | |||
Group 1 - control, Group 2 - DMH, Group 3 - Orientin, Group 4 - Initiation, Group 5 - Post initiation, Group 6 - Entire period. *mmol of 2,6-dichlorophenol indophenol reduced/min/mg protein, #μmol of CDNB-GSH conjugate formed/min/mg protein. Data are presented as the mean ± SD of six rats in each group. Values not sharing a common superscript letter (a–d) differ significantly at P < 0.05 (DMRT). GST-Glutathione-S-transferase.
3.6. Effect of orientin on ACF formation
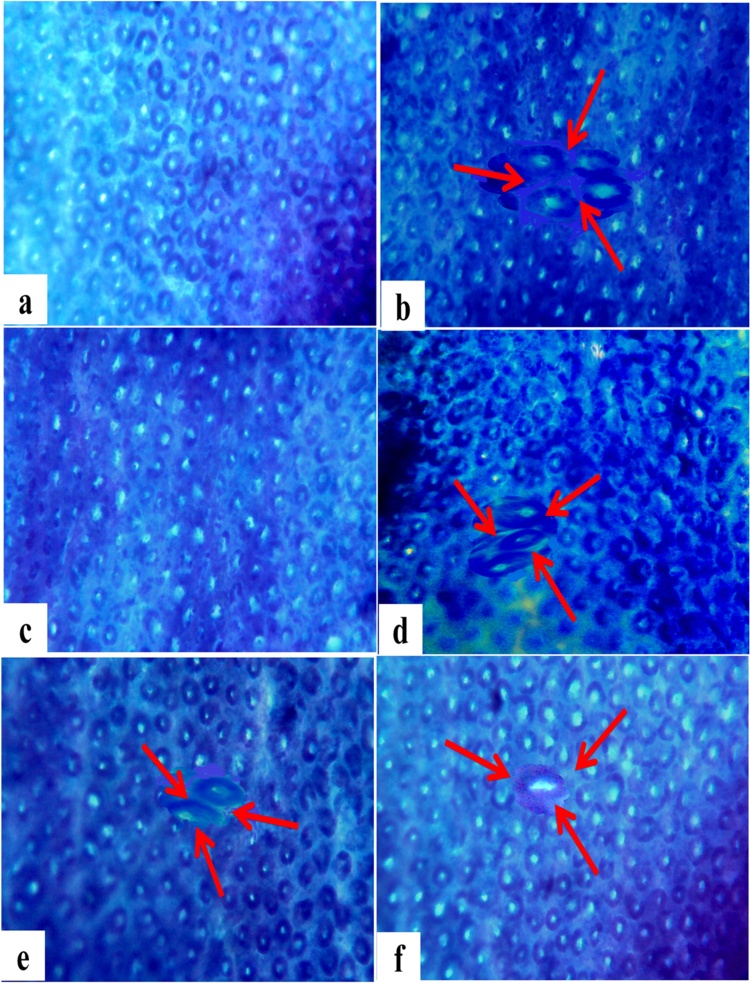
Methylene blue staining of colonic tissue sections (Fig. 4) showed the aberrant colonic crypt formation with distinct morphology, larger size and dense epithelial lining in high fat/DMH induced rats (Group II). Orientin administration to DMH induced rats (Group IV—VI) have shown less intense of methylene blue stain and mere frequency of crypt formation when compared with DMH induced rats (Group II). Normal colonic cellular morphology was observed in orientin alone treated rats similar to that of untreated control rats (Group I). The above histological observations revealed that orientin treatment for entire period significantly limits the occurrence of ACF in DMH induced experimental rats (Table 7).
Fig. 4.
Methylene blue staining of aberrant crypt foci (ACF) formation in the control and treated rats. (x40). (a) Topographical view of normal crypts in control rats. (c) Topographical view of ACF (arrows) with multiple crypts in the colon from rats exposed with 1, 2-dimethylhydrazine (DMH) carcinogen. (c) Topographical view of normal crypts from rats supplemented with orientin alone. (d) Topographical view of ACF (arrow) with 2 and 3 crypts in the colon from rats induced with DMH and treated with orientin (initiation). (E) Topographical view of ACF (arrow) with 2 crypts in the colon from rats under post initiation treatment with orientin (F) Topographical view of ACF (arrow) with no crypt in the colon from rats induced with DMH and treated with orientin for entire period.
Table 7.
Effect of orientin on the incidence of ACF in the colon of control and experimental rats.
| Groups | Number of rats bearing polyps | Number of ACF/colon | Number of aberrant crypt/colon | Number of aberrant crypt/focus |
|---|---|---|---|---|
| Control | 0/6 | 0 | 0 | 0 |
| DMH | 6/6 | 105.33 ± 7.17d | 232.17 ± 14.45d | 2.51 ± 0.18d |
| Orientin | 0/6 | 0 | 0 | 0 |
| Initiation | 6/6 | 79.83 ± 5.84c | 159.83 ± 11.27c | 2.32 ± 0.16c |
| Post initiation | 6/6 | 58.5 ± 5.31b | 95.5 ± 7.39b | 1.45 ± 0.12b |
| Entire period | 6/6 | 36.83 ± 4.26a | 65.0 ± 4.86a | 1.02 ± 0.10a |
Group 1 - control, Group 2 - DMH, Group 3 - Orientin, Group 4 - Initiation, Group 5 - Post initiation, Group 6 - Entire period. Data are presented as the mean ± SD of six rats in each group. Values not sharing a common superscript letter (a–d) differ significantly at P < 0.05 (DMRT). ACF- Aberrant crypt foci.
3.7. Effect of orientin on cell proliferation
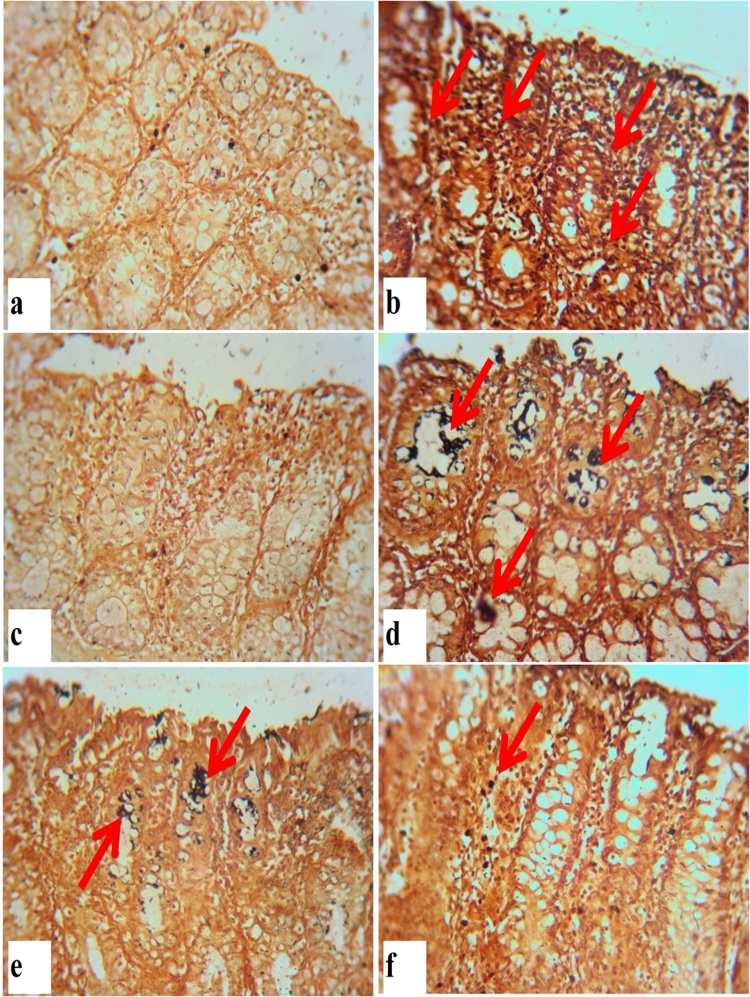
High fat/DMH alone induced Group II rats showed a significant increase in the number of AgNORs/nuclei, however, no such number of AgNORs has been observed in orientin treated groups (Group IV–VI) as shown in Fig. 5. Orientin treated groups showed significant decrease in the number of AgNORs/nuclei when compared to DMH induced rats (Group II) which makes evident that orientin inhibits cell proliferation as shown in Table 8. Group III rats treated with orientin alone showed a similar pattern with that of control rats (Group I).
Fig. 5.
Silver staining of the nucleolar organizer regions in the colonic epithelium of control and treated rats. (x40) (a) Control rats without AgNOR positive nuclei. (b) Increased number and total area of AgNOR dots/nucleus observed in colonic epithelium of DMH induced rats. (c) No such positive nuclei observed in orientin alone treated rats. (d–f) Decrease in the number and total area of AgNOR dots/nucleus in colonic epithelium of orientin treatment under initiation, post initiation and entire period respectively. Arrows represent AgNOR positive cells.
Table 8.
Effect of orientin on the incidence of AgNORs in crypt per nuclei.
| Groups | Number of rats | Number of crypts observed | Mean cells/crypt column | Number of AgNORs/nucleus |
|---|---|---|---|---|
| Control | 6 | 25 | 25.1 ± 3.56 | 1.27 ± 0.11a |
| DMH | 6 | 25 | 28.6 ± 3.46 | 1.63 ± 0.14c |
| Orientin | 6 | 25 | 25.5 ± 3.17 | 1.32 ± 0.11ab |
| Initiation | 6 | 25 | 27.8 ± 3.37 | 1.58 ± 0.13c |
| Post initiation | 6 | 25 | 27.0 ± 3.13 | 1.48 ± 0.11bc |
| Entire period | 6 | 25 | 26.3 ± 2.81 | 1.39 ± 0.18ab |
Group 1 - control, Group 2 - DMH, Group 3 - Orientin, Group 4 - Initiation, Group 5 - Post initiation, Group 6 - Entire period. Data are presented as the mean ± SD of six rats in each group. Values not sharing a common superscript letter (a–d) differ significantly at P < 0.05 (DMRT).
4. Discussion
Colorectal cancer is an intricate process which involves initiation, promotion, progression, and invasive stages of malignant transformation from a normal crypt to an adenoma [39]. The deleterious side effects of clinically approved anticancer agents augment cancer mortality and morbidity and emphasize an urgent need for novel and safer chemotherapeutic agents [40]. Naturally occurring compounds are known to exhibit diverse inhibitory effects in several aberrant signaling pathways took part in all phases of carcinogenesis. Moreover, they are readily available, inexpensive and usually non-toxic agents with both cancer preventive and therapeutic activities [41]. Several retrospective epidemiological studies suggest that some flavonoids that prevent colon cancer enhance the efficacy of chemotherapeutics by modifying the activity of cell proliferation and survival pathways involved in colon cancer [42]. In this regard, the present study was performed to evaluate the chemopreventive efficacy of orientin, a c-glycosyl flavanoid in DMH induced/ high fat diet promoted rat colon carcinogenesis. Animals were fed with high fat diet, which is expected to postulate the number of aberrant crypt foci formation in DMH induced colon carcinogenesis. The increased number of preneoplastic neoplasm formation may be attributed to the metabolic dysfunction and prominent cell proliferation.
Earlier research findings suggest that the body weight and growth rate determines the degree of carcinogenicity in experimental rats induced with carcinogens [7]. The body weight and growth rate were observed to be decreased in DMH alone induced rats when compared with control rats. This may be due to higher tumor burden, appetite loss along with an increase in polyps driven cachexia and anorexia [43]. Treatment with orientin exhibited a significant increase in body weight regardless of the DMH induced metabolic transformations due to their ability to restore the cellular metabolic dysfunctions. Our results were reminiscent of the earlier findings in which plant metabolites have significantly improved body weight by curtailing down the carcinogen induced tumor burden and decreasing the number of polyps in the intestine [44].
The lipid peroxidation and antioxidant status proposed to be useful indicators for determining the risk of oxidative damage induced carcinogenesis [20,10]. In our study, the prominent increase of circulatory TBARS could be due to too much ROS generation and disintegration of cell membranes advancing to epithelial cell transformation [45]. Tumors obtain favorable characteristics and proliferate more rapidly when the lipid peroxidation is lower as observed in DMH exposed rats [46] which mimics earlier reports [43,28,31]. The decreased colonic TBARS could be due to prominent resistance and/or diminished susceptibility of malignant cells to the scavenging defense of ROS.
The natural antioxidants (SOD, CAT, GPx, GR and GSH) scavenge the free radicals and protect cells against oxidative stress. The primary endogenous antioxidants SOD and CAT directly eliminate the free radicals; GPx detoxifies H2O2 and thereby these enzymes playing a noteworthy role in the counteraction of ROS. The non- protein thiol, GSH and dependent enzymes engage in innate antioxidant defense mechanisms. The decreased level of tissue antioxidants in DMH exposed rats could be due to its increased utilization in the detoxification of toxic DMH metabolites by tumor cells. Meanwhile, orientin reinstates the level of antioxidants to exert their scavenging mechanisms and thereby exhibiting their inhibitory role against colorectal tumorigenesis. The free hydroxyl groups in orientin, makes it an effective antioxidant against DMH induced ROS generation.
The subcutaneous administration of 1, 2 dimethyl hydrazine undergoes metabolic activation in the liver upon dehydrogenation to form the metabolic intermediates such as azoxymethane (AOM) and methylazoxymethanol (MAM) [4]. The conversion of carcinogens into DNA reactive metabolites involves the activation and detoxification of xenobiotics by phase I and phase II xenobiotic metabolizing enzymes [47]. Phase I enzymes activate the pro-carcinogen into active carcinogen via the introduction of polar functional groups by oxidation, reduction or hydrolysis whereas the phase II enzymes decreases the toxicity upon conjugation [48].
Cytochrome P450 enzymes, a family of monomeric haemoprotein are capable of instigating the conversion of lipophilic xenobiotics into more hydrophilic metabolites thereby activates pro-carcinogens to the eventual carcinogens [49]. Hence, the considerable increase in hepatic microsomal and colonic epithelial phase I enzymes (CYP450, CYP2E1 (an isoenzyme of CYP450) and cytochrome b) in high fat/DMH induced rats makes evident of carcinogen induced colorectal carcinogenesis. The reactive genotoxic intermediates produced by the metabolic activation of DMH by phase I enzymes covalently bind with DNA to form DNA adducts [50]. However, orientin administration confines the activation of Cytochrome P450 enzymes in corroboration with earlier findings suggesting that flavonoids inhibit CYP450 dependent reactions due to the presence of hydroxyl groups [49].
Phase II enzymes include DT-diaphorase and GST which entail the addition of polar groups to xenobiotics. DT-diaphorase is a flavoprotein containing phase II enzyme detoxifies the quinone and its derivatives to exert protection against carcinogen induced oxidative stress and neoplasia [51]. Glutathione-S-transferase (GST) is another versatile enzyme which detoxifies hydroquinones and neutralizes electrophilic intermediates that initiate carcinogenesis [50]. The increased levels of phase II enzymes are beneficial for chemo protection against carcinogens. Our experimental findings showed significant decrease in the phase II enzyme activities in the liver and colon of DMH induced rats, which could be due to utilization of more detoxifying enzymes to counteract DMH induced neoplastic transformation. Orientin administration to high fat/DMH induced rats increased the level of phase II enzymes in order to detoxify the carcinogens. Thus orientin diminishes the formation of the active DMH metabolites by inhibiting the activity of the DMH metabolizing cytochrome P450 enzymes and elevating the activity of the phase II enzymes, GST and DTD which facilitate possible detoxification and excretion of the carcinogen from the colonic lumen. Orientin exhibits dual action by inhibiting the metabolic activation of DMH and inducing the activities of phase II enzymes to detoxify and excrete carcinogen.
Aberrant crypt foci are putative preneoplastic lesions within the colonic neoplasia considered as surrogate precursor lesions of colorectal cancer distinguished from the normal crypts by their elliptical shape, enlarged size, thickness and intensely stained epithelial cells [52,53]. The aberrant crypt with increased multiplicity (>4 crypts/focus) and the number of ACF correlate with the incidence of colorectal cancer [63]. The carcinogen induced rats fed with high fat diet shows high incidence of ACF and tumor incidence [54]. Earlier evidences suggest that bioactive compounds which inhibit ACF could also promote the anti carcinogenicity of carcinogen induced colorectal cancer models [10,55]. The appearance of ACF in high fat/DMH induced rats indicates the initiation of colorectal carcinogenesis and the increase in number and crypt multiplicity corresponds to the promotion and progression of carcinogenesis. Our results also suggest that orientin inhibits the progression of colonic ACF and suppress the transformation of preneoplasia to malignant neoplasia. Administration of orientin to high fat/DMH treated rats evidently reduced the ACF formation and its multiplicity. The protective activity against ACF development may be attributed to the antioxidant potential of orientin towards the chemical carcinogen induced colon carcinogenesis. Another plausible mechanism could be the inhibitory effect of orientin on the metabolic activation of xenobiotic enzymes.
Cell proliferation is one of the vital mechanisms to maintain integrity of intestinal mucosa and the dysregulation of tumor cell proliferation often results in hyperplasia and tumorigenesis [56]. The Nucleolar Organizer Regions are the nucleolus associated chromosomal region located on the short arms of metaphase acrocentric chromosomes. The silver stained acidic proteins in NORs act as diagnostic and prognostic markers for nucleolar activity and cell proliferation [57]. A positive correlation exists between the increased cell proliferation rate and the higher incidence of AgNORs/nucleus in cancerous cells [55]. The total number of AgNORs/nucleus has been used as a marker for determination of cancer progression and developmental stages. The visualized black dots may correspond to the phase of cell activation and thus used as markers for cell proliferation [41]. In our study, the total number of AgNORs /nucleus of the colonic epithelium was higher in high fat/DMH-induced colon cancer bearing rats, which is in line with earlier studies [38,55]. Reduced AgNORs count/nucleus in orientin treated rats suggest that the decrease in the number of AgNORs/nucleus may be due to culminating effect of orientin on cell proliferation. Corroborating the results of this study, umbelliferone was previously demonstrated to reduce the number of AgNORs/nucleus in enterocytes of rats exposed to DMH [38,58]. These results could be attributed to the antiproliferative effect of orientin.
5. Conclusion
The experimental findings of our study indicate that administration of orientin effectively regulates the antioxidant defense and intervenes in the metabolic activation of DMH by inhibition of phase I biotransformation enzymes and cause elevation in phase II enzymes which augments the detoxification and excretion of carcinogen from the lumen. Orientin prevents the transformation of preneoplastic lesions into malignant neoplasm, inhibits the colonic cell proliferation, and reduces the pathological alterations in high fat/DMH exposed rats. Among the three different treatment phases, the orientin treatment for the entire period showed significant effect on DMH- induced colorectal cancer. The overall experimental findings suggest that bioactive rooibos tea flavonoid, orientin holds promising potential against toxic DMH owing their antioxidant, anti inflammatory and anti proliferative properties.
Conflict of interest
None.
Acknowledgement
The first author immensely acknowledges the financial assistance under University Research Fellowship, Periyar University, Salem, Tamil Nadu, India.
References
- 1.Favoriti P., Carbone G., Greco M., Pirozzi F., Pirozzi R.E.M., Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 2.Goss P.E., Strasser-Weippl K., Lee-Bychkovsky B.L., Fan L., Li J., Chavarri-Guerra Y., Liedke P.E., Pramesh C.S., Badovinac-Crnjevic T., Sheikine Y., Chen Z. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15:489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 3.Karthi N., Kalaiyarasu T., Kandakumar S., Mariyappan P., Manju V. Pelargonidin induces apoptosis and cell cycle arrest via a mitochondria mediated intrinsic apoptotic pathway in HT29 cells. RSC Adv. 2016;6:45064–45076. [Google Scholar]
- 4.Manju V., Nalini N. Effect of ginger on lipid peroxidation and antioxidant status in 1, 2-dimethyl hydrazine induced experimental colon carcinogenesis. J. Biochem. Technol. 2010;2:161–167. [Google Scholar]
- 5.Aranganathan S., Nalini N. Antiproliferative efficacy of hesperetin (Citrus flavanoid) in 1, 2-Dimethylhydrazine-induced colon cancer. Phytother. Res. 2013;27:999–1005. doi: 10.1002/ptr.4826. [DOI] [PubMed] [Google Scholar]
- 6.Pozharisski K.M. The significance of nonspecific injury for colon carcinogenesis in rats. Cancer Res. 1975;35:3824–3830. [PubMed] [Google Scholar]
- 7.Manju V., Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1, 2 dimethylhydrazine-induced colon cancer. Clin. Chim. Acta. 2005;358:60–67. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Sreedharan V., Venkatachalam K.K., Namasivayam N. Effect of morin on tissue lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Invest. New Drugs. 2009;27:21–30. doi: 10.1007/s10637-008-9136-1. [DOI] [PubMed] [Google Scholar]
- 9.Nirmala P., Ramanathan M. Effect of myricetin on 1, 2 dimethylhydrazine induced rat colon carcinogenesis. J. Exp. Ther. Oncol. 2011;9:101–108. [PubMed] [Google Scholar]
- 10.Muthu R., Thangavel P., Selvaraj N., Ramalingam R., Vaiyapuri M. Synergistic and individual effects of umbelliferone with 5-flurouracil on the status of lipid peroxidation and antioxidant defense against 1, 2-dimethylhydrazine induced rat colon carcinogenesis. Biomed. Prev. Nutr. 2013;3:74–82. [Google Scholar]
- 11.Kilari B.P., Kotakadi V.S., Penchalaneni J. Anti-proliferative and apoptotic effects of Basella rubra (L.) against 1, 2-Dimethyl hydrazine-induced colon carcinogenesis in rats. Asian Pacific J. Cancer Prev. 2016;17:73–80. doi: 10.7314/apjcp.2016.17.1.73. [DOI] [PubMed] [Google Scholar]
- 12.Ansil P.N., Prabha S.P., Nitha A., Latha M.S. Chemopreventive effect of Amorphophallus campanulatus (Roxb.) blume tuber against aberrant crypt foci and cell proliferation in 1, 2-dimethylhydrazine induced colon carcinogenesis. Asian Pacific J. Cancer Prev. 2013;14:5331–5339. doi: 10.7314/apjcp.2013.14.9.5331. [DOI] [PubMed] [Google Scholar]
- 13.Lasko C.M., Bird R.P. Modulation of aberrant crypt foci by dietary fat and caloric restriction: the effects of delayed intervention. Cancer Epidemiol. Biomark. Prev. 1995;4:49–55. [PubMed] [Google Scholar]
- 14.Zhu Q.C., Gao R.Y., Wu W., Guo B.M., Peng J.Y., Qin H.L. Effect of a high-fat diet in development of colonic adenoma in an animal model. World J. Gastroenterol. 2014;20:8119–8129. doi: 10.3748/wjg.v20.i25.8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi G., Zeng S., Takashima T., Nozoe K., Shobayashi M., Kakugawa K., Murakami K., Jikihara H., Zhou L., Shimamoto F. Inhibitory effect of various breads on DMH-induced aberrant crypt foci and colorectal tumours in rats. Biomed Res. Int. 2015;2015:1–8. doi: 10.1155/2015/829096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Megaraj V., Ding X., Fang C., Kovalchuk N., Zhu Y., Zhang Q.Y. Role of hepatic and intestinal p450 enzymes in the metabolic activation of the colon carcinogen azoxymethane in mice. Chem. Res. Toxicol. 2014;27:656–662. doi: 10.1021/tx4004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senapathy J.G., Jayanthi S., Viswanathan P., Umadevi P., Nalini N. Effect of gallic acid on xenobiotic metabolizing enzymes in 1, 2-dimethyl hydrazine induced colon carcinogenesis in Wistar rats–A chemopreventive approach. Food Chem. Toxicol. 2011;49:887–892. doi: 10.1016/j.fct.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Sheweita S.A., Tilmisany A.K. Cancer and phase II drug-metabolizing enzymes. Curr. Drug Metab. 2003;4:45–58. doi: 10.2174/1389200033336919. [DOI] [PubMed] [Google Scholar]
- 19.Karthikkumar V., Sivagami G., Viswanathan P., Nalini N. Rosmarinic acid inhibits DMH-induced cell proliferation in experimental rats. J. Basic Clin. Physiol. Pharmacol. 2015;26:185–200. doi: 10.1515/jbcpp-2014-0044. [DOI] [PubMed] [Google Scholar]
- 20.Thangaraj K., Vaiyapuri M. Orientin, a C-glycosyl dietary flavone, suppresses colonic cell proliferation and mitigates NF-κB mediated inflammatory response in 1, 2-dimethylhydrazine induced colorectal carcinogenesis. Biomed. Pharmacother. 2017;96:1253–1266. doi: 10.1016/j.biopha.2017.11.088. [DOI] [PubMed] [Google Scholar]
- 21.Thangaraj K., Arumugasamy K., Natesan K., Ramasamy S., Cyril R., Singh S.K., Vaiyapuri M. In Silico molecular docking analysis of Orientin, a potent glycoside of luteolin against BCL-2 family proteins. J. Chem. Pharm. Res. 2017;9:65–72. [Google Scholar]
- 22.Lam K.Y., Ling A.P., Koh R.Y., Wong Y.P., Say Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016;2016:1–9. doi: 10.1155/2016/4104595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku S.K., Kwak S., Bae J.S. Orientin inhibits high glucose-induced vascular inflammation in vitro and in vivo. Inflammation. 2014;37:2164–2173. doi: 10.1007/s10753-014-9950-x. [DOI] [PubMed] [Google Scholar]
- 24.Law B.N., Ling A.P., Koh R.Y., Chye S.M., Wong Y.P. Neuroprotective effects of orientin on hydrogen peroxide-induced apoptosis in SHSY5Y cells. Mol. Med. Rep. 2014;9:947–954. doi: 10.3892/mmr.2013.1878. [DOI] [PubMed] [Google Scholar]
- 25.Sun A., Ren G., Deng C., Zhang J., Luo X., Wu X., Mani S., Dou W., Wang Z. C-glycosyl flavonoid orientin improves chemically induced inflammatory bowel disease in mice. J. Funct. Foods. 2016;21:418–430. [Google Scholar]
- 26.An F., Wang S., Tian Q., Zhu D. Effects of orientin and vitexin from Trollius chinensis on the growth and apoptosis of esophageal cancer EC-109 cells. Oncol. Lett. 2015;10:2627–2633. doi: 10.3892/ol.2015.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czemplik M., Mierziak J., Szopa J., Kulma A. Flavonoid C-glucosides derived from Flax Straw extracts reduce Human Breast Cancer cell growth in vitro and induce apoptosis. Front. Pharmacol. 2016;7:282–295. doi: 10.3389/fphar.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinothkumar R., Vinothkumar R., Sudha M., Nalini N. Chemopreventive effect of zingerone against colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Eur. J. Cancer Prev. 2014;23:361–371. doi: 10.1097/CEJ.0b013e32836473ac. [DOI] [PubMed] [Google Scholar]
- 29.Siddique A.I., Mani V., Arivalagan S., Thomas N.S., Namasivayam N. Asiatic acid attenuates pre-neoplastic lesions, oxidative stress, biotransforming enzymes and histopathological alterations in 1, 2-dimethylhydrazine-induced experimental rat colon carcinogenesis. Toxicol. Mech. Methods. 2017;27:136–150. doi: 10.1080/15376516.2016.1273422. [DOI] [PubMed] [Google Scholar]
- 30.Thangaraj K., Natesan K., Settu K., Palani M., Govindarasu M., Subborayan V., Vaiyapuri M. Orientin mitigates 1, 2-dimethylhydrazine induced lipid peroxidation, antioxidant and biotransforming bacterial enzyme alterations in experimental rats. J. Cancer Res. Ther. 2018 doi: 10.4103/jcrt.JCRT_1363_16. (In press) [DOI] [PubMed] [Google Scholar]
- 31.Giftson J.S., Jayanthi S., Nalini N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1, 2-dimethyl hydrazine induced rat colon carcinogenesis. Invest. New Drugs. 2010;28:251–259. doi: 10.1007/s10637-009-9241-9. [DOI] [PubMed] [Google Scholar]
- 32.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 33.Omura T. The carbon monoxide-binding pigment of liver microsomes. J. Biol. Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 34.Watt K.C., Plopper C.G., Buckpitt A.R. Measurement of cytochrome P450 2E1 activity in rat tracheobronchial airways using high-performance liquid chromatography with electrochemical detection. Ann. Clin. Biochem. 1997;248:26–30. doi: 10.1006/abio.1997.2109. [DOI] [PubMed] [Google Scholar]
- 35.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 36.Ernster L. DT diaphorase. Meth. Enzymol. 1967;10:309–317. [Google Scholar]
- 37.Ochiai M., Watanabe M., Nakanishi M., Taguchi A., Sugimura T., Nakagama H. Differential staining of dysplastic aberrant crypt foci in the colon facilitates prediction of carcinogenic potentials of chemicals in rats. Cancer Lett. 2005;220:67–74. doi: 10.1016/j.canlet.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Muthu R., Selvaraj N., Vaiyapuri M. Anti-inflammatory and proapoptotic effects of umbelliferone in colon carcinogenesis. Hum. Exp. Toxicol. 2016;10:1041–1054. doi: 10.1177/0960327115618245. [DOI] [PubMed] [Google Scholar]
- 39.Prasad V., Lenzer J., Newman D.H. Why cancer screening has never been shown to" save lives"-and what we can do about it. British Medical Journal (Online) 2016;6:352–355. doi: 10.1136/bmj.h6080. [DOI] [PubMed] [Google Scholar]
- 40.Youns M., Hegazy W.A. The natural flavonoid Fisetin inhibits cellular proliferation of hepatic, colorectal, and pancreatic cancer cells through modulation of multiple signaling pathways. PLoS One. 2017;12:1–18. doi: 10.1371/journal.pone.0169335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arivalagan S., Thomas N.S., Chandrasekaran B., Mani V., Siddique A.I., Kuppsamy T., Namasivayam N. Combined therapeutic efficacy of carvacrol and X-radiation against 1, 2-dimethyl hydrazine-induced experimental rat colon carcinogenesis. Mol. Cell. Biochem. 2015;410:37–54. doi: 10.1007/s11010-015-2536-6. [DOI] [PubMed] [Google Scholar]
- 42.Zamora-Ros R., Guino E., Alonso M.H., Vidal C., Barenys M., Soriano A., Moreno V. Dietary flavonoids, lignans and colorectal cancer prognosis. Sci. Rep. 2015;5:1–5. doi: 10.1038/srep14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinothkumar R., Kumar R.V., Sudha M., Viswanathan P., Balasubramanian T., Nalini N. Modulatory effect of troxerutin on biotransforming enzymes and preneoplastic lesions induced by 1, 2-dimethylhydrazine in rat colon carcinogenesis. Exp. Mol. Pathol. 2014;96:15–26. doi: 10.1016/j.yexmp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Selvam J.P., Aranganathan S., Gopalan R., Nalini N. Chemopreventive efficacy of pronyl‐lysine on lipid peroxidation and antioxidant status in rat colon carcinogenesis. Fundam. Clin. Pharmacol. 2009;23:293–302. doi: 10.1111/j.1472-8206.2009.00670.x. [DOI] [PubMed] [Google Scholar]
- 45.Perse M. Oxidative stress in the pathogenesis of colorectal cancer: cause or consequence? Biomed Res. Int. 2013;2013:1–9. doi: 10.1155/2013/725710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghadi F.E., Ghara A.R., Bhattacharyya S., Dhawan D.K. Selenium as a chemopreventive agent in experimentally induced colon carcinogenesis. World J Gastrointestinal Oncology. 2009;1:74–81. doi: 10.4251/wjgo.v1.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padmavathi R., Senthilnathan P., Sakthisekaran D. Therapeutic effect of propolis and paclitaxel on hepatic phase I and II enzymes and marker enzymes in dimethylbenz (a) anthracene-induced breast cancer in female rats. Comp. Biochem. Physiol. - Part C: Toxicol. Pharmacol. 2006;143:349–354. doi: 10.1016/j.cbpc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Beyerle J., Frei E., Stiborova M., Habermann N., Ulrich C.M. Biotransformation of xenobiotics in the human colon and rectum and its association with colorectal cancer. Drug Metab. Rev. 2015;47:199–221. doi: 10.3109/03602532.2014.996649. [DOI] [PubMed] [Google Scholar]
- 49.Sangeetha N., Viswanathan P., Balasubramanian T., Nalini N. Colon cancer chemopreventive efficacy of silibinin through perturbation of xenobiotic metabolizing enzymes in experimental rats. Eur. J. Pharmacol. 2012;674:430–438. doi: 10.1016/j.ejphar.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Balaji C., Muthukumaran J., Nalini N. Chemopreventive effect of sinapic acid on 1, 2-dimethylhydrazine-induced experimental rat colon carcinogenesis. Hum. Exp. Toxicol. 2014;33:1253–1268. doi: 10.1177/0960327114522501. [DOI] [PubMed] [Google Scholar]
- 51.Mohan K.V., Kumaraguruparan R., Prathiba D., Nagini S. Modulation of xenobiotic-metabolizing enzymes and redox status during chemoprevention of hamster buccal carcinogenesis by bovine lactoferrin. Nutrition. 2006;22:940–946. doi: 10.1016/j.nut.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 52.Bird R.P., Good C.K. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol. Lett. 2000;112:395–402. doi: 10.1016/s0378-4274(99)00261-1. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues M.A., Silva L.A., Salvadori D.M., De Camargo J.L., Montenegro M.R. Aberrant crypt foci and colon cancer: comparison between a short-and medium-term bioassay for colon carcinogenesis using dimethylhydrazine in Wistar rats. Braz. J. Med. Biol. Res. 2002;35:351–355. doi: 10.1590/s0100-879x2002000300010. [DOI] [PubMed] [Google Scholar]
- 54.Baskar A.A., Ignacimuthu S., Michael G.P., Al Numair K.S. Cancer chemopreventive potential of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos Linn. Nutr. Cancer. 2011;63:130–138. doi: 10.1080/01635581.2010.516869. [DOI] [PubMed] [Google Scholar]
- 55.Sengottuvelan M., Viswanathan P., Nalini N. Chemopreventive effect of trans-resveratrol-a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1, 2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis. 2006;27:1038–1046. doi: 10.1093/carcin/bgi286. [DOI] [PubMed] [Google Scholar]
- 56.Lee S.J., Yun C.C. Colorectal cancer cells–proliferation, survival and invasion by lysophosphatidic acid. Int. J. Biochem. Cell Biol. 2010;42:1907–1910. doi: 10.1016/j.biocel.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gundog M., Yildiz O.G., Imamoglu N., Aslan D., Aytekin A., Soyuer I., Soyuer S. Prognostic significance of two dimensional AgNOR evaluation in local advanced rectal cancer treated with chemoradiotherapy. Asian Pacific J. Cancer Prev. 2015;16:8155–8161. doi: 10.7314/apjcp.2015.16.18.8155. [DOI] [PubMed] [Google Scholar]
- 58.Mariyappan P., Kalaiyarasu T., Manju V. Effect of eriodictyol on preneoplastic lesions, oxidative stress and bacterial enzymes in 1, 2-dimethyl hydrazine-induced colon carcinogenesis. Toxicol. Res. 2017;6:678–692. doi: 10.1039/c7tx00074j. [DOI] [PMC free article] [PubMed] [Google Scholar]