Abstract
Introduction
This study aims to evaluate the conceptual relevance of four measures of disease activity in patients with mild/mild-moderate Alzheimer's disease (AD): (1) the Alzheimer's Disease Assessment Scale–Cognitive Subscale; (2) the Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory; (3) the Neuropsychiatry Inventory; and (4) the Dependence Scale.
Methods
A conceptual model depicting patient experience of mild AD was developed via literature review; concepts were compared with the items of the four measures. Relevance of the concepts included in the four measures was evaluated by patients with mild AD in a survey and follow-up interviews.
Results
The four measures assessed few of the symptoms/impacts of mild AD identified within the literature. Measured items addressing emotional impacts were deemed most relevant by participants but were included in the measures only superficially.
Discussion
The four assessment measures do not appear to capture the concepts most relevant to/important to patients with mild/mild-moderate AD.
Keywords: Mild/mild-moderate Alzheimer's disease, Outcome measures, Patient-reported outcome (PRO), Conceptual relevance, Quantitative survey, Qualitative interviews
Highlights
-
•
Many impacts associated with mild Alzheimer's disease were reported by patients.
-
•
The included outcome measures assessed did not provide high conceptual coverage.
-
•
These four measures appeared more suitable for more severe Alzheimer's disease.
-
•
Completion of patient reported outcomes should be possible in this patient population.
-
•
These may be currently used in addition to clinician- and observer-reported outcomes.
1. Introduction
Alzheimer's disease (AD) is a progressive, fatal neurodegenerative disease with no known cure. Although disease etiology is unclear, advancing age is the greatest known risk factor for disease development [1]; approximately 11% of the US population aged ≥65 years has AD, and this increases to nearly 32% in those aged ≥85 years [2]. It is estimated that >5 million individuals in the United States suffer from AD, which is the sixth leading cause of death in the nation [1]; prevalence of AD is increasing with aging populations in the Western Hemisphere, placing an ever-increasing burden on health-care systems [3].
Recent scientific advances in AD mean that the disease can now be thought of as a continuum, with both preclinical (asymptomatic but with evidence of AD pathology) and clinical (symptomatic) phases [4]. The clinical phase is normally associated with signs and symptoms associated with a decline in cognitive and functional abilities [5]. Such symptoms are distinguishable from typical age-related changes [6] and vary greatly depending on where patients are on the disease continuum. These symptoms can include the following: (1) memory loss that disrupts daily life; (2) poor judgment and decision-making; (3) difficulty having conversations; (4) misplacing items and being unable to find them; and (5) changes in mood and personality [5]. Individuals may present with one or more of these symptoms, with varying severity, which can have a huge impact on patients' and caregivers' health-related quality of life [3]. The Food and Drug Administration acknowledges these different stages of AD and that clinical endpoints and associated outcomes will differ for clinical trials, depending on where patients are on the disease continuum [7]. Importantly, more subtle changes observed in the earlier stages of AD may not be as obvious as the symptoms experienced further along the spectrum. The focus of this study was on patients who are within the early clinical phase of AD (mild-moderate).
There are a number of assessment measures used to assess disease severity in patients with AD; however, the sensitivity and suitability of some of these in mild-moderate AD may be limited [2], [8], [9]. Four measures commonly used in clinical trials, but rarely used in clinical practice, are the Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) [10], the Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory (ADCS-ADL) [11], the Neuropsychiatry Inventory (NPI) [12], and the Dependence Scale (DS) [13]. These measures are summarized in Table 1.
Table 1.
Summary of assessment measures used to assess disease activity in patients with AD
| Properties | Assessment measure |
|||
|---|---|---|---|---|
| ADAS-cog | ADCS-ADL | NPI | DS | |
| Aspect of disease activity evaluated | Cognitive impairment | Activities of daily living | Presence of psychopathology | Level of dependency (for daily living activities) |
| Type of assessment measure | Composite (clinician-reported and performance) | Observer-reported | Observer-reported | Observer-reported |
| Number of items | 11 | 23 | 12 | 13 |
| Administered by | Clinician | Caregiver | Caregiver | Someone who lives with patient |
| Recall/evaluation period | None | Previous 4 weeks | Typically 4 weeks of observation | N/S |
Abbreviations: ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale; ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory; DS, Dependence Scale; NPI, Neuropsychiatry Inventory; N/S, not stated.
The ADAS-Cog is a composite measure administered by clinicians, consisting of a clinician-reported assessment measure and a performance assessment measure [10]. The measure consists of four domains, which include memory, language, praxis, and orientation. Variations of the ADAS-Cog exist, each designed to be more sensitive to a different stage of disease. The version used here is the 11-item version designed for mild-to-moderate AD.
The ADCS-ADL is an observer-reported assessment measure typically administered by caregivers [11]. This 23-item measure is designed to evaluate patient's daily living and covers three domains, namely, physical functioning, mental functioning, and independence in self-care.
The NPI is an observer-reported measure used to determine the presence of psychopathology in patients with AD/other dementias [12]. There are 12 items subdivided into a behavioral domain (10 items) and a neurovegetative domain (2 items). The NPI is based on responses from a caregiver and assesses the frequency, severity, and distress experienced for each item.
The DS is a 13-item observer-reported measure adapted from a scale originally developed to assess dependency in community-dwelling older people [13]. The DS assesses patient level of dependency for various daily living activities (e.g., getting dressed).
Although these assessment measures are commonly used with patients who have mild/mild-moderate AD, there is little evidence to show they capture concepts deemed important to these patients [14]. This is compounded by the fact that none of the measures are patient reported. Although patients may have some decreased insight due to their disease, their opinions are important (in addition to clinicians/caregivers) to ensure that relevant concepts are being measured. Therefore, the aim of this study is to evaluate the conceptual relevance of the four outlined assessment measures in the assessment of mild/mild-moderate AD from the patient perspective. The results could help determine the suitability of using these measures in clinical trials involving patients with mild/mild-moderate AD.
2. Methods
2.1. Steering committee
The researchers worked with the Alzheimer's Association to assemble a steering committee to enhance the patient centricity and clinical relevance of the research. The committee comprised a patient representative with mild AD, an informal caregiver of a patient with mild AD, an advocate from the Alzheimer's Association, and a clinical expert with experience in memory problems and AD. The patient representative and informal caregiver were recruited through discussion with the Alzheimer's Association, who suggested suitable members after a full description of study requirements. The clinical expert was recruited based on their expertise with managing patients with AD (minimum of 10 years) and on their experience with outcomes research in this area.
2.2. Assessment of conceptual coverage
2.2.1. Development of a conceptual model for mild AD
A targeted electronic literature review was conducted to identify qualitative publications reporting the patient experience of mild AD. A detailed search strategy combining search terms for AD and qualitative research was developed to identify all relevant publications, (presented in full in the Supplementary Information [Table 5]). Titles and abstracts of publications from the searches were screened using specific inclusion criteria (Table 2). Studies with English-speaking populations and clear definitions of mild AD were included. Included studies were reviewed to extract relevant concepts using qualitative methods facilitated by ATLAS.ti, version 7, software. A conceptual model (CM) of the patient experience of mild AD was subsequently developed to illustrate the relationship(s) between the identified concepts. Concepts (things that were identified as being important) were distinguished as either a symptom (e.g., memory loss) or an impact (e.g., difficulty driving) and grouped into domains of related experiences. The model was reviewed, modified, and approved by the steering committee.
Table 2.
Eligibility criteria for study inclusion in the qualitative literature review
| Criteria | Include | Exclude |
|---|---|---|
| Population |
|
|
| Setting |
|
|
| Study design |
|
|
| Publication language |
|
|
| Year of publication |
|
|
Abbreviation: AD, Alzheimer's disease.
2.2.2. Analysis
Items from the four assessment measures were compared with the concepts identified from the qualitative literature review. Compatibility between the CM and assessment measures was evaluated to determine whether the measures included concepts considered important to patients, and whether any irrelevant concepts were included or not included.
2.3. Assessment of relevance
2.3.1. Patient survey
2.3.1.1. Survey design
A quantitative survey was designed to capture data on patient-reported disease severity and symptom botheration. Survey content was predominantly based on the most relevant concepts extracted from the four assessment measures, with additional concepts deemed to be of significant importance added by the steering committee. Survey development was an iterative process with several review stages and revisions involving the steering committee. Ethics approval was provided by the New England Independent Review Board for the study in December 2016 (#120161008).
The survey comprised 11 main questions that asked predominantly about their AD symptoms and medication. One of the survey questions asked patients to score a list of “bothersome” symptoms (n = 41) on a severity scale ranging from 1 to 5, where 1 was equal to “this does not bother me at all” and 5 was equal to “this bothers me greatly”. Concepts that patients had not experienced could be given a score of 0. In another question, patients were asked how important it is for them to be able to carry out a number of activities and to grade their perceived severity of their AD symptoms overall. There was a 7-day recall period for questions related to bothersome symptoms and most important activities. See Supplementary Information for the full survey.
2.3.1.2. Participant selection
One hundred participants were recruited across five clinical sites at three geographical locations in the United States. Patients aged ≥65 years on the day of survey completion with mild/mild-moderate AD (either confirmed via magnetic resonance imaging scan or suspected via clinical observation and/or measurement) were eligible to participate in the survey. A Mini–Mental State Examination was not administered/required as part of screening; however, if a patient had completed it in the past, a score of 18–25 was required. Participants required an understanding of their condition and sufficient cognitive, reading, and linguistic capabilities to complete a five-page pen-and-paper survey in American English, as determined by their clinician. Participants had to provide informed consent before study admission. Individuals were excluded if they were aged <65 years; had a significant psychiatric/physical comorbid condition that would prevent study participation (clinician determined); had a diagnosis of early onset AD; or had a diagnosis/symptoms suggestive of moderate/severe AD.
Patients completed the survey independently around March to April 2017 at their physician's clinical site. A review of the captured data was carried out to check for any abnormalities after the first five participants had completed the survey. The remaining 95 participants completed the survey once no data collection issues were identified.
2.3.1.3. Analysis
Results were analyzed using descriptive statistics in Microsoft Excel® according to a statistical analysis plan approved before survey implementation.
2.3.2. Patient interviews
2.3.2.1. Interview design
A semistructured patient interview guide was developed to follow up on questions asked in the survey to obtain additional information; all patients were asked the same core set of questions, which aimed to explore patients' feelings about their condition and to better understand their survey answers, e.g., “In the last 7 days, are there any activities or tasks that you have had trouble with that have bothered you?” As with the survey, interview guide development was an iterative process involving the steering committee; questions were developed through discussion among the team. Ethics approval was provided by the New England Independent Review Board in March 2017.
2.3.2.2. Participant selection
Fifteen interview participants were recruited from the group of individuals successfully completing the quantitative survey, thus eligibility criteria remained the same. However, patients were only asked to participate if they completed the survey with ease. Where possible, a range of patients with different demographics were recruited.
One-to-one phone interviews were conducted using the approved interview guide. Interviews were to last approximately 1 hour and took place between March and May 2017. All interviews were audio recorded with patient consent and transcribed verbatim.
2.3.2.3. Analysis
Transcripts were analyzed, according to the approved study protocol, using qualitative methods facilitated by ATLAS.ti, version 7, software [15]; transcripts were uploaded, and quotes from the interviews were sorted by domain using thematic analysis techniques to determine aspects of mild-moderate AD which were important to patients [16].
Results from the survey/interviews were compared against the concepts included in the assessment measures to determine whether the symptoms/impacts of mild-moderate AD reported as bothersome by patients were indeed measured.
3. Results
3.1. Assessment of conceptual coverage
3.1.1. Conceptual model of mild AD
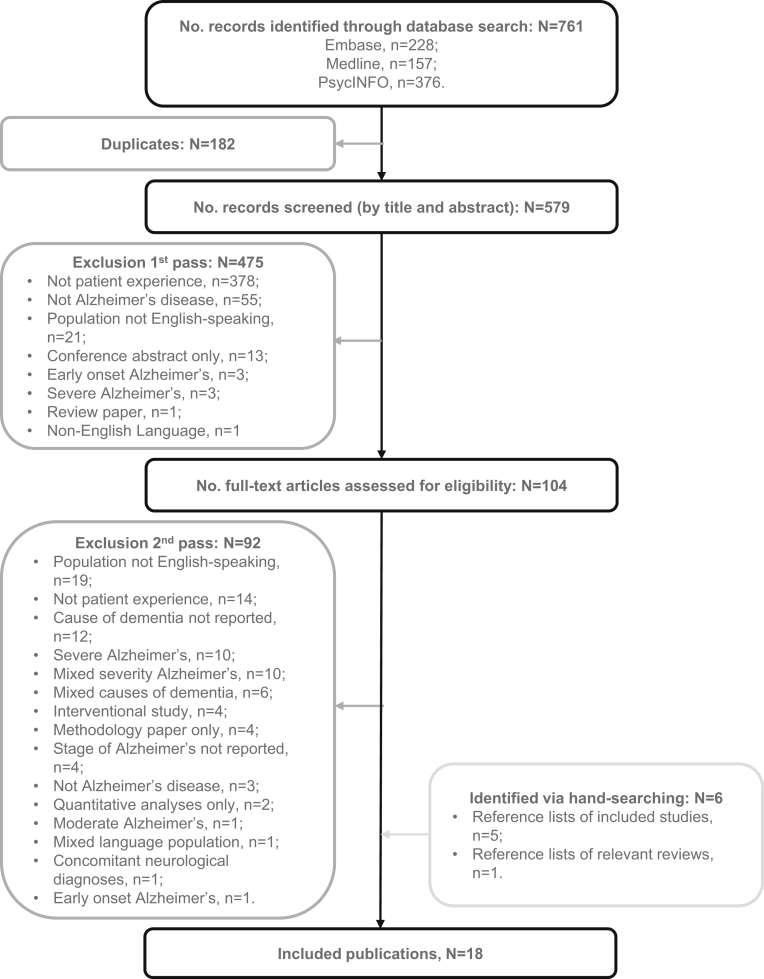
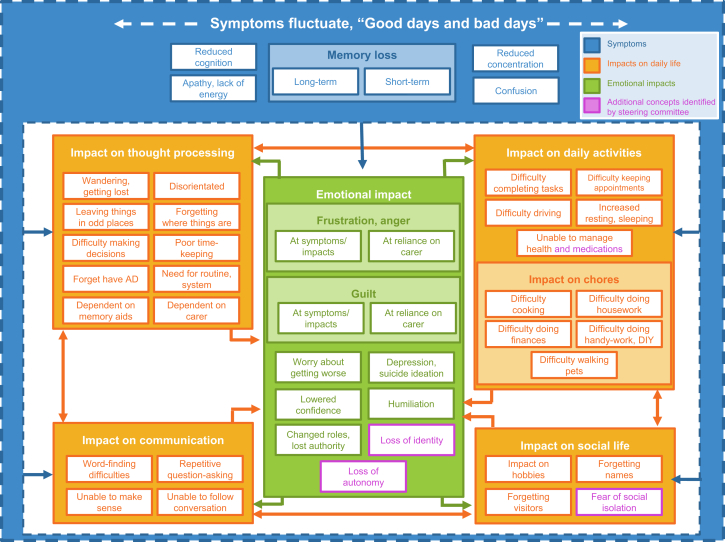
The literature review identified 18 articles (17 unique studies) that were relevant to the target patient population (Fig. 1). After qualitative analysis, 45 concepts were identified which described the patient experience of mild AD, comprising six symptoms experienced directly due to the disease (e.g., short-term memory loss) and 39 impacts experienced because of a disease-related symptom. Impacts were divided into five groups of related experiences (impact on thought processing, impact on daily activities, impact on communication, impact on social life, and emotional impact). An additional four impacts, not identified from the literature review, were proposed by the steering committee as being relevant to the patient population. These four concepts, in addition to those identified during the literature review, were combined into a CM (Fig. 2). The CM displays each concept as a visual representation of patients' symptom experience of mild AD and aims to show any potential relationships.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram for the qualitative literature review. Abbreviation: No., number.
Fig. 2.
Conceptual model visually representing the concepts identified by the literature review and steering committee as being most relevant to patients with mild AD.
3.1.2. Conceptual relevance of assessment measures
The concepts detailed in the CM were mapped to items in the four assessment measures (for compatibility).
The ADAS-Cog covered only two of the six symptoms identified in the CM (short-term memory loss and reduced cognition) and a limited number of impacts, mostly associated with communication. None of the other impact domains (e.g., impact on daily activities and emotional impact) were covered.
The ADCS-ADL covered most domains and concepts identified in the model, including “memory loss” and “reduced concentration” symptoms. However, three symptoms (apathy/lack of energy, reduced cognition, and confusion) were not covered. Impacts associated with thought processing, communication, and daily activities were well covered by the measure (albeit from a caregiver perspective). Emotional impact was not covered.
Out of the measures assessed, the NPI covered the fewest domains and concepts identified by the CM. Only one symptom (apathy) and one emotional impact (depression/suicide ideation) were covered, although it was the only measure to cover any of the emotional impacts.
In terms of overall conceptual coverage, the DS was the only measure that did not include an assessment of identified symptoms. Impacts considered important to patients, especially those associated with daily activities, thought processing, and social life, were covered, but no items covered emotional impacts. The DS contained several items not identified by the CM, including incontinence- and feeding-related impacts.
Twenty concepts identified in the CM were not included in any of the assessment measures (Supplementary Information, Appendix A–Table 6). Two symptoms not assessed by any measure were “reduced energy” and “confusion”. Impacts that were not included were generally emotional rather than physical, including feelings of frustration, anger, and guilt.
3.2. Assessment of relevance
3.2.1. Patient demographics and clinical characteristics
Clinical and demographic characteristics for the 100 survey participants are presented in Table 3. Patients' ages ranged from 65–96 years, (mean age of 77 years), were mostly Caucasian/White (71%) with some being Black/African-American (21%), and were mostly retired (81%) with the majority living with others including their partner or family (73%).
Table 3.
Demographics and clinical characteristics of survey and interview participants
| Characteristic | Survey participants, N = 100 (%) | Interview participants, N = 15 (%) |
|---|---|---|
| Age (years) | ||
| Mean (SD) [range] | 77 (±7) [65–96] | 76 [66–88] |
| Gender | ||
| Male | 49 (49) | 7 (47) |
| Female | 50 (50) | 8 (53) |
| Prefer not to say | 1 (1) | 0 |
| Ethnicity | ||
| Caucasian/White | 71 (71) | 11 (73) |
| Black/African American | 21 (21) | 3 (20) |
| Other/Mixed/Multiethnic | 4 (4) | 1 (7) |
| Hawaiian/Pacific Islander | 3 (3) | 0 |
| Hispanic | 1 (1) | 0 |
| Living situation | ||
| Living with spouse/partner | 58 (58) | 9 (60) |
| Living alone | 26 (26) | 3 (20) |
| Living with family | 15 (15) | 3 (20) |
| Living in sheltered accommodation | 1 (1) | 0 |
| Time since symptom onset | ||
| <6 months | 7 (7) | 2 (13) |
| 6 months–1 year | 14 (14) | 4 (27) |
| 1–2 years | 38 (38) | 3 (20) |
| 3–4 years | 18 (18) | 3 (20) |
| 5–10 years | 8 (8) | 3 (20) |
| >10 years | 10 (10) | 0 |
| Unknown | 5 (5) | 0 |
| Age at diagnosis | ||
| Known | 72 (72) | 10 (67) |
| Mean age in years (SD) [range] | 75 (±7) [63–94] | 74 [66–86] |
| Unknown | 17 (17) | 2 (13) |
| Suspected—no yet confirmed | 11 (11) | 3 (20) |
| Medication usage | ||
| Currently receiving medication | 46 (46) | 7 (47) |
| Mean months on medication (SD) [range] | 25 (±30) [0–153] | 38 [3–98] |
| Not currently receiving medication | 54 (54) | 8 (53) |
| Of which, previously received medication | 3 (6) | 1 (13) |
Abbreviation: SD, standard deviation.
AD symptoms had been experienced for varying lengths of time, from less than six months (7%) to over ten years (10%) with most having experienced symptoms for 1–2 years (38%). Most participants had received a diagnosis of mild AD (83%), a much smaller number had a diagnosis of mild-moderate or moderate disease (4%; 68%), which had been diagnosed using the Mini–Mental State Examination.
Just under half the patients surveyed (46%) were receiving medication to treat the symptoms of AD (mean duration on current medication of 25 months) (Table 3).
3.2.2. Survey and interview results
Patients were able to report their symptom severity and AD disease experience via the survey and, if suitable, through a subsequent interview, which provided patients with the opportunity to further discuss their feelings.
Each assessment measure concept was endorsed as experienced and bothersome by at least one patient. The ten most commonly experienced concepts are outlined in Table 4.
Table 4.
Ten most common concepts (symptoms or problems) experienced by survey participants and relevant quotes extracted from interviews
| Concept | Survey participants experiencing and bothered by symptom, N = 100 | Associated quotes from patient interviews |
|---|---|---|
| Remembering a list of items | 85% |
|
| Remembering names of people and common objects | 82% |
|
| Misplacing things | 80% |
|
| Not being able to find familiar objects | 78% |
|
| Worrying about my disease getting worse | 73% |
|
| Expressing myself verbally, that is, finding the right words and being understood | 71% |
|
| Confusion regarding date and time | 70% |
|
| Change in mood (e.g., being easily irritated in a way that I was not previously) | 69% |
|
| Feeling down or depressed (e.g., feeling sad or tearful) | 65% |
|
| Following instructions | 65% |
|
“Worrying about my disease getting worse,” a concept included by the steering committee and not included in any of the assessment measures, was identified as the most bothersome impact from both the survey (reported as the most bothersome impact by 23% patients) and interview and was experienced by 73% of survey participants. “Loss of identity”, also added by the steering committee; “remembering the names of people and common objects”, assessed by the DS, ADCS-ADL, and ADAS-cog; and “not being able to find familiar objects” assessed by the DS and ADCS-ADL were also among the most bothersome impacts experienced (experienced by 41%, 82%, and 78% of survey participants, respectively).
Patients were also asked to provide any additional symptoms and impacts that they found bothersome because of AD. Six participants described difficulty driving or getting lost while navigating, three described not being fully independent and their need to rely on others, and two mentioned poor organizational skills—these were concepts identified by the qualitative literature review and included in the CM.
Patients rated most activities as being important to do to remain independent. Consequently, it was difficult to determine which single activity was most important to patients; however, “the ability to maintain my health and safety” was reported by the highest number of participants (12%) and yet was not represented by any of the measures. Of the activities not captured within the survey, driving was reported as being important to maintain independence (n = 4). This was corroborated by the interviews, with several patients reporting how important driving was to them.
Overall, survey results appeared to reflect those that were raised in the interviews, that is, the most bothersome symptoms and impacts reported in the survey were also reported in the interviews. However, there were some discrepancies between individuals' responses to the survey questions and answers given in the interview, mainly in relation to self-reported symptom severity where 11 patients answered differently in the survey compared with the interview. Although not a concept or an impact, it is interesting to note and could suggest disease fluctuation. A number of psychological and emotional impacts were reported by patients in greater detail during the interviews; nearly all patients reported one or more bothering feelings including depression, demotivation, frustration, embarrassment, anxiety, and fear.
4. Discussion
This study provides an overview of the conceptual relevance of four assessment measures assessing patients with mild/mild-moderate AD from the patient perspective. The results suggest that the most important symptoms to these patients are related to memory and communication, and the most important impacts are more emotional and psychological than physical. Fear of deterioration (“worrying about my disease getting worse”) was highlighted as being a very important and relevant concept which was not reflected in the reviewed measures. Similarly, “loss of identity” was flagged as a recurrent patient concern also missing from the measures. In addition, patients were concerned about their ability to carry out activities of daily living in relation to remaining independent, such as driving. As seen in the concept matrix (Supplementary Information, Table 6), some of the included assessment measures do include these high-level symptom concepts (e.g., the ADAS-Cog includes an item to capture memory loss). However, it can also be seen that not all of the symptoms or memory issues identified as being important or relevant to patients are captured (e.g., reduced concentration), nor are they patient reported. Issues associated with emotional and psychological impacts were generally poorly covered by the four clinical assessment measures. This could be because emotional concepts are difficult to collect from clinician- or observer-reported measures and are better collected from patients themselves. These existing clinical measures may also not be granular enough to detect subtle changes that occur at mild/mild-moderate disease stages and may be better suited for evaluating individuals with severe AD because they capture impact on basic activities (e.g., toileting independently). Activities requiring a higher level of cognition and ability were considered more important to patients with mild/mild-moderate AD for them to remain independent and are concepts not covered by the measures.
Symptoms reported during interviews and in the survey were in good levels of agreement with one another. The variation noted between the symptom severity score given during the survey and interviews by individual patients could be attributed to variability in symptoms of AD over time. The current research does appear to demonstrate that patients with mild/mild-moderate AD are capable of reporting important issues that affect them and also like to be involved in research.
From the combined results of this research, it can be concluded that the ADAS-Cog, ADCS-ADL, NPI, and DS, all regularly used in clinical trials, do not appear to capture the full breadth of concerns of patients with mild/mild-moderate AD. Ensuring measurement of the concepts that are identified as those that matter to patients may help to distinguish whether new treatments are successful within a trial (as they will be assessing change on concepts that are relevant to patients). Published literature also suggests that these commonly used outcome measures may not be the most suitable to identify reliable change in patients with early AD [9], [17]. A number of high-level concepts assessed by these measures are relevant to patients. A larger number of concepts deemed of greatest importance to patients (including activities such as driving) are not included, nor are they captured directly from the patient. Indeed, published literature also suggests that although commonly used, historical measures, including the ADAS-Cog and NPI, may be less suitable for use in patients with milder AD because of the discrepancy between presenting symptoms and those included in the assessments [18], [19].
Thought should therefore be given to what matters most to these patients before selecting suitable endpoints for clinical trials and, therefore, suitable assessment measures to capture these. Patient and clinician interviews should be conducted before clinical trial endpoint selection. In addition, a full review should be undertaken to identify whether an existing measure can be used (or a composite created) or whether a new patient-reported outcome measure needs to be developed for use in clinical trials of mild AD patients.
4.1. Limitations
Although the overall study was designed appropriately and implemented according to protocol, several limitations are acknowledged. Only studies reporting in English-speaking populations with defined mild AD were included in the literature review. Consequently, relevant studies reported in other languages or with less well-defined descriptions of mild AD may have been excluded. Despite this, the stringent inclusion criteria ensured that all included concepts were highly relevant to the target population.
The inclusion of mixed methodology (quantitative and qualitative) within the study aimed to obtain substantial breadth and depth of data from patients. However, not all concepts identified by the literature review, steering committee, and assessment measures could be included in the survey because of length restrictions. This was mitigated by only including the concepts approved as most relevant by the steering committee and providing patients with an opportunity to discuss symptoms that may have not been included in the survey in the follow-up interviews.
For the interviews, participants were selected if they had completed the survey with ease and if they were deemed “able” enough to complete the interview. This selection process meant that it was more likely that patients with milder AD with the lowest disease activity were chosen for interview. Consequently, the full range of disease experience may not have been represented. Another point to note, as with all qualitative data capture, is that patients may have answered in a way that made their responses socially desirable. This was mitigated by cross-checking responses (where possible) with the quantitative survey results.
No primary data were collected from caregivers or individuals living with the patient who could potentially provide additional insight into the daily problems of the patient. This suggestion was put forward by steering committee and should be considered for future studies.
5. Conclusion
The four assessment measures reviewed in this study do not appear to capture many of the concepts reported as being most important to patients with mild/mild-moderate AD (i.e., emotional and psychological impacts) instead of focusing on physical ability and symptoms. Patient-reported assessment measures may be more suitable for assessing function and ability in patients with mild/mild-moderate AD than clinician or observer reports made in isolation and should therefore be considered for use alongside these reports, providing additional context and understanding. These findings should be considered in clinical trial research involving patients with mild/mild-moderate AD and reporting health-related outcomes with a focus on eliciting what matters most to patients.
Research in Context.
-
1.
Systematic review: The authors reviewed qualitative literature using traditional sources to develop a conceptual model of mild Alzheimer's disease (AD). The authors were aware of the four assessment measures used in clinical trials of mild AD patients and included in this study. It was hypothesized that there may be discordance between concepts that these measures assess and concepts identified as important by patients with mild AD.
-
2.
Interpretation: Findings from the research (literature, quantitative patient survey, and qualitative interviews) confirmed that patients identified additional concepts as being most important compared with those captured in the measures (low conceptual relevance).
-
3.
Future directions: The manuscript proposes that emotional and psychological impacts associated with mild AD are of greatest importance to patients. Some existing measures such as those included in this study do not assess these and therefore may not be able to detect more subtle changes in a clinical trial situation.
Acknowledgments
The authors would like to thank the Alzheimer's Association who provided helpful input and insight into the content of this study survey and qualitative interview guide. The authors would like to also thank in particular Monica Moreno, Bob O'Keefe, and Jeanne Kreiger who were part of the steering committee on this study.
The authors thank all the patients who took part in the quantitative survey and the qualitative interviews for their time and their helpful insight into living with mild/mild-moderate AD.
Funding: This work was financially supported by Lundbeck and Otsuka Pharmaceutical Development & Commercialization, Inc. pharmaceutical companies. DRG Abacus was commissioned by these companies to undertake the work and was paid a fixed fee to do so.
Footnotes
Declaration of interest: S.L.K., N.V.J.A., and T.A.-Z. are all employees of DRG Abacus. A.H. is an employee of Lundbeck US, and M.S. is an employee of Otsuka Pharmaceutical Development & Commercialization, Inc. DRG Abacus is a health outcomes agency that consults with various pharmaceutical companies. Lundbeck and Otsuka are both pharmaceutical companies working to develop new products through research.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.07.006.
Supplementary data
References
- 1.Johnston L., Terp D.M. Dynamics in couples facing early Alzheimer's disease. Clin Gerontol. 2015;38:283–301. [Google Scholar]
- 2.Jekel K., Damian M., Wattmo C., Hausner L., Bullock R., Connelly P.J. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther. 2015;7:17. doi: 10.1186/s13195-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riepe M.W., Mittendorf T., Förstl H., Frölich L., Haupt M., Leidl R. Quality of Life as an outcome in Alzheimer's disease and other dementias- obstacles and goals. BMC Neurol. 2009;9:47. doi: 10.1186/1471-2377-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aisen P.S., Cummings J., Jack C.R., Morris J.C., Sperling R., Frölich L. On the path to 2025: understanding the Alzheimer's disease continuum. Alzheimers Res Ther. 2017;9:60. doi: 10.1186/s13195-017-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Association As. 10 Early Signs and Symptoms of Alzheimer's. 2017. http://www.alz.org/alzheimers_disease_10_signs_of_alzheimers.asp Available at:
- 6.Lanctôt K.L., Amatniek J., Ancoli-Israel S., Arnold S.E., Ballard C., Cohen-Mansfield J. Neuropsychiatric signs and symptoms of Alzheimer's disease: New treatment paradigms. Alzheimers Dement. 2017;3:440–449. doi: 10.1016/j.trci.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(FDA) FaDA . Services USDoHaH. 2018. Early Alzheimer's Disease: Developing Drugs for Treatment Guidance for Industry. [Google Scholar]
- 8.Sikkes S.A., de Lange-de Klerk E.S., Pijnenburg Y.A., Scheltens P., Uitdehaag B.M. A systematic review of instrumental activities of daily living scales in dementia: room for improvement. J Neurol Neurosurg Psychiatry. 2009;80:7–12. doi: 10.1136/jnnp.2008.155838. [DOI] [PubMed] [Google Scholar]
- 9.Podhorna J., Krahnke T., Shear M., E Harrison J. for the Alzheimer's Disease Neuroimaging I. Alzheimer's Disease Assessment Scale–Cognitive subscale variants in mild cognitive impairment and mild Alzheimer's disease: change over time and the effect of enrichment strategies. Alzheimer's Res Ther. 2016;8:8. doi: 10.1186/s13195-016-0170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 11.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11:S33–S39. [PubMed] [Google Scholar]
- 12.Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 13.Stern Y., Albert S.M., Sano M., Richards M., Miller L., Folstein M. Assessing patient dependence in Alzheimer's disease. J Gerontol. 1994;49:M216–M222. doi: 10.1093/geronj/49.5.m216. [DOI] [PubMed] [Google Scholar]
- 14.Siemers E., Holdridge K.C., Sundell K.L., Liu-Seifert H. Function and clinical meaningfulness of treatments for mild Alzheimer's disease. Alzheimers Dement. 2016;2:105–112. doi: 10.1016/j.dadm.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ATLAS.ti ATLAS.ti Qualitative Data Analysis 2018. https://atlasti.com/product/what-is-atlas-ti/ Available at: Accessed July 8, 2018.
- 16.Braun V., Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. [Google Scholar]
- 17.Rockwood K., Fay S., Gorman M., Carver D., Graham J.E. The clinical meaningfulness of ADAS-Cog changes in Alzheimer's disease patients treated with donepezil in an open-label trial. BMC Neurol. 2007;7:26. doi: 10.1186/1471-2377-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knopman D.S. Clinical Trial Design issues in mild to moderate Alzheimer disease. Cogn Behav Neurol. 2008;21:197–201. doi: 10.1097/WNN.0b013e318190cf75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weintraub S., Carrillo M.C., Farias S.T., Goldberg T.E., Hendrix J.A., Jaeger J. Measuring cognition and function in the preclinical stage of Alzheimer's disease. Alzheimers Dement. 2018;4:64–75. doi: 10.1016/j.trci.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.