Abstract
Background
Exercise promotes numerous phenotypic adaptations in skeletal muscle that contribute to improved function and metabolic capacity. An emerging body of evidence suggests that skeletal muscle also releases a myriad of factors during exercise, termed “myokines”. The purpose of this study was to examine the effects of high-intensity interval training (HIIT) on the acute regulation of the mRNA expression of several myokines, including the prototypical myokine interleukin-6 (IL-6), and recently identified myokines fibronectin type III domain-containing protein 5 (FNDC5) (irisin) and meteorin-like protein (METRNL).
Methods
Both before and after a 20-day period of twice-daily high-volume HIIT, 9 healthy males (20.5 ± 1.5 years performed a standardized bout of high-intensity interval exercise (HIIE; 5 × 4 min at ~80% pretraining peak power output) with skeletal muscle biopsy samples (vastus lateralis) obtained at rest, immediately following exercise, and at 3 h recovery.
Results
Before training, a single bout of HIIE increased IL-6 (p < 0.05) and METRNL (p < 0.05) mRNA expression measured at 3 h recovery when compared to rest. Following 20 days of HIIT, IL-6 and FNDC5 mRNA were increased at 3 h recovery from the standardized HIIE bout when compared to rest (both p < 0.05). Resting METRNL and FNDC5 mRNA expression were higher following training (p < 0.05), and there was an overall increase in FNDC5 mRNA post-training (main effect of training, p < 0.05).
Conclusion
In human skeletal muscle (1) an acute bout of HIIE can induce upregulation of skeletal muscle IL-6 mRNA both before and after a period of intensified HIIT; (2) Resting and overall FNDC5 mRNA expression is increased by 20 days of HIIT; and (3) METRNL mRNA expression is responsive to both acute HIIE and short-term intense HIIT. Future studies are needed to confirm these findings at the protein and secretion level in humans.
Keywords: Brown adipose tissue, Exerkines, High-intensity interval training, Intermittent exercise, Myokine, Obesity
1. Introduction
It is well known that exercise promotes positive whole-body phenotypic changes and contributes to a healthy lifestyle. Exercise also offers protection against obesity and metabolic disorders such as type 2 diabetes.1 Recently, myokines have been identified as one of several important factors that might contribute to the widespread benefits of exercise.1, 2 A myokine can be defined as a small protein or molecule being secreted from stimulated muscle tissue.3 These muscle-derived factors may be induced by muscle contraction (exercise) and have been shown to act in conjunction with the immune response to reduce markers of inflammation1, 4 and to induce other phenotypic and metabolic changes.3 For example, the most well-characterized myokine is interleukin-6 (IL-6), which is hypothesized to mediate some of the anti-inflammatory effects of exercise and also act as a potential signal whereby exercising muscle communicates with other metabolic tissues to increase fuel mobilization and delivery.5 Due to their ability to act as mediators of the adaptive response to exercise, myokines are now being studied as potential therapeutic agents for the treatment of a wide range of metabolic diseases.4, 5, 6
Recently, 2 new myokines with therapeutic potential have been identified by Spiegelman's group.4, 6 The first myokine is termed fibronectin type III domain-containing protein 5 (FNDC5), which also may exist as a secreted form, irisin. The second myokine is known as meteorin-like protein (METRNL). It was observed that the overexpression of peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α), as well as exercise, increased the expression of FNDC5 in skeletal muscle.6 Increased FNDC5 production was linked to an increase in brown-fat indicators, such as uncoupling protein-1, in certain adipose deposits both in vitro and in vivo, where the latter was associated with an antiobesity effect in mice.6 The relevance of FNDC5 to human exercise physiology and fat browning has been hotly debated.7, 8, 9 METRNL is proposed to also be induced in skeletal muscle following exercise and have similar fat browning and antiobesity effects.4 Human studies characterizing FNDC5 (irisin), and especially METRNL, are limited, and to our knowledge, the impact of high-intensity interval training (HIIT) on these myokines has not been studied. HIIT involves short repeated bursts of vigorous exercise separated by periods of low-intensity recovery10 and is a form of exercise training used extensively by athletes. In addition, recent research has demonstrated beneficial effects of HIIT in individuals with metabolic disease and type 2 diabetes,11 both of which are conditions where the potential for fat browning may have particular therapeutic value.
Therefore, the purpose of the present study was to examine the impact of HIIT on mRNA expression of IL-6, FNDC5, and METRNL in human skeletal muscle. We explored how a bout of high-intensity interval exercise (HIIE) influenced mRNA expression of these myokines before and after a 20-day period of high-volume HIIT. This design allowed us to examine the impact of a single bout of HIIE performed at the same absolute intensity in the untrained and trained state, while also assessing the impact of intensive training on resting expression of these selected myokines. We hypothesized that all myokines would be up-regulated in skeletal muscle after a single bout of HIIE. We also explored the hypothesis that due to decreased metabolic stress, the induction of these myokines in response to the standardized bout of HIIE would be altered when assessed post-training.
2. Materials and methods
2.1. Informed consent
Participants were informed of the risks, potential benefits, and requirements of the study before providing written informed consent. The study procedures were approved by the Victoria University Human Research Ethics Committee.
2.2. Study design
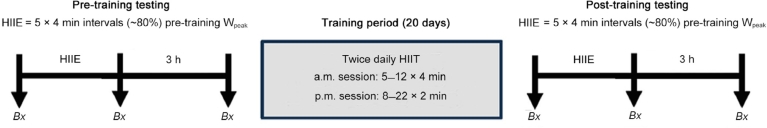
Ten healthy young men (age: 20.5 ± 1.5 years; height: 180 ± 10 cm; body mass: 80 ± 14 kg; peak oxygen uptake (VO2peak): 47 ± 8 mL/kg/min; peak power output (Wpeak): 294 ± 36 W; means ± SD) involved in individual or team sports, who were moderately trained but not currently engaged in a specific interval training protocol, volunteered to participate in the study. The quantity of muscle biopsy material provided by 1 participant was insufficient for RNA extraction; therefore, all samples from this individual were omitted and data is presented for n = 9. Participants were part of a larger previously published study asking separate research questions.12 Participants completed baseline testing consisting of a graded exercise test (GXT) to exhaustion to determine VO2peak, power at lactate threshold (WLT), and Wpeak. Indicators of endurance performance (VO2peak, Wpeak, and WLT) all improved (by ~5%–12%) following high-volume HIIT, as reported previously.12 Indicators of mitochondrial oxidative capacity were also improved after the training period as measured by increases in both mitochondrial respiration in permeabilized fibers and whole muscle citrate synthase activity.12 Before and after 3 weeks of high-volume HIIT, all participants underwent a muscle biopsy trial that consisted of a resting muscle biopsy, a single bout of HIIE at the same absolute intensity (5 × 4 min at ~80% pretraining Wpeak), and muscle biopsies obtained immediately after exercise and at 3 h of recovery. An overview of the study design is provided in Fig. 1.
Fig. 1.
Overview of study design. Participants completed a pre-training muscle biopsy trial involving a single bout of HIIE, 20 days of HIIT comprised of morning (a.m.) and afternoon (p.m.) sessions, and a post-training muscle biopsy trial involving the same bout of HIIE as pre-training. Bx = muscle biopsy; HIIE = high-intensity interval exercise; HIIT = high-intensity interval training; Wpeak = peak power output.
2.3. Baseline testing
2.3.1. GXT
A discontinuously GXT was performed on an electronically-braked cycle ergometer (Lode Excalibur, Version 2.0; Groningen, The Netherlands). Subjects completed baseline GXT to exhaustion to determine VO2peak, WLT, and Wpeak (using the modified Dmax method13). The test involved 4 min stages separated by 30 s of rest (to allow for fingertip capillary blood lactate testing). The test began at 60, 90, or 120 W depending on participants' fitness level and was subsequently increased by 30 W every 4 min until volitional exhaustion. Participants were instructed to maintain a cadence greater than 60 rpm and were given similar verbal encouragement throughout the tests. Only cadence and elapsed time were made available to participants. The test was stopped when a participant reached volitional exhaustion or cadence dropped below 60 rpm. The Wpeak was determined as the power of the last completed stage. If a participant stopped during a stage, Wpeak was determined as the power of the last completed stage plus 7.5 W for every completed minute of the unfinished stage.
2.3.2. GXT gas analysis
A gas analyzer (Moxus modular oxygen uptake system 2010; AEI technologies, Pittsburgh, PA, USA) continually monitored expired air during GXT. The gas analyzers were calibrated immediately before each test using known gas mixtures (A: 21% O2, 0% CO2; B: 16% O2, 4% CO2; BOC, Melbourne, Australia). The 2 highest consecutive 15 s VO2 values were averaged and recorded as the participant's VO2peak.
2.3.3. HIIE trial
On a separate day, at least 72 h following the baseline GXT, a resting muscle biopsy was obtained at rest from the vastus lateralis under local anesthetic (1% Xylocaine, Astra Zeneca) using a Bergstrom biopsy needle adapted with suction at a consistent depth of 2–3 cm. Participants then completed a single session of HIIE involving 5 × 4 min intervals at a power output equal to WLT + 20%Δ (Wpeak − WLT) (i.e., the WLT plus the power that was equal to 20% of the difference between WLT and Wpeak), interspersed with 2 min recovery at 60 W. A second muscle biopsy was obtained immediately after exercise (within 5 s from termination of the last interval). Subjects rested quietly for 3 h, where they were allowed to drink water ad libitum, before a third muscle biopsy was obtained. This trial was repeated at the same absolute intensity following the 3 weeks of HIIT. The 3 biopsies were obtained from the same leg, about 1 cm apart from each other, and the opposite leg was used for the post-HIIT biopsy trial.
2.4. HIIT
Training began the following day and was comprised of 40 HIIT sessions over 20 days, as described previously.12 Training sessions were completed twice per day and consisted of (1) a morning session involving 5–12 repetitions of 4 min intervals with working intensities progressing from WLT + 30%Δ(Wpeak − WLT) to WLT + 80%Δ(Wpeak − WLT); and (2) an afternoon session involving 8–22 repetitions of 2 min intervals, with working intensities progressing from WLT + 50%Δ(Wpeak − WLT) to WLT + 80%Δ(Wpeak − WLT). The goal of this short-term HIIT program was to subject participants to a high volume of HIIT. Approximately 72 h after the final training session subjects completed the post-training muscle biopsy as per the pretraining biopsy procedure.
2.5. Controls for diet and physical activity
Participants were required to refrain from alcohol and any form of exercise 24 h prior to biopsy trials and from food or caffeine for 3 h preceding the muscle biopsies. Each test was performed at the same time of day to limit interference of variables associated with circadian rhythm.
Participants were provided with standardized meals 15 h and 3 h prior to the biopsy trials. Dinner (15 h–13 kcal/kg, 65% carbohydrate, 15% fat, and 20% protein) and breakfast (3 h–11 kcal/kg, 75% carbohydrate, 15% fat, and 10% protein) were the only regulated meals provided to participants. Over the course of the study, participants were encouraged to maintain their normal dietary pattern and maintain a constant routine for physical activity. None of the participants were undergoing any resistance training prior to or during the intervention.
2.6. Muscle preparation
Once biopsies were obtained they were cleaned of excess blood, fat, and connective tissue and immediately flash frozen in liquid N2 and stored at −80°C. Total RNA was extracted from approximately 15 mg of tissue using a modified TRIzol method as described previously.14 Briefly, tissue was homogenized using ceramic/silica beads in TRIzol Reagent (Invitrogen, Melbourne, Australia) via motorized reciprocation (FastPrep FP120 cell disruptor; Thermo Electron Corporation, Milford, MA, USA) for 2 × 20 s bouts. Homogenates were centrifuged (13,000 rpm for 15 min) and the RNA-containing supernatant was removed, combined with chloroform (Sigma-Aldrich, St Louis, MO, USA), and RNA extracted according to the manufacturer's instructions except that RNA precipitation was performed for a minimum of 2 h at −20°C in the presence of 10 µL of 5 mol/L sodium chloride. RNA concentration was quantified spectrophotometrically at 260 nm, DNA digested using the commercially available RQ1 RNase-free DNase kit (Promega Corporations, Madison, WI, USA), and first strand cDNA generated from 0.3 µg of template RNA using the commercially available iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) using random hexamers and oligo dTs. cDNA was stored at −20°C for subsequent analysis.
2.7. qPCR
qPCR was performed using iCycler5 and CFX manager software analyzer (Bio-Rad Laboratories). IL-6, FNDC5, and METRNL primers were custom-designed and purchased from Integrated DNA Technologies (IL-6 forward: 5′ GAC CCA ACC ACA AAT GCC A-3′; reverse: 5′-GTC ATG TCC TGC AGC CAC TG-3′; FNDC5 forward: 5′-AGG TGT CAT TGC CCT CTT CT-3′; reverse: 5′-CTG GTG TGC TGG TTT CTG AT-3′; METRNL: forward 5′-TCC ATC CAG CAA GTT ACC-3′; reverse: 5′-GCT CGA AGA CCC TGC TTT-3′). Forward and reverse primers were diluted separately and combined for the reaction to a final concentration of 5 µmol/L. Beta-actin, which remained stable with acute exercise and training, was utilized as a housekeeping gene. Pooled cDNA from pretraining biopsies of a subject who did not complete the training portion of the study were used to run efficiency curves for IL-6, FNDC5, and METRNL, where all efficiencies were between 90%–110%. qPCR was then performed on a per target basis according to MIQE guidelines.15 Using CFX manager software, the relative amounts of mRNA expression were analyzed using the 2−ΔΔCT method, with mRNA content expressed relative to the resting (baseline) pretraining biopsy.
2.8. Statistical analyses
Two-way repeated measures ANOVA was performed to analyze all qPCR data, with Tukey's HSD post hoc tests used to determine planned contrasts between time points. Specifically, significant main effects of time and interaction were followed up with preplanned contrasts comparing the pretraining biopsy trial separately from post-training biopsy trial. Resting myokine mRNA in the pre- and post-training state was also compared with a preplanned contrast; p ≤ 0.05 was considered statistically significant. The sample size required to detect a 2-fold increase in mRNA expression (considered the smallest meaningful difference for qPCR data15) was 8 participants based on mean ± SD for resting METRNL mRNA expression (1.0 ± 0.8 arbitrary units, Safdar et al., unpublished) assuming a moderate correlation of r = 0.5 between repeated measures with 80% power and an α level of 0.05 (calculated using G*Power Version 3.1).16
3. Results
3.1. IL-6 mRNA expression
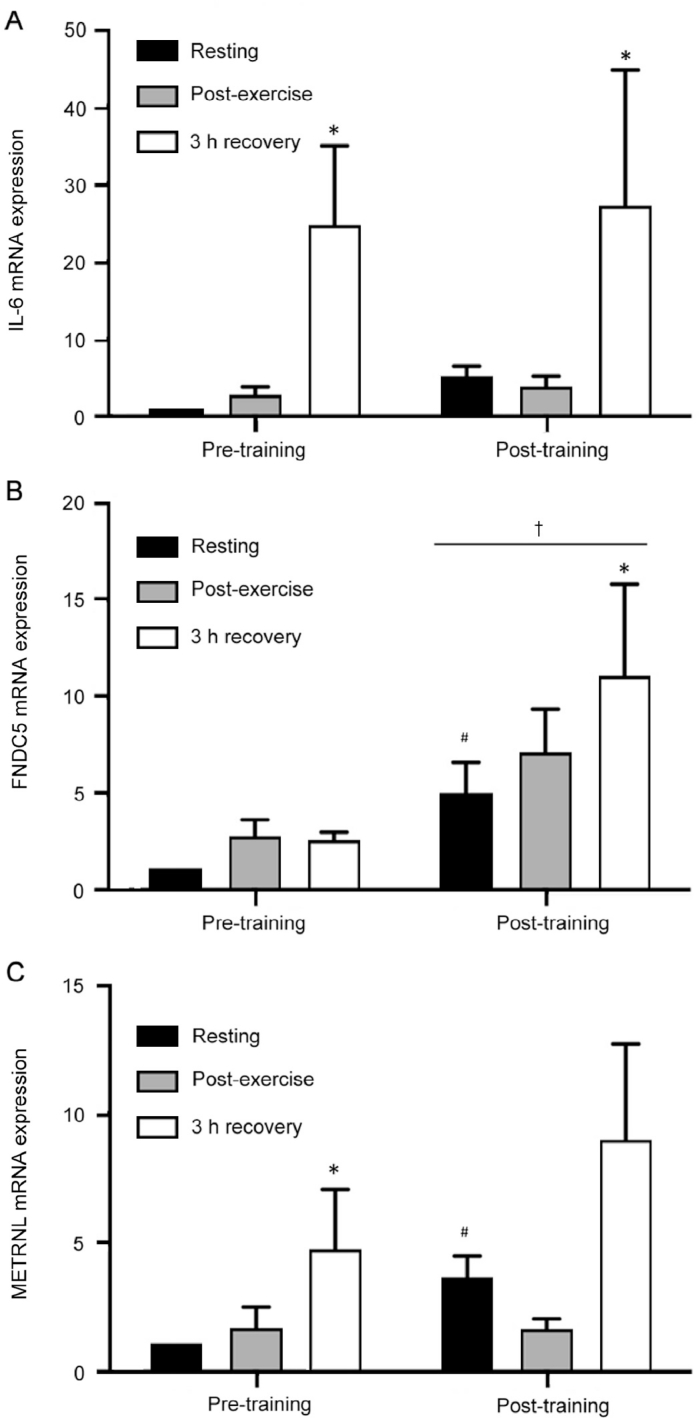
A significant main effect of time was found for IL-6 mRNA levels (p < 0.05) (Fig. 2A). IL-6 mRNA was significantly increased by ~25-fold above resting at the 3 h recovery time point in pre-training muscle biopsy samples (p < 0.05). Post-training IL-6 mRNA was increased by ~5-fold above resting levels at 3 h recovery (p < 0.05). There was no significant change in IL-6 mRNA in the biopsy obtained immediately after an acute bout of HIIE in both pre- and post-training trials (both p > 0.05). Resting IL-6 mRNA expression in response to 20 days of intensified HIIT was not significantly changed (p > 0.05, Fig. 2A).
Fig. 2.
(A) Skeletal muscle interleukin-6 (IL-6) mRNA expression, (B) FNDC5 mRNA expression, and (C) METRNL mRNA expression assessed by qPCR in response to a single bout of high-intensity interval exercise performed at the same absolute intensity before (pre-training) and after (post-training) 20 days of twice daily high-intensity interval training. Vastus lateralis biopsies (n = 9) were obtained prior to exercise (resting), immediately after (post-exercise), and at 3 h of recovery (3-h recovery). *p < 0.05, compared with resting within condition. #p ≤ 0.05, compared with resting in pre-training. †p < 0.05, significant main effect of training.
3.2. FNDC5 mRNA expression
There was no significant effect of an acute bout of HIIE on FNDC5 mRNA assessed pre-training (p > 0.05). Following 20 days of high-volume HIIT overall FNDC5 mRNA expression was greater compared to pre-training (main effect of training, p < 0.05; Fig. 2B). There was also an ~5-fold increase in resting FNDC5 mRNA when comparing post- to pre-training values in the preplanned contrast (p = 0.05). Following HIIT, FNDC5 mRNA increased ~2-fold after 3 h of recovery compared to post-training resting values (p < 0.05).
3.3. METRNL mRNA expression
There was a significant main effect of time for METRNL mRNA (p < 0.05, Fig. 2C). METRNL mRNA was elevated ~5-fold at 3 h recovery in pre-training samples compared to rest (p < 0.05). There was also an ~3.6-fold increase in resting METRNL assessed post-training relative to pre-training resting levels (p < 0.05).
4. Discussion
Our study suggests that a single bout of HIIE can induce an increase in IL-6 and METRNL mRNA in human skeletal muscle. In addition, a 20-day period of high-volume twice-daily interval training led to increased resting FNDC5 and METRNL and an increase in overall FNDC5 mRNA expression. Overall, HIIT appears to up-regulate selected myokine mRNA in human skeletal muscle, with some varying response seen with a single bout of HIIE and 20 days of HIIT. Not all myokines measured responded the same to this type of exercise, suggesting different regulatory mechanisms and time courses for induction.
The majority of findings confirmed our hypotheses. When assessed at 3 h recovery from a single bout of HIIE pre- and post-training, IL-6 mRNA was increased. This is consistent with previous human research examining the IL-6 response to endurance-based exercise.17 Research on IL-6 shows that plasma levels of this myokine can be robustly increased immediately following endurance exercise, which corresponds to release from skeletal muscle.5 The largest increases in IL-6 seem to result from long duration exercises such as marathons or prolonged leg-kicking.5 Fischer18 has also shown that exercise duration is the single most important factor determining the induction of IL-6 levels. Although we did not assess plasma IL-6, our data suggest that HIIE does not immediately induce IL-6 mRNA in human skeletal muscle, but that increases in skeletal muscle IL-6 expression and possible release as a myokine occur later during the recovery phase after interval-style exercise. This is consistent with Williams and colleagues19 who demonstrated that plasma IL-6 was increased above resting values at 3 h but not immediately following Wingate-based HIIT. Interestingly, there were no apparent differences in the ability of a single bout of HIIE to induce IL-6 following training, which suggests that IL-6 is similarly responsive following a period of short, intensified training.
METRNL mRNA expression was also increased at 3 h recovery when assessed pre-training, yet the increased post-training was not statistically significant, possibly due to high interindividual variability and the small sample size. One previous study has reported increased METRNL following a bout of combined endurance and resistance exercise.4 Our novel findings of an increase in METRNL mRNA in the resting state after training suggest that an increase in this purported myokine's expression may be an adaptive response to short-term high-volume HIIT. This is the first study that characterizes the effects of a period of exercise training on METRNL mRNA. Rao et al.4 reported that METRNL mRNA level increased in biopsies obtained after a single bout of combined aerobic and resistance exercise. However, they did not observe any changes in resting METRNL mRNA upon a program of endurance training in mice. It will be interesting to determine if elevated skeletal muscle METRNL mRNA expression is a common adaptive response to other types of exercise training and whether this has any functional effects. Future studies in humans that measure circulating METRNL will be an important and novel step in research on this new myokine. Unfortunately, commercial enzyme-linked immunosorbent assays (ELISAs) are not readily available and in our attempts, we were unable to determine the specificity of existing antibodies to measure METRNL at the protein level in muscle or plasma.
To our knowledge, this is the first study to examine the effects of HIIT on FNDC5 mRNA expression. Prior to training, there were no effects of the single bout of HIIE on muscle FNDC5 mRNA. However, after 20 days of high-volume HIIT, FNDC5 mRNA was increased in resting muscle biopsies, robustly increased overall (i.e., main effect of training), and showed an increase over post-training resting levels at 3 h recovery. These findings indicate that resting FNDC5 mRNA levels in human muscle are responsive to short-term high-volume periods of HIIT and may represent an adaptive skeletal muscle response to HIIT. The impact of acute exercise on FNDC5 mRNA is equivocal in the literature, with some studies showing an increase6, 20 and others showing no effects.8 Our findings of no increases in FNDC5 mRNA in response to acute HIIE pre-training are in agreement with the latter, but the increase above resting values at 3 h recovery post-training suggest that training status may influence the acute response. FNDC5 is purported to be cleaved by an unknown enzyme to produce irisin, which is secreted as a myokine.6 However, some authors have questioned the existence of irisin.8, 9 Our results suggest that further research is warranted because the increase in basal FNDC5 mRNA after training suggests a potential role in the muscle adaptive response.
There are some limitations in our study. Myokines are a relatively new area of study and much of the literature is associative or limited in humans at this point. Our study provides valuable mRNA data in human muscle, and our design was unique in that it compared a bout of HIIE at the same absolute intensity before and after a period of high-volume HIIT. The decision to match absolute intensity in the post-training bout was made based on previous studies,21, 22, 23 which enables interpretation of the skeletal muscle response to the same workload in the trained state. However accounting for individual improvements in performance could provide an alternate approach to standardizing the post-training bout based on relative exercise intensity. A limitation of this study is that we were unable to quantify FNDC5 and METRNL at the protein level due to the lack of validated antibodies (e.g., for METRNL), along with uncertainty over their specificity (particularly for FNDC5 (irisin)). In addition, we did not obtain blood samples so we were unable to confirm if changes in mRNA expression in muscle were paralleled by changes in circulating levels of these purported myokines. Future studies should aim to complement mRNA measures with protein markers, and as antibodies and ELISAs become validated, expansion of these findings will be possible.
5. Conclusion
This study shows that HIIT impacts mRNA expression of IL-6, FNDC5, and METRNL mRNA in human skeletal muscle. A single bout of HIIE increased IL-6 and METRNL mRNA expression at the 3 h time-point in the pre-training state. Following 20 days of high-volume twice-daily HIIT, the same acute bout of HIIE increased IL-6 and FNDC5 as compared to resting levels, and overall FNDC5 mRNA expression was increased after training. Twenty days of HIIT also increased resting levels of METRNL and FNDC5 mRNA. These findings suggest that acute HIIE can increase the expression of putative myokines and that short-term high-volume HIIT may lead to elevated levels of potential fat browning myokines in skeletal muscle. Further human and mechanistic studies are needed to confirm secretion and potential systemic effects of these myokines.
Authors' contributions
ME helped conceive the design, performed the qPCR analyses, analyzed the data, and wrote the first draft of the manuscript; JB and AS assisted with qPCR analyses and helped to draft the manuscript; CG helped conceive the design, supervised experimental trials and training sessions, and helped to draft the manuscript; DB helped conceive the design, supervised the experimental trials, provided funding for the study, and helped draft the manuscript; JPL helped conceive the design, assisted with data analyses, provided funding for the study, and helped to draft the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The authors thank Ms. Svetlana Simtchouk for assistance with qPCR analyses. This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (No. RGPIN 435807-13) to JPL and the ANZ-MASON foundation (to DB). JPL is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Award (No. MSH-141980).
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 2.Vina J., Sanchis-Gomar F., Martinez-Bello V., Gomez-Cabrera M.C. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167:1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raschke S., Eckel J. Adipo-myokines: two sides of the same coin–mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao R.R., Long J.Z., White J.P., Svensson K.J., Lou J., Lokurkar I. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 6.Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmons J.A., Baar K., Davidsen P.K., Atherton P.J. Is irisin a human exercise gene? . Nature. 2012;488:10–11. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- 8.Pekkala S., Wiklund P.K., Hulmi J.J., Ahtiainen J.P., Horttanainen M., Pöllänen E. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? . J Physiol. 2013;591:5393–5400. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atherton P.J., Phillips B.E. Greek goddess or Greek myth: the effects of exercise on irisin/FNDC5 in humans. J Physiol. 2013;591:5267–5268. doi: 10.1113/jphysiol.2013.265371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibala M.J., Little J.P., Macdonald M.J., Hawley J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590:1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little J.P., Gillen J.B., Percival M., Safdar A., Tarnopolsky M.A., Punthakee Z. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111:1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 12.Granata C., Oliveira R.S.F., Little J.P., Renner K., Bishop D.J. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J. 2016;30:3413–3423. doi: 10.1096/fj.201500100R. [DOI] [PubMed] [Google Scholar]
- 13.Bishop D., Jenkins D.G., McEniery M., Carey M.F. Relationship between plasma lactate parameters and muscle characteristics in female cyclists. Med Sci Sports Exerc. 2000;32:1088–1093. doi: 10.1097/00005768-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Cornall L.M., Mathai M.L., Hryciw D.H., McAinch A.J. Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol Biochem. 2011;28:949–958. doi: 10.1159/000335820. [DOI] [PubMed] [Google Scholar]
- 15.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M. The MIQE Guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 16.Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Method. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 17.Febbraio M.A., Pedersen B.K. Muscle-derived interleukin-6: mechanisms for activation. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 18.Fischer C.P. Interleukin-6 in acute exercise and training: what is the biological relevance? . Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 19.Williams C.B., Zelt J.G.E., Castellani L.N., Little J.P., Jung M.E., Wright D.C. Changes in mechanisms proposed to mediate fat loss following an acute bout of high-intensity interval and endurance exercise. Appl Physiol Nutr Metab. 2013;38:1236–1244. doi: 10.1139/apnm-2013-0101. [DOI] [PubMed] [Google Scholar]
- 20.Kurdiova T., Balaz M., Vician M., Maderova D., Vlcek M., Valkovic L. Effects of obesity, diabetes and exercise on FNDC5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592:1091–1107. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee-Young R.S., Canny B.J., Myers D.E., McConell G.K. AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol. 2009;107:283–289. doi: 10.1152/japplphysiol.91208.2008. [DOI] [PubMed] [Google Scholar]
- 22.Stepto N.K., Boubacar B., Wadley G.D., Chibalin A.V., Canny B.J., Eynon N. Short-term intensified cycle training alters acute and chronic responses of PGC1α and Cytochrome C Oxidase IV to exercise in human skeletal muscle. PLoS One. 2012;7:e53080. doi: 10.1371/journal.pone.0053080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgomaster K.A., Howarth K.R., Phillips S.M., Rakobowchuk M., Macdonald M.J., McGee S.L. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]