Abstract
Purpose
The purpose of the present controlled cross-sectional study was to investigate proximal femur and whole-body bone mineral density (BMD), as well as bone turnover profile, in lifelong trained elderly male football players and young elite football players compared with untrained age-matched men.
Methods
One hundred and forty healthy, non-smoking men participated in the study, including lifelong trained football players (FTE, n = 35) aged 65–80 years, elite football players (FTY, n = 35) aged 18–30 years, as well as untrained age-matched elderly (UE, n = 35) and young (UY, n = 35) men. All participants underwent a regional dual-energy X-ray Absorptiometry (DXA) scan of the proximal femur and a whole-body DXA scan to determine BMD. From a resting blood sample, the bone turnover markers (BTMs) osteocalcin, carboxy-terminal type-1 collagen crosslinks (CTX-1), procollagen type-1 amino-terminal propeptide (P1NP), and sclerostin were measured.
Results
FTE had 7.3%–12.9% higher (p < 0.05) BMD of the femoral neck, wards, shaft, and total proximal femur in both legs compared to UE, and 9.3%–9.7% higher (p < 0.05) BMD in femoral trochanter in both legs compared to UY. FTY had 24.3%–37.4% higher (p < 0.001) BMD in all femoral regions and total proximal femur in both legs compared to UY. The whole-body DXA scan confirmed these results, with FTE showing similar whole-body BMD and 7.9% higher (p < 0.05) leg BMD compared to UY, and with FTY having 9.6% higher (p < 0.001) whole-body BMD and 18.2% higher (p < 0.001) leg BMD compared to UY. The plasma concentration of osteocalcin, CTX-1, and P1NP were 29%, 53%, and 52% higher (p < 0.01), respectively, in FTY compared to UY.
Conclusion
BMD of the proximal femur and whole-body BMD are markedly higher in lifelong trained male football players aged 65–80 years and young elite football players aged 18–30 years compared to age-matched untrained men. Elderly football players even show higher BMD in femoral trochanter and leg BMD than untrained young despite an age difference of 47 years.
Keywords: Bone mass, Bone turnover markers, Dual-energy X-ray absorptiometry, Proximal femur bone mineral density, Soccer, Whole-body bone mineral density
1. Introduction
Osteoporosis, which is characterised by decreased bone mineral density (BMD) and increased risk of fractures, is a significant public health concern.1 As the elderly population is increasing at an unprecedented rate2 and daily living is becoming more sedentary due to a large amount of sitting time during work and leisure time,3 the prevalence of osteoporosis and osteoporotic fractures are expected to rise dramatically in the future.4 Indeed, the world population ≥65 years of age is predicted to double from about 506 million in 2008 to 1.3 billion by 2040, at which time it will account for 14% of the world's total population.2 Furthermore, the number of those aged 80 years and above is projected to double from 2010 to 2050. In the European Union, 22 million women and 5.5 million men are estimated to have osteoporosis, and 3.5 million new fragility fractures are sustained annually with hip fractures representing approximately 17% of all fractures.5 The economic burden of incident and prior fragility fractures are estimated at EUR37 billion, and due to the aging of the population, the costs are expected to increase by 25% in 2025.6 In addition, approximately 20% of all patients with a hip fracture do not survive for more than 1 year from diagnosis and more than 50% never completely regain their previous functional status.7 Besides increased mortality and morbidity, physical disabilities and chronic pain after fractures can lead to loss of independence, need for long-term care, and reduced quality of life.1 Preventing osteoporosis and osteoporotic fractures would have a significant positive impact on public health and health economics in the future.
Bone is a dynamic tissue that remodels throughout the life span, and although skeletal characteristics are strongly influenced by genetic factors, with an estimated heritability of BMD of ~60%–80%,8 a healthy lifestyle with a well-balanced diet and regular physical activity can help to prevent age-related bone loss or strengthen already weak bones.9, 10 During physical activity, mechanical forces are exerted on bones through ground reaction forces and through contractile forces from muscles.11 However, osteogenic training effects appear to depend on the type, intensity, and volume of exercise.11, 12, 13 Weight-bearing and resistance exercise producing mechanical strains and high loads, a fast load, or a load affecting the skeleton in an unusual direction, confer the highest anabolic response.12 Even though the specific mechanisms involved are not yet fully understood, it has been suggested that the skeleton accommodates mechanical stimuli over time through a desensitisation of mechanoreceptors in the bone cell membrane.13
Football is an intermittent intensity sport for untrained individuals14, 15 and elite players16, 17 characterised by multiple turns, jumps, and sprints, with accelerations and decelerations causing high rates of force application and large ground reaction forces.13 This loading pattern results in high-impact forces on the bones of the lower limbs and has been shown to improve osteogenesis. Indeed, in untrained individuals, including elderly men,18 premenopausal women,19, 20 middle-aged women21 and men with prostate cancer,22 14–32 weeks of recreational football training performed approximately 2 times a week has resulted in significant and clinically relevant increases in BMD. These improvements in BMD were accompanied by anabolic changes in the concentration of biochemical bone turnover markers (BTMs) in the blood, which may help to explain the time course of osteogenic changes in bone modeling and remodeling in response to odd-impact and high-impact sports.
One of the reasons for the great interest in football is the fact that football is the world's most popular sport with around 300 million registered football players worldwide23 accounting for approximately 4% of the world's total population. Based on existing literature, it is hypothesized that young and elderly football players have significantly higher BMD values particularly in the legs compared to untrained individuals. Thus, football may have the potential to contribute significantly to global prevention of osteoporosis. The purpose of the present controlled cross-sectional study was, therefore, to investigate proximal femur, total body and total leg BMD, as well as bone turnover for lifelong trained elderly football players and young elite football players compared to untrained age-matched controls. Bone turnover was evaluated by the biochemical BTMs osteocalcin, procollagen type-1 amino-terminal propeptide (P1NP), carboxy-terminal type-1 collagen crosslinks (CTX-1), and sclerostin.
2. Methods
2.1. Participants
One hundred and forty participants were recruited for this study, including 70 male football players and 70 untrained men. The football players were recruited through The Danish Football Association (Dansk Boldspil-Union) and coaches in local football clubs near Copenhagen, Denmark. The participants comprised 35 football-trained elderly (FTE) men aged 65–80 years (71.9 ± 2.8 years) with a history of more than 40 years (60.5 ± 8.5 years) of regular football training, and 35 elite football-trained young (FTY) men aged 18–30 years (22.1 ± 3.4 years) playing in the 2nd division or higher and with a history of more than 10 years (16.9 ± 3.6 years) of regular football training. FTE had a total of 2.3 ± 1.1 football training sessions per week, including matches approximately once a week. Besides football training, FTE had 1.2 ± 2.1 non-football-related training sessions per week including jogging, resistance training, cycling, golf, tennis, swimming, dancing, and gymnastics. FTY had a total of 5.4 ± 1.6 football training sessions per week, including matches, and 1.8 ± 1.3 non-football-related training sessions per week including primarily resistance training and running. On average, FTY had played football at a high level (2nd division or higher) for 6.7 ± 3.7 years.
Two control groups consisting of 35 untrained elderly (UE) men aged 65–80 years (69.7 ± 4.1 years) and 35 untrained young (UY) men aged 18–30 years (24.6 ± 3.3 years) were recruited through advertisements in local newspapers and flyers at local institutions. The control groups had not participated in regular physical activity for at least 1 year prior to inclusion, and had not participated in any kind of sport at a high level earlier in life. FTY were slightly younger than UY (2 years; p < 0.05) and FTE were slightly older than UE (2 years; p < 0.05) (Table 1).
Table 1.
Age and anthropometric characteristics in FTY, UY, FTE, and UE (mean ± SD).
| FTY (n = 35) | UY (n = 35) | FTE (n = 35) | UE (n = 35) | |
|---|---|---|---|---|
| Age (year) | 22.1 ± 3.4***,#,††† | 24.6 ± 3.3***,††† | 71.9 ± 2.8* | 69.7 ± 4.1 |
| Height (cm) | 181.3 ± 5.5*,† | 181.4 ± 7.3*,† | 176.3 ± 5.8 | 176.4 ± 6.4 |
| Total body mass (kg) | 77.1 ± 6.3***,# | 85.5 ± 16.5 | 82.9 ± 9.5 | 88.5 ± 12.4 |
| BMI (kg/m2) | 23.5 ± 1.6***,#,††† | 25.9 ± 4.2* | 26.7 ± 3.0 | 28.6 ± 4.5 |
| Total fat percentage (%) | 14.3 ± 3.2***,###,††† | 28.7 ± 8.2* | 29.0 ± 5.7* | 33.2 ± 6.2 |
| Lean body mass (kg) | 63.4 ± 5.4***,###,††† | 57.0 ± 6.7 | 55.9 ± 4.8 | 56.1 ± 5.0 |
Abbreviations: FTE = football-trained elderly men; FTY = football-trained young men; UE = untrained elderly men; UY = untrained young men.
p < 0.05, ***p < 0.001, compared with UE; #p < 0.05, ###p < 0.001, compared with UY; †p < 0.05, †††p < 0.001, compared with FTE.
All participants were non-smoking men without any chronic diseases. Mild hypertension or hyperlipidemia, however, were not an exclusion criteria and the number of subjects who took blood-pressure-lowering medication was 10 and 7 in FTE and UE, respectively, while 10 and 6 participants in these groups, respectively, took cholesterol-lowering medications. Furthermore, 6 subjects in both FTE and UE were medicated with blood thinners, and 5 and 3 subjects, respectively, with glucosamine due to osteoarthritis.
With regard to daily intake of dietary supplements, the participants mainly reported intake of multivitamins (FTE: 12, UE: 6, FTY: 6, UY: 3 subjects), vitamin D (FTE: 11, UE: 6, FTY: 5, UY: 4 subjects), fish oil or omega-3 (FTE: 9, UE: 7, FTY: 6, UY: 2 subjects) and, to a lesser extent, calcium (FTE: 6, UE: 2, FTY: 1, UY: 1 subjects) and magnesium (FTE: 2, UE: 2, FTY: 2, UY: 1 subjects).
All subjects were fully informed of the experimental procedures and possible discomfort associated with participation before giving their written informed consent. The study was carried out in accordance with the guidelines contained in the Declaration of Helsinki and was approved by the National Committee on Health Research Ethics of Region Hovedstaden (journal.nr. H-15009312).
2.2. Testing procedures
To evaluate the potential osteogenic adaptations induced by regular football training, BMD and bone mineral content (BMC) in the proximal femur and in the whole-body were determined together with the plasma concentration of BTMs. Participants attended testing sessions in the morning after an overnight fast (>8 h) and without having performed any strenuous exercise in the 48 h preceding testing. Prior to scanning, participants were asked to use the toilet to empty their bladder. A dual femur and a whole-body dual-energy X-ray Absorptiometry (DXA) scan were performed in accordance with standard procedures (iDXA; GE Healthcare Lunar Corp., Madison, WI, USA). To evaluate regional BMD and BMC in both legs, the whole-body scan was segmented in accordance with standard procedures (GE Healthcare Lunar iDXA user manual). According to manufacturer information, the coefficient of variation for BMD assessment is 1.0%. Body fat percentage and lean body mass were also assessed by the whole-body DXA scanning. All analyses were performed using enCORE software, Version 14.10 (GE Healthcare Lunar). The effective radiation dose for dual femur and whole-body scan in total was 10.84 µSv.
After DXA scanning, a resting blood sample was drawn under standardized conditions from an antecubital vein. The blood was centrifuged at 18,000 g for 2.5 min (centrifuge: Ole Dich, Hvidovre, Denmark) and plasma was collected. Plasma samples were frozen at –80°C and subsequently analyzed for plasma concentrations of osteocalcin, P1NP, CTX-1, and sclerostin, which were evaluated by a fully automated immunoassay system (iSYS; Immunodiagnostic Systems Ltd., Boldon, UK) by method of chemiluminescence. Assay performance, expressed as inter-run coefficients of variation, was 9% for P-OC, 10% for P-CTX-1, and 8% for P-P1NP. The analyses were performed at the Department of Clinical Biochemistry, Rigshospitalet, Glostrup, Denmark.
2.3. Statistical analysis
Between-group differences were evaluated using a one-way analysis of variance (ANOVA) with Holm-Sidak post hoc testing. Effect sizes (ES) were calculated as differences in means divided by the pooled standard deviation (SD)24 and interpreted as suggested by Hopkins et al.:25 ≤0.2 trivial, >0.2 and ≤0.6 small, >0.6 and ≤1.2 moderate, >1.2 and ≤2.0 large, >2.0 very large. Simple linear regression analyses were performed to test correlations between BMD, anthropometrics, and selected training variables within each group. Results are presented as means ± SD. The significance level was set at p < 0.05.
3. Results
3.1. Anthropometrics
FTE had a 12.7% lower total fat percentage compared to UE (p < 0.05, ES = 0.71), but did not differ in any other body composition variables despite a 5.6 kg lower (p > 0.05, ES = 0.51) total body mass. Compared to UY, FTY had 8.4 kg lower body mass (p < 0.05, ES = 0.67), 2.4 kg/m2 lower body mass index (BMI) (p < 0.05, ES = 0.76), 50.2% lower total fat percentage (p < 0.001, ES = 2.31) and 6.4 kg higher lean body mass (p < 0.001, ES = 1.05) (Table 1).
3.2. Femur BMD
In both legs, FTE had 7.3%–12.9% higher BMD in total proximal femur and all femoral regions (p < 0.05, ES = 0.54–0.70) except the trochanter compared to UE. BMD in femoral trochanter (both legs) was 9.3%–9.7% higher for FTE than UY (p < 0.05, ES = 0.65–0.73). FTY had 24.3%–37.4% higher BMD in all femoral regions and total proximal femur in both legs compared to UY (p < 0.001, ES = 2.01–2.68). Compared to FTE and UE, FTY had 23.9%–65.3% (ES = 1.84–3.38) and 30.5%–82.1% (ES = 2.03–3.76) higher BMD in all femoral regions and total proximal femur in both legs (p < 0.001), respectively (Table 2).
Table 2.
Bone mineral density (g/cm2) in proximal femur in FTY, UY, FTE, and UE (mean ± SD).
| Bone region | FTY (n = 35) | UY (n = 35) | FTE (n = 35) | UE (n = 35) |
|---|---|---|---|---|
| Femoral neck | ||||
| Right | 1.389 ± 0.133***,###,††† | 1.089 ± 0.131*** | 1.043 ± 0.143* | 0.953 ± 0.165 |
| Left | 1.376 ± 0.134***,###,††† | 1.094 ± 0.137*** | 1.037 ± 0.111* | 0.948 ± 0.140 |
| Femoral shaft | ||||
| Right | 1.631 ± 0.151***,###,††† | 1.305 ± 0.153* | 1.302 ± 0.133* | 1.198 ± 0.190 |
| Left | 1.639 ± 0.154 ***,###,††† | 1.319 ± 0.164* | 1.298 ± 0.128* | 1.200 ± 0.177 |
| Femoral wards | ||||
| Right | 1.319 ± 0.172***,###,††† | 0.964 ± 0.144***,††† | 0.821 ± 0.163* | 0.727 ± 0.160 |
| Left | 1.326 ± 0.175***,###,††† | 0.965 ± 0.151***,††† | 0.802 ± 0.132* | 0.728 ± 0.141 |
| Femoral trochanter | ||||
| Right | 1.236 ± 0.131***,###,††† | 0.900 ± 0.119 | 0.984 ± 0.140# | 0.921 ± 0.158 |
| Left | 1.232 ± 0.135***,###,††† | 0.906 ± 0.118 | 0.994 ± 0.124# | 0.944 ± 0.148 |
| Total proximal femur | ||||
| Right | 1.422 ± 0.132***,###,††† | 1.097 ± 0.123 | 1.120 ± 0.126* | 1.037 ± 0.161 |
| Left | 1.421 ± 0.130***,###,††† | 1.106 ± 0.129 | 1.124 ± 0.110* | 1.048 ± 0.150 |
Abbreviations: FTE = football-trained elderly men ; FTY = football-trained young men; UE = untrained elderly men; UY = untrained young men.
p < 0.05, ***p < 0.001, compared with UE; #p < 0.05, ###p < 0.001, compared with UY; †††p < 0.001, compared with FTE.
3.3. Whole-body BMD and BMC
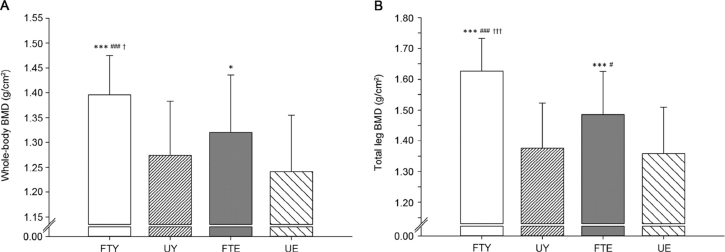
The whole-body scan showed that FTE had 6.4% higher whole-body BMD than UE (p < 0.05, ES = 0.69). Furthermore, FTE had 7.2% (ES = 0.55) and 8.6% (ES = 0.64) higher whole-body BMC compared to both UE and UY (3356 ± 408 g vs. 3132 ± 401 g and 3091 ± 416 g, p < 0.05), respectively. FTY showed 9.6% higher whole-body BMD (ES = 1.28) and 16.7% higher whole-body BMC (3608 ± 297 g vs. 3091 ± 416 g) (ES = 1.43) than UY (p < 0.001). Compared to FTE and UE, FTY had 5.8% (p < 0.05, ES = 0.77) and 12.5% (p < 0.001, ES = 1.58) higher whole-body BMD and 7.5% (p < 0.05, ES = 0.71) and 15.2% (p < 0.001, ES = 1.35) higher whole-body BMC, respectively (Fig. 1A).
Fig. 1.
(A) Whole-body and (B) total leg BMD in elite football-trained young men (FTY), untrained young men (UY), lifelong football-trained elderly men (FTE), and untrained elderly men (UE). *p < 0.05, ***p < 0.001, compared with UE; #p < 0.05, ###p < 0.001, compared with UY; †p < 0.05, †††p < 0.001, compared with FTE.
3.4. Leg BMD and BMC
FTE had 9.4% higher leg BMD (p < 0.001, ES = 0.87) and 10.2% higher leg BMC (1329 ± 164 g vs. 1206 ± 181 g, p < 0.05, ES = 0.71) compared to UE. Furthermore, leg BMD and leg BMC were 7.9% (ES = 0.76) and 11.6% (1329 ± 164 g vs. 1191 ± 188 g, ES = 0.79) higher in FTE than in UY (p < 0.05). FTY had 18.2% higher leg BMD (ES = 1.95) and 22.2% higher leg BMC (1455 ± 115 g vs. 1191 ± 188 g, ES = 1.70) than UY (p < 0.001). Compared to FTE and UE, FTY had 9.5% (ES = 1.13) and 19.7% (ES = 2.05) higher leg BMD (p < 0.001), as well as 9.5% (p < 0.05, ES = 0.89) and 20.6% (p < 0.001, ES = 1.64) higher leg BMC, respectively (Fig. 1B).
3.5. Bone turnover markers
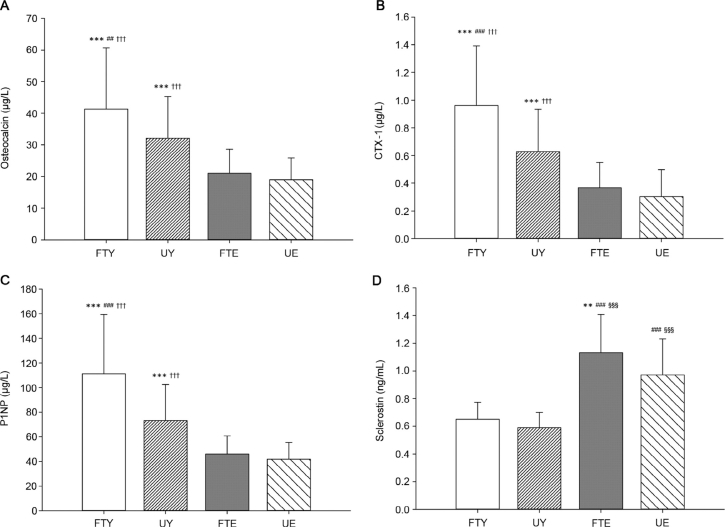
For FTY, the BTM plasma concentrations were 29% higher for osteocalcin (p < 0.01, ES = 0.56), 53% higher for CTX-1 (p < 0.001, ES = 0.88), and 52% higher for P1NP (p < 0.001, ES = 0.95) than for UY. The plasma concentrations of osteocalcin, CTX-1, and P1NP were 53%–117% (ES = 1.03–1.54), 71%–217% (ES = 1.03–1.97), and 59%–166% (ES = 1.17–1.97) higher, respectively, for both FTY and UY compared to FTE and UE (p < 0.001). On the other hand, the plasma concentration of sclerostin was 50%–92% (ES = 1.58–2.54) higher in FTE and UE compared to FTY and UY (p < 0.001). FTE had 17% higher plasma sclerostin (p < 0.01, ES = 0.59) compared to UE (Fig. 2).
Fig. 2.
Concentration of the plasma bone turnover markers (A) osteocalcin, (B) carboxy-terminal type-1 collagen crosslinks (CTX-1), (C) procollagen type-1 amino-terminal propeptide (P1NP), and (D) sclerostin in elite football-trained young men (FTY), untrained young men (UY), lifelong football-trained elderly men (FTE), and untrained elderly men (UE). **p < 0.01, ***p < 0.001, compared with UE; ##p < 0.01, ###p < 0.001, compared with UY; †††p < 0.001, compared with FTE; §§§p < 0.001, compared with FTY.
3.6. Correlations
Within FTY and FTE, lean body mass was the only independent variable correlated with whole-body BMD (p < 0.05 and p < 0.01, respectively). Besides lean body mass (p < 0.001), whole-body BMC was also correlated with body mass (p < 0.001) and height (p < 0.01) in FTY and height (p < 0.01) in FTE. Total proximal femur BMD was only correlated with age (p < 0.05) for FTE. The number of years of regular football training was only correlated with whole-body BMC in FTE (p < 0.05), with increasing years of regular football training resulting in lower BMC. On performing an age-adjusted multiple linear regression, however, this correlation disappeared (p > 0.05). Within UY, age (p < 0.05), height (p < 0.01), lean body mass (p < 0.01), body mass (p < 0.001), and BMI (p < 0.001) were all correlated with whole-body BMD and BMC, but only body mass and lean body mass (p < 0.05) with total proximal femur BMD. Within UE, both body mass (p < 0.05), height, and lean body mass (p < 0.001) were correlated with whole-body BMC (Table 3).
Table 3.
Correlations of BMD and BMC with anthropometric and football training variables in FTY, UY, FTE, and UE.
| Age | Body mass | Height | BMI | Fat mass | Lean body mass | Sessions/week | Years of football | |
|---|---|---|---|---|---|---|---|---|
| Whole-body BMD | ||||||||
| FTY | 0.036 | 0.329 | 0.116 | 0.281 | 0.017 | 0.352* | 0.141 | 0.077 |
| UY | 0.423* | 0.646*** | 0.438** | 0.538*** | 0.185 | 0.528** | – | – |
| FTE | 0.303 | 0.262 | 0.272 | 0.091 | 0.007 | 0.455** | 0.057 | 0.306 |
| UE | 0.193 | 0.245 | 0.332 | 0.053 | 0.218 | 0.282 | – | – |
| Whole-body BMC | ||||||||
| FTY | 0.056 | 0.659*** | 0.535** | 0.302 | 0.119 | 0.690*** | 0.135 | 0.013 |
| UY | 0.370* | 0.727*** | 0.759** | 0.759** | 0.145 | 0.525** | – | – |
| FTE | 0.354* | 0.330 | 0.504** | 0.024 | 0.038 | 0.630*** | 0.022 | 0.383* |
| UE | 0.202 | 0.378* | 0.594*** | 0.051 | 0.202 | 0.595*** | – | – |
| Total proximal femur (right) BMD | ||||||||
| FTY | 0.192 | 0.019 | 0.014 | 0.032 | 0.081 | 0.026 | 0.028 | 0.194 |
| UY | 0.232 | 0.375* | 0.196 | 0.333 | 0.089 | 0.394* | – | – |
| FTE | 0.382* | 0.074 | 0.122 | 0.003 | 0.130 | 0.268 | 0.105 | 0.242 |
| UE | 0.157 | 0.245 | 0.277 | 0.078 | 0.264 | 0.229 | – | – |
Note: linear regression ρ correlation coefficient.
Abbreviations: BMC = bone mineral content; BMD = bone mineral density; BMI = body mass index; FTE = lifelong football-trained elderly men; FTY = elite football-trained young men; UE = untrained elderly men; UY = untrained young men.
p < 0.05; **p < 0.01; ***p < 0.001.
4. Discussion
The main findings of the present study were that FTE men with more than 40 years of regular football training showed BMD values that were significantly higher than in untrained elderly men, with differences of ~7%–13% in proximal femur and ~6% in whole-body BMD (small–moderate effect). FTE even showed ~9% higher whole-body BMC, ~ 8% higher leg BMD, and ~12% higher leg BMC than UY (moderate effect), despite an age difference of more than 47 years. Likewise, young elite football players with a high volume of weekly football training (5.4 sessions per week) showed significantly higher BMD values than age-matched untrained men in the proximal femur BMD (~24%–37%), and whole-body BMD (~10%) and BMC (~17%) (very large effect). The superior BMD values observed in FTY were accompanied by high concentrations of the bone formation markers osteocalcin and P1NP, which were 29% and 52% higher, respectively, in FTY compared to UY.
4.1. Bone remodeling in response to exercise
The finding of a higher bone mass in young and elderly football players compared to controls agrees with other football studies,14, 15 which have shown that football training involves a high number of intermittent, high-intensity, and diverse activities brought about by fast changes in body displacement. Moreover, a significant correlation between increases in leg BMC and the total number of decelerations, total number of accelerations, and total distance covered during small-sided football training has been found in elderly men with prostate cancer.26 Thus, it is reasonable to assume that it is the lifelong participation in regular football training in FTE and FTY that has affected the bones of the lower limbs for many years and accounts for the increased BMD and BMC values observed in the present study.
Other cross-sectional studies comparing football players with untrained controls are in line with our results. Wittich et al.27 reported that professional male first division football players have 12.3% and 18.0% higher whole-body BMD and BMC, respectively, compared to age- and BMI-matched controls, which is comparable to the findings in the present study, where FTY showed ~10% higher whole-body BMD and ~17% higher whole-body BMC compared to UY.
In addition to superior whole-body BMD, regional BMD in hip, leg, spine, and calcaneus has been shown to be significantly higher in elite male football players compared to untrained men aged 20–30 years.28 FTY showed ~24%–37% higher BMD in all femoral regions and ~18% higher leg BMD compared to UY, and likely, spine and calcaneal BMD would have been higher in FTY as well, though this was not investigated. In a study by Fredericson et al.,28 hip, calcaneal, and spine BMD were also found to be significantly higher in elite football players than elite long-distance runners. It has been found that ball sports, including football, generate higher ground reaction forces (>4.0 body weight) than long-distance running (2.0–3.0 body weight)29 and, in contrast to the regular pattern of repetitive loading during running, the large number of forces applied in different directions during football appear to improve BMD at several regional sites, which was not observed after long-distance running.28
In the present study, FTE showed ~7%–13% higher BMD in all femoral regions except the trochanter compared to UE (small-moderate effect). The finding that trochanter BMD was not significantly different between FTE and UE might be due to a type II error, as adaptations in femoral trochanter following football training have been found previously to occur simultaneously with adaptations in other femoral regions. Indeed, in a recent cross-sectional study, it was shown that former professional football players <70 years of age still had significantly higher BMD values at several weight-loaded skeletal regions, including the femoral neck and trochanter, more than 10 years after retirement from their sport compared to non-athletic controls.30 The time after the active career was, however, an independent predictor of BMD at the proximal femur, indicating that advantages in BMD accrued through sports participation diminish with time following retirement from football. Thus, continued exercise training is needed to maintain bone mass achieved through sport.31 Additionally, in a cross-sectional study from the National Senior Games involving 560 Olympic athletes with an average age of ~66 years, it was shown that participation in high-impact sports in old age was a significant predictor of high BMD, even in the oldest athletes.32 In contrast to weight-bearing and strength-based exercises, non-weight-bearing sports, especially those with a cyclic movement pattern, have shown limited osteogenic effects. One cross-sectional study even found that swimmers, whose training is high in volume but of a weight-supporting type, have hip and tibia BMD that are lower than inactive control subjects.33 This result lends credence to the importance of the type of exercise on musculoskeletal adaptations.
A disadvantage of cross-sectional studies is the question of causality. It is difficult to ascertain from a cross-sectional study that regular football training is the cause for improvements in BMD, and that these improvements are not due to confounding factors, such as genetics, selection bias, diet, etc. However, a large number of intervention studies investigating the effects of football training on bone adaptations have confirmed our findings. Following 14–48 weeks of regular football training untrained elderly men had increased proximal femur BMD by up to 1.8% after 4 months and up to 5.4% after 12 months;18 middle-aged women had increased femoral shaft and trochanter BMD by 1.7% and 2.4%, respectively, after 15 weeks of training;21 men with prostate cancer had increased total proximal femur BMD (0.015–0.017 g/cm2) and femoral shaft BMD (0.018–0.024 g/cm2) after 32 weeks of football training compared to controls;22 and premenopausal untrained women had increased BMD in the left and right tibia by 2.6% and 2.1%, respectively, after 14 weeks of football training.19 The differences in proximal femur BMD between FTY and UY (~24%–37%) as well as between FTE and UE (~7%–13%) are well beyond the changes observed after a short period of football training in these intervention studies. This finding indicates that the large improvements in FTY and FTE are the result of many years of regular football training that cannot be achieved with short training interventions. In addition to improvements in the femoral region, increases of 2.3% in whole-body BMD have been observed in premenopausal women after 16 months of recreational football,20 as well as increases in leg BMC of 41 g in healthy untrained men after 12 weeks34 and increases in leg BMD after 64 weeks of football.35 FTY and FTE had 264 g (large effect) and 123 g (moderate effect) higher leg BMC than UY and UE, respectively, which is much more pronounced than improvements observed after a short period of recreational football.
4.2. Bone turnover markers and response to exercise
In order to investigate the mechanisms behind the improvements in BMD and BMC as an effect of lifelong football training, we measured concentrations of the BTMs osteocalcin, P1NP, CTX-1, and sclerostin. Bone turnover is balanced by the coupling of bone formation and bone resorption, which results in a lifelong and continuous remodeling of bone.36 BTMs are products of bone cell metabolism and can be grouped into 2 categories based on the metabolic phase during which they are produced. The International Osteoporosis Foundation has recommended P1NP as a reference bone formation marker and CTX-1 as a reference bone resorption marker. Osteocalcin is a late marker of osteoblastic activity and has thus been used as a bone formation marker as well. Sclerostin is a marker of osteocyte activity, as it is secreted by osteocytes and inhibits bone formation by inhibiting Wnt signaling in the osteoblasts.37 An increase in bone turnover seen with aging and pathologic states such as osteoporosis may lead to deterioration of bone microarchitecture, and thus contribute to increased risk of fractures in addition to low BMD.38
FTY showed significantly higher concentrations of plasma osteocalcin (29%), CTX-1 (53%), and P1NP (52%) compared to UY (small–moderate effect). This is slightly higher compared to findings in male premier league football players, who at the end of the season had plasma osteocalcin and CTX-1 concentrations that were 22% and 34% higher, respectively, compared with a control group.39 In elite female football players, plasma osteocalcin has been shown to be 45% higher than in untrained young women.40 In a recent study investigating the acute changes in BTMs after football training, it has been shown that plasma osteocalcin and P1NP are significantly increased in response to a single bout of small-sided football, with peak values at 13.5 min (~15% increase) for P1NP and 48 h for osteocalcin (~10% increase) after exercise.41 Thus, the increases observed in FTY are expected to be a direct result of the football training. As short (13.5 min) and long (4 × 13.5 min) duration of small-sided football appears to acutely increase plasma osteocalcin and P1NP to a similar degree, exercise duration may not be crucial to the magnitude of the bone turnover response, and as little as 13.5 min of football training might be sufficient to maximize signaling through mechanotransduction pathways and stimulate osteoblast activity.
It is suggested that if these acute changes in bone formation markers are replicated in subsequent exercise bouts, they may contribute to chronic osteogenic adaptations and increased bone mass due to repeated bouts of exercise. In line with this, an association between increases in BMD and increases in BTMs has been proposed in several intervention studies.18, 21, 26 Following a short period of regular football training, significant increases in BTMs have been found repeatedly.18, 21, 26, 40, 42 Thus, increases in osteocalcin of 21%–45% have been shown in elderly men (45%),18 middle-aged women (37%),21 untrained women (37%),40 men with prostate cancer (34%),26 and female hospital employees (21%)42 after 12–16 weeks of recreational football. Increases in P1NP of 41%–52% and CTX-1 of 42%–43% have also been found in some, but not all, of these studies.18, 21, 26 In the study involving men with prostate cancer, the concentration of CTX-1 was unchanged, whereas in the study involving elderly men, CTX-1 was only increased after 12 months. It may be hypothesized that the anabolic response after football training is due to a substantial and early improvement in bone formation, while increases in bone resorption might be less pronounced, delayed, or absent.
In the present study, the 2 young groups had significantly higher plasma osteocalcin, CTX-1, and P1NP than the 2 elderly groups (moderate–large effect), whereas the opposite was the case for sclerostin (large–very large effect). However, this is expected as a large cross-sectional study has shown that P1NP and CTX-1 peak between the ages of 15 and 19 years in men, after which the level of both markers gradually declines,43 whereas osteocalcin seems to peak between the ages of 20 and 29 years before declining and stabilizing.44 The opposite is seen for sclerostin levels, which are increased 4.6-fold in men over the lifespan.45 As sclerostin inhibits bone formation and is proposed to possibly be downregulated by mechanical loading,46 the 17% higher plasma sclerostin in FTE compared to UE was surprising. However, FTE were significantly older than UE, and this age difference might explain the higher sclerostin in FTE.
4.3. Correlations of BMD
To investigate potential correlations between BMD, body composition, and selected training variables, simple linear regression analyses were performed in our study. Lean body mass was the only independent variable correlated with whole-body BMD in the football players. In UY, on the other hand, age, body mass, height, BMI, and lean body mass were all correlated with whole-body BMD and BMC. This was also observed in an earlier study from Argentina comparing professional male football players with age- and BMI-matched untrained controls.27 The correlation between lean body mass and BMD in football players may indicate that exercise is the main cause of the osteogenic adaptations. In UY, age was independently correlated with whole-body BMD, indicating that the skeleton was not fully developed in this group, as whole-body BMD increased with increasing age. The absence of a correlation between age and whole-body BMD in FTY may indicate that elite football players reach their peak bone mass earlier than untrained individuals. Neither the number of football training sessions per week nor the number of years of regular football training were independently correlated with BMD in the football players. It is most likely that BMD in football players is determined by a number of training parameters other than training sessions per week and years of regular training, which could include age of onset, training volume in childhood and adolescence, training intensity, or playing position. It may also be speculated that the BMD of the football players after a certain amount of football training have reached a threshold level that cannot easily be exceeded, which would result in no correlation between age, playing age, etc., and BMD.
4.4. Limitations of the study
Cross-sectional study designs have limitations. Engaging in exercise during skeletal growth is unequivocally more osteogenic than engaging in exercise during skeletal maturity for several reasons, including the fact that periosteal expansion occurs predominantly during growth, providing a window of opportunity to significantly and irreversibly enhance periosteal bone growth and strength through exercise during childhood.47 FTE and FTY had participated in regular football training for approximately 60 and 17 years, respectively. With an average age of 72 and 22 years, respectively, both groups played football during childhood and adolescence. Whether the skeletal adaptations in response to football training have occurred in particular during childhood, adolescence, or adulthood, or throughout the lifespan, remains uncertain. Furthermore, we cannot rule out a role for self-selection, meaning that men with genetically strong muscle and bones may be more likely to choose to play football than men with genetically weaker bones. However, the increased concentrations of BTMs in FTY support the hypothesis that higher BMD is caused by regular training.
4.5. Importance of improved bone mineralization
Despite being merely surrogate measures, BMD and BMC are strongly related to bone strength and thus crucial in regard to whether or not an individual will experience a fracture. Studies in rats have shown that load-induced increases in BMD and BMC in the right ulna of 5.4% and 6.9%, respectively, will lead to a 64% increase in ultimate force (the maximum force the bone could support before failing) and a 94% increase in energy to failure (the amount of energy absorbed by the bone before failure).13 This suggests that even small changes in bone mass can significantly improve bone strength by favorably altering bone geometry, and that superior BMD appears to be a clear indicator of a reduced risk for bone fractures.48 A large community-based cohort study involving approximately 6000 men with a mean age of 73 years has been conducted to determine the epidemiology of osteoporosis and related fractures in men.38 It was shown in that study that lower BMD was associated with a higher risk of fracture, and that each standard deviation decrease in hip BMD increased the risk of hip fracture by 3.2-fold. Thus, the improved BMD in FTE and FTY observed in the present study potentially reduces the risk of hip fractures significantly. Furthermore, BMD loss in the femoral neck during a 4.5-year follow-up was estimated at 0.008, 0.014, and 0.021 g/cm2 for men aged 65, 75, and 85 years, respectively.49 The authors suggested that this bone loss in 85-year-old men may be sufficient to increase the risk of hip fracture by 25% over 4.6 years. FTE had a femoral neck BMD in both legs that was 0.089–0.090 g/cm2 higher than for UE, representing a magnitude of difference way beyond the estimated age-related change over 4.5 years.
It has been proven that fractures beget more fractures, meaning that once an individual has had a fracture, they are at high risk of suffering an additional fracture.4 Osteoporosis-related fractures impose a substantial burden of costs, disability, and mortality in older men. The mortality risk is greatest in the first 3 months after a hip fracture (relative hazards = 7.95), but all-cause mortality risk is still elevated 10 years after a hip fracture (relative hazards = 1.79).50 Generally, the mortality risk is greater in men than in women due to a higher operative risk and more postoperative complications and comorbidities in men. Furthermore, hip fractures can reduce the quality of life as a result of functional impairment and decreased social interactions. Finding ways to prevent fractures in the elderly is a crucial challenge with a major impact on the future of public health. In a study published in The Lancet aimed at investigating the long-term effects of football training after retirement, the authors observed that benefits in leg BMD achieved through regular football training were preserved for 10–20 years after retirement from the sport.31 Indeed, soccer players who had been retired for 15 years had a residual BMD benefit that was about half that found in active soccer players, whereas football players retired for over 35 years had no significant residual benefit in BMD. Consequently, to preserve the large gains in bone mineralization observed in FTY and FTE, continuation of lifelong regular high-impact exercise such as football, basketball,51 and team handball52 training appears to be necessary.
5. Conclusion
Lifelong trained male football players aged 65–80 years and young elite football players aged 18–30 years have proximal femur and whole-body BMD that are markedly higher than in age-matched untrained men. Despite an age difference of more than 47 years, elderly football players have significantly higher femoral trochanter BMD, leg BMD, whole-body BMC, and leg BMC than untrained young men. The superior BMD values observed in young elite football players may partly be explained by increased concentrations of the bone formation markers osteocalcin and P1NP. The present results are promising in regard to the osteogenic effect of football training as a health-enhancing physical activity for both young and elderly men, as lifelong regular football training may diminish age-dependent bone loss and risk of osteoporosis and related fractures.
Acknowledgments
We would like to thank all the participants for their committed participation. We would also like to thank Jon Egelund, Thomas Beck, Andreas Møller, Mikkel Alleslev Larsen, Christian Ritz, Nadia Quardon, and Jens Romlund Halgreen for their excellent practical and technical assistance. The scientific advice given by Morten Bredsgaard Randers Thomsen, Christian M. Werner, and Tim Meyer was also greatly appreciated. Special thanks go to the Danish Football Association (Dansk Boldspil-Union), DBU København and Birgitte Harder in particular, for the irreplaceable assistance with recruiting Danish football players.
Authors' contributions
MH conceived of the study design, applied for funding, carried out the data collection and analysis, interpreted the study results, and drafted the manuscript; EWH carried out data collection, interpreted the study results, and edited the manuscript; TH, BF, JJN, NRJ, JLA, and JWH carried out data collection and edited the manuscript; PK conceived of the study design, applied for funding, interpreted the study results and edited the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Reginster J.Y., Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2006;38(Suppl. 1):S4–9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Kinsella K., Wan H. US Census Bureau, US Government Printing Office; Washington, DC: 2009. International population reports, P95/09-1, an aging world: 2008. [Google Scholar]
- 3.Owen N., Healy G.N., Matthews C.E., Dunstan D.W. Too much sitting: the population-health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38:105–113. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cauley J.A. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68:1243–1251. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernlund E., Svedbom A., Ivergård M., Compston J., Cooper C., Stenmark J. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svedbom A., Hernlund E., Ivergård M., Compston J., Cooper C., Stenmark J. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013;8:137. doi: 10.1007/s11657-013-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonen S., Laan R.F., Barton I.P. Effect of osteoporosis treatments on risk of non-vertebral fractures: review and meta-analysis of intention-to-treat studies. Osteoporos Int. 2005;16:1291–1298. doi: 10.1007/s00198-005-1945-x. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T., Blangero J., Eisman J. Genetic epidemiological approaches to the search for osteoporosis genes. J Bone Miner Res. 2000;15:392–401. doi: 10.1359/jbmr.2000.15.3.392. [DOI] [PubMed] [Google Scholar]
- 9.Bandela V., Munagapati B., Karnati R.K.R., Venkata G.R.S., Nidudhur SR. Osteoporosis: its prosthodontic considerations—A review. J Clin Diagn Res. 2015;9:ZE1–4. doi: 10.7860/JCDR/2015/14275.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.New S.A. Exercise, bone and nutrition. Proc Nutr Soc. 2001;60:265–274. doi: 10.1079/pns200085. [DOI] [PubMed] [Google Scholar]
- 11.Moreira L.D.F., de Oliveira M.L., Lirani-Galvão A.P., Marin-Mio R.V., dos Santos R.N., Lazaretti-Castro M. Physical exercise and osteoporosis: effects of different types of exercises on bone and physical function of postmenopausal women. Arq Bras Endocrinol Metab. 2014;58:514–522. doi: 10.1590/0004-2730000003374. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Cabello A., Ara I., González-Agüero A., Casajús J.A., Vicente-Rodríguez G. Effects of training on bone mass in older adults. Sports Med. 2012;42:301–325. doi: 10.2165/11597670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Turner C.H., Robling A.G. Designing exercise regimens to increase bone-strength. Exerc Sports Sci Rev. 2003;31:45–50. doi: 10.1097/00003677-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Randers M.B., Nybo L., Petersen J., Nielsen J.J., Christiansen L., Bendiksen M. Activity profile and physiological response to football training for untrained males and females, elderly and youngsters: influence of the number of players. Scand J Med Sci Sports. 2010;20 (Suppl. 1):S14–23. doi: 10.1111/j.1600-0838.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- 15.Randers M.B., Nielsen J.J., Bangsbo J., Krustrup P. Physiological response and activity profile in recreational small-sided football: no effect of the number of players. Scand J Med Sci Sports. 2014;24 (Suppl. 1):S130–7. doi: 10.1111/sms.12232. [DOI] [PubMed] [Google Scholar]
- 16.Krustrup P., Mohr M., Ellingsgaard H., Bangsbo J. Physical demands during an elite female soccer game: importance of training status. Med Sci Sports Exerc. 2005;37:1242–1248. doi: 10.1249/01.mss.0000170062.73981.94. [DOI] [PubMed] [Google Scholar]
- 17.Stølen T., Chamari K., Castagna C., Wisløff U. Physiology of soccer: an update. Sports Med. 2005;35:501–536. doi: 10.2165/00007256-200535060-00004. [DOI] [PubMed] [Google Scholar]
- 18.Helge E.W., Andersen T.R., Schmidt J.F., Jørgensen N.R., Hornstrup T., Krustrup P. Recreational football improves bone mineral density and bone turnover marker profile in elderly men. Scand J Med Sci Sports. 2014;24(Suppl. 1):S98–104. doi: 10.1111/sms.12239. [DOI] [PubMed] [Google Scholar]
- 19.Helge E.W., Aagaard P., Jakobsen M.D., Sundstrup E., Randers M.B., Karlsson M.K. Recreational football training decreases risk factors for bone fractures in untrained premenopausal women. Scand J Med Sci Sports. 2010;20(Suppl. 1):S31–9. doi: 10.1111/j.1600-0838.2010.01107.x. [DOI] [PubMed] [Google Scholar]
- 20.Krustrup P., Hansen P.R., Andersen J.L., Jakobsen M.D., Sundstrup E., Randers M.B. Long-term musculoskeletal and cardiac health effects of recreational football and running for premenopausal women. Scand J Med Sci Sport. 2010;20 (Suppl. 1):S58–71. doi: 10.1111/j.1600-0838.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- 21.Mohr M., Helge E.W., Petersen L.F., Lindenskov A., Weihe P., Mortensen J. Effects of soccer vs. swim training on bone formation in sedentary middle-aged women. Eur J Appl Physiol. 2015;115:2671–2679. doi: 10.1007/s00421-015-3231-8. [DOI] [PubMed] [Google Scholar]
- 22.Uth J., Hornstrup T., Christensen J.F., Christensen K.B., Jørgensen N.R., Schmidt J.F. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteoporos Int. 2016;27:1507–1518. doi: 10.1007/s00198-015-3399-0. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak J., Junge A. Twenty years of the FIFA Medical Assessment and Research Centre: from ‘Medicine for Football’ to ‘Football for Health’. Br J Sports Med. 2015;49:561–563. doi: 10.1136/bjsports-2015-094805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. 2nd ed. Lawrence Erlbaum; Hillsdale, NJ: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 25.Hopkins W., Marshall S., Batterham A., Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sport Exerc. 2009;41:3–13. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 26.Uth J., Hornstrup T., Christensen J.F., Christensen K.B., Jørgensen N.R., Helge E.W. Football training in men with prostate cancer undergoing androgen deprivation therapy: activity profile and short-term skeletal and postural balance adaptations. Eur J Appl Physiol. 2016;116:471–480. doi: 10.1007/s00421-015-3301-y. [DOI] [PubMed] [Google Scholar]
- 27.Wittich A., Mautalen C.A., Oliveri M.B., Bagur A., Somoza F., Rotemberg E. Professional football (soccer) players have a markedly greater skeletal mineral content, density and size than age- and BMI-matched controls. Calcif Tissue Int. 1998;63:112–117. doi: 10.1007/s002239900499. [DOI] [PubMed] [Google Scholar]
- 28.Fredericson M., Chew K., Ngo J., Cleek T., Kiratli J., Cobb K. Regional bone mineral density in male athletes: a comparison of soccer players, runners and controls. Br J Sports Med. 2007;41:664–668. doi: 10.1136/bjsm.2006.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crossley K., Bennel K., Wringley T., Oakes B.W. Ground reaction forces, bone characteristics, and tibial stress fracture in male runners. Med Sci Sports Exerc. 1999;31:1088–1093. doi: 10.1097/00005768-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Uzunca K., Birtane M., Durmus-Altun G., Ustun F. High bone mineral density in loaded skeletal regions of former professional football (soccer) players: what is the effect of time after active career? . Br J Sports Med. 2005;39:154–158. doi: 10.1136/bjsm.2003.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlsson M.K., Linden C., Karlsson C., Johnell O., Obrant K., Seeman E. Exercise during growth and bone mineral density and fractures in old age. The Lancet. 2000;355:469–470. doi: 10.1016/s0140-6736(00)82020-6. [DOI] [PubMed] [Google Scholar]
- 32.Leigey D., Irrgang J., Francis K., Cohen P., Wright V. Participation in high-impact sports predicts bone mineral density in senior Olympic athletes. Sports Health. 2009;1:508–513. doi: 10.1177/1941738109347979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferry B., Duclos M., Burt L., Therre P., Le Gall F., Jaffré C. Bone geometry and strength adaptations to physical constraints inherent in different sports: comparison between elite female soccer players and swimmers. J Bone Miner Metab. 2011;29:342–351. doi: 10.1007/s00774-010-0226-8. [DOI] [PubMed] [Google Scholar]
- 34.Krustrup P., Nielsen J.J., Krustrup B.R., Christensen J.F., Pedersen H., Randers M.B. Recreational soccer is an effective health-promoting activity for untrained med. Br J Sports Med. 2009;43:825–831. doi: 10.1136/bjsm.2008.053124. [DOI] [PubMed] [Google Scholar]
- 35.Randers M.B., Nielsen J.J., Krustrup B.R., Sundstrup E., Jakobsen M.D., Nybo L. Positive performance and health effects of a football training program over 12 weeks can be maintained over a 1-year period with reduced training frequency. Scand J Med Sci Sports. 2010;20 (Suppl. 1):S80–9. doi: 10.1111/j.1600-0838.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 36.Shetty A., Kapoor N., Bondu J.D., Thomas N., Paul T.V. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocr Metab. 2016;20:846–852. doi: 10.4103/2230-8210.192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheater G., Elshahaly M., Tuck S.P., Datta H.K., van Laar J.M. The clinical utility of bone marker measurements in osteoporosis. J Transl Med. 2013;11:201. doi: 10.1186/1479-5876-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cawthon P.M., Shahnazari M., Orwoll E.S., Lane N.E. Osteoporosis in men: findings from the osteoporotic fractures in men study (MrOS) Ther Adv Musculoskel Dis. 2016;8:15–27. doi: 10.1177/1759720X15621227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlsson K.M., Karlsson C., Ahlborg H.G., Valdimarsson O., Ljunghall S., Obrant K.J. Bone turnover responses to changed physical activity. Calcif Tissue Int. 2003;72:675–680. doi: 10.1007/s00223-002-2052-z. [DOI] [PubMed] [Google Scholar]
- 40.Jackman S.R., Scott S., Randers M.B., Orntoft C., Blackwell J., Zar A. Musculoskeletal health profile for elite female footballers versus untrained young women before and after 16 weeks of football training. J Sports Sci. 2013;31:1468–1474. doi: 10.1080/02640414.2013.796066. [DOI] [PubMed] [Google Scholar]
- 41.Bowtell J.L., Jackman S.R., Scott S., Connolly L.J., Mohr M., Ermidis G. Short duration small sided football and to a lesser extent whole body vibration exercise induce acute changes in markers of bone turnover. Biomed Res Int. 2016;2016:3574258. doi: 10.1155/2016/3574258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barene S., Krustrup P., Jackman S.R., Brekke O.L., Holtermann A. Do soccer and Zumba exercise improve fitness and indicators of health among female hospital employees? A 12-week RCT. Scand J Med Sci Sports. 2014;24:990–999. doi: 10.1111/sms.12138. [DOI] [PubMed] [Google Scholar]
- 43.Li M., Li Y., Deng W., Connolly L.J., Mohr M., Ermidis G. Chinese bone turnover marker study: reference ranges for C-Terminal telopeptide of type I collagen and procollagen I N-Terminal peptide by age and gender. PLoS One. 2014;9:e103841. doi: 10.1371/journal.pone.0103841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gundberg C.M., Looker A.C., Nieman S.D., Calvo M.S. Patterns of osteocalcin and bone specific alkaline phosphatase by age, gender, and race or ethnicity. Bone. 2002;31:703–708. doi: 10.1016/s8756-3282(02)00902-x. [DOI] [PubMed] [Google Scholar]
- 45.Mödder U.I., Hoey K.A., Amin S., McCready L.K., Achenbach S.J., Riggs B.L. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–379. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ardawi M.M., Rouzi A.A., Qari M.H. Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J Clin Endocrinol Metab. 2012;97:3691–3699. doi: 10.1210/jc.2011-3361. [DOI] [PubMed] [Google Scholar]
- 47.Kannus P., Haapasalo M., Sankelo M., Sievänen H., Pasanen M., Heinonen A. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123: 27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 48.Karlsson M.K., Nordqvist A., Karlsson C. Sustainability of exercise-induced increases in bone density and skeletal structure. Food Nutr Res. 2008;52 doi: 10.3402/fnr.v52i0.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cawthon P.M., Ewing S.K., McCulloch C.E., Ensrud K.E., Cauley J.A., Cummings S.R. Loss of hip BMD in older men: the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res. 2009;24:1728–1735. doi: 10.1359/JBMR.090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abrahamsen B., van Staa T., Ariely R., Olson M., Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20:1633–1650. doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- 51.Randers M.B., Hagman M., Brix J., Christensen J.F., Nielsen J.J., Pedersen M.T. Effects of 3 months of full-court and half-court street basketball training on health profile in untrained men. J Sport Health Sci. 2018;7:132–138. doi: 10.1016/j.jshs.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hornstrup T., Wikman J.M., Fristrup B., Póvoas S., Helge E.W., Nielsen S.H. Fitness and health benefits of team handball training for young untrained women—a cross-disciplinary RCT on physiological adaptations and motivational aspects. J Sport Health Sci. 2018;7: 139–148. doi: 10.1016/j.jshs.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]