Abstract
Purpose
Although exercise and sleep duration habits are associated with cognitive function, their beneficial effects on cognitive function remain unclear. We aimed to examine the effect of sleep duration and daily physical activity on cognitive function, elucidating the neural mechanisms using near-infrared spectroscopy (NIRS).
Methods
A total of 23 healthy young adults (age 22.0 ± 2.2 years) participated in this study. Exercise amount was assessed using a uniaxial accelerometer. We evaluated total sleep time (TST) and sleep efficiency by actigraphy. Cognitive function was tested using the N-back task, the Wisconsin Card Sorting Test (WCST), and the Continuous Performance Test–Identical Pairs (CPT-IP), and the cortical oxygenated hemoglobin levels during a word fluency task were measured with NIRS.
Results
Exercise amount was significantly correlated with reaction time on 0- and 1-back tasks (r = −0.602, p = 0.002; r = −0.446, p = 0.033, respectively), whereas TST was significantly correlated with % corrects on the 2-back task (r = 0.486, p = 0.019). Multiple regression analysis, including exercise amount, TST, and sleep efficiency, revealed that exercise amount was the most significant factor for reaction time on 0- and 1-back tasks (β = −0.634, p = 0.002; β = −0.454, p = 0.031, respectively), and TST was the most significant factor for % corrects on the 2-back task (β = 0.542, p = 0.014). The parameter measured by WCST and CPT-IP was not significantly correlated with TST or exercise amount. Exercise amount, but not TST, was significantly correlated with the mean area under the NIRS curve in the prefrontal area (r = 0.492, p = 0.017).
Conclusion
Exercise amount and TST had differential effects on working memory and cortical activation in the prefrontal area. Daily physical activity and appropriate sleep duration may play an important role in working memory.
Keywords: Cortical oxygenation, Executive function, Exercise, Sustained attention, Total sleep time, Working memory
1. Introduction
Exercise is known to be associated with cognitive function, but its effects differ depending on the type of exercise and methodology in cognitive function tests.1, 2, 3 Our recent study on left ventricle responses to exercise in athletes suggested that habitual exercise may play an important role in effective hemodynamics.4 Regular aerobic exercise alleviated the age-related reduction in brain hemodynamics by more than a decade in healthy men aged 18–79 years.5 Exercise increases the level of oxyhemoglobin (OxyHb) in the frontal cortices and enhances advantages in cognition.6, 7 The benefits of exercise training, such as spatial learning and recall, hippocampal cell activity, and brain-derived neurotrophic factors, are impaired after chronic moderate sleep restriction.8 Sleep and exercise influence each other through complex, reciprocal interactions including multiple physiological and psychological pathways;9 however, their beneficial interaction on cognitive function has yet to be clarified.
Poor sleep quality increases affective symptomatology as well as the interaction between stress and performance on an emotional memory test and sustained attention task. It is also associated with a negative cognitive bias, with a concomitant decrease in sustained attention to non-emotional stimuli in undergraduate students.10, 11 Sleep restriction represents an important facet of modern life that leads to cognitive performance declines, including lapses of attention, slowed working memory, reduced cognitive throughput, and perseveration of thought.12, 13 An epidemiological study demonstrated that fatigue and short and long sleep duration were associated with both objectively assessed and self-reported decreases in cognitive function in the general population.14 Given the importance of early detection and prevention of sleep-related cognitive impairment, sleep management may be clinically beneficial.
The N-back task, Wisconsin Card Sorting Test (WCST), and Continuous Performance Test–Identical Pairs (CPT-IP) are widely used in studies of cognitive function in young adults.15, 16 The N-back task has been used in many human studies to investigate the neural basis of the prefrontal cortex on working memory processes.16, 17 Working memory refers to a brain system that provides temporary storage and manipulation of the information necessary for such complex cognitive tasks as language comprehension, learning, and reasoning.18 The WCST, a neurocognitive test, is frequently used for evaluating executive function.19, 20 Executive functions are the high-level cognitive processes that facilitate new ways of behaving and that optimize one's approach to unfamiliar circumstances.21 The CPT-IP measures sustained attention or vigilance that requires identification of identical stimulus pairs within a continuously presented series of stimuli22 and have been used extensively in psychiatric research, especially in studies concerned with the role of sustained attention in schizophrenia.23 These cognitive functions are related to the frontal lobe.16, 21, 24
Near-infrared spectroscopy (NIRS) allows for noninvasive measurements of regional cerebral blood flow by assessing the relative concentration of OxyHb with high temporal resolution.25 The approach has been applied to the examination of psychiatric patients as well as healthy individuals.25, 26 Psychiatric patients had slower and reduced increase in prefrontal activation when compared to healthy controls.25 A recent NIRS study revealed an increase in frontal brain activation during walking in healthy young adults.27 We have previously examined the effects of 1 night of insufficient sleep (i.e., sleep duration less than 4 h) on cognitive function using N-back task (2-back), WCST, and CPT-IP, and cortical oxygenation as assessed by NIRS in healthy adults. Our findings suggested that 1 night of insufficient sleep lowered sustained attention, vigilance, and vigor using the Profile of Mood States, which was accompanied by decreased cortical OxyHb levels in the frontal lobe.28, 29 NIRS offers several advantages over functional magnetic resonance imaging (fMRI), such as feasibility for measurement of concentration changes in oxygenated hemoglobin, finer temporal resolution, and ease of administration, in addition to being noninvasive and portable.30 Thus, evaluation of the cortical oxygenation response using NIRS may facilitate our understanding of the role of exercise and/or sleep in cognitive function related to daily life and industrial safety.
Accordingly, we hypothesized that daily physical activity (PA) and sleep duration would have differential involvement on working memory, executive function, and sustained attention. In this study, we investigated the effects of daily PA and sleep duration on these cognitive functions using NIRS to elucidate neural mechanisms in healthy young adults.
2. Methods
2.1. Study participants
A total of 23 healthy young adults (13 males and 10 females; age 22.0 ± 2.2 years) participated in this study. We excluded those who were taking any medications or had a history of major physical illness, neurologic disorder, substance abuse, smokers, alcohol abuse, and severe head trauma. The subjects exhibited no problems in their social lives, and all were healthy, which was confirmed by a medical doctor who conducted structured clinical interviews testing for Diagnostic and Statistical Manual of Mental Disorders-IV axis I disorders. The nocturnal sleep duration on the night immediately preceding the test was adjusted to 6–8 h, because cortical oxygenation and cognitive function are known to be significantly lower following a night of insufficient sleep versus a night of sufficient sleep.28 For NIRS measurements, the participants were examined in a natural sitting position, without any surrounding distraction. The recording environment was a sound-attenuated room. Participants were tested individually.
The Chubu University and the Nagoya University Ethics Committees approved all procedures associated with the study. Written informed consent was obtained from all participants after the nature of the study and the procedures involved were explained to them.
2.2. Assessment of PA
During measurements, participants wore a uniaxial accelerometer (Lifecorder GS/Me; Suzuken Co. Ltd., Nagoya, Japan) on their belts.31, 32 Measurements were taken over the course of a day; the number of steps walked and exercise amount per day were also evaluated.
2.3. Actigraphy
Actigraphy was performed in 5−7 days for all participants. We measured total sleep time (TST), sleep efficiency (calculated as TST/time spent in bed × 100%), bedtime, and wake-up time. The actigraph (Ambulatory Monitoring Inc., New York, NY, USA) was worn around the wrist of the nondominant hand and was set to store data in 1 min increments. We analyzed actigraphy data using the algorithm supplied by the ActionW-2 clinical sleep analysis software package for Windows (Ambulatory Monitoring Inc.) and a sleep diary. Sleep and activity levels were scored according to the Cole–Kripke formula.33, 34 Diary-derived sleep parameters of bedtime and wake-up time were used to ascertain and set the analysis interval for the actigraphy device.35
2.4. Questionnaires
2.4.1. Pittsburgh Sleep Quality Index (PSQI)
We evaluated sleep quality using the PSQI, a questionnaire that assesses sleep quality and quantity over a 1-month period.10 The PSQI contains 19 items in 7 component domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. The component scores are combined to produce a global sleep quality score ranging from 0 to 27. We considered participants with scores of 6 or greater to be poor sleepers. Participants completed this questionnaire once at baseline.
2.4.2. Epworth Sleepiness Scale (ESS)
In this test, participants are asked to rate on a scale of 0–3 how likely they would be to doze off or fall asleep in each of 8 different situations, based on their usual way of life in recent time.36 The total ESS score is the sum of all responses and ranges from 0 to 24. A score of 11 or greater reflects excessive daytime sleepiness. Participants completed this questionnaire once at baseline.
2.5. Cognitive performance tests
We measured aspects of cognitive function that have been studied extensively in neuroscience.37 Cognitive function was measured at baseline using the Trail Making Test (TMT).38 The N-back task,17 WCST,20 and CPT-IP22 were conducted to evaluate individual differences in working memory, executive function, and sustained attention. Cognitive function was assessed by the N-back task, WCST, and CPT-IP in the morning (from 10:00 a.m. to noon) after the evaluation of PA and sleep.
2.5.1. TMT
The TMT consists of 2 parts (A and B)38 and provides information on speed of processing, visual attention, and executive function. Part A required participants to draw lines as quickly as possible to connect the numbers from 1 to 25 in an ascending pattern, with the numbers distributed in random order on a sheet of paper. In Part B, participants drew lines to connect numbers and letters in alternating patterns by connecting the first number with the first letter, continuing to connect the number–letter pairs until the last number, 13, was reached. Participants were required to connect these numbers sequentially as quickly as possible. Time to completion (Scores, in seconds) was recorded for each part of the TMT.
2.5.2. N-back task
The N-back task was used to measure working memory via software that required participants to update their mental set continually while responding to previously seen stimuli (i.e., numbers).17, 39, 40 Each test comprised 14 trials, each of which had a stimulus duration of 0.4 s, an inter-stimulus interval of 1.4 s, and 0-, 1-, and 2-back conditions. Participants responded to stimuli using the numeric keypad of a computer. Performance was measured as % correct (0-back task = the number correct/14 × 100%; 1-back task = the number correct/13 × 100%; 2-back task = the number correct/12 × 100%) and the mean reaction time for correct hits. To perform N-back working memory tasks, participants monitored a series of number stimuli and were asked to indicate when the presented number was the same as previously presented number. The stimuli consisted of numbers (2, 4, 6, or 8) shown in random sequence and displayed at the points of a diamond-shaped box.40
2.5.3. WCST
The WCST (WCST-Keio F-S version; Japanese Stroke Data Bank, Osaka, Japan) measured executive functions such as abstract reasoning ability and the ability to shift cognitive strategies in response to changing environmental contingencies.19, 41 In this study, we measured category achievement and total errors. Category achievement was the number of categories for which 6 consecutive correct responses were achieved (8 was the maximum number of categories that could be achieved). The sum of the total errors was the number of perseverative error scores (Nelson types) and non-perseverative error scores.42
2.5.4. CPT-IP
The CPT-IP (Biobehavioral Technologies, Inc., New York, NY, USA) was used to measure sustained attention or vigilance, as described previously.22 A series of 4-digit stimuli were presented for a period of 50 ms, with an inter-stimulus interval of 950 ms. Each session consisted of 150 trials, of which 30 target trials required a response. Two sessions (first and second tests) were conducted, and results from the second test were adopted. Performance was measured as the percentage of correct responses (% corrects) and the signal detection index d prime, a measure of discriminability computed from “hits” and “false alarms”.
2.6. NIRS
Relative concentrations of OxyHb were measured with a 22-channel NIRS recorder (Foire-3000; Shimadzu Co., Kyoto, Japan) while the participant performed a word fluency task. The word fluency task consisted of a 30 s pre-test baseline, a 60 s word fluency task, and a 70 s post-task baseline.25 First, participants were asked to repeat the syllables /a/, /i/, /u/, /e/, /o/, then to generate as many words as they could with the initial syllable /ka/, /sa/, etc. The 3 initial syllables were changed every 20 s during the 60 s task. To conclude the task, participants were asked to repeat the syllables /a/, /i/, /u/, /e/, /o/. NIRS probes (3 cm distance between emitter probes and detector probes) were placed over regions of each hemisphere, the lowest probes positioned along the Fp1–Fp2 line according to the international 10–20 system used in electroencephalography (Fig. 1). The absorption of near-infrared light was measured with a temporal resolution of 0.1 s.
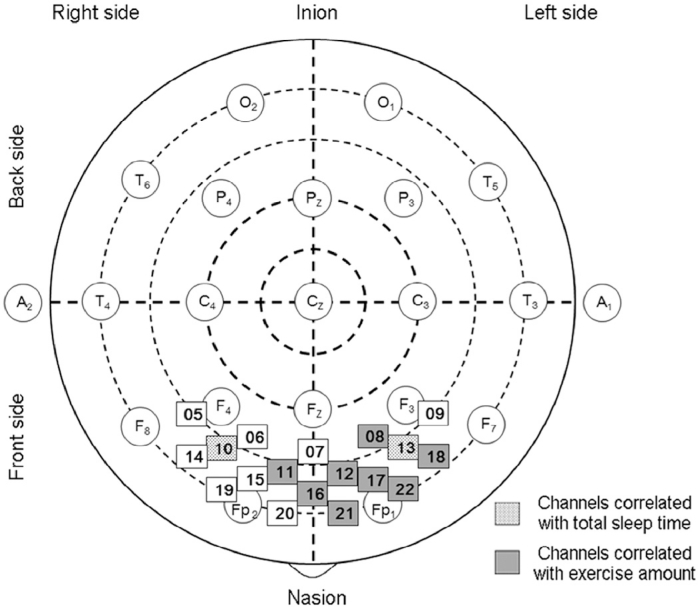
Fig. 1.
Position of near-infrared spectroscopy channels in prefrontal area according to the international 10–20 system. In regional analysis, dotted and gray squares correspond to channels for which total sleep time and exercise amount were significantly correlated with the area under the near-infrared spectroscopy curve, respectively.
The values recorded from 18 channels in the prefrontal area (Channels 5–22) were averaged (Fig. 1). OxyHb data in Channels 1, 2, 3, and 4 that showed low signal-to-noise ratios were excluded from the analysis. The OxyHb was found to be the more sensitive indicator of changes in oxygenation in NIRS measurements,43 and OxyHb has been shown to reflect corticalactivation by simultaneous measurements of glucose metabolism or cerebral blood flow with other methodologies such as positron emission tomography or fMRI.44, 45, 46
We measured peak OxyHb and time to the peak OxyHb from task start. The peak OxyHb has been used in many human studies.27, 47 Time to the peak OxyHb is the time from the start of the task to the peak OxyHb, indicating cortical activation.28, 29 The area under the NIRS curve was measured using the trapezium rule, which approximates the region under the NIRS curve described by the function f(x).28, 29, 48 The reaction time is indicated by the time (s), which is indicated by a perpendicular line from the centroid of a NIRS signal-change area (calculated with positive change) throughout all the task periods.48
In healthy young adults, OxyHb increased during the task period and the time-course patterns of the changes differed depending on the measuring channel.28 In frontal channels, OxyHb increased immediately after the start of the task period, was maintained at the activated level during the task period, and decreased gradually after the task was finished.28, 29 Measuring the peak OxyHb, the area under the NIRS curve, time to the peak OxyHb, and the reaction time was useful for detecting abnormal brain activation.28, 29, 48, 49, 50 NIRS was performed in the morning (from 10:00 a.m. to noon).
2.7. Statistical analysis
All results are presented as mean ± SD. Comparison by gender was performed using a non-paired t test. We performed Pearson's correlation analyses followed by stepwise multiple regression analysis to determine independent parameters that correlated with 0-, 1-, and 2-back tasks; WCST; CPT-IP; and NIRS parameters in relation to age, education, gender, TMT, exercise amount, TST, sleep efficiency, bedtime, and wake-up time. We examined the correlation between exercise amount, TST, sleep efficiency, and the area under the NIRS curve for OxyHb at Channels 5–22 using Pearson's correlation and multiple regression analyses for each channel. A value of p < 0.05 was considered significant. Statistical analyses were performed using SPSS Version 21.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Correlations of PA or sleep duration and cognitive function
Participant characteristics are shown in Table 1. Exercise amount was significantly correlated with reaction time on 0- and 1-back tasks (r = −0.602, p = 0.002; r = −0.446, p = 0.033; respectively) (Table 2), but not with % corrects on 0- and 1-back tasks, % corrects and reaction time on 2-back task; category achievement and total errors on WCST, and % corrects and d prime on CPT-IP. Multiple regression analysis revealed that exercise amount was the most significant factor correlated with reaction time on 0- and 1-back tasks (β = −0.634, p = 0.002; β = −0.454, p = 0.031; respectively) versus TST and sleep efficiency (Table 2).
Table 1.
Participant characteristics (mean ± SD).
| Total (n = 23) | Male (n = 13) | Female (n = 10) | |
|---|---|---|---|
| Age (year) | 22.0 ± 2.2 | 21.6 ± 0.8 | 22.5 ± 3.3 |
| Height (cm) | 166.5 ± 9.0 | 171.8 ± 8.0 | 159.6 ± 4.3 |
| Weight (kg) | 58.2 ± 8.2 | 62.4 ± 7.4 | 52.7 ± 5.7 |
| BMI (kg/m2) | 21.0 ± 2.3 | 21.2 ± 2.5 | 20.7 ± 2.0 |
| Physical activity | |||
| Step number (steps/day) | 7937.9 ± 1961.3 | 7789.8 ± 2107.1 | 8130.4 ± 1846.8 |
| Exercise amount (kcal/day) | 203.5 ± 58.2 | 213.1 ± 61.6 | 191.1 ± 54.0 |
| Total sleep time (min) | 369.5 ± 59.4 | 383.3 ± 54.4 | 351.6 ± 63.7 |
| Sleep efficiency (%) | 96.6 ± 2.7 | 96.9 ± 2.7 | 96.3 ± 2.9 |
| Bedtime | 01:25 ± 00:53 | 01:19 ± 00:51 | 01:33 ± 00:56 |
| Wake-up time | 07:50 ± 00:58 | 07:55 ± 01:00 | 07:43 ± 00:57 |
| PSQI | 4.8 ± 1.9 | 5.3 ± 1.9 | 4.3 ± 1.8 |
| ESS | 7.9 ± 3.6 | 6.9 ± 3.5 | 9.1 ± 3.6 |
| Education (year) | 15.6 ± 1.4 | 15.4 ± 0.7 | 15.9 ± 2.0 |
| Cognitive function tests | |||
| Trail Making Test | |||
| Total score, Part A (s) | 22.4 ± 6.6 | 23.5 ± 7.1 | 20.9 ± 5.7 |
| Total score, Part B (s) | 47.5 ± 10.5 | 48.0 ± 12.4 | 46.7 ± 7.5 |
| N-back task | |||
| 0-back task | |||
| % corrects | 98.6 ± 3.4 | 97.8 ± 4.3 | 99.6 ± 1.1 |
| Reaction time (s) | 513.1 ± 88.9 | 506.2 ± 110.2 | 522.1 ± 54.5 |
| 1-back task | |||
| % corrects | 96.3 ± 6.4 | 96.0 ± 7.1 | 96.8 ± 5.7 |
| Reaction time (s) | 437.6 ± 200.3 | 408.8 ± 183.3 | 475.1 ± 224.7 |
| 2-back task | |||
| % corrects | 91.3 ± 14.4 | 96.1 ± 6.1 | 85.0 ± 19.5 |
| Reaction time (s) | 480.6 ± 255.1 | 440.2 ± 212.5 | 533.3 ± 305.6 |
| WCST | |||
| Category achievement | 5.9 ± 0.3 | 5.9 ± 0.3 | 5.8 ± 0.4 |
| Total errors | 9.6 ± 2.7 | 9.3 ± 3.3 | 9.9 ± 1.7 |
| CPT-IP | |||
| % corrects | 87.4 ± 12.4 | 86.7 ± 13.2 | 88.3 ± 11.9 |
| Signal detection index (d prime) | 2.5 ± 0.8 | 2.7 ± 0.8 | 2.1 ± 0.7 |
| NIRS | |||
| Peak OxyHb (mmol/L) | 0.035 ± 0.023 | 0.042 ± 0.024 | 0.026 ± 0.018 |
| Time to the peak OxyHb (s) | 37.2 ± 17.3 | 39.0 ± 15.7 | 34.9 ± 19.9 |
| Area under the NIRS curve | 17.3 ± 20.5 | 21.5 ± 23.8 | 12.8 ± 15.0 |
| Reaction time (s) | 44.7 ± 17.5 | 48.8 ± 14.9 | 39.5 ± 20.0 |
| Number of words generated (words) | 13.7 ± 5.0 | 13.2 ± 4.7 | 14.5 ± 5.5 |
Abbreviations: BMI = body mass index; CPT-IP = Continuous Performance Test–Identical Pairs; ESS = Epworth Sleepiness Scale; NIRS = near-infrared spectroscopy; OxyHb = oxyhemoglobin; PSQI = Pittsburgh Sleep Quality Index; WCST = Wisconsin Card Sorting Test.
Table 2.
Relationships among the parameters by N-back task, WCST, CPT-IP, and NIRS versus physical activity, TST, and sleep efficiency.
| Simple correlation |
Multiple regression analysis |
Simple correlation |
Multiple regression analysis |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p | β | p | r | p | β | p | |
| 0-back task | % corrects | Reaction time | ||||||
| Exercise amount | −0.129 | 0.558 | −0.139 | 0.554 | −0.602 | 0.002* | −0.634 | 0.002* |
| TST | 0.040 | 0.856 | 0.030 | 0.900 | 0.006 | 0.980 | −0.029 | 0.876 |
| Sleep efficiency | 0.038 | 0.862 | 0.052 | 0.831 | 0.092 | 0.678 | 0.204 | 0.293 |
| 1-back task | % corrects | Reaction time | ||||||
| Exercise amount | 0.327 | 0.127 | 0.334 | 0.133 | −0.446 | 0.033* | −0.454 | 0.031* |
| TST | 0.234 | 0.282 | 0.250 | 0.268 | −0.314 | 0.145 | −0.333 | 0.113 |
| Sleep efficiency | 0.030 | 0.893 | −0.099 | 0.662 | −0.044 | 0.841 | 0.128 | 0.536 |
| 2-back task | % corrects | Reaction time | ||||||
| Exercise amount | 0.195 | 0.372 | 0.209 | 0.292 | −0.113 | 0.607 | −0.108 | 0.626 |
| TST | 0.486 | 0.019* | 0.542 | 0.014* | −0.332 | 0.121 | −0.343 | 0.143 |
| Sleep efficiency | 0.024 | 0.913 | −0.218 | 0.293 | −0.067 | 0.760 | 0.052 | 0.823 |
| WCST | Category achievement | Total errors | ||||||
| Exercise amount | 0.080 | 0.719 | 0.119 | 0.604 | −0.249 | 0.252 | −0.212 | 0.336 |
| TST | −0.037 | 0.866 | 0.030 | 0.899 | −0.246 | 0.257 | −0.184 | 0.416 |
| Sleep efficiency | −0.216 | 0.322 | −0.245 | 0.312 | −0.271 | 0.211 | −0.182 | 0.428 |
| CPT-IP | % corrects | d prime | ||||||
| Exercise amount | −0.163 | 0.458 | −0.148 | 0.525 | −0.036 | 0.869 | −0.074 | 0.747 |
| TST | −0.084 | 0.704 | −0.054 | 0.820 | −0.034 | 0.876 | −0.107 | 0.652 |
| Sleep efficiency | −0.121 | 0.584 | −0.080 | 0.740 | 0.212 | 0.333 | 0.255 | 0.293 |
| NIRS | Peak OxyHb | Time to the peak OxyHb | ||||||
| Exercise amount | 0.407 | 0.054 | 0.394 | 0.067 | 0.326 | 0.130 | 0.373 | 0.091 |
| TST | 0.286 | 0.186 | 0.267 | 0.218 | 0.004 | 0.984 | 0.080 | 0.714 |
| Sleep efficiency | 0.154 | 0.483 | 0.011 | 0.960 | −0.223 | 0.307 | −0.308 | 0.176 |
| NIRS | Area under the NIRS curve | Reaction time | ||||||
| Exercise amount | 0.492 | 0.017* | 0.486 | 0.023* | 0.605 | 0.002* | 0.576 | 0.004* |
| TST | 0.227 | 0.297 | 0.211 | 0.311 | 0.171 | 0.436 | 0.102 | 0.586 |
| Sleep efficiency | 0.131 | 0.550 | −0.010 | 0.960 | 0.279 | 0.197 | 0.155 | 0.417 |
Abbreviations: CPT-IP = Continuous Performance Test–Identical Pairs; NIRS = near-infrared spectroscopy; OxyHb = oxyhemoglobin; TST = total sleep time; WCST = Wisconsin Card Sorting Test.
p < 0.05.
TST was significantly correlated with % corrects on the 2-back task (r = 0.486, p = 0.019), but not with % corrects and reaction time on 0- and 1-back tasks, reaction time on 2-back task, category achievement and total errors on WCST, or % corrects and d prime on CPT-IP. Multiple regression analysis showed that TST was the most significant factor correlated with % corrects on the 2-back task (β = 0.542, p = 0.014) versus exercise amount and sleep efficiency (Table 2). Age, gender, education, sleep efficiency, wake-up time, and ESS were not significant factors for cognitive function. Bedtime was significantly correlated with % corrects on the 2-back task (r = −0.445, p = 0.033), but multiple regression analysis revealed that bedtime and wake-up time were not significant contributing factors for cognitive function. TMT Part A score at baseline was significantly correlated with category achievement on WCST (r = −0.521, p = 0.013), but not with % corrects and reaction time on N-back task, total errors on WCST, % corrects and d prime on CPT-IP, daily PA, or TST. TMT Part B score at baseline showed no significant correlations with cognitive function, daily PA, or TST. We observed no significant difference between TST on Days 5–7 or on the night immediately preceding the tests (372.5 ± 66.8 min vs. 397.5 ± 45.9 min, p = 0.086).
3.2. Correlations of PA or sleep duration and NIRS parameters
Exercise amount was significantly correlated with the area under the NIRS curve and reaction time on NIRS (r = 0.492, p = 0.017; r = 0.605, p = 0.002; respectively), but not with peak OxyHb and time to the peak OxyHb on NIRS. Multiple regression analysis showed that exercise amount was the most significant factor correlated with the area under the NIRS curve and reaction time on NIRS (β = 0.486, p = 0.023; β = 0.576, p = 0.004; respectively) versus TST and sleep efficiency (Table 2). There were also no significant correlations among TST, sleep efficiency, ESS, peak OxyHb, time to the peak OxyHb, area under the NIRS curve, and reaction time on NIRS. Gender was not a significant factor in the NIRS parameters.
Exercise amount was significantly correlated with the area under the NIRS curve (r = 0.502, p = 0.015; r = 0.551, p = 0.006; r = 0.540, p = 0.008; r = 0.481, p = 0.020; r = 0.546, p = 0.007; r = 0.482, p = 0.023; r = 0.430, p = 0.046; r = 0.507, p = 0.016; respectively) in Channels 8, 11, 12, 16, 17, 18, 21, and 22 (Table 3) (Fig. 1). Multiple regression analysis showed that exercise amount was the most significant factor correlated with the area under the NIRS curve (β = 0.466, p = 0.021; β = 0.541, p = 0.011; β = 0.517, p = 0.015; β = 0.470, p = 0.029; β = 0.538, p = 0.009; β = 0.542, p = 0.016; β = 0.456, p = 0.045; β = 0.513, p = 0.025; respectively) in Channels 8, 11, 12, 16, 17, 18, 21, and 22 versus TST and sleep efficiency (Table 3).
Table 3.
Relationships between area under the NIRS curve, exercise amount, TST, and sleep efficiency for Channels 5–22.
| Simple correlation |
Multiple regression analysis |
Simple correlation |
Multiple regression analysis |
Simple correlation |
Multiple regression analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | β | p | r | p | β | p | r | p | β | p | |
| Area under the NIRS curve | Channel 5 | Channel 6 | Channel 7 | |||||||||
| Exercise amount | −0.016 | 0.941 | −0.002 | 0.994 | 0.363 | 0.089 | 0.347 | 0.100 | 0.025 | 0.930 | 0.061 | 0.838 |
| TST | 0.133 | 0.544 | 0.172 | 0.475 | 0.367 | 0.085 | 0.350 | 0.107 | 0.109 | 0.699 | 0.152 | 0.609 |
| Sleep efficiency | −0.081 | 0.713 | −0.132 | 0.589 | 0.169 | 0.442 | 0.009 | 0.968 | −0.301 | 0.275 | −0.334 | 0.273 |
| Area under the NIRS curve | Channel 8 | Channel 9 | Channel 10 | |||||||||
| Exercise amount | 0.502 | 0.015* | 0.466 | 0.021* | 0.244 | 0.261 | 0.201 | 0.347 | 0.335 | 0.118 | 0.324 | 0.110 |
| TST | 0.320 | 0.137 | 0.253 | 0.201 | 0.325 | 0.131 | 0.257 | 0.247 | 0.454 | 0.030* | 0.455 | 0.034* |
| Sleep efficiency | 0.313 | 0.146 | 0.162 | 0.414 | 0.310 | 0.150 | 0.202 | 0.367 | 0.141 | 0.522 | −0.047 | 0.820 |
| Area under the NIRS curve | Channel 11 | Channel 12 | Channel 13 | |||||||||
| Exercise amount | 0.551 | 0.006* | 0.541 | 0.011* | 0.540 | 0.008* | 0.517 | 0.015* | 0.370 | 0.082 | 0.375 | 0.051 |
| TST | 0.123 | 0.577 | 0.090 | 0.656 | 0.077 | 0.728 | 0.017 | 0.933 | 0.505 | 0.014* | 0.537 | 0.009* |
| Sleep efficiency | 0.153 | 0.486 | 0.038 | 0.852 | 0.222 | 0.308 | 0.133 | 0.519 | 0.061 | 0.784 | −0.159 | 0.409 |
| Area under the NIRS curve | Channel 14 | Channel 15 | Channel 16 | |||||||||
| Exercise amount | 0.253 | 0.255 | 0.255 | 0.284 | 0.035 | 0.108 | 0.340 | 0.138 | 0.481 | 0.020* | 0.470 | 0.029* |
| TST | 0.094 | 0.676 | 0.090 | 0.708 | 0.218 | 0.330 | 0.197 | 0.393 | 0.220 | 0.313 | 0.194 | 0.354 |
| Sleep efficiency | 0.032 | 0.886 | −0.038 | 0.877 | 0.119 | 0.599 | 0.003 | 0.989 | 0.158 | 0.472 | 0.024 | 0.910 |
| Area under the NIRS curve | Channel 17 | Channel 18 | Channel 19 | |||||||||
| Exercise amount | 0.546 | 0.007* | 0.538 | 0.009* | 0.482 | 0.023* | 0.542 | 0.016* | 0.377 | 0.084 | 0.367 | 0.113 |
| TST | 0.268 | 0.217 | 0.248 | 0.212 | 0.197 | 0.379 | 0.285 | 0.191 | 0.125 | 0.578 | 0.096 | 0.676 |
| Sleep efficiency | 0.155 | 0.479 | −0.006 | 0.977 | 0.048 | 0.833 | −0.180 | 0.415 | 0.117 | 0.603 | 0.026 | 0.911 |
| Area under the NIRS curve | Channel 20 | Channel 21 | Channel 22 | |||||||||
| Exercise amount | 0.408 | 0.059 | 0.439 | 0.052 | 0.430 | 0.046* | 0.456 | 0.045* | 0.507 | 0.016* | 0.513 | 0.025* |
| TST | −0.198 | 0.376 | −0.193 | 0.383 | −0.096 | 0.670 | −0.059 | 0.798 | 0.089 | 0.695 | 0.116 | 0.598 |
| Sleep efficiency | −0.088 | 0.697 | −0.109 | 0.625 | −0.093 | 0.681 | −0.146 | 0.533 | 0.164 | 0.465 | −0.001 | 0.998 |
Abbreviations: NIRS = near-infrared spectroscopy; TST = total sleep time.
p < 0.05.
TST was significantly correlated with the area under the NIRS curve (r = 0.454, p = 0.030; r = 0.505, p = 0.014; respectively) in the region of Channels 10 and 13 (Table 3) (Fig. 1). Multiple regression analysis showed that TST was the most significant factor correlated with the area under the NIRS curve (β = 0.455, p = 0.034; β = 0.537, p = 0.009; respectively) in Channels 10 and 13 versus exercise amount and sleep efficiency (Table 3). Age, education, TMT score, bedtime, and wake-up time were not significantly correlated with NIRS parameters. Gender was not a significant factor for prefrontal changes on NIRS parameters.
3.3. Correlations between cognitive function and NIRS parameters
Reaction time on the 0-back task correlated with reaction time on NIRS (r = −0.424, p = 0.044). The percentage of correct responses on the CPT-IP (% corrects) correlated with peak OxyHb on NIRS (r = 0.466, p = 0.025) (Table 4). Cognitive function was not correlated with time to the peak OxyHb and area under the NIRS curve. NIRS parameters were not significantly correlated with the number of words generated.
Table 4.
Correlations between NIRS parameters and cognitive function.
| Peak OxyHb |
Time to the peak OxyHb |
Area under the NIRS curve |
Reaction time |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| 0-back task | ||||||||
| % corrects | −0.108 | 0.622 | −0.055 | 0.804 | 0.139 | 0.536 | −0.134 | 0.543 |
| Reaction time | −0.099 | 0.654 | −0.293 | 0.175 | −0.141 | 0.531 | −0.424 | 0.044* |
| 1-back task | ||||||||
| % corrects | 0.368 | 0.084 | 0.210 | 0.336 | 0.407 | 0.060 | 0.401 | 0.058 |
| Reaction time | −0.331 | 0.123 | −0.291 | 0.179 | −0.344 | 0.117 | −0.396 | 0.062 |
| 2-back task | ||||||||
| % corrects | 0.194 | 0.376 | 0.254 | 0.243 | 0.131 | 0.561 | 0.036 | 0.872 |
| Reaction time | −0.260 | 0.231 | −0.134 | 0.541 | −0.135 | 0.550 | −0.156 | 0.476 |
| WCST | ||||||||
| Category achievement | −0.161 | 0.462 | 0.217 | 0.319 | −0.186 | 0.408 | −0.180 | 0.411 |
| Total errors | 0.014 | 0.949 | −0.175 | 0.425 | −0.040 | 0.860 | 0.059 | 0.788 |
| CPT-IP | ||||||||
| % corrects | 0.466 | 0.025* | −0.127 | 0.564 | 0.367 | 0.093 | 0.084 | 0.705 |
| d prime | 0.301 | 0.162 | −0.169 | 0.440 | 0.183 | 0.415 | 0.083 | 0.708 |
Abbreviations: CPT-IP = Continuous Performance Test–Identical Pairs; NIRS = near-infrared spectroscopy; OxyHb = oxyhemoglobin; WCST = Wisconsin Card Sorting Test.
p < 0.05.
4. Discussion
In the present study, daily PA and TST were significantly correlated with N-back working memory, which could be relevant to a cortical network including the dorsolateral prefrontal cortex. Daily PA, but not TST, was significantly correlated with the mean area under the NIRS curve based on 18 channels for the prefrontal area. Daily PA and TST were not significant factors for executive function on WCST and sustained attention on CPT-IP. Our findings suggest that appropriate daily PA and sleep duration may be important factors for maintaining working memory and increasing prefrontal activity in healthy young adults.
4.1. Correlations of PA or sleep duration and working memory
We found that daily PA was closely correlated with reaction time on 0- and 1-back tasks but not with % corrects on the 0-, 1-, or 2-back tasks. In these tests, reaction time on the N-back task possibly reflects visual-speed processing and synchrony with motor output commands from the premotor area, whereas accuracy on the N-back task likely reflects working memory performance.51 We therefore speculate that daily PA influences visual-speed processing.
TST was associated with % corrects on the 2-back task. The N-back task requires attention, rehearsal, response selection, and behavioral inhibition as well as holding increasing amounts of information on line (1 and 2).52 The presumed mental demands increased from the 1-back to the 2-back task condition. When task load becomes difficult, sleep duration may influence these results. Moreover, bedtime significantly correlated with % corrects on the 2-back task. A recent animal study demonstrated that sleep–wake rhythm was involved in the cognitive impairment and pretreatment with melatonin had a positive effect on circadian normalization and cognition reversal.53 There is also evidence regarding the influence of circadian disruption with irregular bedtime on cognitive development in children.54 Sleep loss, in turn, results in compromised cognitive performance, memory deficits, depressive mood, and involuntary sleep episodes during the day.55 From a clinical perspective, short sleep duration or circadian disruption may reduce working memory performance.
4.2. Correlations of PA or sleep duration and executive function
We found no significant correlation between daily PA or TST and the parameters on the WCST. In a previous study, acute aerobic exercise did not appear to influence executive function, as assessed by the WCST, indicating that this classical neuropsychological test of executive function may not be sufficiently sensitive to identify the effect of acute exercise.56 One night of total sleep deprivation (34–36 h) did not appear to impair performance on WCST, which was designed to assess higher cortical functioning.57 The category achievement was 6 in 87.0% of all participants, which may have created a ceiling effect due to the ease of completing the WCST in young adults. Both WCST and the N-back task were frontal tasks. However, the N-back task was a test for working memory processes,16, 17, 18 whereas the WCST reflected executive function,19, 20 which may explain the differences in our results for the 2 tasks.
4.3. Correlations of PA or sleep duration and sustained attention
Daily PA and TST were not associated with CPT-IP, which measures sustained attention. According to a study using accelerometry data, free-living PA was not related to the TMT,1 which is consistent with our findings. In our previous study, the % corrects on CPT-IP after insufficient sleep (<4 h) was significantly lower than after sufficient sleep (≥8 h).28 In the present study, the TST ranged from 4.0 h to 8.4 h. If TST is less than 4 h, it may affect sustained attention.
4.4. Effects of PA and sleep duration on cognitive function and NIRS parameters
In the present study, daily PA was related to the mean area under the NIRS curve and reaction time on NIRS in 18 channels, as well as the area under the NIRS curve in the left prefrontal area in the regional analysis. Reaction times for the 0-back task were related to NIRS parameters. During the acceleration period immediately preceding a steady walking or running speed, levels of OxyHb reportedly increase in the frontal cortices.6 NIRS activation (OxyHb) was found to be related to working memory in young adults.58 On the N-back task, the frontal cortical regions were activated robustly.16 These findings support our findings. Potential mechanisms by which PA may improve cognitive function include increased cortical oxygenation, stimulation of neurotransmitter (acetylcholine and dopamine) release in the brain, elevation of nerve growth factors, and increase in activated neurons.3 Our present findings suggest that PA may be involved in short-term memory via increased prefrontal cortical oxygenation.
We demonstrated that TST was not correlated with the mean area under the NIRS curve for the prefrontal area based on 18 channels on NIRS. In the regional analysis, we found TST to be correlated with the area under the NIRS curve for Channels 10 and 13, which are adjacent to the left and right dorsolateral prefrontal cortex. One night of sleep deprivation reduced participant performance on neuropsychological tasks that are believed to rely heavily on the prefrontal cortex.28, 59 Exercise amount correlated with the area under the NIRS curve in the left (dominant) prefrontal area in the regional analysis. PA and sleep duration affected cortical activation in the prefrontal area differently as measured by NIRS. To the best of our knowledge, this is the first study to report on the combination of sleep duration and PA and their impact on cognitive function and brain activity.
We found that the % corrects on CPT-IP were related to NIRS parameters. A previous fMRI study reported that cerebral blood flow at rest was associated with speed and attention in younger and older subjects.60 Our recent NIRS study demonstrated that after the first and third nights of insufficient sleep, peak OxyHb levels significantly decreased, as did the number of correct responses to the CPT-IP when compared to the number of correct responses after a sufficient night of sleep in young adults.28 These studies and our present NIRS findings suggest that sustained attention may be related to increased cortical oxygenation.
4.5. Effect of gender on cognitive function and NIRS parameters
In a study of 36 healthy subjects aged 17–28 years, males were found to be significantly more accurate than females on the spatial and object versions of the N-back task, and they performed the same as females on the verbal version of the task.61 In another study, among 39 healthy subjects aged 23–52 years, increases in frontal OxyHb concentration were greater in males than in females during a word fluency task using NIRS.62 Thus, gender was found to influence frontal cognitive function in previous studies. However, in the present study gender was not a significant factor impacting cognitive function and frontal activation.
One fMRI study found that verbal working memory was not affected behaviorally or neurologically by gender in 50 healthy individuals aged 18–58 years.63 In addition, no significant gender differences were identified in the WCST conducted in 116 healthy subjects aged 17–22 years.64 A previous NIRS study did not find any gender differences with respect to brain oxygenation in 44 healthy young subjects aged 19–31 years.65 These findings may support our own findings. Further research is needed to understand the relationship between gender and frontal cognitive performance and frontal neural activity, particularly with regard to daily PA or sleep duration.
4.6. Limitations
Our study has several limitations. First, we examined only correlations between PA, TST, and cognitive function. Second, while neuroimaging techniques such as fMRI and positron emission tomography detect subtle regional changes in the internal cerebrovascular system,66 NIRS measures cortical surface oxygenation. Third, our study population was relatively small. Intervention trials involving a larger sample population will be required to elucidate how brain activity and cognitive function are impacted by the combination of PA and sleep habits, the latter of which should include sleep time and sleep–wake cycle.
5. Conclusion
Daily PA and sleep duration were differentially associated with working memory and cortical activation as measured by NIRS in the prefrontal area. Our findings suggest that daily PA along with sufficient sleep duration may have beneficial effects on working memory in healthy young adults.
Authors' contributions
AN conceived of the study, participated in its design and coordination, carried out acquisition of data, performed the statistical analysis, and drafted the manuscript; KK carried out acquisition of data, performed the statistical analysis, and drafted the manuscript; NK carried out NIRS study; KI, YN, and NO helped to acquire data and draft the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science, KAKENHI (25282210,15H05935), and by Grants-in-Aid from the Comprehensive Research on Disability Health and Welfare, the Ministry of Health, Labor and Welfare of Japan; the Academic Frontier Project for Private Universities; Comparative Cognitive Science Institutes; and Meijo University.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Loprinzi P.D., Kane C.J. Exercise and cognitive function: a randomized controlled trial examining acute exercise and free-living physical activity and sedentary effects. Mayo Clin Proc. 2015;90:450–460. doi: 10.1016/j.mayocp.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 2.Pontifex M.B., Hillman C.H., Fernhall B., Thompson K.M., Valentini T.A. The effect of acute aerobic and resistance exercise on working memory. Med Sci Sports Exerc. 2009;41:927–934. doi: 10.1249/MSS.0b013e3181907d69. [DOI] [PubMed] [Google Scholar]
- 3.Prakash R.S., Voss M.W., Erickson K.I., Kramer A.F. Physical activity and cognitive vitality. Annu Rev Psychol. 2015;66:769–797. doi: 10.1146/annurev-psych-010814-015249. [DOI] [PubMed] [Google Scholar]
- 4.Sugiura K.M., Noda A., Miyata S., Kojima J., Hara Y., Minoshima M. The effect of habitual physical training on left ventricular function during exercise assessed by three-dimensional echocardiography. Echocardiography. 2015;32:1670–1675. doi: 10.1111/echo.12934. [DOI] [PubMed] [Google Scholar]
- 5.Ainslie P.N., Cotter J.D., George K.P., Lucas S., Murrell C., Shave R. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586:4005–4010. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki M., Miyai I., Ono T., Oda I., Konishi I., Kochiyama T. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. Neuroimage. 2004;23:1020–1026. doi: 10.1016/j.neuroimage.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Cotman C.W., Berchtold N.C., Christie L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Zielinski M.R., Davis J.M., Fadel J.R., Youngstedt S.D. Influence of chronic moderate sleep restriction and exercise training on anxiety, spatial memory, and associated neurobiological measures in mice. Behav Brain Res. 2013;250:74–80. doi: 10.1016/j.bbr.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chennaoui M., Arnal P.J., Sauvet F., Léger D. Sleep and exercise: a reciprocal issue? . Sleep Med Rev. 2015;20:59–72. doi: 10.1016/j.smrv.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 11.Gobin C.M., Banks J.B., Fins A.I., Tartar J.L. Poor sleep quality is associated with a negative cognitive bias and decreased sustained attention. J Sleep Res. 2015;24:535–542. doi: 10.1111/jsr.12302. [DOI] [PubMed] [Google Scholar]
- 12.Zohar D., Tzischinsky O., Epstein R., Lavie P. The effects of sleep loss on medical residents' emotional reactions to work events: a cognitive-energy model. Sleep. 2005;28:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Banks S., Dinges D.F. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 14.Kronholm E., Sallinen M., Suutama T., Sulkava R., Era P., Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–446. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 15.Ittig S., Studerus E., Papmeyer M., Uttinger M., Koranyi S., Ramyead A. Sex differences in cognitive functioning in at-risk mental state for psychosis, first episode psychosis and healthy control subjects. Eur Psychiatry. 2015;30:242–250. doi: 10.1016/j.eurpsy.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callicott J.H., Bertolino A., Mattay V.S., Langheim F.J., Duyn J., Coppola R. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 18.Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez J.A., Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 20.Kashima H., Handa T., Kato M., Sakuma K., Yokoyama N., Murakami M. Neuropsychological investigation on chronic schizophrenia. Aspects of its frontal functions. In: Takahashi R., Flor-Henry P., Gruzelier J., Niwa S., editors. Cerebral dynamics, laterality and psychopathology. 1st ed . Elsevier; Amsterdam: 1987. pp. 337–345. [Google Scholar]
- 21.Gilbert S.J., Burgess P.W. Executive function. Curr Biol. 2008;18:110–114. doi: 10.1016/j.cub.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Cornblatt B.A., Risch N.J., Faris G., Friedman D., Erlenmeyer-Kimling L. The continuous performance test, identical pairs version (CPT-IP): I. new findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- 23.Nuechterlein K.H., Dawson M.E. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 24.Keilp J.G., Herrera J., Stritzke P., Cornblatt B.A. The continuous performance test, identical pairs version (CPT-IP): III: brain functioning during performance of numbers and shapes subtasks. Psychiatry Res. 1997;74:35–45. doi: 10.1016/s0925-4927(96)02881-8. [DOI] [PubMed] [Google Scholar]
- 25.Kameyama M., Fukuda M., Yamagishi Y., Sato T., Uehara T., Ito M. Frontal lobe function in bipolar disorder: a multichannel near-infrared spectroscopy study. Neuroimage. 2006;29:172–184. doi: 10.1016/j.neuroimage.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Kohmura K., Iwamoto K., Aleksic B., Sasada K., Kawano N., Katayama H. Effects of sedative antidepressants on prefrontal cortex activity during verbal fluency task in healthy subjects: a near-infrared spectroscopy study. Psychopharmacology (Berl) 2013;226:75–81. doi: 10.1007/s00213-012-2885-8. [DOI] [PubMed] [Google Scholar]
- 27.Mirelman A., Maidan I., Bernad-Elazari H., Nieuwhof F., Reelick M., Giladi N. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J Neuroeng Rehabil. 2014;11:85. doi: 10.1186/1743-0003-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyata S., Noda A., Ozaki N., Hara Y., Minoshima M., Iwamoto K. Insufficient sleep impairs driving performance and cognitive function. Neurosci Lett. 2010;469:229–233. doi: 10.1016/j.neulet.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Miyata S., Noda A., Iwamoto K., Kawano N., Banno M., Tsuruta Y. Impaired cortical oxygenation is related to mood disturbance resulting from three nights of sleep restriction. Sleep Biol Rhythms. 2015;13:387–394. [Google Scholar]
- 30.Cui X., Bray S., Bryant D.M., Glover G.H., Reiss A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54:2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumahara H., Schutz Y., Ayabe M., Yoshioka M., Yoshitake Y., Shindo M. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235–243. doi: 10.1079/BJN20031033. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama Y., Kawamura T., Tamakoshi A., Noda A., Hirai M., Saito H. Comparison of accelerometry and oxymetry for measuring daily physical activity. Circ J. 2002;66:751–754. doi: 10.1253/circj.66.751. [DOI] [PubMed] [Google Scholar]
- 33.Floam S., Simpson N., Nemeth E., Scott-Sutherland J., Gautam S., Haack M. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J Sleep Res. 2015;24:296–304. doi: 10.1111/jsr.12259. [DOI] [PubMed] [Google Scholar]
- 34.Cole R.J., Kripke D.F., Gruen W., Mullaney D.J., Gillin J.C. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 35.Morgenthaler T., Alessi C., Friedman L., Owens J., Kapur V., Boehlecke B. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 36.Johns M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 37.Ira E., Zanoni M., Ruggeri M., Dazzan P., Tosato S. COMT, neuropsychological function and brain structure in schizophrenia: a systematic review and neurobiological interpretation. J Psychiatry Neurosci. 2013;38:366–380. doi: 10.1503/jpn.120178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tombaugh T.N. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 39.Jacola L.M., Willard V.W., Ashford J.M., Ogg R.J., Scoggins M.A., Jones M.M. Clinical utility of the N-back task in functional neuroimaging studies of working memory. J Clin Exp Neuropsychol. 2014;36:875–886. doi: 10.1080/13803395.2014.953039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callicott J.H., Mattay V.S., Bertolino A., Finn K., Coppola R., Frank J.A. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 41.Tomida K., Takahashi N., Saito S., Maeno N., Iwamoto K., Yoshida K. Relationship of psychopathological symptoms and cognitive function to subjective quality of life in patients with chronic schizophrenia. Psychiatry Clin Neurosci. 2010;64:62–69. doi: 10.1111/j.1440-1819.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- 42.Banno M., Koide T., Aleksic B., Yamada K., Kikuchi T., Kohmura K. A case control association study and cognitive function analysis of neuropilin and tolloid-like 1 gene and schizophrenia in the Japanese population. PLoS One. 2011;6:e28929. doi: 10.1371/journal.pone.0028929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoshi Y. Functional near-infrared spectroscopy: potential and limitations in neuroimaging studies. Int Rev Neurobiol. 2005;66:237–266. doi: 10.1016/S0074-7742(05)66008-4. [DOI] [PubMed] [Google Scholar]
- 44.Hock C., Müller-Spahn F., Schuh-Hofer S., Hofmann M., Dirnagl U., Villringer A. Age dependency of changes in cerebral hemoglobin oxygenation during brain activation: a near-infrared spectroscopy study. J Cereb Blood Flow Metab. 1995;15:1103–1108. doi: 10.1038/jcbfm.1995.137. [DOI] [PubMed] [Google Scholar]
- 45.Mehagnoul-Schipper D.J., van der Kallen B.F., Colier W.N., van der Sluijs M.C., van Erning L.J., Thijssen H.O. Simultaneous measurements of cerebral oxygenation changes during brain activation by near-infrared spectroscopy and functional magnetic resonance imaging in healthy young and elderly subjects. Hum Brain Mapp. 2002;16:14–23. doi: 10.1002/hbm.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strangman G., Culver J.P., Thompson J.H., Boas D.A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage. 2002;17:719–731. [PubMed] [Google Scholar]
- 47.Matsuo K., Watanabe A., Onodera Y., Kato N., Kato T. Prefrontal hemodynamic response to verbal-fluency task and hyperventilation in bipolar disorder measured by multi-channel near-infrared spectroscopy. J Affect Disord. 2004;82:85–92. doi: 10.1016/j.jad.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Takizawa R., Fukuda M., Kawasaki S., Kasai K., Mimura M., Pu S. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage. 2014;85:498–507. doi: 10.1016/j.neuroimage.2013.05.126. [DOI] [PubMed] [Google Scholar]
- 49.Suto T., Fukuda M., Ito M., Uehara T., Mikuni M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biol Psychiatry. 2004;55:501–511. doi: 10.1016/j.biopsych.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Nishimura Y., Tanii H., Fukuda M., Kajiki N., Inoue K., Kaiya H. Frontal dysfunction during a cognitive task in drug-naive patients with panic disorder as investigated by multi-channel near-infrared spectroscopy imaging. Neurosci Res. 2007;59:107–112. doi: 10.1016/j.neures.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 51.Kuriyama K., Mishima K., Suzuki H., Aritake S., Uchiyama M. Sleep accelerates the improvement in working memory performance. J Neurosci. 2008;28:10145–10150. doi: 10.1523/JNEUROSCI.2039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lythe K.E., Williams S.C., Anderson C., Libri V., Mehta M.A. Frontal and parietal activity after sleep deprivation is dependent on task difficulty and can be predicted by the fMRI response after normal sleep. Behav Brain Res. 2012;233:62–70. doi: 10.1016/j.bbr.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 53.Xia T., Cui Y., Chu S., Song J., Qian Y., Ma Z. Melatonin pretreatment prevents isoflurane-induced cognitive dysfunction by modulating sleep-wake rhythm in mice. Brain Res. 2016;1634:12–20. doi: 10.1016/j.brainres.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 54.Kelly Y., Kelly J., Sacker A. Time for bed: associations with cognitive performance in 7-year-old children: a longitudinal population-based study. J Epidemiol Community Health. 2013;67:926–931. doi: 10.1136/jech-2012-202024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porkka-Heiskanen T., Zitting K.M., Wigren H.K. Sleep, its regulation and possible mechanisms of sleep disturbances. Acta Physiol. 2013;208:311–328. doi: 10.1111/apha.12134. [DOI] [PubMed] [Google Scholar]
- 56.Wang C.C., Shih C.H., Pesce C., Song T.F., Hung T.M., Chang Y.K. Failure to identify an acute exercise effect on executive function assessed by the Wisconsin Card Sorting Test. J Sport Health Sci. 2015;4:64–72. [Google Scholar]
- 57.Binks P.G., Waters W.F., Hurry M. Short-term total sleep deprivations does not selectively impair higher cortical functioning. Sleep. 1999;22:328–334. doi: 10.1093/sleep/22.3.328. [DOI] [PubMed] [Google Scholar]
- 58.Ogawa Y., Kotani K., Jimbo Y. Relationship between working memory performance and neural activation measured using near-infrared spectroscopy. Brain Behav. 2014;4:544–551. doi: 10.1002/brb3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muzur A., Pace-Schott E.F., Hobson J.A. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- 60.Steffener J., Brickman A.M., Habeck C.G., Salthouse T.A., Stern Y. Cerebral blood flow and gray matter volume covariance patterns of cognition in aging. Hum Brain Mapp. 2013;34:3267–3279. doi: 10.1002/hbm.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lejbak L., Crossley M., Vrbancic M. A male advantage for spatial and object but not verbal working memory using the N-back task. Brain Cogn. 2011;76:191–196. doi: 10.1016/j.bandc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Kameyama M., Fukuda M., Uehara T., Mikuni M. Sex and age dependencies of cerebral blood volume changes during cognitive activation: a multichannel near-infrared spectroscopy study. Neuroimage. 2004;22:1715–1721. doi: 10.1016/j.neuroimage.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt H., Jogia J., Fast K., Christodoulou T., Haldane M., Kumari V. No gender differences in brain activation during the N-back task: an fMRI study in healthy individuals. Hum Brain Mapp. 2009;30:3609–3615. doi: 10.1002/hbm.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCormick C.M., Lewis E., Somley B., Kahan T.A. Individual differences in cortisol levels and performance on a test of executive function in men and women. Physiol Behav. 2007;91:87–94. doi: 10.1016/j.physbeh.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 65.Herrmann M.J., Walter A., Ehlis A.C., Fallgatter A.J. Cerebral oxygenation changes in the prefrontal cortex: effects of age and gender. Neurobiol Aging. 2006;27:888–894. doi: 10.1016/j.neurobiolaging.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Montagne A., Nation D.A., Pa J., Sweeney M.D., Toga A.W., Zlokovic B.V. Brain imaging of neurovascular dysfunction in Alzheimer's disease. Acta Neuropathol. 2016;131:687–707. doi: 10.1007/s00401-016-1570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]