Abstract
Background
Floorball training offers a motivating and socially stimulating team activity for older adults, and 12 weeks of floorball training twice a week among men aged 65–76 years have been shown to have positive effects on a number of physiological parameters important for health. However, the effect of long-term participation in floorball training among male elderly has not been investigated. The aim of the present study was to examine the effect of 26-month self-organized regular participation in floorball training on cardiovascular fitness, body composition, blood lipids, glucose control, and physical function among recreationally active men aged 66–78 years.
Methods
After completing a 12-week randomized and controlled intervention with floorball and petanque training in the autumn 2014 or spring 2015, 15 subjects chose to participate in floorball training (floorball group, FG), whereas 16 subjects resumed their usual lifestyle (control group, CG). FG took part in self-organized floorball training 1.7 sessions of 40 min/week, and CG continued their normal recreationally active lifestyle during a 26-month follow-up period. At baseline and after the follow-up period subjects were tested for cardiovascular fitness, glucose control (resting blood samples), body composition dual-energy X-ray absorptiometry (DXA-scanning), and functional capacity.
Results
In FG, the decline in maximal oxygen uptake (VO2max) during the follow-up period was lower (242 ± 379 mL/min, p = 0.01), blood glycosylated hemoglobin (HbA1c) increased less (−1.6 ± 2.9 mmol/L, p = 0.02), and leg bone mineral density increased more (0.03 ± 0.05 g/cm2, p = 0.02) than those in CG. The effects on body mass, total lean body mass, fat mass, blood lipids, and physical function were similar in FG and CG.
Conclusion
Approximately twice weekly floorball sessions with 40 min/session over 26-month appear to reduce age-related decline in cardiovascular fitness and glucose control and improve leg bone mineral density, suggesting that long-term participation in floorball training can be considered as a health-enhancing activity in recreationally active male elderly.
Keywords: Bone mineral density, Elderly, Floorball, Glucose control, VO2max
1. Introduction
Increasing age is associated with a decline in physical function and health, including adverse cardiovascular events and development of type 2 diabetes.1, 2 Age-related changes also involve loss of muscle strength and coordination, metabolic dysregulation of blood lipids, accumulation of body fat and insulin resistance.3, 4, 5 However, regular exercise can postpone the inevitable decline in physical capacity with age in older adults.6, 7, 8, 9
It is important to be physically active regularly, especially for older adults. A 3-year longitudinal study showed that physically active older adults had higher muscle mass and less total and truncal fat.10 Furthermore, a cohort study showed that sustained physical activity in older age was associated with improved health.11 Significant health benefits were also seen among participants who became physically active relatively late in life.11
In the past decade, a number of studies have shown that soccer training organized as small-sided games led to a broad spectrum of positive health outcomes in both young and older adults,12 as well as in people with lifestyle diseases.13, 14, 15, 16, 17, 18 Recently, other team games such as team handball,19 basketball,20 and floorball21, 22 have been shown to demonstrate positive health effects on untrained young women, post-menopausal women and middle-aged men. Vorup et al.9 showed that floorball training in older adults aged 65–76 years had positive effects on a number of physiological parameters important for health, including reduced plasma low density lipoprotein cholesterol (LDL-C), triglycerides, body fat content, heart rate (HR) at rest and during submaximal cycling, as well as elevated muscle force and improved physical performance. These positive health effects of small-sided floorball and other ball games are intriguing, in particular since the small-sided ball games have been shown to be highly motivating due to the social connectedness and playful elements of the ball games.23, 24 However, the majority of studies examining health effects of small-sided ball games have been of moderate durations, typically 12–16 weeks.
Only 2 studies have investigated the long-term (>1 year) health effects of small-sided ball games training. One study showed that 1 year of soccer training, conducted as small-sided games, reduced body mass index (BMI) and improved maximal oxygen uptake (VO2max), heart function, anti-oxidative capacity, leg strength, rapid force development of leg muscles, and functional ability among older healthy men aged 63–74 years.25, 26, 27 In a other study, 12 weeks of floorball training 2 times a week of 60 min increased bone mineral density (BMD), intermittent exercise capacity, and VO2max of pre- and post-menopausal women, and these adaptations were maintained after additional 40 weeks of floorball training with a reduced frequency to 60 min/week.22 Nevertheless, the long-term health effects of training with small-sided floorball games of older men are not known. Furthermore, little information exists about training interventions lasting more than 1 year in older adults. Especially we want to study the effect of long time real life implementations of a controlled study without the interference of researchers in the implementation.
Thus, the aim of the present study was to examine the effect of 26-month regular participation in floorball training on cardiovascular fitness, body composition, blood lipids, glucose control, and physical function, in recreationally active men aged 66–78 years. It was hypothesized that regular floorball training would result in physiological adaptions considered important for health and functional capacity compared to a recreationally active lifestyle.
2. Methods
2.1. Subjects
This study is part of a parent study. The subjects were recruited from a study by Vorup et al.9 (referred to as the original study), which examined the effect of 12 weeks of floorball training or petanque training on blood lipids, muscle strength, body composition, and functional capacity of men aged 65–76 years.
Thirty-one subjects from the original study aged 72.9 ± 3.5 years (mean ± SD; range: 66–78 years) with a height, body mass, and BMI of 1.78 ± 0.05 m, 81.5 ± 11.9 kg, and 25.7 ± 3.9 kg/m2, respectively, volunteered to take part in follow-up investigations about 26 months after completing the original study.
Before the original study (baseline), the subjects were recreationally active (walking or cycling for transportation on a daily basis, and some had gymnastic, fitness or swimming activities on a weekly basis), but none had been involved in any type of regular (>1 weekly sessions) physical training for at least 10 years. This was supported by accelerometer measurements (AX3; Axivity Ltd., Hoults Yard, UK) showing that weekly running activity before the intervention period was low (7.0 ± 25.4 min/week), and walking was the most preferred physical activity (611 ± 205 min/week). Subjects were taking 9578 ± 3099 steps/day, which places this group in the highest quintile in men aged >65 years with regard to steps per day.28 For exclusion criteria readers are refered to the original study.9 At baseline, 7 subjects took medicine to lower blood pressure, 1 subject was prescribed medicine to lower blood cholesterol and 3 subjects took medicine in connection with type 2 diabetes. During the 2-year study period subjects on medication did not change their intake of medicine.
The study was approved by the Committee on Health Research Ethics, Region of Copenhagen (H-2-2013-149) and conducted in accordance with the guidelines of the Declaration of Helsinki. Subjects were informed of any risks and discomforts associated with the experiments before giving their written informed consent to participate in the study.
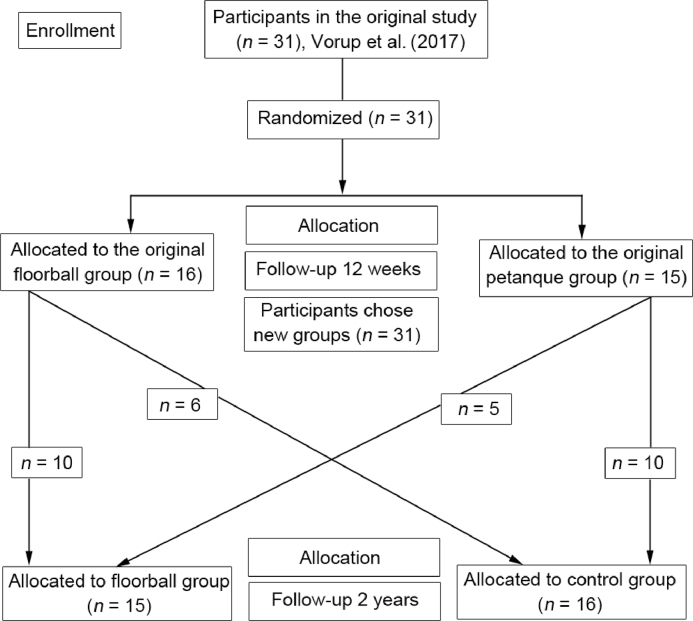
2.2. Design
Twelve subjects were enrolled in the original study in September 2014 (first recruitment round). To increase power, another 19 subjects were enrolled in March 2015 (second recruitment round). The follow-up examination was conducted in March 2017. Thus, the mean follow-up time from baseline was 26 ± 3 months. At the end of the original 12-week training intervention in December 2014 or June 2015,9 the subjects in the floorball and petanque group were given the opportunity to participate in floorball training 3 sessions weekly. A flow diagram of the recruitment process including the original study is presented in Fig. 1. Fifteen subjects chose to participate in floorball training (floorball group, FG), whereas 16 subjects did not want to participate in the floorball training (control group, CG), and resumed their usual lifestyle. FG consisted of 10 subjects from the original floorball group and 5 subjects from the original petanque group (including 1 drop-out from the petanque group). CG consisted of 6 subjects from the original floorball group and 10 subjects from the original petanque group (including 3 drop-outs from the petanque group) (Fig. 1).
Fig. 1.
Flow diagram showing the recruitment process during the 2-year follow-up study. Only subjects included in the 2-year follow-up study are depicted in the flow diagram.
2.3. Training
In FG, training sessions consisted of floorball games performed indoor on a wooden surface sized 13 m × 20 m. Floorball is a team sport like field hockey, but played indoor and with plastic sticks (http://www.floorball.org). A training session lasted 60 min preceded by a 10-min warm-up period. The training session consisted of 5 small-sided games with duration of 8 min separated by a rest period of 1–8 min, and the small-sided games were played 5 vs. 5 or 6 vs. 6. The work: rest ratio was 2:1 resulting in 40 min of effective floorball playing time at each training session. All training was self-organized without interference from the researchers. The subjects had the opportunity to play 3 times a week on Mondays, Wednesdays, and Fridays. Interviews with the subjects showed that 6 subjects in CG performed regular exercise, mainly activities like running, strength training, or fitness training for 1–2 h/week. In FG, 7 subjects performed strength and fitness training for 1–2 h/week in addition to the floorball training.
Training compliance was registered electronically by the subjects. In FG, compliance was 55% ± 15% corresponding to 1.7 ± 0.5 training sessions a week. The compliance for the last 10 and 4 weeks before follow-up examinations was 56% ± 19%, corresponding to 1.7 ± 0.6 training sessions a week, and 64% ± 20%, corresponding to 1.9 ± 0.6 training sessions a week, respectively.
2.4. Measuring and test procedures
Subjects were instructed to refrain from strenuous exercise for at least 36 h before reporting to the laboratory, and the first experimental day was preceded by an overnight fast. Subjects on medicine were instructed to take their habitual medicine as usual on the experimental day.
2.4.1. Experimental Day 1: body composition, resting blood values, blood pressure
Subjects reported to the laboratory between 07:00 and 10:00 in the morning. A blood sample was taken from the cubital vein for determination of fasting blood lipoproteins, triglycerides, glucose, insulin, and glycated hemoglobin (HbA1c). Body composition was determined from whole-body dual-energy X-ray absorptiometry (DXA) scanning (Lunar Prodigy Advance; GE-medical Systems) and software (enCORE v15; GE-medical Systems, Madison, WI, USA), and subjects rested at least 15 min in a supine position before blood pressure was measured 6 consecutive times by an automatic upper arm blood pressure monitor (M7; OMRON, Vernon Hills, IL, USA).
2.4.2. Experimental Day 2: maximal voluntary knee extension contraction and VO2max
Subjects reported to the laboratory between 07:00 and 12:00 in the morning. Subjects performed a maximal isometric voluntary knee extension contraction (MVC) sitting in a special-designed chair with one ankle strapped to an isometric strain gauge. The highest force output of 3 trials was used as the test result. If the highest force output was performed in the final attempt, another trial was carried out. After another ∼15 min of rest, subjects performed a standardized ergometer cycle exercise test (Monark, Varberg, Sweden) to determine VO2max and submaximal heart rate. The cycle exercise test protocol consisted of a 4 min submaximal bout at 60 W ∼85 revolutions per minute (rpm), followed by a 2 min rest period and an incremental test starting with 2 min at 60 W after which the load was increased 20 W every second minute until volitional fatigue. Oxygen uptake (CPX; Viasys Healthcare, Jaeger, Germany) and HR (Polar Team System; Polar Electro Oy, Kempele, Finland) were measured continuously during the exercises.
2.4.3. Functional capacity tests
On a separate day, 6 standardized functional exercises were performed, including (a) maximal sit-to-stand repetitions in 30 s, (b) time to sit-to-stand 5 repetitions, (c) time to sit-to-stand interspersed by a 2 × 2.45 m walk out and back around a cone, (d) maximal repetitions of biceps-curls with an 8 kg dumbbell, (e) maximal hand-grip strength with an adjustable hydraulic hand dynamometer (JAMAR; North Coast Medical, Oakleigh, VIC, Australia), and (f) maximal distance in a 6 min walking test.29 All tests were performed indoors on a wooden surface.
2.4.4. Physiological response during training
At selected training sessions, subjects were wearing an HR monitor (Polar Team System) and the training was recorded on video to measure HR response and activity profile during floorball training. HR and video data were subsequently analyzed using appropriate software (‘Polar ProTrainer 5’; Polar Electro Oy, and ’Time Motion’; App for IPAD, Grant Abt, 2015).
2.4.5. Blood analysis
Whole blood samples were analyzed at the clinical biochemical unit at the Copenhagen main hospital (Rigshospitalet) using an automatic analyzer with enzymatic kits (COBAS 8000; Roche Diagnotics International Ltd, Rotkreuz, Switzerland) for total plasma cholesterol, plasma LDL-C, plasma high density lipoprotein cholesterol (HDL-C), plasma triglycerides, glucose, and HbA1c using turbidimetric immunoassay (Tosoh G7; Tosoh Bioscience Inc, South San Francisco, CA, USA).
Blood samples for collection of plasma were drawn into tubes containing ethylenediamine tetraacetic acid (EDTA) as anticoagulant. Blood was then immediately centrifuged for 60 s 1500 g, and plasma was then stored at 80°C for later analysis. Plasma concentrations of insulin were measured using an enzyme immunoassay insulin kit (DAKO 0.5−180 µU/mL, Dako Cytomation, Cambridgeshire, UK).
2.4.6. Daily physical activity
Weekly level of physical activity was determined by accelerometer measurements (Axivity, Newcastle, UK). The accelerometer was placed on the thigh, and used for 8 consecutive days. ACTi 4, Version 14.09 (ACTi Corp., Copenhagen, Denmark) was used to discriminate between physical activity types, including sitting, standing, walking, fast walking (more than 99 steps per min), running, cycling, sit-to-stand movements (i.e., transitions from sitting to upright stand), and number of steps based on threshold values of standard deviation of acceleration and the derived inclination.30 The activity move was defined as a left-over activity used if none of the other activities were detected. It will normally correspond to a standing posture that is neither detected as standing still nor walking, such as tripping or trying to dribble the opponent during floorball. Daily time as well as changes over time (before. during intervention) for the above mentioned activity types were calculated.
2.5. Statistics
Comparisons of baseline outcome measures between FG and CG were performed using a two-tailed unpaired test. Chi-square (χ2) test of distribution of frequencies was used to look for differences and changes in medicine intake between FG and CG. For the considered outcome measures, the effects of floorball training compared to control were evaluated using analysis of covariance (ANCOVA) including the groups (FG, CG) as a categorical independent variable while adjusting for baseline values of the outcome, age and medicine intake. Contrast estimates (CE), 95% confidence interval (CI), and values were reported for FG and CG (see Results). All analyses of changes were based on comparisons of baseline before the 12-week training intervention9 and follow-up data. Distribution of the data was checked for normality before applying the t tests or ANCOVA. IBM SPSS statistics Version 22.0 (IBM Corp., New York, NY, USA) was used for all tests, p < 0.05 was chosen as the level of significance and all data are presented as means ± SD.
3. Results
At baseline there were no significant differences between groups for any measurements of daily physical activity, body composition, blood lipids and lipoproteins, glucose and HbA1c, VO2max, HR, blood pressure, maximal voluntary contraction and functional capacity except for fasting insulin and calculated homeostatic model assessment insulin resistance (HOMA-IR), which were lower in CG (23 ± 13 pmol/L and 0.88 ± 0.46) compared to FG (47 ± 32 pmol/L and 2.07 ± 1.55); and CG spent more time cycling (0.18 ± 0.24 h/day) than FG (0.10 ± 0.13 h/day). There were no significant differences in medicine intake between groups.
3.1. HR and activity profile during training
The activity profile, including number of intense actions and duration of running, fast walking and move during 1 h of floorball training, is presented in Table 1. Mean HR during floorball training was 77% ± 7% of maximal heart rate (HRmax) with 10 ± 8 min exceeding 90% of HRmax. The number of steps was 3500 ± 1578.
Table 1.
Activity profile and number of intense actions and HR during 1 h of floorball training (n = 14) (mean ± SD).
| Parameter | Valuea |
|---|---|
| Type of activity | |
| Sitting | 14 ± 8 |
| Standing | 9 ± 5 |
| Move | 11 ± 5 |
| Walking | 27 ± 12 |
| Slow walk | 5 ± 2 |
| Fast walk | 22 ± 10 |
| Running | 2 ± 1 |
| Steps | 3500 ± 1578 |
| Type of intense action | |
| Fast run | 21 ± 25 |
| Shots or throws | 58 ± 15 |
| Rapid turns > 90° | 32 ± 13 |
| Total | 111 ± 18 |
| HR zone (% of HRmax) | |
| >90 | 10 ± 8 |
| >80 | 20 ± 7 |
| >70 | 28 ± 9 |
| >60 | 37 ± 9 |
Notes: a values are presented as duration (min) for types of activity and HR zone, and as number of actions for steps and type of intense action.
Abbreviations: HR = heart rate; HRmax = maximal heart rate.
3.2. Body composition
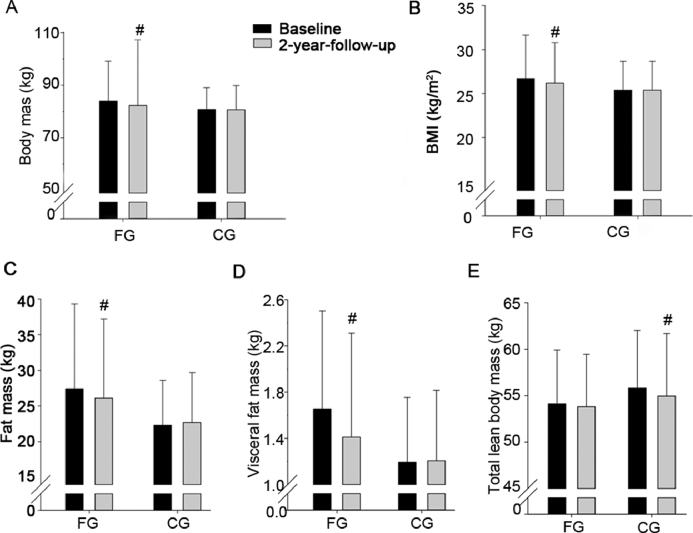
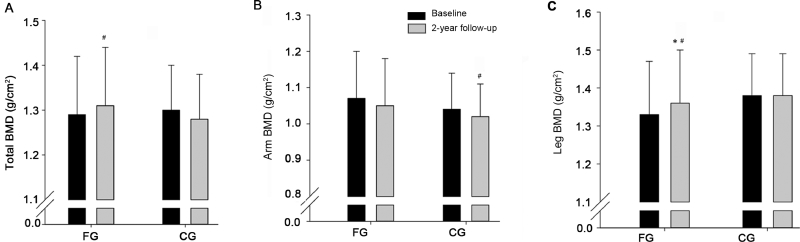
In FG, the increase in leg BMD during the follow-up period was higher (0.03 ± 0.05 g/cm2, p = 0.047) than in CG. No significant effects of the intervention were observed on total BMD, arm BMD, body mass, BMI, fat mass, visceral fat mass, and lean body mass (Table 2 and Figs. 2 and 3).
Table 2.
CE, 95%CI, and p values for comparing outcome measures for a group playing floorball twice a week (FG) and a recreationally active control group (CG).
| FG |
||
|---|---|---|
| CE (95%CI) | p | |
| Body mass (kg) | −1.7 (−3.8 to 0.4) | 0.12 |
| BMI (kg/m2) | −0.5 (−1.2 to 0.2) | 0.14 |
| Fat mass (kg) | −1.8 (−3.7 to 0.2) | 0.07 |
| Visceral fat mass (kg) | −0.2 (−0.7 to 0.3) | 0.33 |
| Total lean body mass (kg) | 0.6 (−0.5 to 1.8) | 0.27 |
| Leg lean body mass (kg) | −0.03 (−0.4 to 0.4) | 0.89 |
| Arm lean body mass (kg) | −0.01 (−0.4 to 0.4) | 0.97 |
| Total BMD (g/cm2) | 0.03 (−0.003 to 0.06) | 0.08 |
| Leg BMD (g/cm2) | 0.03 (0.00 to 0.06) | 0.047* |
| Arm BMD (g/cm2) | 0.01 (−0.04 to 0.05) | 0.77 |
| Total cholesterol (mmol/L) | −0.1 (−0.6 to 0.3) | 0.57 |
| HDL-C (mmol/L) | −0.01 (−0.2 to 0.1) | 0.24 |
| LDL-C (mmol/L) | −0.2 (−0.7 to 0.2) | 0.35 |
| Triglycerides (mmol/L) | 0.20 (0.002 to 0.4) | 0.047* |
| Fasting glucose (mmol/L) | 0.1 (−0.2 to 0.4) | 0.43 |
| HbA1c (mmol/L) | −1.6 (−3.1 to -0.02 ) | 0.047* |
| Fasting insulin (pmol/L) | 3 (−10 to 20) | 0.57 |
| HOMA-IR | 100 (−46 to 66) | 0.71 |
| VO2max (mL/min) | 242 (40 to 244) | 0.02* |
| VO2max (mL/min/kg) | 3.60 (0.4 to 6.7) | 0.03* |
| HRmax (bpm) | 1.4 (−6.3 to 9.0) | 0.72 |
| Submaximal HR (bpm) | −6.1 (0.8 to 13.1) | 0.08 |
| Resting HR (bpm) | 1.35 (−4.7 to 7.4) | 0.65 |
| Systolic BP (mmHg) | −3.3 (−12.2 to 5.6) | 0.45 |
| Diastolic BP (mmHg) | 0.1 (−3.5 to 3.7) | 0.96 |
| Maximal distance in 6 min (m) | 23 (−18 to 63) | 0.27 |
| 30 s sit-to-stand (rep) | 1.3 (−0.9 to 3.5) | 0.24 |
| 5 times sit-to-stand (s) | −0.5 (−1.4 to 0.4) | 0.28 |
| Hand-grip strength (kg) | 1.7 (−0.7 to 4.1) | 0.16 |
| Arm curls (rep) | −0.6 (−4.4 to 3.3) | 0.77 |
| 2.45 m up-and-go (s) | −1.3 (−0.5 to 0.2) | 0.48 |
| MVC (N) | −12 (−64 to 39) | 0.62 |
p < 0.05, compared with CG.
Abbreviations: BMD = bone mineral density; BMI = body mass index; BP = blood pressure; bpm = beats/min; CE = contrast estimates; CI = confidence interval; HbA1c = glycosylated hemoglobin; HDL-C = high density lipoprotein cholesterol; HOMA-IR = homeostatic model assessment insulin resistance; LDL-C = low density lipoprotein cholesterol; MVC = maximal isometric voluntary knee extension contraction; rep = repetition; VO2max = maximal oxygen uptake.
Fig. 2.
Body mass (A), body mass index (BMI) (B), fat mass (C), visceral fat mass (D), and total lean body mass (E) at baseline and after a 2-year follow-up period for a group playing floorball twice a week (FG) and a recreationally active control group (CG). Means ± SD are presented. #p < 0.05, significant within-group change from baseline to follow-up.
Fig. 3.
Total bone mineral density (BMD) (A), arm BMD (B), and leg BMD (C) at baseline and after a two-year follow-up period for a group playing floorball twice a week (FG) and a recreationally active control group (CG). Means ± SD are presented. * p < 0.05, significant effect compared to CG. #p < 0.05, significant within-group change from baseline to follow-up examination.
3.3. Blood lipids and lipoproteins
In FG, triglycerides at rest increased more (0.20 ± 0.34 mmol/L, p = 0.047) during the follow-up period than in CG. No significant intervention effects were observed on total cholesterol, HDL-C and LDL-C (Tables 2 and 3).
Table 3.
Values for blood pressure, blood lipids, lean body mass, and maximal voluntary contraction force before (baseline) and after a 2-year follow-up period for FG and CG (mean ± SD).
| FG |
CG |
|||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| Systolic BP (mmHg) | 142 ± 15 | 145 ± 17 | 132 ± 15 | 138 ± 18# |
| Diastolic BP (mmHg) | 83 ± 8 | 83 ± 7 | 79 ± 8 | 80 ± 8 |
| Total cholesterol (mmol/L) | 5.35 ± 0.96 | 5.38 ± 0.88 | 5.74 ± 0.67 | 5.81 ± 0.72 |
| HDL-C (mmol/L) | 1.58 ± 0.45 | 1.62 ± 0.48 | 1.64 ± 0.31 | 1.74 ± 0.36# |
| LDL-C (mmol/L) | 3.25 ± 0.90 | 3.20 ± 0.81 | 3.70 ± 0.64 | 3.80 ± 0.71 |
| Triglycerides (mmol/L) | 1.20 ± 0.49 | 1.28 ± 0.44* | 1.01 ± 0.31 | 0.98 ± 0.23 |
| Leg lean body mass (kg) | 18.1 ± 2.8 | 17.9 ± 2.8 | 18.9 ± 2.7 | 18.8 ± 2.8 |
| Arm lean body mass (kg) | 6.3 ± 1.0 | 6.1 ± 1.1 | 6.3 ± 0.8 | 6.0 ± 0.9# |
| MVC knee extension (N•m) | 136 ± 32 | 147 ± 32 | 158 ± 48 | 168 ± 57 |
Abbreviations: BP = blood pressure; CG = control group; FG = floorball group; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; MVC = model view controller
p < 0.05, compared with CG.
p < 0.05, compared with baseline within group.
3.4. Blood glycosylated hemoglobin, glucose and insulin
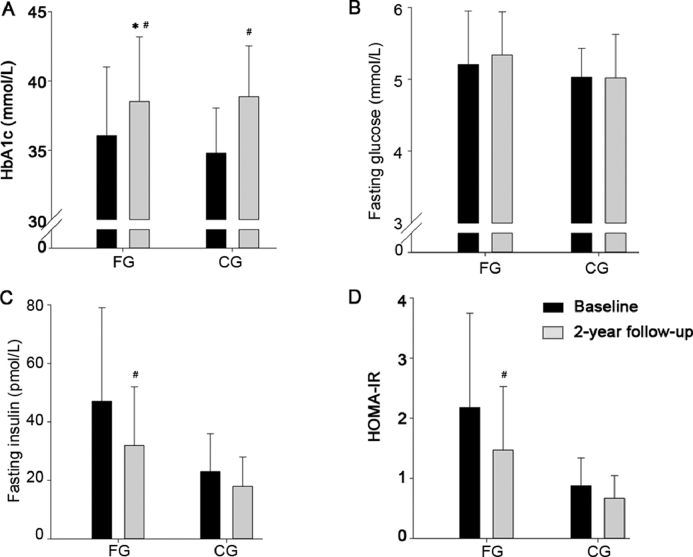
In FG, blood HbA1c increased less (−1.6 ± 2.9 mmol/L, p = 0.047) during the follow-up period compared to CG. No significant intervention effects were observed on fasting plasma glucose, insulin, and HOMA-IR (Table 2 and Fig. 4).
Fig. 4.
Plasma glycosylated hemoglobin (HbA1c) (A), fasting glucose (B), fasting insulin (C), and calculated homeostatic model assessment insulin resistance (HOMA-IR) (D) at baseline and after a 2-year follow-up period for a group playing floorball twice a week (FG) and a recreationally active control group (CG). Means ± SD are presented. * p < 0.05, significant effect compared to CG; #p < 0.05, significant within-group change from baseline to follow-up examination.
3.5. VO2max, HR, and blood pressure
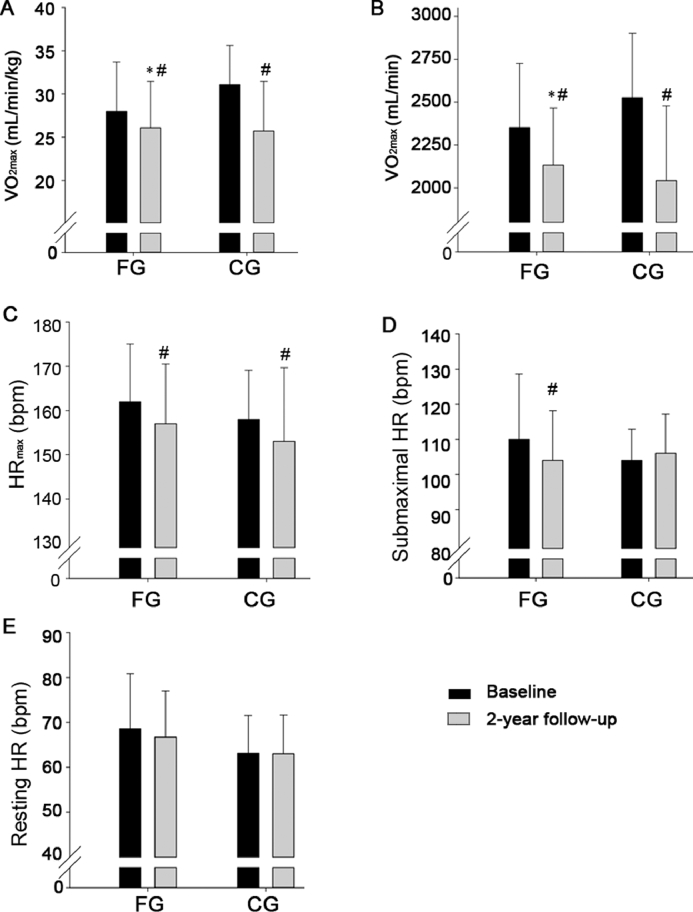
In FG, the decline in absolute and relative VO2max during the follow-up period was lower (242 ± 379 mL/min, p = 0.02; 3.60 ± 5.93 mL/min/kg, p = 0.03) than in CG. No significant effects of the intervention were observed on HRmax, HR during rest and submaximal cycling, and blood pressure at rest (Tables 2 and 3, Fig. 5).
Fig. 5.
VO2max per body weight (A), VO2max (B), peak HR during maximal exercise (C), HR during submaximal cycling (D), and HR at rest (E) at baseline and after a 2-year follow-up period for a group playing floorball twice a week (FG) and a recreationally active control group (CG). Means ± SD are presented. * p < 0.05, significant effect compared to CG; #p < 0.05, significant within-group change from baseline to follow-up examination. bpm = beats/min; HR = heart rate; VO2max = maximal oxygen uptake.
3.6. Maximal voluntary contraction and functional capacity
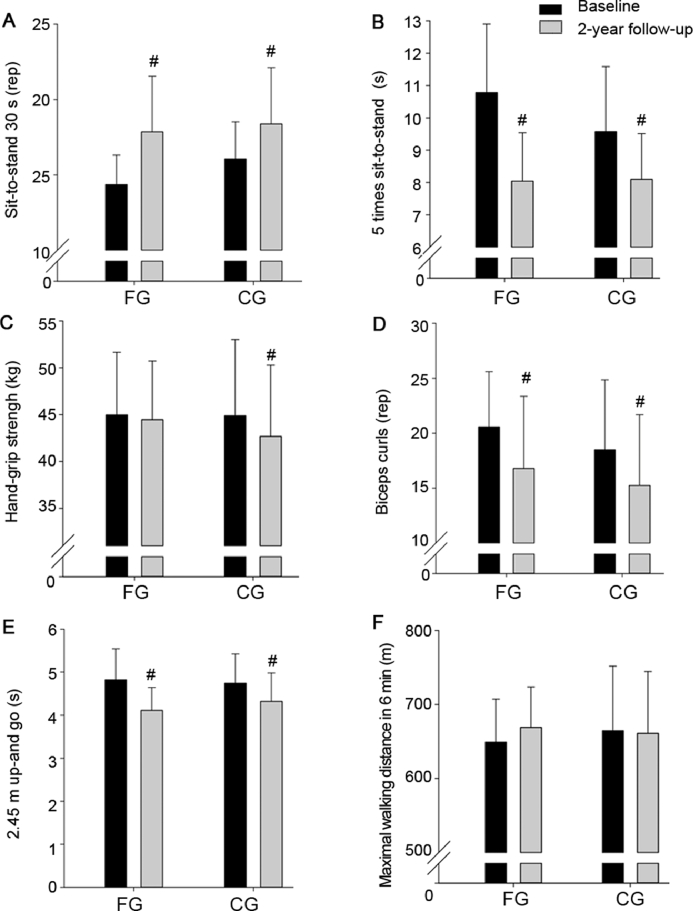
No significant intervention effects were observed on MVC knee extension, number of repetitions in sit-to-stand during 30 s, time to perform 5 times sit-to-stand repetitions, hand-grip strength, number of arm curls during 30 s, and time to conduct 2.45 m up-and-go and maximal distance in 6 min (Tables 2 and 3, Fig. 6).
Fig. 6.
Number of sit-to-stand repetitions in 30 s (A), time to perform 5 times sit-to-stand (B), hand-grip strength (C), maximal biceps curls repetitions (D), time to perform 2.45 m up-and-go (E), and maximal walking distance in 6 min (F) at baseline and after a 2-year follow-up period (grey bars) for a group playing floorball twice a week (FG) and a recreationally active control group (CG). Means ± SD are presented. #p < 0.05, significant within-group change from baseline to follow-up. rep = repetition.
3.7. Daily physical activity
FG had higher increase in fast walking (0.34 ± 0.26 h/day, p = 0.02) than CG at the follow-up examination. Compared to FG, CG decreased move (0.17 ± 0.47 h/day, p = 0.01) and spend less time standing (0.44 ± 0.96 h/day, p = 0.04) after compared to before the follow-up period (Table 4).
Table 4.
Daily physical activity before (baseline) and after a 2-year follow-up period for FG and CG (mean ± SD).
| FG |
CG |
|||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| Sitting (h/day) | 9.5 ± 1.5 | 9.3 ± 3.6 | 9.2 ± 1.1 | 10.0 ± 1.3# |
| Standing (h/day) | 2.8 ± 0.9 | 2.7 ± 0.9* | 3.3 ± 1.1 | 2.8 ± 1.1# |
| Move (h/day) | 1.3 ± 0.5 | 1.3 ± 0.5* | 1.4 ± 0.4 | 1.2 ± 0.5# |
| Walking (h/day) | 1.3 ± 0.5 | 1.5 ± 0.5 | 1.5 ± 0.4 | 1.5 ± 0.4 |
| Running (min/day) | 0.2 ± 0.2 | 1.4 ± 0.2# | 2.2 ± 5.5 | 5.0 ± 10.9 |
| Stair climbing (min/day) | 5.4 ± 4.8 | 6.6 ± 5.4 | 6.0 ± 2.4 | 5.4 ± 2.4 |
| Cycling (min/day) | 6.0 ± 7.8 | 4.8 ± 4.2 | 10.8 ± 14.4 | 8.4 ± 9.0 |
| Steps (number/day) | 8878 ± 3281 | 9808 ± 1822 | 10,209 ± 2951 | 10,784 ± 3064 |
| Slow walking (min/day) | 25.2 ± 12.0 | 24.6 ± 7.8 | 34.2 ± 19.2 | 36.0 ± 18.0 |
| Fast walking (min/day) | 52.2 ± 22.8 | 57.6 ± 11.4* | 53.4 ± 18.0 | 53.4 ± 14.4 |
| Rise Sit (number/day) | 54 ± 14 | 55 ± 13 | 69 ± 15 | 63 ± 16 |
p < 0.05, compared with CG.
p < 0.05, compared with baseline within group.
Abbreviations: CG = control group; FG = floorball group.
4. Discussion
The main findings of the present study were that 26-month floorball training performed about twice a week reduced the decline in VO2max, reduced the increase in HbA1c, and increased leg BMD and triglycerides at rest in older men.
VO2max declined less in the group playing floorball (∼7%) compared to the control group (∼18%) during the intervention period of about 26 months. Thus, although the floorball training appears to have a beneficial effect on VO2max, the study also demonstrated that playing floorball twice a week is not sufficient to maintain VO2max. In accordance, we observed that 12 weeks of floorball training 2 times 24 min a week did not change VO2max,9 which may be due to the moderate training volume. In support, Schmidt et al.26 found increases in VO2max of 16% after 4 months and 18% after 12 months of small-sided soccer training, comparable in HR response and intervention duration, with a higher training volume (84–126 min/week compared to ∼68 min/week in the present study) in untrained older individuals. The 7% and 18% decline in VO2max over about 2 years in FG and CG respectively, are higher than observed in other studies.31 It may be related to the moderate to high baseline levels as well as an age effect reflected in the decline in HRmax of ∼3.5% and to a marginally lower compliance and mean HR compared to the original study.9 Nevertheless, the reduced decline in VO2max in the floorball group during the 2-year follow-up period is considered important, since a reduction of 3.5 mL/min/kg in VO2max increases the risk of cardiovascular mortality by about 20% in men.32
As insulin sensitivity is reduced with age, this may contribute to higher levels of blood glucose in older men.1, 2 The floorball training led to a smaller increase in HbA1c (∼7%) compared to the groups living a recreationally active lifestyle (12%), indicating that the long-term participation in floorball training was beneficial for glucose control. In accordance, studies examining small-sided soccer training in older men and small-sided floorball training in pre- and post-menopausal women have found that 52 months of training 2–3 h per week can improve blood glucose regulation.14, 22 In addition to the lower increase in HbA1c in the present study, the HOMA-IR data suggest an improvement in insulin resistance. However, conclusions should be interpreted with caution since the lowering of HOMA-IR for the floorball players was not significantly different from the control subjects, and baseline values were higher in the floorball group compared to the control group. Nevertheless, the lower increase in HbA1c is considered clinically relevant since it may reduce the risk of developing type 2 diabetes.33
The floorball training led to a higher leg BMD and larger increase than in the control group. In accordance, studies examining recreational football training among older men found higher leg BMD after 1 year of training.34, 35 The multiple intense actions during the floorball training, including sprints, turns, and rapid change of direction may explain the increase in BMD despite a moderate training volume (∼68 min/week). It should be noted that in order to achive bone adaptations, a rather long period of training (>6 months) appears to be needed.35 Accordingly, no adaptations were observed in older men after 12 weeks of floorball training.9 The increase of 2.3% in leg BMD in the floorball group is important as a 5%–8% increase in BMD leads to an improved bone strength of about 75%.35 Furthermore, age-related decline in BMD is estimated to 0.5% per year for men older than 40 years, which stresses the importance of efficient bone stimulating training activities with old age.36 Thus, the higher leg BMD after the floorball training period is likely to protect the older men better from bone injuries in case of falling.
The higher plasma triglyceride concentration (0.08 mmol/L) in the floorball group after the follow-up period compared to the control group was surprising, since 12 weeks of floorball training in older men led to an 8% decrease.9 In addition, periods of training are typically associated with reduced or unchanged levels of plasma triglycerides.37 Thus, other factors such as the diet, may explain the increase in plasma triglycerides after the floorball intervention.38 Thus, to reduce triglyceride levels, focus may be on the diet in combination with the floorball training. Indeed, an increase of 1 mmol/L does elevate the risk for cardiovascular disease by 30% in men.39
Lean body mass was not affected by the floorball training, which is in line with studies examining the effect of small-sided floorball, soccer and resistance training for 12–16 weeks as well as small-sided soccer and resistance training for 52 weeks in older men.9, 14, 25, 26 Aging is associated with loss of muscle mass of ∼0.7% per year,40 which is supported by the present data in which lean body mass was lower (∼1.5%) in the recreationally active lifestyle group after the 26-month follow-up period, whereas no change in lean body mass was observed in the floorball group. Apparently, the floorball training can provide sufficient anabolic stimuli to maintain muscle mass.41 To increase muscle mass in older adults, protein after training may be needed. Thus, ingesting protein immediately and 3 h after small-sided ball training has been shown to promote gain in muscle mass in older individuals.42 It is possible that muscle hypertrophy would have been induced if the subjects had ingested proteins after the floorball training during the follow-up period. However, the diet of the subjects in the floorball group was not controlled, nor was it recorded, so it remains unknown to what extent protein was ingested immediately after training sessions.
The positive effects of floorball training on the physical function tests, and muscle force except for hand grip strength, were not larger than observed for the control group subjects. This finding may be related to the relatively high physical activity level in the control group during the follow-up period, which was supported by accelerometer measurements and participant interviews, showing that a considerable part of the control subjects performed training activities like running, strength training, or fitness training for 1–2 h/week. Other studies have reported better physical function and maximal muscle strength after a period of small-sided soccer training or resistance training for 12–16 weeks.41, 43 In the study by Vorup et al.,9 12 weeks of floorball was associated with better physical function, whereas a group conducting petanque training did not have any changes. Thus, floorball training appears to stimulate physical function positively in older men.
A limitation of the present study is that the subjects were not randomized into the 2 study groups, i.e., the subjects themselves chose the group. Nevertheless, significant differences at baseline were only seen for 2 out of 32 outcomes. For ethical reasons, we did not restrict the activity level of the control group. Another issue is that the effects were assessed as the difference between baseline and the 26 months follow-up examination, in spite of the fact that some subjects in both groups initially played floorball for 12 weeks. The reason for comparing the 2-year follow-up examination with baseline was that FG and CG consisted of a mix of floorball players and petanque players as well as drop-outs from the original 12-week intervention study. Thus, a comparison with the 12-week examination was irrelevant since both FG and CG was a mix of subjects originally allocated to floorball and petanque training. It cannot be ruled out that the original 12-week training intervention may have impacted the results in the 2-year follow-up examination. On the other hand, training adaptations typically return to baseline values within a few weeks or months after terminating a period of exercise in untrained individuals. Thus, we chose to ignore the effect of the original 12 weeks of exercise as it was considered to be minimal after more than 2 years.
5. Conclusion
The present study is an example of a successful implementation based on a 12-week training study. The subjects have managed to maintain participation without the interference of the researchers for more than 2 years, and floorball training has become a permanent activity, which is probably due to the social interaction and intrinsic motivation related to team play.23, 24 In terms of health promotion, the floorball training in the present study could be considered a maintenance activity, i.e., the floorball training protected against age-related declines in physiological health, except for bone health which improved. It is likely that a higher training compliance or intensity in the floorball training could lead to more pronounced effects important for health. In addition, conducting floorball training concurrent with other training forms, for example, strength training or running, may be even more beneficial for the participants’ physiological health, but this speculation needs further investigation. Taken together, recreational small-sided team sports, including floorball training, should be considered as part of future health enhancing strategies for older adults. It will be of interest to study how the subjects in the 2 groups are developing in the following years.
Acknowledgments
Authors’ contributions
MTP coordinated the study, carried out all the measurements, performed the statistical analyses and drafted the manuscript; JV participated in the coordination, measurements and drafting of the manuscript; JB participated in its design and coordination and helped to draft the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Abdelhafiz AH, Sinclair AJ. Diabetes, nutrition, and exercise. Clin Geriatr Med. 2015;31:439–451. doi: 10.1016/j.cger.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Houterman S, Boshuizen HC, Verschuren WM, Giampaoli S, Nissinen A, Menotti A. Predicting cardiovascular risk in the elderly in different European countries. Eur Heart J. 2002;23:294–300. doi: 10.1053/euhj.2001.2898. [DOI] [PubMed] [Google Scholar]
- 3.Artero EG, Lee DC, Lavie CJ, España-Romero V, Sui X, Church TS. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32:351–358. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 5.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 6.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(Suppl. 1):S3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl. 3):S1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 9.Vorup J, Pedersen MT, Melcher PS, Dreier R, Bangsbo J. Effect of floorball training on blood lipids, body composition, muscle strength, and functional capacity of elderly men. Scand J Med Sci Sports. 2017;27:1489–1499. doi: 10.1111/sms.12739. [DOI] [PubMed] [Google Scholar]
- 10.Raguso CA, Kyle U, Kossovsky MP, Roynette C, Paoloni-Giacobino A, Hans D. A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin Nutr. 2006;25:573–580. doi: 10.1016/j.clnu.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Hamer M, Lavoie KL, Bacon SL. Taking up physical activity in later life and healthy ageing: the English longitudinal study of ageing. Br J Sports Med. 2014;48:239–243. doi: 10.1136/bjsports-2013-092993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bangsbo J, Hansen PR, Dvorak J, Krustrup P. Recreational football for disease prevention and treatment in untrained men: a narrative review examining cardiovascular health, lipid profile, body composition, muscle strength and functional capacity. Br J Sports Med. 2015;49:568–576. doi: 10.1136/bjsports-2015-094781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen LJ, Randers MB, Hansen PR, Hornstrup T, Schmidt JF, Dvorak J. Structural and functional cardiac adaptations to 6 months of football training in untrained hypertensive men. Scand J Med Sci Sports. 2014;24(Suppl. 1):S27–S35. doi: 10.1111/sms.12237. [DOI] [PubMed] [Google Scholar]
- 14.Andersen TR, Schmidt JF, Thomassen M, Hornstrup T, Frandsen U, Randers MB. A preliminary study: effects of football training on glucose control, body composition, and performance in men with type 2 diabetes. Scand J Med Sci Sports. 2014;24(Suppl. 1):S43–S56. doi: 10.1111/sms.12259. [DOI] [PubMed] [Google Scholar]
- 15.Knoepfli-Lenzin C, Sennhauser C, Toigo M, Boutellier U, Bangsbo J, Krustrup P. Effects of a 12-week intervention period with football and running for habitually active men with mild hypertension. Scand J Med Sci Sports. 2010;20(Suppl. 1):S72–S79. doi: 10.1111/j.1600-0838.2009.01089.x. [DOI] [PubMed] [Google Scholar]
- 16.Krustrup P, Skoradal MB, Randers MB, Weihe P, Uth J, Mortensen J. Broad-spectrum health improvements with one year of soccer training in inactive mildly hypertensive middle-aged women. Scand J Med Sci Sports. 2017;27:1893–1901. doi: 10.1111/sms.12829. [DOI] [PubMed] [Google Scholar]
- 17.Mohr M, Lindenskov A, Holm PM, Nielsen HP, Mortensen J, Weihe P. Football training improves cardiovascular health profile in sedentary, premenopausal hypertensive women. Scand J Med Sci Sports. 2014;24(Suppl. 1):S36–S42. doi: 10.1111/sms.12278. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt JF, Andersen TR, Horton J, Brix J, Tarnow L, Krustrup P. Soccer training improves cardiac function in men with type 2 diabetes. Med Sci Sports Exerc. 2013;45:2223–2233. doi: 10.1249/MSS.0b013e31829ab43c. [DOI] [PubMed] [Google Scholar]
- 19.Hornstrup T, Wikman JM, Fristrup B, Póvoas S, Helge EW, Nielsen SH. Fitness and health benetits of team handball training for young untrained women – a cross-disciplinary RCT on physiological adaptions and motivational aspects. J Sports Health Sci. 2018;7:139–148. doi: 10.1016/j.jshs.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randers MB, Hamer M, Brix J, Christensen JF, Pedersen MT, Nielsen JJ. Effect of 3 months of full-court and half-court street basketball training on health profile in untrained men. J Sports Health Sci. 2018;7:132–138. doi: 10.1016/j.jshs.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyberg M, Seidelin K, Andersen TR, Overby NN, Hellsten Y, Bangsbo J. Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: effect of exercise training. Am J Physiol Regul Integr Comp Physiol. 2014;306:R510–R517. doi: 10.1152/ajpregu.00539.2013. [DOI] [PubMed] [Google Scholar]
- 22.Seidelin K, Nyberg M, Piil P, Jørgensen NR, Hellsten Y, Bangsbo J. Adaptations with intermittent exercise training in post- and premenopausal women. Med Sci Sports Exerc. 2017;49:96–105. doi: 10.1249/MSS.0000000000001071. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen MT, Vorup J, Nistrup A, Wikman JM, Alstrøm JM, Melcher PS. Effect of team sports and resistance training on physical function, quality of life, and motivation in older adults. Scand J Med Sci Sports. 2017;27:852–864. doi: 10.1111/sms.12823. [DOI] [PubMed] [Google Scholar]
- 24.Wikman JM, Nistrup A, Petersen JV, Pedersen MT, Melcher PGS, Bangsbo J. et al. The effect of floorball training on health status, psychological haelth and social capital in older men. AIMS Public Health. 2017;4:364–382. doi: 10.3934/publichealth.2017.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen TR, Schmidt JF, Pedersen MT, Krustrup P, Bangsbo J. The effects of 52 weeks of soccer or resistance training on body composition and muscle function in +65-year-old healthy males—a randomized controlled trial. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt JF, Hansen PR, Andersen TR, Andersen LJ, Hornstrup T, Krustrup P. Cardiovascular adaptations to 4 and 12 months of football or strength training in 65- to 75-year-old untrained men. Scand J Med Sci Sports. 2014;24(Suppl. 1):S86–S97. doi: 10.1111/sms.12217. [DOI] [PubMed] [Google Scholar]
- 27.Sundstrup E, Jakobsen MD, Andersen LL, Andersen TR, Randers MB, Helge JW. Positive effects of 1-year football and strength training on mechanical muscle function and functional capacity in elderly men. Eur J Appl Physiol. 2016;116:1127–1138. doi: 10.1007/s00421-016-3368-0. [DOI] [PubMed] [Google Scholar]
- 28.Tudor-Locke C, Schuna Jr JM, Barreira TV, Mire EF, Broyles ST, Katzmarzyk PT. Normative steps/day values for older adults: NHANES 2005–2006. J Gerontol A Biol Sci Med Sci. 2013;68:1426–1432. doi: 10.1093/gerona/glt116. [DOI] [PubMed] [Google Scholar]
- 29.Rikli RE, Jones CJ. Human Kinetics; Champaign, IL: 2001. Senior fitness test manual. [Google Scholar]
- 30.Skotte J, Korshøj M, Kristiansen J, Hanisch C, Holtermann A. Detection of physical activity types using triaxial accelerometers. J Phys Act Health. 2014;11:76–84. doi: 10.1123/jpah.2011-0347. [DOI] [PubMed] [Google Scholar]
- 31.Rogers MA, Hagberg JM, Martin 3rd WH, Ehsani AA, Holloszy JO. Decline in VO2max with aging in master athletes and sedentary men. J Appl Physiol. 1990;68:2195–2199. doi: 10.1152/jappl.1990.68.5.2195. [DOI] [PubMed] [Google Scholar]
- 32.Nes BM, Vatten LJ, Nauman J, Janszky I, Wisløff U. A simple nonexercise model of cardiorespiratory fitness predicts long-term mortality. Med Sci Sports Exerc. 2014;46:1159–1165. doi: 10.1249/MSS.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 33.Umpierre D, Ribeiro PA, Schaan BD, Ribeiro JP. Volume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: a systematic review with meta-regression analysis. Diabetologia. 2013;56:242–251. doi: 10.1007/s00125-012-2774-z. [DOI] [PubMed] [Google Scholar]
- 34.Helge EW, Andersen TR, Schmidt JF, Jørgensen NR, Hornstrup T, Krustrup P. Recreational football improves bone mineral density and bone turnover marker profile in elderly men. Scand J Med Sci Sports. 2014;24(Suppl. 1):S98–104. doi: 10.1111/sms.12239. [DOI] [PubMed] [Google Scholar]
- 35.Kohrt WM. Bloomfield SA, Little KD, Nelson ME, Yingling VR, American College of Sports Medicine. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–1996. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 36.Boonen S, Vanderschueren D, Callewaeert F, Haentjens P. Aging and bone loss. In: Orwoll ES, Bilezikian JP, Vanderschueren D, editors. Osteoporosis in men: the effects of gender on skeletal health. 2nd ed. Elsevier Inc.; London: 2010. pp. 207–219. [Google Scholar]
- 37.Durstine JL, Grandjean PW, Cox CA, Thompson PD. Lipids, lipoproteins, and exercise. J Cardiopulm Rehabil. 2002;22:385–398. doi: 10.1097/00008483-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Kris-Etherton PM, Taylor DS, Zhao G. Is there an optimal diet for the hypertriglyceridemic patient. J Cardiovasc Risk. 2000;7:333–337. doi: 10.1177/204748730000700506. [DOI] [PubMed] [Google Scholar]
- 39.Evans M, Roberts A, Davies S, Rees A. Medical lipid-regulating therapy: current evidence, ongoing trials and future developments. Drugs. 2004;64:1181–1196. doi: 10.2165/00003495-200464110-00003. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narici MV, Reeves ND, Morse CI, Maganaris CN. Muscular adaptations to resistance exercise in the elderly. J Musculoskelet Neuronal Interact. 2004;4:161–164. [PubMed] [Google Scholar]
- 42.Vorup J, Pedersen MT, Brahe LK, Melcher PS, Alstrøm JM, Bangsbo J. Effect of small-sided team sport training and protein intake on muscle mass, physical function and markers of health in older untrained adults: a randomized trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bangsbo J, Junge A, Dvorak J, Krustrup P. Executive summary: football for health - prevention and treatment of non-communicable diseases across the lifespan through football. Scand J Med Sci Sports. 2014;24(Suppl. 1):S147–S150. doi: 10.1111/sms.12271. [DOI] [PubMed] [Google Scholar]