Abstract
Background
Glomus tumors are rare benign neoplasms that usually occur in the upper and lower extremities. Oral cavity involvement is exceptionally rare, with only a few cases reported to date.
Case presentation
A 24-year-old woman with complaints of swelling in the left floor of her mouth for 6 months was referred to our institution. Her swallowing function was slightly affected; however, she did not have pain or tongue paralysis. Enhanced computed tomography revealed a 2.8 × 1.8 × 2.1 cm-sized well-defined, solid, heterogeneous nodule above the mylohyoid muscle. The mandible appeared to be uninvolved. The patient underwent surgery via an intraoral approach; histopathological examination revealed a glomus tumor. The patient has had no evidence of recurrence over 4 years of follow-up.
Conclusions
Glomus tumors should be considered when patients present with painless nodules in the floor of the mouth.
Keywords: Glomus tumor, Floor of mouth, Oral surgery
Background
The glomus body is a special arteriovenous anastomosis and functions in thermal regulation. Glomus tumors are rare, benign, mesenchymal tumors that originate from modified smooth muscle cells of the normal glomus body [1]. Glomus tumors account for only 1.6% of all soft tissue tumors and typically present as blue-red nodules (sized < 1 cm) that occur in the deep dermis or subcutis region [2]. These tumors are relatively common in the upper and lower extremities, particularly in the subungual site, but rarely occur in mucinous regions or the viscera [3]. Oral cavity involvement is exceptionally rare, with very few cases having been reported to date. Here, we present a case of an unusual glomus tumor that originated from the left floor of the mouth.
Case presentation
A 24-year-old woman with a 6-month history of swelling in the left floor of her mouth was referred to our institution. Although she experienced slight difficulty in swallowing, she did not experience pain or tongue paralysis. Her medical and family histories were unremarkable.
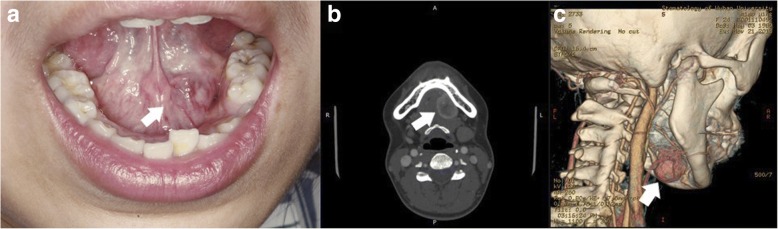
Intraoral examination revealed a well-defined 3.5 × 3 × 2 cm-sized solid, spherical submucosal nodule adjacent to the sublingual gland; the nodule was covered with light bluish smooth mucus (Fig. 1a). The patient experienced slight pain when pressure was applied to the tumor. Mobility and sensory functions of the tongue were normal, and no lymphadenopathy in the submandibular region was detected on palpation. All relevant laboratory test results were normal. Enhanced computed tomography revealed a 2.8 × 1.8 × 2.1 cm-sized well-defined, solid, heterogeneous nodule that did not appear to involve the mandible (Fig. 1b). In addition, a three-dimensionally reconstructed image showed a nodular lesion occupying the left floor of the mouth with abundant blood flow (Fig. 1c). No enlarged lymph nodes were found in the submental or submandibular regions.
Fig. 1.
Physical examination and computed tomography findings. a A light bluish, solid neoplastic lesion measuring 3 cm in size was palpated in the left floor of the mouth, and the covered mucosa had no ulcers (arrow). b A computed tomography scan of the head and neck shows a well-defined, solid, heterogeneous enhanced nodule in the left floor of the mouth. There was no obvious infiltration in the surrounding soft tissue, and the lingual cortical bone of mandible was not involved (arrow). c A three-dimensional image showing the lesion was located lateral to the hyoid bone and adjacent to the left sublingual fossa of the mandible with abundant blood flow (arrow)
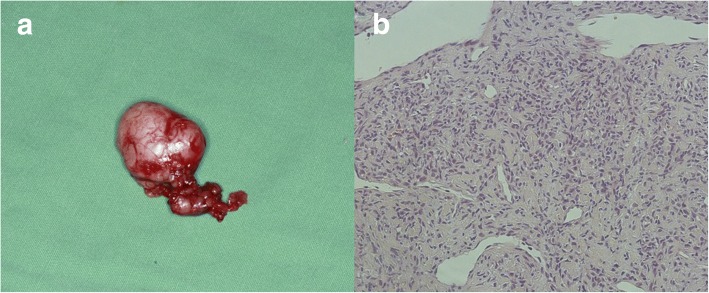
The initial clinical impression was a benign salivary gland tumor, dermoid cyst, or benign connective tissue neoplasm. The patient was scheduled for surgery via an intraoral approach. First, an elliptical incision was made around the periphery of the sublingual gland through only the oral mucosa, and a full-thickness tissue flap was prepared along the lingual aspect of the sublingual gland. After the sublingual gland was freed from its surrounding tissue with blunt dissection, a well-circumscribed tumor without capsular extension was found beneath the body of the sublingual gland and located above the submandibular gland duct and lingual nerve. The submandibular gland duct and lingual nerve were carefully freed from the tumor surface, and the complete tumor was excised along with the sublingual gland (Fig. 2a). The tissue sample was fixed with 10% formalin and submitted for histopathological diagnosis.
Fig. 2.
Macroscopic and histopathological examinations of the resected specimen (hematoxylin and eosin staining, magnification × 200). a The resected specimen was grayish white, with a diameter of approximately 3 cm, and was soft, compressible, covered with capsule, and had a firm attachment to the sublingual gland. b Tumor cells were round, oval, polyhedral, or fusiform arranged in organoid and sheet-like patterns with vascular lumens. Nuclei were round or ovoid with eosinophilic cytoplasm and had no obvious atypical and active mitotic activity. Cell borders were not clearly delineated
Microscopically, the tumor cells were round, oval, polyhedral, or fusiform and were arranged in organoid and sheet-like patterns with vascular lumens. Most of their nuclei were small and round within an amphiphilic or slightly eosinophilic cytoplasm. Nuclear atypia was rare (Fig. 2b).
Immunohistochemistry revealed that the tumor cells yield positive results for vimentin and alpha-smooth muscle actin, but negative results for desmin, anti-cytokeratin (AE)1 or AE3, cluster of differentiation (CD)31 and CD34, and S-100, and exhibited a Ki-67 index of 5%. These findings were consistent with those for a glomus tumor.
After surgery, the patient had an uneventful recovery with primary healing and had no evidence of recurrence over 4 years of follow-up.
Discussion and conclusions
Glomus tumors are rare mesenchymal tumors that occur due to glomus body hyperplasia or hamartomatous development, and they appear to originate from modified smooth muscle cells [1]. These tumors are categorized into three types based on their histological appearances: glomangiomas (20% of cases), solid glomus tumors (75%), and glomangiomyomas (5%) [4]. In our patient, the solid-type tumor was predominant.
Such tumors mainly arise in the dermis or subcutaneous tissues of the hands and feet, especially the tips of fingers and toes. Extradigital glomus tumors are rare; fewer than 1% of glomus tumors are found in the head region [5], and a lesion located in the floor of the mouth has not been reported previously. Recently published case reports regarding glomus tumors of the oral cavity were reviewed [3, 6–39]. The patient characteristics of all 37 cases (including our case) are shown in Table 1. The neoplasms developed in 23 women and 14 men (male to female, 1.6:1), and patient age ranged from 10 to 85 years (median, 52 years). In most cases, the tumors were located on the lip (n = 14, lower 4, upper 10), followed by the buccal mucosa (n = 7), tongue (n = 5), hard palate (n = 6), gingiva (n = 2), maxilla (n = 1), the floor of the mouth (n = 1), and multiple locations (n = 1). Unfortunately, many of the cases documented had an unknown clinical presentation or medical history. Due to inadequate figures or illustrations in these articles, the histologic types of the tumors were not included in our table. Of the cases with available information, the size of the glomus tumor ranged from 0.3 to 4.5 cm. Some lesions were painful, and most were asymptomatic. All lesions were completely excised. Follow-up information was only available in 11 cases ranging from 2 months to 7 years; local recurrence was noted in two cases. Owing to the low incidence rate of glomus tumors in the head and neck region, accurate information on the peak incidence period and sex ratio remain unclear.
Table 1.
Clinical treatment and outcome features of cases of glomus tumors in the oral cavity
| Case | Authors | Year | Gender | Age(years) | Site | Size(cm) | Clinical presentation | Treatment | Follow-up time | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Langer [6] | 1949 | M | 52 | Hard palate | NA | NA | NA | NA | NA |
| 2 | King [7] | 1954 | M | 32 | Gingiva | NA | No symptoms | NA | NA | NA |
| 3 | Kirschner and Strassburg [8] | 1962 | M | 56 | Gingiva/alveolar mucosa | NA | NA | NA | NA | NA |
| 4 | Frenkel [9] | 1965 | M | 13 | Buccal mucosa | NA | NA | NA | NA | NA |
| 5 | Harris and Griffin [10] | 1965 | F | 35 | Maxilla | 0.5 × 0.25 cm | Pain | Surgery | 2 years | NED |
| 6 | Sidhu [11] | 1967 | F | 10 | Hard palate | NA | Painless neoplasm | NA | NA | NA |
| 7 | Charles [12] | 1976 | F | 17 | Hard palate | NA | NA | NA | NA | NA |
| 8 | Lele [13] | 1977 | M | 35 | Hard palate | 1.5 × 1 cm | Painless neoplasm | Surgery | 6 months | NED |
| 9 | Sato et al. [14] | 1979 | M | 29 | Tongue | NA | Painless neoplasm | NA | NA | NA |
| 10 | Tajima et al. [15] | 1981 | F | 63 | Tongue | NA | Painless neoplasm | NA | NA | NA |
| 11 | Saku et al. [16] | 1985 | M | 45 | Buccal mucosa | 4.5 × 3 × 3.5 cm | No symptoms | Surgery | NA | NA |
| 12 | Ficarra et al. [3] | 1986 | F | 51 | Upper lip | NA | No symptoms | NA | NA | NA |
| 13 | Moody et al. [17] | 1986 | F | 65 | Upper lip | 1 × 0.5 × 0.5 cm | No symptoms | Surgery | NA | NA |
| 14 | Stajcic and Bojic [18] | 1987 | M | 55 | Tongue | NA | Painless neoplasm | Surgery | NA | NA |
| 15 | Geraghty et al. [19] | 1992 | M | 71 | Hard palate | 1.5 cm | No symptoms | Surgery | NA | NA |
| 16 | Kusama et al. [20] | 1995 | M | 57 | Upper lip | NA | Painless swelling | Surgery | 4 years | NED |
| 17 | Savaci et al. [22] | 1996 | M | 55 | Buccal mucosa | 1 cm | Pain | Surgery | NA | NA |
| 18 | Sakashita et al. [23] | 1997 | M | 54 | Upper lip | 1.2 × 1 cm | Painless swelling | Surgery | NA | NA |
| 19 | Yu et al. [24] | 2000 | F | 54 | Left mandibular area, lip, anterior buccal mucosa | NA | Painless neoplasm | Surgery | NA | NA |
| 20 | Kessaris et al. [25] | 2001 | F | 46 | Hard palate | 1.8 cm | Painless swelling | Surgery | 3 years | NED |
| 21 | Rallis et al. [27] | 2004 | F | 85 | Upper lip | 1.3 × 1 × 1 cm | Painful swelling | Surgery | 1.5 years | NED |
| 22 | Quesada et al. [26] | 2004 | M | 61 | Tongue | 3 cm | Painless neoplasm | Surgery | 7 years | Recurrence |
| 23 | Lanza et al. [28] | 2005 | M | 65 | Lower lip | NA | Painful mass | NA | NA | NA |
| 24 | Ide et al. [30] | 2008 | M | 57 | Upper lip | 0.8 cm | NA | Surgery | NA | NA |
| 25 | Ide et al. [30] | 2008 | M | 54 | Upper lip | 1.2 cm | NA | Surgery | NA | NA |
| 26 | Wang et al. [31] | 2008 | M | 51 | Buccal mucosa | NA | NA | Surgery | NA | NA |
| 27 | Wang et al. [31] | 2008 | F | 58 | Buccal mucosa | NA | NA | Surgery | NA | NA |
| 28 | Boros et al. [32] | 2010 | M | 34 | Lower lip | NA | NA | Surgery | NA | NA |
| 29 | Yoruk et al. [34] | 2010 | F | 30 | Buccal mucosa | 2 × 1.1 × 0.5 cm | Painless neoplasm | Surgery | 1 years | NED |
| 30 | Dérand et al. [33] | 2010 | F | 11 | Lower lip | 0.3 cm | No symptoms | Surgery | 7 years | NED |
| 31 | Veros et al. [35] | 2012 | F | 24 | Buccal mucosa | 1 × 1 cm | Painful mass | Surgery | 2 months | Recurrence |
| 32 | Chou et al. [36] | 2015 | M | 39 | Upper lip | NA | NA | NA | NA | NA |
| 33 | Monaghan [37] | 2017 | M | 73 | Upper lip | 1 cm | No symptoms | Surgery | NA | NA |
| 34 | Vasconcelos et al. [39] | 2018 | M | 67 | Upper lip | 1 cm | Painful swelling | Surgery | 3.3 years | NED |
| 35 | Smith et al. [38] | 2018 | M | 26 | Lower lip | 1.5 × 0.5 × 0.5 cm | Painful mass | Surgery | NA | NA |
| 36 | Smith et al. [38] | 2018 | F | 58 | Tongue | 2 × 1 cm | No symptoms | Surgery | NA | NA |
| 37 | Our case | M | 24 | The floor of the mouth | 2.8 × 1.8 × 2.1 cm | Painful swelling | Surgery | 4 years | NED |
F female, M male, NA not available, NED no evidence of death
Glomus tumors in the dermis or subcutaneous tissues of the hands and feet are usually < 1 cm in size [21]. However, glomus tumors in the head and neck region are larger, with average diameters of 1–1.5 cm [5]. There is no evidence that the tumor volume influences the patients’ prognoses [25]. Although cases of malignant glomus tumors have been documented, malignancies in the head and neck region are very rare [29], and no characteristic symptoms or imaging features have yet been reported. The diagnosis of malignant transformation of a glomus tumor still depends on its pathological examination. Tumors > 2 cm in size with atypical mitotic figures, moderate-to-high nuclear grade, and > 5 mitotic figures per 50 high-power fields are considered highly suspicious for malignancy [40]. A recent study found that BRAF V600E mutations may be associated with a malignant phenotype in glomus tumors [41]; however, larger cohorts and multicenter studies are required to confirm this finding.
Atypical performance may be the main reason why patients with head and neck glomus tumors postpone visiting the maxillofacial surgery clinic. Although cold sensitivity, spontaneous intermittent pain, and pinpoint tenderness are hallmarks of extraoral glomus tumors, few patients with oral glomus tumors who are referred to the maxillofacial clinic have these symptoms [32]. The lack of such sensations may be attributable to the varying distribution of nerve fibers in different anatomical regions; this notion remains to be explored.
The accurate preoperative diagnosis of intraoral glomus tumors remains challenging. Inaccurate diagnoses are largely attributed to this tumor’s rarity and the lack of distinguishing clinico-morphologic characteristics. Furthermore, such lesions have nonspecific and heterogeneous appearances on radiologic images. A glomus tumor may initially be diagnosed as a salivary tumor, sebaceous cyst, neurofibromatosis, dermoid cyst, teratoid tumor, vascular malformation, or another type of mesenchymal neoplasm [42]. Although vascular malformations and cystic soft tissue lesions can usually be ruled out using color duplex ultrasonography, the differential diagnosis of solid tumors remains challenging. Recently, 18fluorodopa (F-DOPA) positron emission tomography was used for detecting glomus tumors [43]; however, the validity and specificity of this technique for tumors in the head and neck region requires verification. As formal diagnostic guidelines are absent, histologic examination and immunohistochemical analysis remain the gold standards.
Histologically, the appearance of glomus tumors depends on their cellular compositions and differentiation levels. A typical solid glomus tumor is composed of small vascular channels surrounded by clusters of well-defined round cells with lightly eosinophilic cytoplasm, and a large central round or oval nucleus with no atypia. The immunohistochemical profile of glomus tumor cells includes positivity for vimentin, smooth muscle actin, and muscle-specific actin; moreover, positivity for desmin, CD34, and BRAF mutations has been identified in some cases [44]. Conversely, these tumors yield negative results for S-100, myoglobin, neurofilaments, and factor VIII-related antigen [27].
Glomus tumors should first be differentiated from tumors originating from the sublingual gland, where acinar and ductal structures can be observed histologically in such neoplasia [45]; these structures were not observed in our cases. Meanwhile, the sublingual gland is a common site for epithelial tumors; we found that epithelial markers were negative in this case, indicating that the tumor was not a neoplasm of epithelial original.
Secondly, the differential diagnosis for glomus tumors includes vascular tumors such as hemangioma, hemangioendothelioma, epithelioid hemangioma, kaposiform angiodermatitis, reactive angioendotheliomatosis, and angiosarcoma [25]. These tumors can be easily excluded based on histomorphologic features and the expression of endothelial cell markers [46].
Glomus cells, myopericytes, vascular smooth muscle cells, and myofibroblasts are derivatives of pericytes [47]; therefore, glomus tumors should also be distinguished from the most common of the perivascular tumors, including myofibromas, glomangiopericytoma, and myopericytoma. These tumors share many histologic, immunophenotypic, and cytogenetic features, and it is difficult to distinguish them from one another solely by immunohistochemical examination [30]. However, myopericytoma can be differentiated from the glomus tumor based on concentric perivascular growth of spindle neoplastic cells [16]. Myofibromas have a biphasic zonation pattern with light staining fascicles to whorls of myofibroblastic cells, and dark-staining zones of polygonal cells associated with hemangiopericytoma-like vessels [48]. Glomus tumors are composed of cuboidal cells with distinct cell borders, and a round, centrally located nucleus, and they lack spindle cell morphology. The absence of both a multinodular and biphasic pattern would help to exclude myofibroma. Angioleiomyomas have a predominant vascular smooth muscle cell component. On histopathological examination, proliferation of vascular channels was noted, along with thick walls of circumferentially arranged spindle cells [32]. The histomorphological characteristics and positive expression of both actin and desmin can be used to positively identify a glomus tumor.
The majority of glomus tumors are entirely benign; hence, en bloc resection is an effective treatment. While incomplete resection may result in recurrence, local recurrence is very uncommon. Malignant glomus tumors are very rare and require multimodal integrated treatments [26].
We report the rare case of a glomus tumor in the floor of a patient’s mouth that showed no marked symptoms, which complicated its early diagnosis. Glomus tumors should be included in the initial differential diagnosis in patients presenting with painless nodules in the floor of the mouth. En bloc resection is an effective treatment, and patients should receive long-term counseling regarding the risk of recurrence.
Acknowledgements
We would like to thank the patient for agreeing to the publication of this case report and Editage for the English language editing.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AE1
Anti-cytokeratin 1
- AE3
Anti-cytokeratin 3
- CD
Cluster of differentiation
- F-DOPA
18Fluorodopa
Authors’ contributions
All authors provided intellectual contribution to this manuscript. HZ wrote the manuscript. LS and MJ analyzed and interpreted the patient data. LW confirmed the histopathological examination results. YS reviewed the clinical notes and edited the document. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the Hospital of Stomatology of Wuhan University.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the patient’s written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Haixiao Zou, Email: haixiao_zou@whu.edu.cn.
Li Song, Email: nedfy91009@ncu.edu.cn.
Mengqi Jia, Email: jmengqi1992@163.com.
Li Wang, Email: 417331907@qq.com.
Yanfang Sun, Phone: 0086-27-87686215, Email: sunyanfang@whu.edu.cn.
References
- 1.Liapiavgeri G, Karabelabouropoulou V, Agnanti N. Glomus tumor. A histological, histochemical and immunohistochemical study of the various types. Pathol Res Pract. 1994;190(1):2–10. doi: 10.1016/S0344-0338(11)80490-5. [DOI] [PubMed] [Google Scholar]
- 2.Soule EH. Primary soft-tissue tumors. Am J Clin Pathol. 1955;25(25):1392–1394. doi: 10.1093/ajcp/25.12.1392. [DOI] [PubMed] [Google Scholar]
- 3.Ficarra G, Merrell PW, Johnston WH, Hansen LS. Intraoral solitary glomus tumor (glomangioma): case report and literature review. Oral surg oral med oral pathol. 1986;62(3):306–311. doi: 10.1016/0030-4220(86)90013-7. [DOI] [PubMed] [Google Scholar]
- 4.Gombos Z, Zhang PJ. Glomus tumor. Archiv Pathol Lab Med. 2008;132(9):1448–1452. doi: 10.5858/2008-132-1448-GT. [DOI] [PubMed] [Google Scholar]
- 5.Makino Y. A clinicopathological study on soft tissue tumors of the head and neck. Acta Pathol Jpn. 1979;29:389–408. doi: 10.1111/j.1440-1827.1979.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 6.Langer R. Glomustumor des harten Gaumens. Monatsschr Ohrenheilkd Laryngorhinol. 1948;82(7):324. [PubMed]
- 7.King ESJ. Glomus tumour. Australian & New Zealand J Surg. 1954;23(4):280–295. doi: 10.1111/j.1445-2197.1954.tb05057.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirschner H, Strassburg M. Ein am zhanlosen Alveolarfortsatz des Unterkiefers lokalisierter Glomustumor, vol. 17. 1962. [Google Scholar]
- 9.Frenkel G. Auftreten eines Leiomyofibroangioms in Wangen- und Jochbogenbereich, vol. 20. 1965. [PubMed] [Google Scholar]
- 10.Harris R, Griffin CJ. Glomus tumour of the periodontal tissues. Aust Dent J. 1965;10(1):33–37. doi: 10.1111/j.1834-7819.1965.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 11.Sidhu SS. Glomus tumour of palate. J Indian Dental Assoc. 1967;39(10):167. [PubMed] [Google Scholar]
- 12.Charles NC. Multiple glomus tumors of the face and eyelid. Arch Ophthalmol. 1976;94(8):1283–1285. doi: 10.1001/archopht.1976.03910040155005. [DOI] [PubMed] [Google Scholar]
- 13.Lele DN. Glomus tumour of the hard palate. Indian J Otolaryngol. 1977;29(3):136–137. [Google Scholar]
- 14.Sato M, Shirasuna K, Sakuda M, Yanagawa T, Yoshida H, Imai J, Maeda N, Kubo K, Yura Y, Miyazaki T. Fine structure of a glomus tumor of the tongue and expression of C type virus in its tumor cells. Int J Oral Surg. 1979;8(3):199–204. doi: 10.1016/S0300-9785(79)80019-8. [DOI] [PubMed] [Google Scholar]
- 15.Tajima Y, Weather DR, Neville BW, Benoit PW, Pedley DM. Glomus tumor (golomangioma) of the tongue. A light and electron microscopic study. Oral surg oral med oral pathol. 1981;52(3):288–293. doi: 10.1016/0030-4220(81)90268-1. [DOI] [PubMed] [Google Scholar]
- 16.Saku T, Okabe H, Matsutani K, Sasaki M. Glomus tumor of the cheek: an immunohistochemical demonstration of actin and myosin. Oral Surg Oral Med Oral Pathol. 1985;60(1):65–71. doi: 10.1016/0030-4220(85)90218-X. [DOI] [PubMed] [Google Scholar]
- 17.Moody GH, Myskow M, Musgrove C. Glomus tumor of the lip. A case report and immunohistochemical study. Oral Surg Oral Med Oral Pathol. 1986;62(3):312–318. doi: 10.1016/0030-4220(86)90014-9. [DOI] [PubMed] [Google Scholar]
- 18.Stajcic Z, Bojic P. Intraoral glomus tumour. A case report. J Cranio-Maxillofac Surg. 1987;15(6):376–378. doi: 10.1016/S1010-5182(87)80087-2. [DOI] [PubMed] [Google Scholar]
- 19.Geraghty JM, Thomas RW, Robertson JM, Blundell JW. Glomus tumour of the palate: case report and review of the literature. British J Oral Maxillofacial Surg. 1992;30(6):398–400. doi: 10.1016/0266-4356(92)90210-A. [DOI] [PubMed] [Google Scholar]
- 20.Kusama K, Chu L, Kidokoro Y, Kouzu M, Uehara T, Honda M, Ohki H, Sekiwa T, Terakado M, Sato H, et al. Glomus tumor of the upper lip. J Nihon Univ School Dentistry. 1995;37(2):97–101. doi: 10.2334/josnusd1959.37.97. [DOI] [PubMed] [Google Scholar]
- 21.Pulitzer DR, Martin PC, Reed RJ. Epithelioid glomus tumor. Hum Pathol. 1995;26(9):1022–1027. doi: 10.1016/0046-8177(95)90093-4. [DOI] [PubMed] [Google Scholar]
- 22.Savaci N, Emiroǧlu M, Gümren M, Güngör S. A rare case of glomus tumour; buccal localization. Br J Oral Maxillofac Surg. 1996;34(2):199–200. doi: 10.1016/S0266-4356(96)90391-5. [DOI] [PubMed] [Google Scholar]
- 23.Sakashita H, Miyata M, Nagao K. Glomus tumor in the upper lip. A case report. Int J Oral Maxillofac Surg. 1997;26(4):301–302. doi: 10.1016/S0901-5027(97)80876-4. [DOI] [PubMed] [Google Scholar]
- 24.Yu HJ, Kwon SJ, Bahn JY, Park JM, Park YW. Localized multiple glomus tumors of the face and oral mucosa. J Dermatol. 2000;27(3):211–213. doi: 10.1111/j.1346-8138.2000.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 25.Kessaris P, Klimis T, Zanakis S. Glomus tumour of the hard palate: case report and review. Br J Oral Maxillofac Surg. 2001;39(6):478–479. doi: 10.1054/bjom.2001.0721. [DOI] [PubMed] [Google Scholar]
- 26.Quesada R, González-Lagunas J, Raspall G. Aggressive glomus tumor of the tongue. report of a case. 2004;9(4):350. [PubMed] [Google Scholar]
- 27.Rallis G, Komis C, Mahera H. Glomus tumor: a rare location in the upper lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2004;98(3):327–336. doi: 10.1016/j.tripleo.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Lanza A, Moscariello A, Villani R, Colella G. Glomus tumor of the lower lip. A case report. Minerva Stomatol. 2005;54(11–12):687. [PubMed] [Google Scholar]
- 29.Mehrotra S, Sharma R. Glomus tumour : a rare presentation. Med J Armed Forces India. 2007;63(4):378–379. doi: 10.1016/S0377-1237(07)80027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ide F, Mishima K, Yamada H, Saito I, Horie N, Shimoyama T, Kusama K. Perivascular myoid tumors of the oral region: a clinicopathologic re-evaluation of 35 cases. J Oral Pathol Med. 2008;37(1):43–49. doi: 10.1111/j.1600-0714.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Wang J, Shehan J, Sarma DP. Glomus tumor of the cheek. Int J Dermatol. 2008;6(2):5. [Google Scholar]
- 32.Boros AL, Davis JP, Sedghizadeh PP, Yamashita DD. Glomus tumor: report of a rare case affecting the oral cavity and review of the literature. J Oral Maxillofac Surg. 2010;68(9):2329–2334. doi: 10.1016/j.joms.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Dérand P, Warfvinge G, Thor A. Glomangioma: a case presentation. J Oral Maxillofac Surg. 2010;68(1):204–207. doi: 10.1016/j.joms.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Yoruk O, Ucuncu H, Aktan B, Calik M, Kilic K. Glomuvenous malformations in the buccal area. J craniofacial surg. 2010;21(6):2001–2003. doi: 10.1097/SCS.0b013e3181f535a2. [DOI] [PubMed] [Google Scholar]
- 35.Veros K, Markou K, Filitatzi C, Kyrmizakis DE. Glomus tumor of the cheek: a case report. Case Rep Med. 2012;2012:307294. doi: 10.1155/2012/307294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou T, Pan SC, Shieh SJ, Lee JW, Chiu HY, Ho CL. Glomus tumor: twenty-year experience and literature review. Ann Plast Surg. 2016;76(Suppl 1):S35–S40. doi: 10.1097/SAP.0000000000000684. [DOI] [PubMed] [Google Scholar]
- 37.Monaghan L. Glomus tumour presenting in the upper lip - a case report. Br J Oral Maxillofac Surg. 2017;55(10):e156–e157. doi: 10.1016/j.bjoms.2017.08.205. [DOI] [Google Scholar]
- 38.Smith MH, Bhattacharyya I, Cohen DM, Hinze SR, Islam MN. Glomus tumor: a comprehensive review of the clinical and histopathologic features with report of two intraoral cases. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology; 2018. 10.1016/j.oooo.2018.07.056. [Epub ahead of print]. [DOI] [PubMed]
- 39.Vasconcelos ACU, Loyola AM, Gomes APN, de Araújo VC, Tarquínio SBC, Silveira FM, de Aguiar MCF. A symptomatic swelling of the upper lip. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(2):107–111. doi: 10.1016/j.oooo.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Folpe AL, Fanburgsmith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1–12. doi: 10.1097/00000478-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Karamzadeh ND, Bahrami A, Lee SJ, Jenkins SM, Rodriguez FJ, Folpe AL, Boland JM. BRAF V600E mutations occur in a subset of glomus tumors, and are associated with malignant histologic characteristics. Am J Surg Pathol. 2017;41(11):1532–1541. doi: 10.1097/PAS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 42.Lee DW, Yang JH, Chang S, Won CH, Lee MW, Choi JH, Moon KC. Clinical and pathological characteristics of extradigital and digital glomus tumours: a retrospective comparative study. J Europ Acad Dermatol Venereol. 2011;25(12):1392–1397. doi: 10.1111/j.1468-3083.2011.03979.x. [DOI] [PubMed] [Google Scholar]
- 43.Hoegerle S, Ghanem N, Altehoefer C, Schipper J, Brink I, Moser E, Neumann HP. 18F-DOPA positron emission tomography for the detection of glomus tumours. Europ J Nuclear Med Molec Imaging. 2003;30(5):689–694. doi: 10.1007/s00259-003-1115-3. [DOI] [PubMed] [Google Scholar]
- 44.Chakrapani A, Warrick A, Nelson D, Beadling C, Corless CL. BRAF and KRAS mutations in sporadic glomus tumors. Am J Dermatopathol. 2012;34(5):533–535. doi: 10.1097/DAD.0b013e31823931b4. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Madrigal F, Micheau C. Histology of the major salivary glands. Am J Surg Pathol. 1989;13(10):879–899. doi: 10.1097/00000478-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Requena L, Sangueza OP. Cutaneous vascular proliferation. Part II. Hyperplasias and benign neoplasms. J Am Acad Dermatol. 1997;37(6):887. doi: 10.1016/S0190-9622(97)70065-3. [DOI] [PubMed] [Google Scholar]
- 47.Mentzel T, Dei Tos AP, Sapi Z, Kutzner H. Myopericytoma of skin and soft tissues: clinicopathologic and immunohistochemical study of 54 cases. Am J Surg Pathol. 2006;30(1):104–113. doi: 10.1097/01.pas.0000178091.54147.b1. [DOI] [PubMed] [Google Scholar]
- 48.Venkatesh V, Kumar BP, Kumar KAJ, Mohan AP. Myofibroma—a rare entity with unique clinical presentation. J Maxillofacial Oral Surg. 2015;14(1):64–68. doi: 10.1007/s12663-011-0299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.