Summary
Objective
There are conflicting data regarding the association between body mass index (BMI) and health‐related quality of life (HRQoL), especially among certain population subgroups and for mental and physical health domains.
Methods
This study analysed the relationship between BMI and HRQoL (Patient‐Reported Outcomes Measurement Information System mental and physical health scales) using ordinary least squares regression. Each model allowed for the possibility of a non‐linear relationship between BMI and the outcome, adjusting for age, gender, comorbidities, diet and physical activity.
Results
A total of 10,133 respondents were predominantly female (71.7%), White (84.1%), median age of 52.1 years (interquartile range 37.2–63.3) and median BMI of 27.9 (interquartile range 24.0–33.2). In adjusted models, BMI was significantly associated with physical and mental HRQoL (p < 0.001). For physical HRQoL, there was a significant interaction with age (p = 0.02). For mental HRQoL, there was a significant interaction with sex (p = 0.0004) but not age (p = 0.7).
Conclusions
This study demonstrates a non‐linear association of variable clinical relevance between BMI and HRQoL after adjusting for demographic factors and comorbidities. The relationship between BMI and HRQoL is nuanced and impacted by gender and age. These findings challenge the idea of obesity as a main driver of reduced HRQoL, particularly among women and with respect to mental HRQoL.
Keywords: Body mass index (BMI), epidemiology, public health, quality of life
Introduction
Health‐related quality of life (HRQoL) is a multidimensional concept used to describe physical, mental, emotional and social functioning and generally focuses on an individual's perception of his or her own health status 1, 2. With overweight and obesity now affecting nearly 70% of adults in the USA, understanding the multifaceted consequences of obesity, including its impact on HRQoL, will serve to inform decisions for policymakers, physicians and patients 1, 2.
The current body of literature suggests a complex relationship between body mass index (BMI) and HRQoL. In many previous studies, obesity has been associated with reduced quality of life, even when controlling for comorbid illness. However, overweight has been associated with improved quality of life 2, 3, 4, 5, 6, 7, 8, 9, 10, 11. HRQoL is made up of both physical and mental components, and the relationship between BMI and HRQoL may differ with respect to these two domains. Most studies demonstrate a greater deleterious impact of obesity on the physical domains of HRQoL as compared with mental domains, including studies examining individuals with obesity undergoing bariatric surgery or weight loss therapy 5, 12, 13, 14, 15. The relationship between BMI and mental HRQoL appears to be more complex, and results in the current literature are inconsistent. Some studies have found a negative association between obesity and mental HRQoL, while others have demonstrated no association 8, 9, 16, 17. Furthermore, the effects of obesity on quality of life are not uniform for population subgroups. For example, obesity appears to have a differential impact on HRQoL on the basis of gender and age. Previous studies have indicated that women with obesity have greater impairment in physical domains of HRQoL than their male counterparts, and the association between BMI and HRQoL has a variable association with age 9, 18, 19, 20, 21, 22, 23.
While a mounting evidence base points to the adverse influence of obesity on HRQoL, many areas of uncertainty about their association still exist 9, 12. For example, several studies have lacked comprehensive assessments of comorbidities that could potentially confound the relationship between obesity and quality of life 11, 24, 25. Many studies have focused on highly specific and sometimes homogenous geographic or demographic populations, limiting the generalizability of their findings 5, 22, 26, 28. Additionally, most studies to date have examined the relationship between BMI and HRQoL without investigating potentially non‐linear associations 3, 6, 8, 11, 24, 25, 29, 30.
The purpose of this study was to test for associations between BMI and both physical and mental quality of life, controlling for comorbid illnesses, among a geographically diverse adult population in the USA. Because of the large sample size, this study is able to evaluate for potential non‐linear effects while specifically investigating for interactions by age and gender. The authors hypothesized that higher BMI would be associated with lower values of both physical and mental HRQoL, with physical domains, females, and middle‐aged individuals more significantly impacted than their respective counterparts.
Methods
Study design and data sources
A cross‐sectional analysis of data obtained from an online survey was conducted. Study participants were recruited from the Mid‐South Clinical Data Research Network, which integrates a clinical data infrastructure across the USA, consisting of (i) Vanderbilt University Medical Center partnering with Meharry Medical College, (ii) the Vanderbilt Healthcare Affiliated Network, (iii) Greenway Health and (iv) the Carolinas Collaborative, a consortium of four academic health systems and multiple community health systems across North Carolina and South Carolina 31.
Study population
A sample of potentially eligible patients from Vanderbilt University Medical Center, Vanderbilt Healthcare Affiliated Network and Greenway Health was contacted to complete a survey consisting of 72 items that queried participants about demographics and health behaviors (Figure 1). Survey participants were recruited using one of four approaches: face‐to‐face recruitment in medical clinics, an email sent directly from a patient's medical provider, an email sent from the research team or an email sent from a clinic's medical director 32. Survey data collection occurred between August 2014 and November 2015. The primary mode of survey administration was via an electronic survey delivered using REDCap (administered using a tablet computer in person or through an emailed survey link) 33. Participants recruited in person had the option to complete a paper survey. All participants signed informed consent prior to participating and received a $10 gift card for completing the survey. This study received approval from the Vanderbilt University Institutional Review Board.
Figure 1.
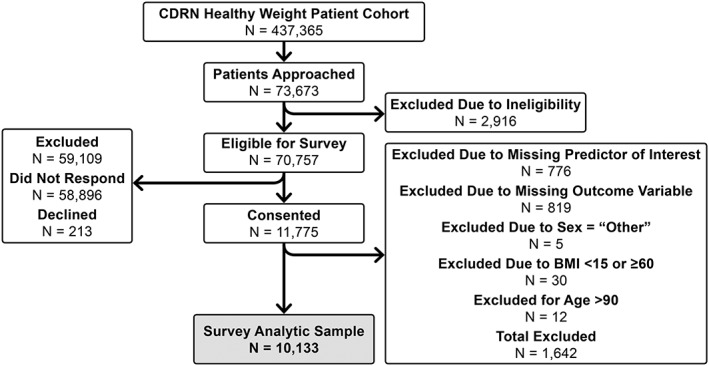
Flow diagram of survey participants. BMI, body mass index; CDRN, Clinical Data Research Network.
Eligibility criteria were determined from structured data available in the electronic medical record and were as follows: survey participants had to (i) be ≥18 years old; (ii) have ≥1 clinic note in the electronic health record (EHR) since 30 April 2009; (iii) have ≥2 weight measures in the EHR since 30 April 2009; and (iv) have ≥1 height measurement in the EHR after age 18. Participants were excluded from survey participation if they had visual acuity or a mental condition that precluded their ability to participate. The survey was offered only in English, so English reading proficiency was also required of participants. Inclusion in this analysis also required that individuals have data for both the predictor and outcome variables of interest, have BMI ≥15 and <60 and be ≤90 years old. In addition, individuals who reported their gender as ‘Other’ were excluded due to the extremely small number of respondents in this category. An in‐depth analysis of sociodemographic characteristics of survey responders and non‐responders has previously been conducted, using inverse probability weighting. These analyses suggest that there is not a sampling bias in the survey responses 32.
The primary independent variable was BMI. For the purposes of this analysis, BMI was calculated using weight in kilograms divided by height in metres squared using self‐reported height and weight data from the survey. The World Health Organization's obesity classification parameters were used to classify participants as underweight, normal weight, overweight, obese or extremely obese 34.
The primary dependent variable was HRQoL, as measured by the Patient‐Reported Outcomes Measurement Information System (PROMIS) Global Health metric, a validated, non‐obesity‐specific tool for assessment of HRQoL 35. PROMIS was developed through an initiative of the National Institutes of Health in an effort to enhance clinical outcomes research by providing a means of measurement for patient‐reported outcomes across a broad range of demographic characteristics and disease processes. The PROMIS Global Health instrument is a 10‐item questionnaire that assesses self‐reported overall health. The PROMIS Global Health instrument yields two scores: a global physical health score and a global mental health score, each of which is based on four items and is standardized using population norms 36. Physical HRQoL is captured using survey items to assess physical health and functioning, pain intensity and fatigue with a potential range of 16.2 to 67.7. Mental HRQoL encompasses overall quality of life, mental health, satisfaction with social interactions and relationships and emotional problems and has a potential range of 21.2 to 67.6 36. Raw scores in each category were converted to standardized T‐scores with the potential ranges as shown previously. As a point of reference, the mean T‐score for both physical and mental domains among American adults is 50 with a standard deviation of 10 points 36. On both physical and mental scales, higher scores indicate better quality of life. Two of the included PROMIS Global items are not used to produce the physical or mental health scores (general health and social roles). In order to comment upon the relationship between the independent variables and overall HRQoL, a validated equation was used to convert the respondents' PROMIS raw scores into a EuroHRQL‐5D (EQ 5D) score, which is a measure of general quality of life commonly utilized in obesity research 37.
Demographic characteristics, which were included as covariates, included age, gender, race/ethnicity, employment status, household income, education, marital status, current smoking status and number of children living in the household. Demographic characteristics were summarized using median and interquartile range (IQR) for continuous variables and using proportions for categorical variables. Self‐reported physical activity and diet quality were also included as covariates with single items that ranged from 1 = ‘I am very inactive’ to 5 = ‘I am active most days’ and 1 = ‘Poor quality diet’ to 5 = ‘Excellent quality diet’, respectively. Spirituality, assessed with a single item ranging from 1 = ‘Not spiritual’ to 5 = ‘Highly spiritual’, was also included as a covariate.
Statistical analysis
This study analysed the relationship between BMI and each outcome (PROMIS mental health score and PROMIS physical health score) using ordinary least squares regression. Each model allowed for the possibility of a non‐linear relationship between BMI and the outcome by modeling BMI using a restricted cubic spline with four knots. Both models were adjusted for age (flexibly modeled using a four‐knot restricted cubic spline), gender, race/ethnicity (4 levels), income (7 levels), educational history (5 levels), employment status (4 levels), marital status (4 levels), number of people under age 19 living in the home (6 levels), current smoking status (yes/no), physical activity (5 levels), diet (5 levels), self‐reported history of high blood pressure (yes/no), self‐reported history of high cholesterol (yes/no), self‐reported history of diabetes (3 levels) and spirituality (4 levels). To allow for the possibility that the relationship between BMI and the PROMIS scores differed by age and/or gender, interactions between gender and BMI and between age and BMI were also included. To evaluate the effect of important potential confounders, three models for each outcome were performed, including unadjusted analyses, partially adjusted analysis controlling for sociodemographics and fully adjusted models including all covariates in the previous models plus diet and physical activity behaviours.
Multiple imputation was used to address missing data. Of the 10,133 respondents who had data on the primary exposure and outcome variables, 1,921 (19%) were missing one or more covariates. The most common missing covariate was income (920 missing, 9%). The authors first imputed 10 sets of missing covariate values using predictive mean matching and then conducted the statistical analyses in each of the 10 partially imputed datasets. Final results arise from the average of the 10 sets of coefficient estimates and from an imputation‐corrected variance–covariance matrix. A sensitivity analysis with complete cases was also conducted.
Because the utilized models allowed the relationship between BMI and the PROMIS scores to be non‐linear, single regression‐coefficient estimates are not helpful in model interpretation, and single‐coefficient significance tests are not helpful in interpreting results. Instead, for each model, F‐tests were conducted for the joint significance of all of the BMI‐related coefficients taken together, as well as for the joint significance of the interaction terms. Model‐based estimates from the resulting predictive models with 95% confidence intervals are also reported. All analyses were conducted using r version 3.4.3 41.
Results
Population characteristics
The response rate as defined by the Council of American Survey Research Organizations at each of the sites varied substantially by recruitment method used 32. While in‐person recruitment achieved up to 94% response rate, recruitment approaches using traditional paper mail resulted in a response rate between 3% and 6%. The overall response rate was 16.4%.
The analytic sample (N = 10,133) was predominantly female (71.7%), White (84.1%) and had a median age of 52.1 years (IQR 37.2–63.3). The median BMI of the analytic sample was 27.9 (IQR 24.0–33.2). Underweight individuals represented 1.2% of the sample, normal weight 30%, overweight 30%, obese 19% and extremely obese 20%. The median score on the PROMIS physical health scale was 50.8 (IQR 42.3–54.1), and the median score on the PROMIS mental health scale was 50.8 (IQR 45.8–56.0).
Demographic and clinical characteristics of patients included in the primary analysis are presented in Table 1, stratified by BMI category. All baseline sociodemographic characteristics were associated with obesity status, where being overweight, obese or extremely obese was associated with higher age, lower socioeconomic status, worse diet quality and lower levels of physical activity. Similarly, overweight and obesity were associated with higher levels of comorbid conditions including hypertension, diabetes and hyperlipidaemia.
Table 1.
Demographic and clinical characteristics of the study population
| Underweight N = 124 | Healthy weight N = 3,073 | Overweight N = 3,039 | Obese N = 1,923 | Morbidly obese N = 1,974 | |
|---|---|---|---|---|---|
| Age* (N = 10,118) | 39.1 (30.1–61.3) | 44.9 (31.5–61.3) | 55.5 (40.9–66.1) | 54.7 (42.9–64.5) | 51.3 (40.1–60.5) |
| Gender* (N = 10,099) | |||||
| Male | 12.2% (15) | 19.0% (584) | 38.3% (1,160) | 33.1% (634) | 23.4% (460) |
| Female | 87.8% (108) | 81.0% (2,483) | 61.7% (1,868) | 66.9% (1,281) | 76.6% (1,506) |
| Race/ethnicity* (N = 9,959) | |||||
| White, non‐Hispanic | 82.1% (101) | 88.0% (2,657) | 86.3% (2,580) | 80.9% (1,521) | 77.9% (1,516) |
| Black, non‐Hispanic | 4.9% (6) | 5.1% (153) | 8.4% (250) | 13.8% (259) | 18.8% (365) |
| Hispanic | 3.3% (4) | 2.1% (63) | 2.0% (60) | 1.9% (36) | 1.4% (27) |
| Other, non‐Hispanic | 9.8% (12) | 4.9% (148) | 3.3% (98) | 3.5% (65) | 2.0% (38) |
| Annual income* (N = 9,213) | |||||
| less than $10,000 | 2.0% (2) | 3.4% (94) | 2.2% (62) | 4.2% (73) | 4.7% (86) |
| $10,000 to $19,999 | 1.0% (1) | 4.6% (126) | 5.1% (142) | 6.5% (114) | 8.6% (158) |
| $20,000 to $34,999 | 15.8% (16) | 11.4% (314) | 12.5% (347) | 12.8% (224) | 17.8% (326) |
| $35,000 to $49,999 | 11.9% (12) | 12.3% (338) | 14.2% (394) | 15.2% (266) | 18.0% (330) |
| $50,000 to $74,999 | 17.8% (18) | 20.6% (567) | 21.5% (598) | 22.5% (393) | 22.0% (403) |
| $75,000 to $99,999 | 20.8% (21) | 16.0% (440) | 18.1% (502) | 16.1% (282) | 13.9% (255) |
| $100,000 or more | 30.7% (31) | 31.7% (873) | 26.4% (732) | 22.7% (398) | 15.0% (275) |
| Highest level of education* (N = 10,073) | |||||
| Less than HS degree | 2.5% (3) | 1.2% (38) | 1.7% (50) | 1.8% (34) | 2.3% (44) |
| HS graduate or GED | 13.1% (16) | 8.4% (257) | 12.8% (386) | 14.2% (271) | 17.3% (338) |
| Some college or 2‐year degree | 20.5% (25) | 22.2% (678) | 29.6% (894) | 33.4% (639) | 36.5% (714) |
| College degree | 25.4% (31) | 31.0% (948) | 25.7% (776) | 23.8% (455) | 23.2% (453) |
| More than college degree | 38.5% (47) | 37.2% (1,138) | 30.4% (918) | 26.9% (514) | 20.8% (406) |
| Employment status* (N = 10,120) | |||||
| Unemployed/homemaker/stay at home | 25.2% (31) | 17.5% (536) | 9.1% (277) | 7.4% (143) | 8.9% (176) |
| Caregiver/full‐time student | |||||
| Unable to work (disabled) | 13.0% (16) | 5.1% (155) | 7.0% (212) | 9.0% (173) | 14.1% (278) |
| Retired | 15.4% (19) | 16.6% (509) | 24.6% (748) | 22.5% (433) | 13.7% (270) |
| Employed (full‐time, part‐time or self‐employed) | 46.3% (57) | 60.9% (1,869) | 59.2% (1,798) | 61.0% (1,173) | 63.3% (1,247) |
| Marital status* (N = 10,074) | |||||
| Never married | 26.4% (32) | 18.4% (563) | 10.2% (309) | 9.2% (176) | 15.0% (294) |
| Divorced/separated | 8.3% (10) | 8.9% (273) | 11.1% (336) | 14.2% (271) | 15.9% (312) |
| Widowed | 3.3% (4) | 4.0% (123) | 4.4% (134) | 4.0% (76) | 4.3% (84) |
| Married/living with partner | 62.0% (75) | 68.6% (2,098) | 74.2% (2,245) | 72.6% (1,388) | 64.8% (1,271) |
| Number of children <19 years living in household** (N = 10,020) | |||||
| 0 | 70.7% (87) | 68.2% (2,076) | 70.4% (2,115) | 69.6% (1,323) | 65.2% (1,271) |
| 1 | 11.4% (14) | 14.6% (443) | 13.7% (411) | 14.4% (273) | 17.9% (350) |
| 2 | 11.4% (14) | 11.9% (363) | 10.5% (315) | 11.5% (218) | 11.6% (226) |
| 3 | 4.1% (5) | 4.0% (123) | 3.6% (108) | 3.3% (63) | 3.8% (74) |
| 4 | 1.6% (2) | 0.9% (28) | 1.3% (40) | 0.8% (16) | 1.2% (24) |
| 5 or more | 0.8% (1) | 0.3% (9) | 0.5% (14) | 0.5% (9) | 0.3% (5) |
| Current smoking status** (N = 9,898) | |||||
| No | 87.5% (105) | 92.4% (2,773) | 90.4% (2,674) | 90.1% (1,694) | 90.1% (1,748) |
| Yes | 12.5% (15) | 7.6% (228) | 9.6% (283) | 9.9% (186) | 9.9% (192) |
| Physical activity* (N = 10,118) | |||||
| I am very inactive | 8.9% (11) | 7.8% (238) | 10.2% (309) | 13.0% (249) | 22.7% (448) |
| I am active a couple times a month | 10.5% (13) | 10.1% (309) | 13.3% (404) | 17.9% (343) | 24.4% (481) |
| I am active most weeks | 18.5% (23) | 16.9% (520) | 18.5% (562) | 22.0% (423) | 20.0% (394) |
| I am active several days a week | 24.2% (30) | 26.2% (805) | 26.0% (788) | 23.4% (449) | 18.6% (367) |
| I am active most days | 37.9% (47) | 39.0% (1,196) | 32.0% (972) | 23.8% (456) | 14.3% (281) |
| Diet quality* (N = 10,115) | |||||
| Poor | 2.4% (3) | 0.9% (28) | 1.9% (59) | 3.9% (75) | 9.1% (179) |
| Fair | 14.5% (18) | 8.2% (250) | 14.6% (443) | 24.2% (464) | 33.9% (668) |
| Good | 27.4% (34) | 32.6% (1,000) | 43.1% (1,309) | 45.8% (880) | 42.7% (842) |
| Very good | 37.9% (47) | 42.8% (1,311) | 32.6% (989) | 21.7% (417) | 12.8% (252) |
| Excellent | 17.7% (22) | 15.5% (476) | 7.8% (236) | 4.4% (84) | 1.5% (29) |
| High blood pressure* (N = 10,010) | |||||
| No | 84.2% (101) | 78.5% (2,387) | 57.7% (1,733) | 45.9% (872) | 38.7% (753) |
| Yes | 15.8% (19) | 21.5% (652) | 42.3% (1,270) | 54.1% (1,029) | 61.3% (1,194) |
| High cholesterol* (N = 9,953) | |||||
| No | 77.0% (94) | 73.7% (2,227) | 52.4% (1,564) | 46.8% (885) | 51.0% (986) |
| Yes | 23.0% (28) | 26.3% (795) | 47.6% (1,421) | 53.2% (1,005) | 49.0% (948) |
| Diabetes mellitus type I or II* (N = 10,073) | |||||
| No | 96.0% (119) | 92.9% (2,839) | 84.0% (2,542) | 71.6% (1,367) | 62.1% (1,217) |
| Yes | 2.4% (3) | 5.0% (154) | 11.9% (361) | 19.7% (377) | 26.8% (525) |
| Pre‐diabetes or borderline diabetes | 1.6% (2) | 2.0% (62) | 4.0% (122) | 8.7% (166) | 11.1% (217) |
| Spirituality* (N = 9,835) | |||||
| Very | 40.5% (49) | 43.2% (1,281) | 47.2% (1,402) | 48.6% (908) | 49.4% (941) |
| Fairly | 26.4% (32) | 29.4% (874) | 31.8% (946) | 33.3% (622) | 33.1% (631) |
| Slightly | 16.5% (20) | 15.9% (472) | 13.9% (413) | 12.6% (235) | 11.8% (224) |
| Not at all | 16.5% (20) | 11.5% (341) | 7.1% (212) | 5.5% (103) | 5.7% (109) |
Demographic characteristics, psychosocial variables and comorbid illness stratified by obesity status among the 10,133 survey respondents included in the analytic sample.
p < 0.001.
p < 0.05.
GED, general educational development; HS, high school.
Unadjusted estimates
Unadjusted linear regression using a restricted cubic spline showed a non‐linear relationship between BMI and physical HRQoL, with little relationship between BMI and physical HRQoL for respondents with healthy weights, but a pronounced negative association between BMI and physical HRQoL for overweight and obese respondents. There was a similar pattern observed in unadjusted models comparing BMI and mental HRQoL. The addition of covariates to the models substantially changed both the shape and magnitude of the associations (Figure 2).
Figure 2.
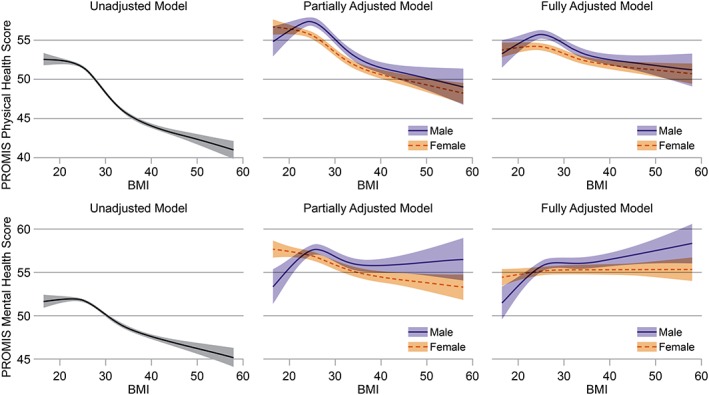
Model‐based estimates showing the association between body mass index (BMI) and health‐related quality of life in both physical and mental domains. We show model‐based estimates and 95% confidence intervals for unadjusted models. Subsequent models are partially adjusted, controlling for age, gender, income, education, employment, marital status, race/ethnicity, smoking status, number of people age < 19 years living in the home, history of high blood pressure, history of diabetes, history of high cholesterol and spirituality. Fully adjusted models control for all of the previous covariates, with the addition of diet and physical activity. The F‐tests of the overall association between BMI and quality of life were statistically significant for both physical health quality of life (p < 0.0001) and mental health quality of life (p < 0.0001). Estimates from the partially and fully adjusted models are shown for subjects with the sample median value for age (52) and with values of all other covariates equal to the sample mode. PROMIS, Patient‐Reported Outcomes Measurement Information System.
Adjusted estimates
After controlling for potential confounders, multivariable linear regression still suggested non‐linear relationships between BMI and both physical and mental HRQoL scores (Figure 2). The F‐tests of the overall association between BMI and quality of life were statistically significant for both physical health quality of life (p < 0.0001) and mental health quality of life (p < 0.0001). To evaluate the association between BMI and overall HRQoL, similar models using the EQ 5D scores were conducted and are presented as model‐based estimates in Table 2. A complete‐case sensitivity analysis was also conducted, and results did not substantively differ from the multiply‐imputed dataset (results not shown).
Table 2.
BMI and general health‐related quality of life
| BMI | Gender | Age 30 | Age 50 | Age 70 |
|---|---|---|---|---|
| 25 | Female | 0.80 (0.79, 0.81) | 0.79 (0.79, 0.80) | 0.80 (0.79, 0.80) |
| Male | 0.82 (0.81, 0.83) | 0.81 (0.80, 0.82) | 0.81 (0.80, 0.82) | |
| 28 | Female | 0.80 (0.80, 0.81) | 0.79 (0.78, 0.80) | 0.79 (0.78, 0.80) |
| Male | 0.82 (0.81, 0.83) | 0.81 (0.80, 0.81) | 0.81 (0.80, 0.82) | |
| 30 | Female | 0.80 (0.80, 0.81) | 0.79 (0.78, 0.79) | 0.78 (0.78, 0.79) |
| Male | 0.82 (0.81, 0.83) | 0.80 (0.80, 0.81) | 0.80 (0.79, 0.81) | |
| 35 | Female | 0.80 (0.80, 0.81) | 0.78 (0.78, 0.79) | 0.77 (0.76, 0.78) |
| Male | 0.82 (0.81, 0.83) | 0.79 (0.79, 0.80) | 0.78 (0.78, 0.79) | |
| 40 | Female | 0.80 (0.79, 0.81) | 0.78 (0.77, 0.78) | 0.76 (0.76, 0.77) |
| Male | 0.82 (0.81, 0.83) | 0.79 (0.78, 0.80) | 0.78 (0.77, 0.79) |
Model‐based estimates of a general health‐related quality of life measure (EQ 5D), from adjusted ordinary least squares regression with non‐linear BMI term as the primary predictor, controlling for age, gender, income, education, employment, marital status, race/ethnicity, smoking status, number of people age < 19 years living in the home, physical activity, diet, history of high blood pressure, history of diabetes, history of high cholesterol and spirituality. Model‐based estimates are given for a range of BMI values, stratified by age and gender. The model‐based estimate for EQ 5D is given plus the 95% confidence interval.
BMI, body mass index.
For physical HRQoL, this study found a significant interaction between BMI and age (p = 0.02) and a trend toward significance for the interaction between BMI and gender (p = 0.0579). For mental HRQoL, there was a significant interaction between gender and BMI (p = 0.0004) but not between age and BMI (p = 0.7). For both physical and mental health quality of life, the relationship between BMI and HRQoL was more pronounced in men. Among women, BMI was not associated with mental HRQoL, but a steady, nearly linear association between higher BMI and lower physical HRQoL was observed. Figure 3 shows model‐based estimates for men and women across a range of ages, suggesting that older individuals have a stronger association between BMI and HRQoL.
Figure 3.
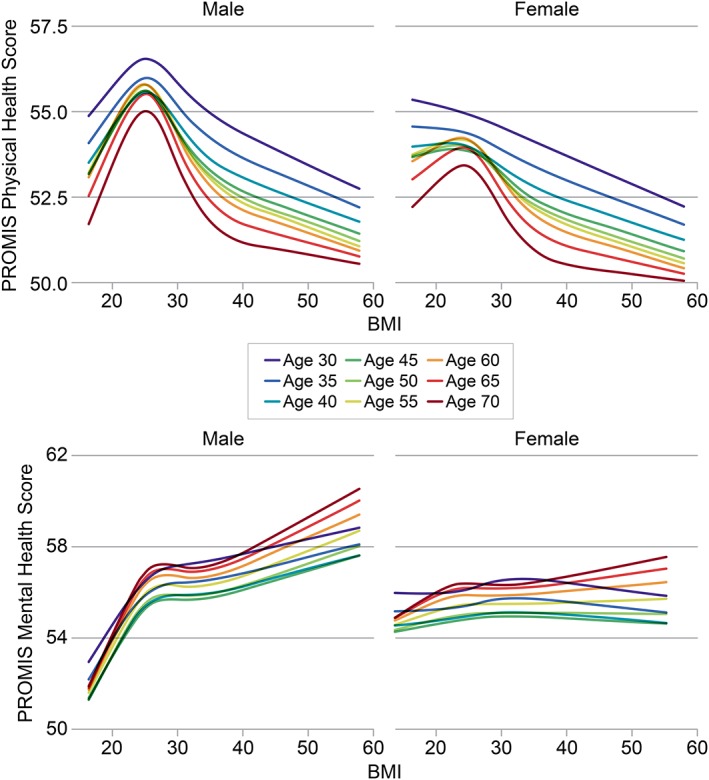
Associations between body mass index (BMI) and health‐related quality of life (HRQoL), with interations by age and gender. Model‐based estimates showing the association between BMI and HRQoL in both physical and mental domains are shown separately by gender, across a range of age values. Models are adjusted for age, gender, income, education, employment, marital status, race/ethnicity, smoking status, number of people age < 19 years living in the home, physical activity, diet, history of high blood pressure, history of diabetes, history of high cholesterol and spirituality. For physical HRQoL, there was a significant interaction with age (p = 0.02) and a trend towards significance for the interaction with gender (p = 0.0579). For mental HRQoL, there was a significant interaction with gender (p = 0.0004) but not with age (p = 0.7).
Both diet and physical activity also showed significant associations with HRQoL (p < 0.001) in these analyses. Because the causal relationship between these two covariates, HRQoL and obesity, is likely bidirectional, however, the data using partially adjusted models that controlled for all covariates except diet and physical activity were also analysed. Figure 2 shows that the exclusion of these covariates did not substantially alter the shape or magnitude of associations between BMI and HRQoL in either mental or physical health domains.
Discussion
This study of a large, geographically diverse sample demonstrated a statistically significant and nuanced association between BMI and both mental and physical domains of HRQoL. In some population subgroups, the effect of BMI on HRQoL is quite small: specifically, for women, the clinical relevance of associations between BMI and mental HRQoL is unclear. In contrast, older men had a clinically significant association between BMI and mental HRQoL. Because the associations are so complex, it is difficult to make sweeping generalizations about the nature and magnitude of the associations. However, this complexity fits within the paradigm of personalized medicine, which pushes the medical community to identify important individual differences that can shape both clinical recommendations and research agenda. Consequently, the main application of these results is to recognize that for some individuals, BMI will likely be a primary driver of HRQoL, while for others, the association may be minimal.
In general, these findings are in agreement with the current literature in that increased BMI is related to decreased overall HRQoL 6, 14, 21, 39. However, it is important to note that the majority of previous studies have assumed that the relationship between BMI and HRQoL is linear 3, 6, 8, 11, 24, 25, 29, 30. The non‐linear nature of the associations revealed here, as well as the presence of multiple interactions by age and gender, points to a more complex relationship between BMI and HRQoL than has previously been described. When looking at overall measures of HRQoL (i.e. EQ 5D), other studies have consistently demonstrated small effect sizes 9. For example, a 2011 study by Bentley et al., reported a decrease of 0.06 points in the EQ 5D score between the normal weight category and the obese category 11. The present study found a decrease of 0.03 points on the model‐based EQ 5D score between a 70‐year‐old male with a BMI of 25 and a 70‐year‐old male with a BMI of 40.
The current study found that the impact of BMI on physical HRQoL was greater among men than women, and that for women, there was very little association between BMI and mental HRQoL. These findings are consistent with those of Sarwer et al., who have examined the relationship between sexual dysfunction, quality of life and BMI in individuals with extreme obesity seeking bariatric surgery 40, 41, 42. These previous studies have demonstrated a positive association between weight loss and physical HRQoL among men and women, which was sustained up to 4 years following bariatric surgery. However, in these same cohorts, for women with extreme obesity undergoing bariatric surgery, measures of mental HRQoL initially improved but eventually returned to pre‐surgery baseline by 4 years after surgery 42. These findings support the concept that the relationship between mental health and BMI is complex and multifactorial, particularly among women. Taken together, these data suggest several areas of future research, including evaluation of HRQoL among individuals with low BMI, while our models suggest a potential association, the sample size among underweight individuals was limited.
This study advances the current literature by using a novel measure in the PROMIS scale, which focuses predominantly on aspects of HRQoL that are important to patients. In addition, one area of significant strength of this study is the inclusion of multiple covariates in the modelling approach. As demonstrated by the change in the shape and directionality of the associations when comparing unadjusted to fully adjusted models, it is important to recognize the potentially complex relationships between HRQoL, BMI, comorbid illness and diet/physical activity behaviors. Another important contribution of these analyses is to suggest that while there is a statistically significant association between both diet and physical activity and HRQoL even after adjusting for other covariates and BMI, this study's overall conclusions about the associations between BMI and HRQoL do not depend on whether diet and physical activity are included in the model. While one cannot draw causal inference because of the cross‐sectional nature of this study, this study points to the need for future research aimed at elucidating the causal pathway in obesity and quality of life.
To evaluate potential sources of bias that would explain these findings, one may consider the following limitations. In addition to the cross‐sectional nature of the analysis and the potential for residual confounding, another limitation is the generalizability of the findings. This study's participants were all healthcare users, and as such, the results may lack generalizability to non‐healthcare users. The study population was predominantly middle‐aged, female, White, affluent, well‐educated and fluent in English. In addition, it is possible that generic measures of HRQoL, such as PROMIS, may not be as sensitive to obesity‐specific impairment in HRQoL, which may be better ascertained using an obesity‐specific tool 43. The self‐reports of height and weight, used to calculate BMI, may have introduced a differential misclassification bias; however, the association between BMI and other comorbid medical conditions (like diabetes and hypertension) limits that potential concern. While there were low survey response rates, prior in‐depth analyses suggest no selection bias 32.
Conclusions
This study demonstrates a non‐linear association between BMI and HRQoL after adjusting for demographic factors and comorbid illness, regardless of whether we controlled for diet and physical activity. The relationship between BMI and HRQoL is nuanced and significantly impacted by gender and age. These findings challenge the idea of obesity as a main driver of reduced HRQoL, particularly among women and with respect to mental HRQoL. Future studies using flexible modeling techniques with longitudinal follow‐up are needed to further our understanding of the potentially bidirectional associations between BMI and HRQoL and to inform clinical and research practices in the fields of obesity and quality of life.
Conflict of Interest Statement
The authors declare no competing financial interests. The Mid‐South CDRN was initiated and funded by Patient‐Centered Outcomes Research Institute (PCORI) through the contract CDRN‐1306‐04869, the Vanderbilt Institute for Clinical and Translational Research with grant support from ULTR000445 from NCATS/NIH and institutional funding.
Apple, R. , Samuels, L. R. , Fonnesbeck, C. , Schlundt, D. , Mulvaney, S. , Hargreaves, M. , Crenshaw, D. , Wallston, K. A. , and Heerman, W. J. (2018) Body mass index and health‐related quality of life. Obesity Science & Practice, 4: 417–426. 10.1002/osp4.292.
References
- 1. Guyatt GH, Feeny DH, Patrick DL. Measuring health‐related quality of life. Annals of Internal Medicine 1993; 118: 622–629. [DOI] [PubMed] [Google Scholar]
- 2. Milder IE, de Hollander EL, Picavet HS, Verschuren WM, de Groot LC, Bemelmans WJ. Changes in weight and health‐related quality of life. The Doetinchem cohort study. Journal of Epidemiology and Community Health 2014; 68: 471–477. [DOI] [PubMed] [Google Scholar]
- 3. Wang R, Wu MJ, Ma XQ, et al. Body mass index and health‐related quality of life in adults: a population based study in five cities of China. European Journal of Public Health 2012; 22: 497–502. [DOI] [PubMed] [Google Scholar]
- 4. Hassan MK, Joshi AV, Madhavan SS, Amonkar MM. Obesity and health‐related quality of life: a cross‐sectional analysis of the US population. International Journal of Obesity and Related Metabolic Disorders 2003; 27: 1227–1232. [DOI] [PubMed] [Google Scholar]
- 5. Fontaine KR, Barofsky I. Obesity and health‐related quality of life. Obesity Reviews 2001; 2: 173–182. [DOI] [PubMed] [Google Scholar]
- 6. Kearns B, Ara R, Young T, Relton C. Association between body mass index and health‐related quality of life, and the impact of self‐reported long‐term conditions – cross‐sectional study from the south Yorkshire cohort dataset. BMC Public Health 2013; 13: 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolotkin RL, Crosby RD, Gress RE, Hunt SC, Engel SG, Adams TD. Health and health‐related quality of life: differences between men and women who seek gastric bypass surgery. Surgery for Obesity and Related Diseases 2008; 4: 651–658. discussion 658‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Zwaan M, Petersen I, Kaerber M, et al. Obesity and quality of life: a controlled study of normal‐weight and obese individuals. Psychosomatics 2009; 50: 474–482. [DOI] [PubMed] [Google Scholar]
- 9. Ul‐Haq Z, Mackay DF, Fenwick E, Pell JP. Meta‐analysis of the association between body mass index and health‐related quality of life among adults, assessed by the SF‐36. Obesity (Silver Spring) 2013; 21: E322–E327. [DOI] [PubMed] [Google Scholar]
- 10. Ul‐Haq Z, Mackay DF, Fenwick E, Pell JP. Impact of metabolic comorbidity on the association between body mass index and health‐related quality of life: a Scotland‐wide cross‐sectional study of 5,608 participants. BMC Public Health 2012; 12: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bentley TG, Palta M, Paulsen AJ, et al. Race and gender associations between obesity and nine health‐related quality‐of‐life measures. Quality of Life Research 2011; 20: 665–674. [DOI] [PubMed] [Google Scholar]
- 12. Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health‐related quality of life. Clin Obes 2017; 7: 273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen JR, Aasprang A, Karlsen TI, Natvig GK, Vage V, Kolotkin RL. Health‐related quality of life after bariatric surgery: a systematic review of prospective long‐term studies. Surgery for Obesity and Related Diseases 2015; 11: 466–473. [DOI] [PubMed] [Google Scholar]
- 14. Huang IC, Frangakis C, Wu AW. The relationship of excess body weight and health‐related quality of life: evidence from a population study in Taiwan. International Journal of Obesity 2006; 30: 1250–1259. [DOI] [PubMed] [Google Scholar]
- 15. Hachem A, Brennan L. Quality of life outcomes of bariatric surgery: a systematic review. Obesity Surgery 2016; 26: 395–409. [DOI] [PubMed] [Google Scholar]
- 16. Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. Journal of Psychosomatic Research 2008; 64: 97–105. [DOI] [PubMed] [Google Scholar]
- 17. Fontaine KR, Bartlett SJ, Barofsky I. Health‐related quality of life among obese persons seeking and not currently seeking treatment. The International Journal of Eating Disorders 2000; 27: 101–105. [DOI] [PubMed] [Google Scholar]
- 18. Mannucci E, Petroni ML, Villanova N, et al. Clinical and psychological correlates of health‐related quality of life in obese patients. Health and Quality of Life Outcomes 2010; 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health‐related quality of life in patients with chronic illness. Journal of General Internal Medicine 2000; 15: 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lean ME, Han TS, Seidell JC. Impairment of health and quality of life using new US federal guidelines for the identification of obesity. Archives of Internal Medicine 1999; 159: 837–843. [DOI] [PubMed] [Google Scholar]
- 21. Ford ES, Moriarty DG, Zack MM, Mokdad AH, Chapman DP. Self‐reported body mass index and health‐related quality of life: findings from the behavioral risk factor surveillance system. Obesity Research 2001; 9: 21–31. [DOI] [PubMed] [Google Scholar]
- 22. Lopez‐Garcia E, Banegas Banegas JR, Gutierrez‐Fisac JL, Perez‐Regadera AG, Ganan LD, Rodriguez‐Artalejo F. Relation between body weight and health‐related quality of life among the elderly in Spain. International Journal of Obesity and Related Metabolic Disorders 2003; 27: 701–709. [DOI] [PubMed] [Google Scholar]
- 23. Busutil R, Espallardo O, Torres A, Martinez‐Galdeano L, Zozaya N, Hidalgo‐Vega A. The impact of obesity on health‐related quality of life in Spain. Health and Quality of Life Outcomes 2017; 15: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hopman WM, Berger C, Joseph L, et al. The association between body mass index and health‐related quality of life: data from CaMos, a stratified population study. Quality of Life Research 2007; 16: 1595–1603. [DOI] [PubMed] [Google Scholar]
- 25. Renzaho A, Wooden M, Houng B. Associations between body mass index and health‐related quality of life among Australian adults. Quality of Life Research 2010; 19: 515–520. [DOI] [PubMed] [Google Scholar]
- 26. Kolotkin RL, Head S, Hamilton M, Tse CK. Assessing impact of weight on quality of life. Obesity Research 1995; 3: 49–56. [DOI] [PubMed] [Google Scholar]
- 27. Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non‐surgical treatment for obesity: a systematic review and meta‐analysis of randomised controlled trials. BMJ 2013; 347: f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fabricatore AN, Wadden TA, Sarwer DB, Faith MS. Health‐related quality of life and symptoms of depression in extremely obese persons seeking bariatric surgery. Obesity Surgery 2005; 15: 304–309. [DOI] [PubMed] [Google Scholar]
- 29. Doll HA, Petersen SE, Stewart‐Brown SL. Obesity and physical and emotional well‐being: associations between body mass index, chronic illness, and the physical and mental components of the SF‐36 questionnaire. Obesity Research 2000; 8: 160–170. [DOI] [PubMed] [Google Scholar]
- 30. Marchesini G, Solaroli E, Baraldi L, et al. Health‐related quality of life in obesity: the role of eating behaviour. Diabetes, Nutrition & Metabolism 2000; 13: 156–164. [PubMed] [Google Scholar]
- 31. Rosenbloom ST, Harris P, Pulley J, et al. The Mid‐South Clinical Data Research Network. Journal of the American Medical Informatics Association 2014; 21: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heerman WJ, Jackson N, Roumie CL, et al. Recruitment methods for survey research: findings from the Mid‐South Clinical Data Research Network. Contemporary Clinical Trials 2017; 62: 50–55. [DOI] [PubMed] [Google Scholar]
- 33. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Committee WE. Physical Status: The Use and Interpretation of Anthropometry, Technical Report Series, Vol. 854 WHO: Geneva, 1995. [PubMed] [Google Scholar]
- 35. Garcia SF, Cella D, Clauser SB, et al. Standardizing patient‐reported outcomes assessment in cancer clinical trials: a patient‐reported outcomes measurement information system initiative. Journal of Clinical Oncology 2007; 25: 5106–5112. [DOI] [PubMed] [Google Scholar]
- 36. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the Patient‐Reported Outcomes Measurement Information System (PROMIS) global items. Quality of Life Research 2009; 18: 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Revicki DA, Kawata AK, Harnam N, Chen WH, Hays RD, Cella D. Predicting EuroQol (EQ‐5D) scores from the patient‐reported outcomes measurement information system (PROMIS) global items and domain item banks in a United States sample. Quality of Life Research 2009; 18: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. R: A Language and Environment for Statistical Computing. [computer program]. R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- 39. Sarwer DB, Lavery M, Spitzer JC. A review of the relationships between extreme obesity, quality of life, and sexual function. Obesity Surgery 2012; 22: 668–676. [DOI] [PubMed] [Google Scholar]
- 40. Sarwer DB, Spitzer JC, Wadden TA, et al. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA Surgery 2014; 149: 26–33. [DOI] [PubMed] [Google Scholar]
- 41. Sarwer DB, Spitzer JC, Wadden TA, et al. Sexual functioning and sex hormones in men who underwent bariatric surgery. Surgery for Obesity and Related Diseases 2015; 11: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sarwer DB, Wadden TA, Spitzer JC, et al. 4‐Year changes in sex hormones, sexual functioning, and psychosocial status in women who underwent bariatric surgery. Obesity Surgery 2018; 28: 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Le Pen C, Levy E, Loos F, Banzet MN, Basdevant A. “Specific” scale compared with “generic” scale: a double measurement of the quality of life in a French community sample of obese subjects. Journal of Epidemiology and Community Health 1998; 52: 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]


