Summary
Background/aim
Exercise can be used as a strategy to attenuate hyperglycaemia experienced during gestational diabetes mellitus (GDM). To maximize its use for clinical management, the most effective modality should be identified. The purpose of this review is to elucidate the most effective modality of exercise on insulin sensitivity and blood glucose control in pregnant women with or at risk of GDM.
Methods
A search was undertaken in MEDLINE, PubMed, Scopus, CINAHL, the Cochrane Library, Embase and the Maternity & Infant Healthcare Database. Studies that met inclusion criteria were randomized controlled trials and case‐controlled studies, which compared exercise interventions with standard care during pregnancy in women with or at risk of GDM.
Results
Two interventions using resistance training, eight using aerobic exercise and two using a combination of both modalities were included. The interventions showed consistently that requirements of insulin therapy, dosage, and latency to administration were improved in the exercise groups. Less consistent results were observed for capillary blood glucose measurements; however, both modalities and combination of modalities were effective at improving blood glucose control in already diagnosed patients and pregnant women with obesity. Discrepancies in the timing of intervention, GDM diagnostic criteria, and the different measures used to assess glucose metabolism make it difficult to draw clear recommendations.
Conclusion
Exercising three times per week for 40–60 min at 65–75% age‐predicted heart rate maximum using cycling, walking or circuit training as a modality improved glycaemic control in GDM patients and reduced incidence of GDM in pregnant women with obesity. Further studies looking specifically at the effects of different modalities of exercise on glucose metabolism with combined strategies to enhance insulin sensitivity should be explored to maximize benefits for GDM pregnancies. Consistency in design and delivery of exercise‐only interventions is required to make recommendations on a suitable exercise prescription in this population. In practice, adherence to consensus in diagnostic cut‐offs for GDM diagnosis is fundamental for standardizing future research.
Keywords: Exercise, gestational diabetes mellitus, glycaemic control, insulin sensitivity
List of abbreviations
- GDM
gestational diabetes mellitus
- RCT
randomized controlled trial
- HAPO
Hyperglycaemia and Adverse Pregnancy Outcomes
- GLUT‐4
glucose transporter type 4
- OGTT
oral glucose tolerance test
- HRR
heart rate reserve
- HRmax
heart rate maximum
- OGIS
Oral Glucose Insulin Sensitivity index
- HOMA‐IR
homeostatic model assessment‐insulin resistance
- MET‐h/week
metabolic equivalent hours per week
- HbA1c
glycated haemoglobin
- RT
resistance training
- AER
aerobic exercise
- IADPSG
International Association of the Diabetes and Pregnancy Study Groups
Introduction
Gestational diabetes mellitus (GDM) is a carbohydrate intolerance resulting in hyperglycaemia of variable severity with onset or first recognition during pregnancy and with resolution post‐partum 1. It is recognized that overt diabetes during pregnancy is associated with significant levels of perinatal morbidity, such as macrosomia, neonatal hypoglycaemia, shoulder dystocia and other birth injuries 2, as well as more recently respiratory, neurological, digestive and cardiac disorders such as cardiac malformations and hypertrophic cardiomyopathy 3.
In addition, exposure to GDM pregnancy in utero has also been shown to induce long‐term effects in offspring 4, 5, such as increased incidence of type II diabetes, cardiovascular alterations such as hypertension 6, metabolic syndrome 7 and obesity 8 in the offspring later in adulthood, as well as increased risk of developing long‐standing diabetes in the mother 9. Complications for pregnancies subsequent to GDM are well established and carry serious consequences 10.
Stringent new diagnostic criteria have been adopted as usual practice in centres globally following findings from the prominent Hyperglycaemia and Adverse Pregnancy Outcomes (HAPO) study 11, which showed that small degrees of hyperglycaemia have significant effects on pregnancy and neonatal outcomes. These findings have led to improved uniformity in the diagnosis of GDM internationally. Use of the newly established criteria 12 has also resulted in a rise of prevalence of GDM from 2.7% using previous criteria for diagnosis 13 to figures between 9.3% and 25% across the continents using the newly adopted and more stringent diagnostic criteria 14, 15. This threefold increase in prevalence is accompanied by a concurrent rise in specialist medical referrals and has therefore become a significant burden on the healthcare system.
Medical therapy during gestation, through nutritional therapy and pharmacological intervention to obtain glycaemic control, has had positive results in the management of this condition and attenuation of complications 16. The importance of prenatal glycaemic control and weight management through exercise and nutrition manipulation is recognized in practice. The Royal College of Obstetricians and Gynaecologists and the American College of Obstetricians and Gynaecologist both endorse the participation of pregnant women in aerobic and strength‐conditioning exercise, with the goal of maintaining a good fitness level, as part of a healthy lifestyle during pregnancy 17, 58. Despite multiple interventions over the last decade, the most effective form of lifestyle management composed of dietary and physical activity behaviours for the prevention of GDM remains undetermined 18. A Cochrane review of lifestyle interventions for the treatment of GDM reported that women exposed to lifestyle interventions were less likely to have post‐natal depression and were more likely to achieve post‐partum weight goals 19. Exposure was also associated with a decreased risk of the neonate being born large for gestational age and decreased neonatal adiposity. Despite these positive findings, the contribution of individual components of lifestyle could not be assessed owing to study design limitations 19.
Exercise has long been accepted as an adjunctive therapy in the management of type II diabetes mellitus in non‐pregnant individuals, owing to its ability to improve insulin sensitivity and insulin‐stimulated muscle glucose uptake, both of which improve glycaemic control 35. The adaptations to exercise occur at the skeletal muscle level, and owing to similarities with GDM, the findings may translate to this population group 36. Modality, frequency, and duration of exercise are important components of exercise prescription and need to be defined in order to be of practical use to be prescribed in pregnancies both ‘at risk’ and those with a clear diagnosis of GDM.
The purpose of this literature review was threefold: (1) to identify exercise intervention studies implemented specifically during pregnancies complicated by diagnosed GDM or ‘at risk’ of GDM, (2) to determine which exercise modality was the most effective at improving insulin sensitivity and glycaemic control and (3) to make recommendations for future exercise intervention studies in this population.
Methods
Data sources and search strategy
A systematic search of the literature was performed to identify journals articles that examined the insulin and glycaemic effects of exercise intervention during pregnancy on women at risk or diagnosed with GDM. The search strategy ‘gestational diabetes’ AND ‘exercise’ AND ‘intervention’ AND ‘glycaemic control’ NOT ‘type II diabetes’ was applied to the following seven databases: MEDLINE (EBSCO), PubMed (NCBI), Scopus, CINAHL, the Cochrane Library, Embase and the Maternity and Infant Healthcare Database (Ovid). No date or limits were set; language limits were set for English. RSS notifications were set up for each database. In addition, bibliographies of existing reviews, eligible studies, key journals and conference proceedings were manually scanned. Scholars of various articles were contacted to enquire about protocol. Publications that did not have follow‐up publication of the corresponding trial results were followed‐up. The literature search was conducted in April 2018.
Study selection
All journal articles retrieved from the databases were independently reviewed in a two‐stage process by three reviewers. In the first stage, the titles and abstracts of articles from the database search were merged into EndNote™ and duplications removed. Studies that met review inclusion criteria and studies where there was uncertainty about meeting inclusion criteria were reviewed in full text by the main author. In the second stage, the full text of the study was read to determine if the study would be included in the review. The eligible studies were then reviewed by a second independent reviewer. Ambiguity was resolved by discussion with a third reviewer (A. D.). Inclusion criteria consisted of the following: (1) a study population of women diagnosed with GDM or considered at risk, with clearly defined risk factors; (2) an intervention of exercise (on multiple occasions) including any modality (aerobic, resistance, aquatic, etc.); (3) comparisons of exercise interventions with standard care; (4) outcome measures of insulin sensitivity or blood glucose control; and (5) a randomized controlled trial study design or (6) a case‐controlled trial study design. Studies were excluded if they included a dietary aspect to the intervention (unless this was part of standard medical therapy), participants presented with co‐morbidities or used medication to control hyperglycaemia, and they investigated the response to one bout of exercise.
Data extraction
Data from articles were extracted onto an Excel© spreadsheet. Data extracted on the details of participants included the following: number of participants in each intervention and control; nature of intervention; and timing of intervention, duration and type. Outcome measures relevant to the review such as glycaemic measures and measures of insulin sensitivity were included. Other reported outcomes were listed. Inclusion/exclusion criteria, setting (supervised or home based) and compliance/adherence methods of objectively measuring intensity of exercise were included.
Assessment of risk of bias, data synthesis, and analysis
The main author and a second assessor independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions 23. Any disagreement was resolved by a third assessor (A. D.). When eligible studies did not have combinable outcomes for meta‐analysis, a narrative review was thus undertaken. The eligible articles were summarized and discussed.
Results
Description of the studies
The initial search yielded 685 abstracts, of which 13 studies met inclusion and exclusion criteria (Figure 1). Twelve of these were included in this review after reviewing for quality (a summary is shown in Tables S1a and b): two interventions looked at resistance exercise 24, 25, eight studies looked at the effect of an aerobic exercise intervention 26, 27, 28, 29, 30, 62, 64, 68 and two studies utilized a combination of aerobic and resistance exercises 63, 65. One study was omitted owing to poor adherence to the intervention, with only 16.4% of people attending half the sessions 31. This study was therefore not included, as the results did not reflect the effect of the exercise trial, which was a combination of resistance and aerobic training.
Figure 1.
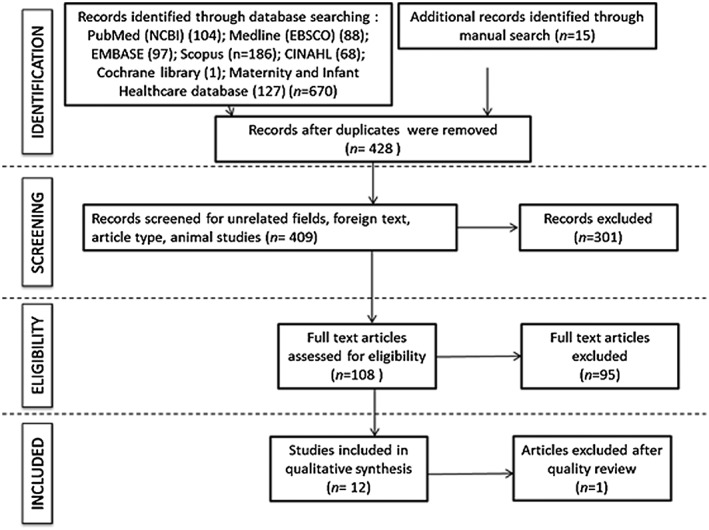
PRISMA showing inclusion/exclusion of journal articles throughout screening procedures.
Risk of bias
Allocation
Methods to generate the random sequence were judged to be adequate in 10 of the 12 included randomized controlled trials, and two were unclear risk (see Table S1a). Various techniques were used for randomization; these methods included use of random number table 24, computer‐generated random series produced by a person not related to the protocol 25 and block randomization 32; others stated they randomized participants but did not detail how this was performed 26, 29, 30.
Five trials were judged to have used adequate methods for allocation concealment 24, 25, 26, 30, 65, 68. Of these five trials, three used concealed opaque envelopes; a separate researcher allocated patients according to a randomization list and one trial allocation was conducted by a third party at another location outside the hospital 30. For the remaining four trials, the risk of bias was judged to be unclear owing to inadequate allocation concealment as no methods were detailed 29, 32, 62, 64.
Blinding
For 10 trials, the risk of performance bias due to inadequate blinding of participants and personnel was judged to be high 24, 26, 27, 29, 62, 63, 64, 68; one trial did not state details of blinding 30, and one study successfully blinded the personnel 25. However, owing to the nature of the interventions, blinding participants is not possible (participants are required to perform exercise).
All trials were considered at high risk of detection bias owing to patient‐reported outcomes being self‐monitored and also the end‐point being insulin administration.
Outcome data
All studies were considered at low risk of attrition bias with clearly reported attrition rates, and all trials had low risk of reporting bias as they included data from these participants in their analysis, with the exception of one trial 24. Oostdam et al. 31 had a low adherence of 16.5%, and as a result, much of the follow‐up data were missing. They used a statistical technique, bootstrapping, to analyse estimates of missing data.
Case‐controlled trial
One included study was a case‐controlled trial 28 that was assessed using a tool specific for its design 23. The risk of bias was judged to be minimal. Details of this can be seen in Table S1b.
Characteristics of studies
Table 1 summarizes the studies selected, showing author, number of participants (n), nature of the population diagnosed or at risk of GDM, the exercise modality and details of the timing of the intervention.
Table 1.
Characteristics of studies meeting inclusion criteria
| Article | n | Mode | Population | Timing of intervention | Duration (weeks) | |||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Diagnosed with GDM | At risk of GDM | Start point | End point | |||
| Brankston et al. 24 | 16 | 16 | RT | X | From GDM diagnosis (26–32) | Till end of gestation | ~5 | |
| De Barros et al. 25 | 32 | 32 | RT | X | From GDM diagnosis (24–34 weeks) | Till end of gestation | ~5 | |
| Halse et al. 26 | 20 | 20 | AER | X | From GDM diagnosis (week 28.8 ± week of gestation) | Till week 34 gestation | ~5 | |
| Ruchat et al. 27 | 6 | 6 | AER | X | Between 16 and 20 weeks | 34–36 weeks' gestation | 14–20 | |
| Davenport et al. 28 | 10 | 20 | AER | X | From diagnosis (24–28 weeks) | To delivery | ≥6 | |
| Ong et al. 29 | 6 | 6 | AER | X | From week 18 gestation | 28 weeks' gestation | 10 | |
| Callaway et al. 30 | 25 | 25 | AER | X | From 12 weeks' gestation | 36 weeks' gestation | 24 | |
| Avery et al. 62 | 16 | 17 | AER | X | From GDM diagnosis (from 34 weeks or less) | Till end of gestation | 4–6 | |
| Guelfi et al. 64 | 85 | 87 | AER | X | Between 13 to 15 weeks' gestation | 28 weeks' gestation | 14 | |
| Wang et al. 68 | 150 | 150 | AER | X | <12+6 weeks' gestation | 36 weeks' gestation | ~24 | |
| Garnaes et al. 63 | 46 | 45 | COMB | X | 12–18 weeks' gestation | 36 weeks' gestation | 18–24 | |
| Sklempe Kokic et al. 65 | 20 | 22 | COMB | X | 28 weeks | Till end of gestation | 6–10 | |
AER, aerobic exercise; COMB, combination; GDM, gestational diabetes mellitus; RT, resistance training.
Resistance exercise
Two studies examining the effects of a resistance exercise programme during GDM pregnancy were identified 24, 25. The interventions both took place from diagnosis at circa week 24 until the end of gestation, for a period of at least 10 weeks. Details of the design of the intervention, outcome measures taken and their main findings are summarized in Table 2, with a more comprehensive table in Table S2a.
Table 2.
Abridged summary table for resistance exercise interventions, their main outcomes and findings
| Article | Intervention | Main outcome measures | Main findings (intervention group vs. control) |
|---|---|---|---|
| Brankston et al. 24 | Circuit session: 3× per week. 3 rounds of 8 exercises × 15 repetitions | Requirement for insulin | ↔ No difference |
| Amount of insulin | ↓ Improved | ||
| Latency of administration of insulin | ↑ improved | ||
| Pooled post‐meal glucose | ↓ improved | ||
| De Barros et al. 25 | Circuit session 3× per week: 3 rounds of 8 exercises × 15 repetitions | Requirement for insulin | ↓ Improved |
| Amount of insulin | ↔ No difference | ||
| Latency of administration of insulin | ↔ No difference | ||
| Pooled capillary glucose levels | ↔ No difference |
Both studies had similar exercise interventions, each consisting of a circuit format of eight exercises working up to 15 repetitions of each exercise using a resistance band, three times a week. Both showed positive results, and these differed; Brankston et al. 24 showed that the exercise group required less insulin during gestation (43.8%) in comparison with diet alone (56.3%), but this was not statistically significant (p = 0.48). The amount of insulin required (in units per kilogram) was less in the exercise intervention group, 0.22 ± 0.2 vs. 0.48 ± 0.3 (p < 0.05), and women in the intervention group required insulin later in pregnancy, 3.71 ± 3.1 vs. 1.11 ± 0.8 weeks after diagnosis (p < 0.05). No detectable difference in blood glucose levels, with the exception of pooled post‐meal (2 h) glucose, which was lower in the exercise group, 6.0 ± 0.29 vs. 6.4 ± 0.81 mmol L−1 (p < 0.05). De Barros et al. 25 found that fewer patients in the exercise group 21.9% vs. 56.3% required insulin during gestation (p = 0.005). Moreover, patients in the exercise intervention who used insulin continued to present adequate glycaemic control according to the target established for a longer percent period of weeks compared with control patients who used insulin (0.63 ± 0.30 vs. 0.41 ± 0.30 [p = 0.006]). No difference was detected between groups in mean glucose levels, amount of insulin required and latency to insulin requirement in those patients requiring insulin. Mean glucose levels were observed between patients of the two groups who used insulin; however, these were not found to be different (control: 5.9 ± 0.4 vs. intervention: 6.1 ± 0.5 mmol L−1; p = 0.342).
Aerobic exercise
Eight intervention studies were identified that used aerobic exercise as their exercise intervention 26, 27, 28, 29, 30, 62, 64, 68. The details of the design and findings of the outcome measures are summarized in Table 3 with a more comprehensive table in Table S2b.
Table 3.
Abridged summary table for aerobic exercise interventions, their main outcomes and findings
| Article | Intervention | Main outcome measures | Main findings (control vs. intervention group) |
|---|---|---|---|
| Halse et al. 26 | Cycling 5× per week. 3× a week: supervised 45 min moderate intensity and short bouts of higher intensity, 2× a week 30 min moderate cycling unsupervised | Mean capillary blood glucose pre‐exercise and post‐exercise (exercise group only) | ↓ Improved |
| HbA1c | Increased in both groups, with no difference between groups | ||
| OGTT | ↔No difference | ||
| Insulin sensitivity | ↔No difference | ||
| Pooled capillary glucose levels | ↓ Improved | ||
| Ruchat et al. 27 | Supervised walking programme 3–4× per week: 40 min in total with 30 min at target HR of 30 or 70% HRR according to group allocation | Capillary blood glucose pre‐exercise and post‐exercise | ↓ Improved in all groups and durations. Longer durations of exercise (40 min). Improvements in capillary glucose attenuated with longer durations of exercise |
| Davenport et al. 28 | 3–4 walking sessions a week of 40 min at 30% HRR | Capillary blood glucose | ↓ Improved |
| Requirement for insulin | ↓ Improved | ||
| Amount of insulin | ↓ Improved | ||
| Ong et al. 29 | 3× per week 45 min cycling ergometer at 50–60% HRmax | Blood glucose response (OGTT) | ↓ Improved OGTT at 1 h |
| Insulin sensitivity (OGIS) | ↔ No difference | ||
| Callaway et al. 30 | Individualized exercise plan, to reach recommendation of 7.5–12.5 MET‐h/week of moderate to vigorous intensity activity | Insulin resistance (HOMA‐IR) | ↔ No difference |
| Fasting glucose | ↓ Improved | ||
| Fasting insulin | ↓ Improved | ||
| Avery et al. 62 | 2 supervised session per week 30‐ and 5‐min warm‐up, 20 min 70% HRmax, 5‐min cool down on cycle ergometer. In addition, 1–2 unsupervised sessions at the same intensity walking | Fasting glucose | ↔ No difference |
| HbA1c | ↔ No difference | ||
| Use of insulin therapy | ↔ No difference | ||
| Guelfi et al. 64 | 3× per week at home supervised on cycle ergometer. Warm‐up for 5 min at 55–65% HRmax, intervals alternating between 65 and 75% HRmax and 75–85% HRmax. Sessions progressed by increasing in duration by 5 min every 2–3 weeks so that they started at 20 min up to a maximum of 60 min | Pre‐intervention and post‐intervention OGTT | ↔ No difference |
| HOMA‐IR | ↔ No difference | ||
| OGIS | ↔ No difference | ||
| HbA1c | ↔ No difference | ||
| Wang et al. 68 |
3× per week supervised exercise sessions on cycle ergometer. 5‐min warm‐up (55–65% HRmax) 30‐s sprint at 75–85% HRmax every 2 min for 3–5 intervals, followed by 5 min at 60–70% HRmax. 3 × 1 min at 75–85% HRmax (increased resistance) 2 min at 65–75% HRmax. 5‐min cool down at 55–65% HRmax. Exercise period start at 45 min and increased to 60 min progressively |
Incidence of GDM | ↓ Improved |
| Gestational weight gain |
↓ Improved Insulin levels ↓ |
||
| Insulin resistance |
Improved Insulin resistance ↔ remained the same |
||
| OGTT: fasted | ↓ Improved | ||
| 1 h post‐ingestion | ↓ Improved | ||
| 2 h post‐ingestion | ↓ Improved | ||
GDM, gestational diabetes mellitus; HbA1c, glycated haemoglobin; HOMA‐IR, homeostatic model assessment‐insulin resistance (method to quantify insulin resistance 38); HRmax, heart rate maximum established from predicted formula or sub‐maximal exercise testing; HRR, heart rate reserve (target heart rate was determined using the HRR equation by Karvonen et al. 36); MET‐h/week, metabolic equivalents‐hours per week; OGIS, Oral Glucose Insulin Sensitivity index, which determines insulin sensitivity from the OGTT 37; OGTT, oral glucose tolerance test (the specific test used is outlined in each study).
The exercise interventions were completely supervised in some cases 27, 28, 29, 64, 68, partially supervised in others 26, 62 and not supervised at all in one study 30. The methods used and outcome measures taken vary greatly in each trial, making it difficult to compare them directly (see Table S2b); however, there seems to be a positive impact of exercise in outcome measures of insulin sensitivity and glycaemic control across those studies that had at least three supervised sessions per week lasting 40–45 min. Two studies found no differences in various measures of blood glucose control and surrogate measures of insulin sensitivity between intervention and control groups. The first study 62 had two supervised sessions lasting 20 min, and outcome measures were limited to fasting glucose, glycated haemoglobin (HbA1c) and use of insulin therapy. The second study 64 started at 20 min and increased in duration; the outcome measures used were oral glucose tolerance test (OGTT), homeostatic model assessment‐insulin resistance (HOMA‐IR), Oral Glucose Insulin Sensitivity and HbA1c.
Three of these studies recruited a population already diagnosed with GDM, two of which reported improvements in outcome measurements 26, 28, whereas Avery et al. 62 did not detect any changes in outcome measures. The difference between these studies was the type of exercise: Halse et al. 26 used cycling as a modality and Davenport et al. 28 walking, whereas Avery et al. 62 used an arm ergometer. Frequency and duration of exercise were also less: twice per week for 20 min 62 as opposed to three times per week for 40 min 26, 28.
Five of the studies engaged a population at risk of gestational diabetes 27, 29, 30, 64, 68. The duration of these interventions ranged from 10 to 20 weeks in comparison with the 4–6 weeks' duration of intervention in those studies that engaged a population diagnosed with GDM 26, 28, 62. Improvements were found in capillary blood glucose levels 27, blood glucose response to OGTT 29, 68, insulin resistance 68 and gestational weight gain; and incidence of GDM was reportedly improved in one study 68. One study in particular 64 reported no difference in all outcome measures, despite similar frequency, intensity, type and duration of exercise intervention. This study differed from the aforementioned studies in the population recruited, where women with previous GDM were recruited as opposed to women with obesity. These women had a lower body mass index (BMI) than those women engaged in the other studies, with 44% in the exercise group and 55% in the control group within a healthy BMI. It is also worth noting that this had a sample size powered to gestational weight gain and not measure of glucose control or insulin sensitivity.
Combined aerobic and strength exercise
Two studies included both aerobic and resistance training modalities of exercise 63, 65. A summary can be found in Table 4 and more in‐depth details in Table S2b. Both studies had supervised and non‐supervised elements. Garneas and colleagues 63 reported that an incidence of GDM was less in exercise group vs. control group (6.1% vs. 27.3%, p = 0.04); however, no difference was observed in OGTT, insulin, HbA1c, and HOMA2‐IR. Sklempe and colleagues 65 found an improvement in post‐intervention average of three postprandial measures (4.66 ± 0.46 vs. 5.30 ± 0.47, p < 0.001), but no difference in fasting glucose between the two groups. The two interventions varied in duration and population characteristics, with Garnaes et al. 63 intervening for 18–24 weeks in pregnant women at risk of GDM (BMI ≥ 28 kg m−2) and Sklempe et al. 65 between 6 and 10 weeks following a GDM diagnosis.
Table 4.
Abridged summary table for combined aerobic and strength exercise interventions, their main outcomes and findings
| Article | Intervention | Main outcome measures | Main findings (intervention group vs. control) |
|---|---|---|---|
| Garnaes et al. 63 |
3× per week supervised. 35‐min aerobic exercise (walking/jogging) at ~80% HRmax. 3× 10 reps squats, push‐ups, diagonal lifts, oblique abdominal crunches. 3× 30‐s plank at the end. Pelvic floor exercise 10 sets of 6–8 s hold. 50‐min home programme 1× per week (same structure as supervised session). Pelvic floor exercises daily |
Gestational weight gain | ↔ No difference |
| Incidence of GDM | ↓ Improved | ||
| OGTT | ↔ No difference | ||
| Insulin | ↔ No difference | ||
| HbA1c | ↔ No difference | ||
| HOMA2‐IR | ↔ No difference | ||
| Sklempe et al. 65 |
2× per week supervised session. (50–55 min) 20‐min treadmill walking at 65–75% HRmax. Resistance exercise using body weight, elastic bands and 0.5‐kg handheld weight. 6 exercises × 3 sets of 10–15 reps. 3 different routines were used and interchanged. Exercise group was also asked to perform 30‐min brisk walk per day |
Post‐intervention average of 3 postprandial measures | ↓ Improved |
| Fasting glucose | ↔ No difference | ||
| Insulin therapy | No participants required insulin therapy | ||
HbA1c, glycated haemoglobin; HOMA‐IR, homeostatic model assessment‐insulin resistance; HRmax, heart rate maximum; HRR, heart rate reserve; OGTT, oral glucose tolerance test.
Discussion
Twelve intervention studies met the inclusion criteria for this systematic review and were included in this review 24, 25, 26, 27, 28, 29, 30, 62, 63, 64, 65, 68. Modalities of these interventions were resistance exercise 24, 25 and aerobic exercise 26, 27, 28, 29, 30, 62, 64, 68. Some interventions showed that requirement of insulin therapy 25, 28, dosage 24, 28 and latency to administration 24 improved in the exercise groups. Capillary blood glucose measurements also improved 26, 27, 28, as well as post‐meal glucose 24 and blood glucose response 29. Other outcomes measured showed no difference in insulin sensitivity 26, 29, 64, insulin resistance 30, 68, requirement of insulin 24, amount of insulin required 25 and latency of administration 25. Of note is that no studies reported any negative outcomes of exercise on blood glucose control. Discrepancies in the timing of intervention, GDM diagnostic criteria and the variety in outcome measures used to assess glucose metabolism make it difficult to draw clear recommendations but have useful considerations for the design of future exercise interventions in this patient population.
The details of the exercise (modality, time, intensity) are of high importance during GDM, as diagnosis occurs around weeks 24–28 of gestation, allowing for 8–10 weeks' opportunity for intervention before parturition. In the studies included in this review, the time frame exposed to the exercise intervention and degree of hyperglycaemia of the participants varied as a product of different GDM diagnostic criteria used by the study to define a starting point 37, 38, 39, 40. These criteria are less stringent than the current guidelines by the World Health Organization 1 derived from the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) as a result of findings from the HAPO study. This could potentially have an effect of the outcomes of the exercise interventions, as it still needs to be established at which specific point prior or during GDM can an exercise intervention be most effective.
Interventions delivered in ‘at risk’ population 27, 29, 30, 63, 64, 68 commenced earlier in gestation and lasted 10–24 weeks. Ong and colleagues 29 recruited pregnant women with obesity otherwise not at risk of GDM, and the length of time of intervention was over 6 weeks. Commencing exercise intervention earlier gave positive results to glycaemic control in all cases, barring a population who had previous GDM. This is in line with exercise interventions in type II diabetic patients, where positive outcomes were attributed to the benefits of metabolic control and adaptation over 15 weeks or more 41, with even 1 week of aerobic training known to improve whole body insulin sensitivity in obese individuals with type II diabetes 42. In light of this, it is worth considering at which point to intervene with an exercise intervention, even though it is recognized that exercise prior to pregnancy is effective at reducing the risk of GDM 43; the most effective strategies to maximize results have not been identified.
The modality of exercise also needs to be considered in terms of the longer‐term aspects of the effects that it may have. Most of the studies included in this literature review did not follow up the women or infants post‐partum, with the exception of Halse et al. 26 who reported follow‐up data separately 60. This is very valuable, as they reported a reduced incidence of macrosomia in the offspring and less maternal weight gain over the intervention period in the group who engaged in the exercise intervention. No other improvements in obstetric or neonatal outcomes were observed, despite that it is also positive that no adverse effects were reported as a result of the exercise intervention. These data are relevant in understanding what benefits aerobic activity confer in the longer term. Specifically, this population group is at higher risk of developing type II diabetes following gestation 44, and the benefits of various exercise modalities can extend beyond the acute phase post‐partum 22, 45.
The tests used to assess glycaemic control in the interventions are typically 75‐g OGTTs at diagnosis and later in pregnancy, postprandial blood glucose, random blood glucose and insulin measures, HbA1c, indirect measures of insulin sensitivity (Oral Glucose Insulin Sensitivity) based on OGTT 33, HOMA‐IR 34 and need for insulin treatment and others as indicators of progression of hyperglycaemia; however, additionally, treatment criteria depend on which criteria the health centre practises. Sensitivity of these measures needs to be considered 61, 67, and can explain the variation in results, sometimes seeing a positive outcome in one outcome with no change in another, all within the same study. Postprandial plasma glucose excursions have been found to be as important 46 in achieving HbA1c goals in type II diabetic patients, and owing to the limited time frame of pregnancy, this may be a more relevant marker than HbA1c, as blood renews itself after 8–12 weeks, therefore missing out on the period of the acute intervention delivery. The lack of homogeneity in measurements across studies makes them difficult to compare.
Adherence was measured in each of the trials, with the use of attendance logs 24, 26, 27, 29, pedometer readings 28, self‐monitored exercise diaries 26 and a combination of attendance and logbook 24, 27. All included trials reported high attendance with over 90% exercise sessions attended by intervention groups. Trials involving supervised components of at least three times per week with at least 40 min of exercise had better outcomes in glycaemic management than those who engaged in less.
When lifestyle changes do not normalize blood glucose levels, pharmacological treatment can be utilized, progressing to insulin treatment as a final course of action. Delaying and minimizing treatment with insulin are of clinical importance not only as an indicator of progression of hyperglycaemia but also owing to its association with vascular damage 47. Both resistance interventions took measures of insulin administration 24, 25, and although these specific interventions were not statistically powered for this outcome measure, they showed improvements as a result of the intervention. Future studies should incorporate these measures within their trials.
The mechanisms behind impaired insulin sensitivity during GDM are not completely understood and, therefore, remain a very fertile ground for research. Exercise may be an effective strategy to optimize glucose homeostasis as it can lower blood glucose levels, thereby improving insulin sensitivity during pregnancy, reducing the burden on the compensating β‐cells 20. Exercise lowers blood glucose concentration via two distinct mechanisms: the contraction‐mediated pathway and the insulin‐stimulated pathway 21. The physiological mechanisms involved in increasing insulin sensitivity include increased number of insulin‐sensitive glucose transporters (GLUT‐4), enhanced response of GLUT‐4 to insulin and increased glycogen synthase activity, all within the skeletal muscle. These work in combination to lower capillary glucose concentrations. The underlying mechanisms surrounding this are described in depth elsewhere 22. As the skeletal muscle is the major source of insulin‐stimulated glucose uptake, any treatment targeted to improve glucose uptake in this tissue will improve whole‐body insulin sensitivity. The metabolic benefits of exercise, specifically during GDM pregnancy, are thought to be due to changes affecting pathways, which influence insulin sensitivity, adipokines and reduction–oxidation reactions 22. Aerobic and resistance exercises trigger various metabolic pathways to elicit metabolic benefits when performed prior to pregnancy 18 and as part of medical therapy for glycaemic management in type II diabetic patients 48. Some research has shown that the metabolic benefits and protective effects are dose dependent 49, directing some studies to turn their focus to energy expenditure 30, 59. However, studies investigating the effects of differing modality of exercise on several metabolic markers and compartmental changes in body composition show that the metabolic benefits are specific and diverse 50, 51, 52, 53, 54.
Aerobic exercise may work best for increased uptake of glucose into the muscle and reducing fat mass (reduced adipokine and leptin production). However, resistance exercise may be more effective at increasing lean muscle, and thus basal metabolic rate, and therefore may have its place in the management of GDM pregnancies, in terms of long‐term maternal outcomes and their risk of developing type II diabetes mellitus 44. Previous studies have suggested that the maternal environment, in particular reduction in maternal insulin sensitivity, contributes significantly to foetal growth 69. Regular aerobic exercise, through an effect on maternal insulin sensitivity, may influence offspring size by regulating nutrient supply to the foetus.
The discrepancies in the results of the resistance exercise interventions 24, 25 included in this review may be due to the higher numbers recruited in the study of De Barros et al. 25. Also of note is the difference in delivery of interventions. Brankston et al. 24 supervised three sessions per week, including a weekly phone call to ensure adherence. This was in contrast to De Barros et al. 25, where one session per week was supervised and phone contact was made with participants to encourage adherence for the other two sessions that took place at participants' home unsupervised. In both trials, resistance was adjusted via the length of the elastic band to increase tension, and even though it is speculated that this modality is self‐limiting unlike free weights, a short‐term study in women has found elastic bands to produce the same benefits in body composition changes as free weights 55. The aerobic interventions had varying results, as the delivery of their intervention and outcome measures were different in each study. Therefore, as previously alluded to, it is suggested that future study designs be homogenized in order to make comparisons between effectiveness of exercise modality on glycaemic parameters.
Studies have previously shown that greater exercise intensity yields greater glucose uptake by skeletal muscle cells acutely, and over time through the contraction‐mediated and insulin‐stimulated pathways, to increase insulin sensitivity 56. Exercise prior to pregnancy is known to reduce the risk of developing GDM 35, 43. In an overweight/obese non‐diabetic population, it has been shown that aerobic exercise was more effective at reducing fat mass, and resistance training was more effective at increasing lean mass. However, performing both, and hence doubling the time committed to exercise by participants, did not double the benefits 57. This, as well as the limited time frame between diagnosis of GDM and parturition (~8 weeks), further highlights the importance of establishing the most effective modality of exercise as a treatment for hyperglycaemia in GDM patients during pregnancy, in order to maximize strategies for minimizing hyperglycaemia in the antenatal period.
Conclusion for practice
This systematic review recommends that patients with GDM and pregnant women with obesity can improve glycaemic management and incidence of GDM during pregnancy through exercise. Evidence collated in this review suggests that women diagnosed with GDM benefit from exercise performed a minimum of three times per week, resistance exercise consisting of eight exercises of 15–20 repetitions each using major muscle groups or aerobic exercise using major muscle groups such as cycling and walking, performed at a rate of perceived exertion (RPE 66) of 12–14 (equivalent to 65–75% age‐predicted HRmax) for 40–60 min. For adherence purposes, supervising sessions and making these sessions interesting using brief intervals of increased intensity such as RPE 13–15 (75–85% age‐predicted HRmax) using resistance or speed can be undertaken safely with suitable monitoring and realistic increments of time and intensity according to the patients' previous ability and progress. Combinations of aerobic and resistance exercises also confer such benefits to glycaemic control, in line with the 7.5–12.5 MET‐h/week (~900 kcal) of moderate to vigorous exercise guidelines set out for this population group. Women at risk of GDM due to high BMI (>28 kg m−2) would benefit from a similar intervention; however, those at high risk of GDM due to previous exposure to GDM without obesity do not seem to have improvement in glycaemic control with such interventions.
Further research on the effectiveness of exercise interventions needs to take place, in a standardized manner, in order to compare results and answer what is the most effective exercise intervention in this population. This includes timing and duration of intervention, as well as methods of measuring glucose control and indices of insulin sensitivity. It is recommended that dietary intake and physical activity are measured as confounding factors, in order to isolate and observe the effects of specific exercise interventions. Future studies should also focus on measurements of hyperglycaemia, as confirmed by the large HAPO study, that small degrees of hyperglycaemia have a significant effect on pregnancy and neonatal outcomes. The clinical significance of the outcome measures used should be considered. Interventions should aim to follow up participants post‐partum to understand longer‐term benefits of antenatal exercise intervention.
Well‐controlled exercise interventions, which are homogenous in the measures used, specific gestational period when intervention is implemented and clinical population (i.e. all diagnosed at the same diagnostic threshold) are required to understand which modality, intensity and duration of exercise are most effective in this population.
Conclusions
There is a paucity of literature on exercise interventions during pregnancy on women with GDM, specifically including measures of glycaemic control. The studies included in this review showed an improvement in blood glucose measures in two modalities of exercise: aerobic and resistance exercises. Future studies looking at exercise strategies to maximize non‐insulin stimulated uptake of glucose through are needed to counteract the increase in insulin resistance observed during pregnancy, and especially of GDM.
Studies examining specific exercise interventions in this particular population are of importance on several levels: to understand the mechanisms behind the exercise being performed; for public health policy, to discern which modality and duration are most effective in order to make recommendations and promote these to this specific population; and economically, as effective interventions may reduce the medical burden this condition constitutes to both mother and infant.
There needs to be a shift in paradigm, similar to the nutrition adage of ‘eating for two’ having been dispelled in recent years through education. It is important to emphasize to pregnant women that moderate‐intensity exercise during pregnancy is safe, healthy and indeed beneficial to both mother and child, when performed in line with guidelines 58. In recent years, nutrition has taken the role of being the ‘cornerstone of therapy – also referred to as medical nutritional therapy’; however, exercise has not quite caught up to this reputation, despite the effects it has on multiple metabolic mechanisms in the body 56. In the months during pregnancy, mothers are known to be very receptive to behaviour change and have many ‘teachable moments’. This is certainly supported by the studies included with high adherence rates in these studies and should be considered a good opportunity for behavioural change to be maximized by the allied health professions.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Supporting information
Table S1 (a) Cochrane assessment of risk of bias for randomized control trials summary figure. ‘+’ low risk, ‘−’ high risk ‘?’ unclear risk. (b) Quality assessment for case‐controlled trial.
Table S2 (a) Details of exercise intervention, the outcome measures taken and main findings for the interventions using a resistance exercise intervention. (b) Details of exercise intervention, the outcome measures taken and main findings for the interventions using an aerobic exercise intervention. (c) Details of exercise intervention, the outcome measures taken and main findings for the interventions using a combination of aerobic and resistance exercise intervention.
Acknowledgements
This research project was supported by the University of Limerick through an Education & Health Sciences Faculty Postgraduate Deans Scholarship, Graduate Entry Medical School Strategic Research Fund and Health Research Institute Seed Funding.
Cremona, A. , O'Gorman, C. , Cotter, A. , Saunders, J. , and Donnelly, A. (2018) Effect of exercise modality on markers of insulin sensitivity and blood glucose control in pregnancies complicated with gestational diabetes mellitus: a systematic review. Obesity Science & Practice, 4: 455–467. 10.1002/osp4.283.
References
- 1. World Health Organization . Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. WHO Press: Geneva, Switzerland, 2013. [PubMed] [Google Scholar]
- 2. Hod M, Rabinerson D, Kaplan B, et al. Perinatal complications following gestational diabetes mellitus' how ‘sweet’ is ill? Acta Obstet Gynecol Scand 1996; 75: 809–815. [DOI] [PubMed] [Google Scholar]
- 3. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes 2015; 6: 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 2011; 60: 1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carolan‐Olah M, Duarte‐Gardea M, Lechuga J. A critical review: early life nutrition and prenatal programming for adult disease. J Clin Nurs 2015; 24: 3716–3729. [DOI] [PubMed] [Google Scholar]
- 6. Simeoni U, Barker DJ. Offspring of diabetic pregnancy: long‐term outcomes. Semin Foetal Neonatal Med 2009; 14: 119–124. [DOI] [PubMed] [Google Scholar]
- 7. Clausen TD, Mathiesen ER, Hansen T, et al. Overweight and the metabolic syndrome in adult offspring of women with diet‐treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 2009; 94: 2464–2470. [DOI] [PubMed] [Google Scholar]
- 8. Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P. Gestational diabetes: a clinical update. World J Diabetes 2015; 6: 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Sullivan JB. Body weight and subsequent diabetes mellitus. JAMA 1982; 248: 949–952. [PubMed] [Google Scholar]
- 10. Ornoy A, Reece EA, Pavlinkova G, Kappen C, Miller RK. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res C Embryo Today 2015; 105: 53–72. [DOI] [PubMed] [Google Scholar]
- 11. Metzger B, Lowe L, Dyer A, et al. Hyperglycemia and adverse pregnancy outcomes. NEJM 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 12. Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for the diagnosis and management of diabetes mellitus. Acta Bioquim Clin Latinoam 2012; 46: 701–741. [Google Scholar]
- 13. Griffin ME, Coffey M, Johnson H, et al. Universal vs. risk factor‐based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabet Med 2000; 17: 26–32. [DOI] [PubMed] [Google Scholar]
- 14. Sacks DA, Coustan DR, Hadden DR, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel‐recommended criteria: The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Diabetes Care 2012; 35: 526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Sullivan EP, Avalos G, O'Reilly M, et al. Atlantic DIP: the prevalence and consequences of gestational diabetes in Ireland. Ir Med J 2012; 105: 13–15. [PubMed] [Google Scholar]
- 16. Tieu J, McPhee AJ, Crowther CA, et al. Screening and subsequent management for gestational diabetes for improving maternal and infant health. Cochrane Database Syst Rev 2014; 2: CD007222. [DOI] [PubMed] [Google Scholar]
- 17. Royal College of Obstetricians and Gynaecologists (RCOG) . Exercise in Pregnancy. Statement No 4, 2006.
- 18. Bain E, Crane M, Tieu J, et al. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev 2015: CD010443. [DOI] [PubMed] [Google Scholar]
- 19. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev 2017. CD011970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005; 115: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hawley JA, Lessard SJ. Exercise training‐induced improvements in insulin action. Acta Physiol (Oxf) 2008; 192: 127–135. [DOI] [PubMed] [Google Scholar]
- 22. Golbidi S, Laher I. Potential mechanisms of exercise in gestational diabetes. J Nutr Metab 2013; 285948: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions. Wiley‐Blackwell: England, 2011. [Google Scholar]
- 24. Brankston GN, Mitchell BF, Ryan EA, Okun NB. Resistance exercise decreases the need for insulin in overweight women with gestational diabetes mellitus. Am J Obstet Gynecol 2004; 190: 188–193. [DOI] [PubMed] [Google Scholar]
- 25. de Barros MC, Lopes MAB, Francisco RPV, et al. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am J Obstet Gynecol 2010; 203: 556. [DOI] [PubMed] [Google Scholar]
- 26. Halse RE, Wallman KE, Newnham JP, et al. Home‐based exercise training improves capillary glucose profile in women with gestational diabetes. Med Sci Sports Exerc 2014; 46: 1702–1709. [DOI] [PubMed] [Google Scholar]
- 27. Ruchat SM, Davenport MH, Giroux I, et al. Effect of exercise intensity and duration on capillary glucose responses in pregnant women at low and high risk for gestational diabetes. Diabetes Metab Res Rev 2012; 28: 669–678. [DOI] [PubMed] [Google Scholar]
- 28. Davenport MH, Mottola MF, McManus R, Gratton R. A walking intervention improves capillary glucose control in women with gestational diabetes mellitus: a pilot study. Appl Physiol Nutr Metab 2008; 33: 511–517. [DOI] [PubMed] [Google Scholar]
- 29. Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home‐based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab 2009; 35: 418–421. [DOI] [PubMed] [Google Scholar]
- 30. Callaway LK, Colditz PB, Byrne NM, et al. Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care 2010; 33: 1457–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oostdam N, Van Poppel MNM, Wouters MGAJ, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG 2012; 119: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 32. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn 1957; 35: 307–315. [PubMed] [Google Scholar]
- 33. Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model‐based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001; 24: 539–548. [DOI] [PubMed] [Google Scholar]
- 34. Kirwan JP, Huston‐Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy – validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 2001; 24: 1602–1607. [DOI] [PubMed] [Google Scholar]
- 35. Ruchat SM, Mottola MF. The important role of physical activity in the prevention and management of gestational diabetes mellitus. Diabetes Metab Res Rev 2013; 29: 334–346. [DOI] [PubMed] [Google Scholar]
- 36. Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010; 33: e147‐e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop‐Conference on Gestational Diabetes Mellitus. Diabetes Care 2007; 30: S251–S260. [DOI] [PubMed] [Google Scholar]
- 38. Hoffman L, Nolan C, Wilson JD, et al. Gestational diabetes mellitus – management guidelines. The Australasian Diabetes in Pregnancy Society. Med Aust 1998; 169: 93–97. [DOI] [PubMed] [Google Scholar]
- 39. Meltzer S, Leiter L, Daneman D, et al. Clinical practice guidelines for the management of Diabetes in Canada: Canadian Diabetes Association. CMAJ 1998; 159: S1–S29. [PMC free article] [PubMed] [Google Scholar]
- 40. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee . Gestational diabetes mellitus. CMAJ 2003; 169: S99–S105. [Google Scholar]
- 41. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. JAMA 2001; 286: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 42. Winnick JJ, Sherman WM, Habash DL, et al. Short‐term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole‐body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab 2008; 93: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus – a Cochrane review. J Paediatr Child Health 2012; 48: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes. Diabetes Care 2002; 25: 1862–1868. [DOI] [PubMed] [Google Scholar]
- 45. Ruchat SM, Allard C, Doyon M, et al. Timing of excessive weight gain during pregnancy modulates newborn anthropometry. J Obstet Gynaecol Can 2016; 38: 108–117. [DOI] [PubMed] [Google Scholar]
- 46. Sorkin JD, Muller DC, Fleg JL, Andres R. The relation of fasting and 2‐h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care 2005; 28: 2626–2632. [DOI] [PubMed] [Google Scholar]
- 47. Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA 2000; 283: 221–228. [DOI] [PubMed] [Google Scholar]
- 48. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev 2006; 3: CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. CMAJ 2006; 174: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E‐BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006; 576: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 2010; 199: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ku YH, Han KA, Ahn H, et al. Resistance exercise did not alter intramuscular adipose tissue but reduced retinol‐binding protein‐4 concentration in individuals with type 2 diabetes mellitus. J Int Med Res 2010; 38: 782–791. [DOI] [PubMed] [Google Scholar]
- 53. Ibanez J, Izquierdo M, Arguelles I, et al. Twice‐weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care 2005; 28: 662–667. [DOI] [PubMed] [Google Scholar]
- 54. Rattarasarn C. Physiological and pathophysiological regulation of regional adipose tissue in the development of insulin resistance and type 2 diabetes. Acta Physiol (Oxf) 2006; 186: 87–101. [DOI] [PubMed] [Google Scholar]
- 55. Colado JC, Triplett NT. Effects of a short‐term resistance program using elastic bands versus weight machines for sedentary middle‐aged women. J Strength Cond Res 2008; 22: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 56. Keshel TE, Coker RH. Exercise training and insulin resistance: a current review. J Obes Weight Loss Ther 2015; 5: S5–S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Willis LH, Slentz CA, Bateman LA, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol 2012; 113: 1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. The American College of Obstetricians and Gynaecologists (ACOG) . Physical activity and exercise during pregnancy and the postpartum period. 2015; Committee Opinion No. 650.
- 59. Kumareswaran K, Elleri D, Allen JM, et al. Physical activity energy expenditure and glucose control in pregnant women with type 1 diabetes: is 30 minutes of daily exercise enough? Diabetes Care 2013; 36: 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Halse RE, Wallman KE, Dimmock JA, et al. Home‐based exercise improves fitness and exercise attitude and intention in women with GDM. Med Sci Sports Exerc 2015; 47: 1698–1704. [DOI] [PubMed] [Google Scholar]
- 61. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 62. Avery MD, Leon AS, Kopher RA. Effects of a partially home‐based exercise program for women with gestational diabetes. Obstet Gynecol 1997; 89: 10–15. [DOI] [PubMed] [Google Scholar]
- 63. Garnaes KK, Morkved S, Salvesen O, Moholdt T. Exercise training and weight gain in obese pregnant women: a randomized controlled trial (ETIP Trial). PLoS Med 2016; 13: e1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guelfi KJ, Ong MJ, Crisp NA, et al. Regular exercise to prevent the recurrence of gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol 2016; 128: 819–827. [DOI] [PubMed] [Google Scholar]
- 65. Sklempe Kokic I, Ivanisevic M, Biolo G, et al. Combination of a structured aerobic and resistance exercise improves glycaemic control in pregnant women diagnosed with gestational diabetes mellitus. A randomised controlled trial. Women Birth 2017: S1871–S5192. 30271–2. [DOI] [PubMed] [Google Scholar]
- 66. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377‐81. [PubMed] [Google Scholar]
- 67. Lapolla A, Dalfrà MG, Mello G, et al. Early detection of insulin sensitivity and β‐cell function with simple tests indicates future derangements in late pregnancy. J Clin Endocrinol Metabol 2008; 93: 876–880. [DOI] [PubMed] [Google Scholar]
- 68. Wang C, Wei Y, Zhang X, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol 2017; 216: 340–351. [DOI] [PubMed] [Google Scholar]
- 69. Scholl TO, Sowers MF, Chen X, et al. Maternal glucose concentration influences fetal growth, gestation, and pregnancy complications. Am J Epidemiol 2001; 154: 514–520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 (a) Cochrane assessment of risk of bias for randomized control trials summary figure. ‘+’ low risk, ‘−’ high risk ‘?’ unclear risk. (b) Quality assessment for case‐controlled trial.
Table S2 (a) Details of exercise intervention, the outcome measures taken and main findings for the interventions using a resistance exercise intervention. (b) Details of exercise intervention, the outcome measures taken and main findings for the interventions using an aerobic exercise intervention. (c) Details of exercise intervention, the outcome measures taken and main findings for the interventions using a combination of aerobic and resistance exercise intervention.


