Summary
Objective
Fructose consumption is a risk factor for metabolic disease. We recently demonstrated that fibroblast growth factor 21 (FGF21), a metabolic hormone involved in lipid and glucose metabolism, is acutely stimulated in humans by 75 g oral fructose, with peak levels occurring 2 h after consumption. This study reports on the dose dependency and reproducibility of the FGF21 response to fructose.
Methods
Lean, healthy adults drank either five different doses of fructose dissolved in water, each separated by 2 weeks, or the same dose on three occasions, each separated by 1 week.
Results
Fibroblast growth factor 21 levels peaked at 2 h in a dose‐dependent manner. No significant increase in FGF21 was seen after consumption of 10 g fructose, while robust increases were seen after drinking solutions containing 30, 50 and 75 g. At 2 h, the minimal fold change of FGF21 was highest following a 75 g fructose drink, and all subjects demonstrated at least a doubling of FGF21 levels following consumption of this dose.
Conclusions
The increase in FGF21 following an oral fructose challenge is dose dependent, with levels peaking at 2 h independent of dose. The FGF21 response to 75 g fructose is also highly reproducible within individuals.
Clinical Implications
By demonstrating that the FGF21 response to fructose is dose dependent and reproducible, this study deepens current understanding of FGF21 fructose dynamics and physiology in humans. This is an important area of clinical interest given associations between fructose intake and a wide variety of metabolic derangements.
Keywords: Dose response, Fibroblast growth factor 21, Fructose
Introduction
Fibroblast growth factor 21 (FGF21) is a member of the endocrine FGF family. It is released into the circulation in states of metabolic stress and requires both an FGF receptor and an obligate co‐receptor, beta klotho, in order to exert endocrine, autocrine and paracrine effects. Administration of exogenous FGF21 to rodents leads to insulin‐independent glucose uptake into adipocytes, thereby improving glycaemic control 1, 2, 3, 4. It also increases energy expenditure and protects against diet‐induced obesity 1, 2, 3, 4. FGF21 is stimulated in rodent models through nutritional manipulations including fasting and ketogenic diet 1, 2; however, there is currently limited understanding of the regulation of FGF21 in humans. Several cross‐sectional studies have shown that FGF21 levels are increased in obesity and metabolic disease 5, 6, 7, 8, 9, 10, suggesting the possibility of an FGF21‐resistant state, similar to obesity‐induced insulin and leptin resistance. Elevated FGF21 levels have also been reported in other acute inflammatory states including sepsis 11 and pancreatitis 12.
Identifying factors that regulate FGF21 in humans is an important and active area of investigation. In contrast to nutritional manipulations in rodents, until recently, there have been no known dietary manipulations that consistently and robustly increase FGF21 levels in humans. It has since been demonstrated that intake of a 75 g fructose beverage leads to a robust increase in FGF21 levels in humans at 2 h, a response that is analogous to the effect of glucose ingestion on insulin secretion 13. A comparable increase in FGF21 levels has also been observed after ingestion of a 75 g oral sucrose load 14; similar changes have not been seen with an oral glucose load 13. A 3 d high‐carbohydrate diet (80% carbohydrates, 52 g d−1 fructose) significantly increased FGF21 levels in lean humans 15. Furthermore, baseline fasting FGF21 levels are higher, and the FGF21 response to fructose is exaggerated in humans with metabolic syndrome compared with lean counterparts 13. Understanding the physiological impact of fructose consumption in humans is particularly germane given that fructose consumption has been implicated in the growing obesity epidemic and has been associated with metabolic derangements including insulin resistance and fatty liver 16, 17. FGF21 has also been implicated in sweet preference, macronutrient intake and alcohol consumption in humans 14, 18, possibly through impacts on the reward system, and has potential to be a future target for modifying human eating behaviours.
In humans, baseline (unstimulated fasting) FGF21 levels vary within individuals, across individuals and across assays3, 12. It has also been showed that the FGF21 response to 75 g of fructose is quite variable across individuals 13, 14, 19, raising questions about what dose of fructose should be used in large populations to assess the FGF21 response. Reasons for this variability are unclear and are possibly related to differences in gender, age, genetic variants or chronic fructose intake. Furthermore, FGF21 circulates as both a total and active (intact) protein 20; triggers for inactivation of FGF21 are also poorly understood and may account for some of the inter‐individual variability 21. A better understanding of the FGF21 fructose response across a range of doses of fructose is critical for understanding the in vivo role of FGF21 as an endocrine hormone and metabolic regulator. This manuscript examines the dose response of FGF21 to oral fructose beverages in humans, as well as the reproducibility of the FGF21 response to a specific dose of fructose and the stability of the ratio of intact (active) to total FGF21 levels in lean humans. We hypothesize that stimulated FGF21 levels will increase correspondingly with greater doses of fructose and that intra‐individual variability of the FGF21 response to 75 g of fructose will be low despite differences between individuals.
Methods
Subjects
Subjects were recruited through local advertisement to participate in two different protocols. Written informed consent was obtained from each subject. The protocols were approved by the Beth Israel Deaconess Medical Center Institutional Review Board (http://clinicaltrials.gov; Identifiers: NCT00968747, NCT2884791). All visits were conducted at the Harvard Catalyst Clinical Research Center at the Beth Israel Deaconess Medical Center in Boston, Massachusetts, in accordance with the Declaration of Helsinki.
Eight lean, healthy subjects were enrolled in the dose response study. The initial screening visit included a medical history, physical exam, baseline laboratory tests and a standard 2 h 75 g oral glucose tolerance test (OGTT). Inclusion criteria were age 18–60, body mass index 19–25 kg m−2, absence of chronic medical illness and absence of long‐term medication use other than oral contraceptive pills and thyroid hormone (stable dose for more than 6 months and Thyroid Stimulating Hormone (TSH) in the normal range at the screening visit). Subjects were excluded if they had impaired glucose tolerance, diabetes, hyperlipidaemia, hypertension, liver disease, kidney disease or a history of fructose intolerance. Using the same inclusion and exclusion criteria, 12 lean, healthy subjects were enrolled in a study examining the reproducibility of the FGF21 response to a 75 g fructose beverage.
Dose response protocol
Following an overnight fast of at least 8 h, subjects consumed five different doses of oral fructose ranging from 10 to 75 g dissolved in 225 mL of water. Each subject consumed every dose of fructose. Study visits were separated by at least 2 weeks, and the order in which the subjects consumed each fructose beverage was randomly assigned. Blood was drawn prior to drinking the fructose beverage and hourly thereafter for 5 h. Subjects also completed an online dietary questionnaire (Vioscreen, Viocare Inc., NJ, USA) that provides data on food frequency consumption over the previous 90 d and allows for assessment of average daily carbohydrate and fructose consumption.
Reproducibility protocol
The dose response protocol yielded data identifying the optimal dose of fructose to be used to assess the reproducibility of the FGF21 response to fructose. Based on these data, a second cohort of subjects enrolled in the reproducibility of the FGF21 response study drank a 75 g fructose beverage on three occasions, each separated by at least 1 week. All other measurements were the same as those in the dose response protocol.
Biochemical analysis
Serum total FGF21 levels were measured using a commercially available ELISA assay (R&D Systems, Inc., MN, USA), and serum intact FGF21 levels were measured using an intact FGF21 ELISA kit (Eagle Biosciences, Nashua, NH, USA). Samples were collected in aprotinin‐treated tubes and stored at −80°C until analysis. Evaluation of baseline screening labs including glucose, triglycerides, total cholesterol, high‐density lipoprotein and low‐density lipoprotein was performed at LabCorp using standard protocols.
Calculations and statistical analysis
Fibroblast growth factor 21 levels are presented as mean ± standard error. Post‐ingestion FGF21 levels were compared with fasting values using a linear mixed effects with random‐intercept term to account for the within‐subject correlations. The time and dose were included as fixed effects with appropriate indicator variables in the model, and subsequently, appropriate contrast statements were constructed to obtain the estimate of differences and their standard errors between different timing and dosing levels. A two‐tailed paired t‐test was used to compare peak FGF21 fold change at the 2 h time point for each fructose dose. Area under the curve (AUC) for FGF21 was calculated by the trapezoidal method, with incremental AUC (iAUC) calculated by subtracting the area accounted for by baseline values. Correlations between fructose doses and iAUC were calculated using a one‐way anova with Tukey's post hoc analysis. Results for one subject after 30 g fructose consumption were excluded as peak FGF21 levels were greater than two standard deviations above average, concerning for inaccurate measurement. Reproducibility of the FGF21 response to 75 g fructose was calculated using repeated measures anova. A p value of ≤0.05 was considered statistically significant.
Results
Dose response study
Baseline characteristics of the study population are summarized in Table 1a. All subjects were lean and without biochemical or clinical evidence of metabolic disease. Baseline (unstimulated, after an overnight fast) FGF21 levels were highly variable, with a mean level of 105.5 ± 32 pg mL−1, range 3–411 pg mL−1.
Table 1.
(a) Baseline characteristics of the study population for the dose response study. Values are mean ± SE. (b) Baseline characteristics of the study population for the dose reproducibility study. Values are mean ± SE.
| (a) | |
|---|---|
| Subjects (M/F) | 8(3/5) |
| Age (years) | 34 ± 4.9 |
| Body mass index (kg m−2) | 23.0 ± 0.5 |
| Waist circumference (cm) | 83.4 ± 2.0 |
| Total cholesterol (mg dL−1) | 156.6 ± 8.5 |
| LDL cholesterol (mg dL−1) | 82.0 ± 5.0 |
| HDL cholesterol (mg dL−1) | 59.4 ± 4.5 |
| Triglycerides (mg dL−1) | 76.1 ± 8.9 |
| Systolic blood pressure (mmHg) | 118.5 ± 4.3 |
| Diastolic blood pressure (mmHg) | 75.0 ± 3.3 |
| Fasting plasma glucose (mg dL−1) | 83.1 ± 1.7 |
| 2 h OGTT plasma glucose (mg dL−1) | 94.3 ± 6.7 |
| Plasma FGF21 (pg mL−1) | 105.5 ± 32.4 |
| Average fructose consumption (g d−1) | 29.6 ± 6.9 |
| (b) | |
| Subjects (M/F) | 12(5/7) |
| Age (years) | 33.1 ± 3.9 |
| Body mass index (kg m−2) | 23.7 ± 0.4 |
| Waist circumference (cm) | 85.9 ± 2.1 |
| Total cholesterol (mg dL−1) | 182.4 ± 6.3 |
| LDL cholesterol (mg dL−1) | 98.6 ± 5.8 |
| HDL cholesterol (mg dL−1) | 67.9 ± 4.4 |
| Triglycerides (mg dL−1) | 80.6 ± 9.8 |
| Systolic blood pressure (mmHg) | 117.3 ± 5.8 |
| Diastolic blood pressure (mmHg) | 74.3 ± 3.9 |
| Fasting plasma glucose (mg dL−1) | 87.3 ± 2.3 |
| 2 h OGTT plasma glucose (mg dL−1) | 115.7 ± 6.2 |
| Plasma FGF21 (pg mL−1) | 182.9 ± 38.2 |
| Average fructose consumption (g d−1) | 28.4 ± 6.6 |
FGF21, fibroblast growth factor 21; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
To evaluate whether the FGF21 response to an oral fructose load is dose dependent, subjects consumed beverages containing 10, 20, 30, 50 and 75 g pure fructose powder dissolved in water. In agreement with previously published data, it was observed that the peak FGF21 level occurred 2 h after ingestion of fructose (Figure 1A–E). There was no significant increase in FGF21 levels 2 h after ingestion of 10 g of fructose (Figure 1A), but there were increases in peak FGF21 levels with higher fructose concentrations (Figure 1B–E). The minimum fructose concentration that led to a statistically significant increase in FGF21 levels from baseline to 2 h peak was 20 g; further increases were seen with 30, 50 and 75 g fructose solutions (p < 0.001). The absolute peak FGF21 response following consumption of 30, 50 and 75 g of fructose was significantly greater than the peak response observed with 10 g (p < 0.05); additionally, the peak response at 75 g was significantly greater than the response seen with 20 g (p < 0.002).
Figure 1.
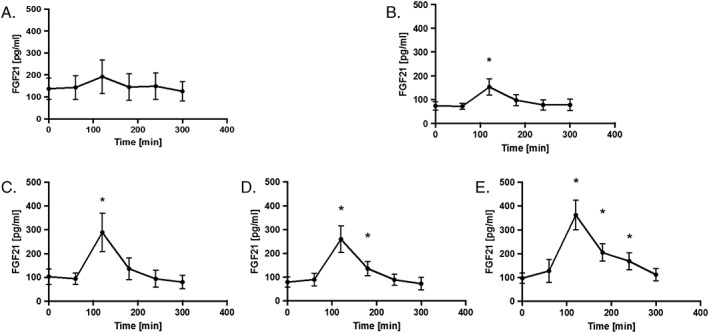
The serum FGF21 response to various doses of fructose consumption in healthy adults. (A) Average serum FGF21 response to consumption of 10 g fructose. (B) Average serum FGF21 response to consumption of 20 g fructose. (C) Average serum FGF21 response to consumption of 30 g fructose. (D) Average serum FGF21 response to consumption of 50 g fructose. (E) Average serum FGF21 response to consumption of 75 g fructose. *p < 0.05. FGF21, fibroblast growth factor 21
To account for individual variability in unstimulated FGF21 levels, we evaluated the fold change from baseline to 2 h peak. There was a dose‐dependent increase in the fold change of FGF21, with a 0.3‐fold increase in FGF21 following 10 g of fructose, 1.2‐fold increase following 20 and 30 g, 2.8‐fold increase following 50 g, and 3.3‐fold increase following 75 g (Figure 2A).
Figure 2.
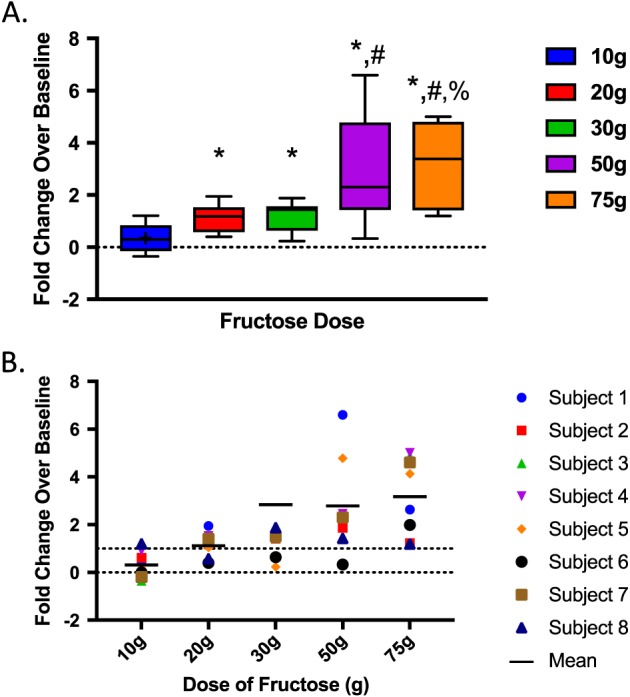
(A) Maximal fold change of FGF21 levels with 75 g fructose stimulation. *p < 0.05 versus 10 g. #p < 0.05 versus 20 g; %p < 0.05 versus 30 g. (B) Fold change of FGF21 for each individual subject by fructose dose. Mean fold change is represented by solid lines at each dose. Hash‐line represents 100% increase.
The fold increase seen following the 10 g fructose beverage was significantly lower than that seen with 20 (p = 0.01), 30 (p = 0.006), 50 (p = 0.01) or 75 g (p = 0.004). The peak fold change following 50 g fructose was significantly greater than that of 20 g (p = 0.05), while the response following 75 g was significantly greater than that following 20 (p = 0.009) and 30 g (p = 0.036). Figure 2B and Table 2 demonstrate the incremental response to higher doses of fructose for each individual subject, rather than for the study group as a whole (total study group represented in Figure 1). As demonstrated, the only dose of fructose for which all subjects had at least a 100% increase in FGF21 levels following stimulation was the 75 g dose.
Table 2.
Average and range fold change of fibroblast growth factor 21 response to fructose by dose
| Dose (g) | Average fold change | Range fold change |
|---|---|---|
| 10 | 0.4 ± 0.6 | −0.4 to 1.2 |
| 20 | 1.2 ± 0.5 | 0.4 to 1.9 |
| 30 | 2.9 ± 4.7 | 0.2 to 14.3 |
| 50 | 2.8 ± 2.1 | 0.3 to 6.6 |
| 75 | 3.2 ± 1.6 | 1.2 to 5.0 |
Figure 3 demonstrates the increase in iAUC with each incremental fructose dose. The iAUC of FGF21 response after consumption of 75 g of fructose was significantly greater than that with 10 (p = 0.002), 20 (p = 0.011) or 30 g (p = 0.03). The iAUC with 50 and 75 g were not statistically significantly different despite nearly a twofold absolute difference.
Figure 3.
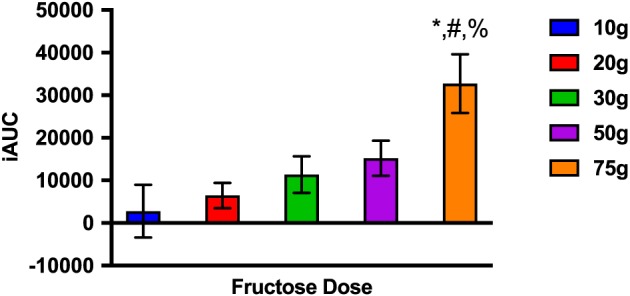
The incremental area under the curve (iAUC) of the FGF21 response to each fructose dose. *p < 0.05 compared with 10 g. #p < 0.05 compared with 20 g. %p < 0.05 compared with 30 g.
Reproducibility of the fibroblast growth factor 21 response to 75 g fructose
Baseline characteristics of the participants in the reproducibility study are summarized in Table 1b. All subjects were lean and without biochemical or clinical evidence of metabolic disease. Mean baseline FGF21 levels were 182.9 ± 38.2 pg mL−1, range 15.7 to 1037.0 (average CV% = 49.87). We found that the FGF21 fold change response to repeated ingestion of the 75 g dose of fructose was also highly reproducible (average CV% = 44.82%). Across all individuals, there was no statistically significant difference in baseline or stimulated fold change FGF21 values, although the amount of inter‐individual variability was greater with FGF21 compared with insulin, another secreted peptide (Table 3). To further characterize FGF21/fructose dynamics following ingestion of 75 g fructose, we measured the ratio of intact to total FGF21 in both baseline and stimulated states. The ratio of intact to total FGF21 was approximately 50% and consistent across three separate baseline assessments (44%, 51%, 51%; p = 0.79) and also across three separate stimulated observations (55%, 48%, 49%; p = 0.91).
Table 3.
Reproducibility of FGF21 baseline and fold change of FGF21. Data are mean ± SEM.
| Baseline total FGF21 (pg mL−1) | Peak total FGF21 (pg mL−1) | Baseline insulin (mU L−1) | Peak insulin (mU L−1) | |
|---|---|---|---|---|
| 75 g 1 | 155.20 ± 63.82 | 564.14 ± 197.00 | 5.55 ± 0.54 | 11.44 ± 1.76 |
| 75 g 2 | 176.52 ± 60.89 | 737.24 ± 215.38 | 6.30 ± 0.83 | 12.00 ± 2.14 |
| 75 g 3 | 216.87 ± 77.05 | 679.80 ± 207.55 | 6.26 ± 0.96 | 11.31 ± 1.64 |
| p value | 0.14 | 0.25 | 0.70 | 0.92 |
FGF21, fibroblast growth factor 21; SEM, standard error of the mean.
Discussion
Many cross‐sectional studies report that FGF21 levels are increased in obesity and metabolic disease 5, 6, but very few studies have prospectively investigated regulation of FGF21 in humans through nutritional manipulation 18, 22. An oral fructose load is one of the few interventions that has been shown to impact FGF21 levels in humans. Initial data evaluating the fructose response was limited to a 75 g challenge, analogous to the 75 g oral glucose tolerance test. The present study confirms that consumption of 75 g of oral fructose leads to a significant and highly reproducible increase in FGF21 levels in humans 2 h following intake, with a return to baseline levels after 5 h 13.
However, daily dietary consumption of fructose amongst adults in the USA ranges from 30 to 75 g; 80–90% of this is in the form of added sugar 23, 24. In this particular cohort, ad libitum daily fructose consumption was 29.9 ± 6 g. Because of highly variable dietary habits, it was not clear whether 75 g of fructose was the ideal dose to assess the in vivo FGF21 response, prompting the present dose response study.
Herein, it is demonstrated for the first time that the 2 h peak in FGF21 levels following an oral fructose beverage is consistent across a range of doses of fructose, and the magnitude of the peak appears dose dependent. There was only a minimal change in FGF21 following a 10 g fructose beverage, with increasing responses through the 75 g dose. Given that baseline FGF21 levels show considerable inter‐individual variability, there was concern that looking solely at peak values may over or underestimate the response for those individuals with very high or low fasting values. Fold change of total FGF21 levels were calculated to account for this variability, and it demonstrated a similar pattern to peak FGF21 responses. Using a threshold of 100% increase (twofold change) in FGF21 as the minimal threshold for significance 15, a 75 g fructose challenge appears to be the optimal dose for studying regulation of FGF21 by fructose in humans, as this was the only dose for which all subjects achieved at least a twofold change above baseline. A twofold increase in FGF21 was seen in only 86% of subjects following consumption of the 50 g fructose solution, raising concern that the 50 g dose may not be provocative enough to detect an impaired response in some individuals.
Once the 75 g fructose dose was established, the focus was shifted to study the reproducibility of the fold change of FGF21 following consumption of a 75 g fructose beverage. As there is known to be variability in baseline FGF21 levels between individuals 5, 19, which was confirmed in this study, it was hypothesized that similar inter‐individual variability would be seen in the stimulated FGF21response to 75 g of fructose. The data, however, suggest that the fold change FGF21 response to a 75 g fructose beverage is indeed reproducible, insofar as the fold change of FGF21 following 75 g of fructose was similar on three separate occasions. Additionally, the stable and consistent ratio of intact to total FGF21 levels in both the baseline and unstimulated states indicates that fructose ingestion increases secretion and not just cleavage of the FGF21 protein. The consistency of intact to total FGF21 levels in a stimulated state has not previously been reported.
Minimal side effects were noted with the 75 g fructose beverage; however, nausea, vomiting and diarrhoea were observed with higher doses of fructose (unpublished data), thereby limiting the feasibility of a fructose beverage containing greater than 75 g. These findings therefore suggest that a 75 g fructose challenge is an appropriate dose for future studies designed to assess FGF21/fructose dynamics. Indeed, much larger studies will be helpful for definitively determining whether there are significant differences between 50 and 75 g oral fructose challenges. Larger studies are needed to define more precisely the threshold increase, both absolute and relative, of FGF21 following fructose ingestion in order to assess present or future risk for metabolic disease.
This study has several limitations. First, the sample size is small. Given the large variation seen in response to an oral fructose tolerance test, the small sample size may limit our ability to draw conclusions that apply to larger populations. Further studies are required to understand the mechanisms underlying this variability and implications for metabolic health. It remains unclear whether a robust (or weak) FGF21 response is adaptive and physiologically beneficial or whether it is an early warning signal for risk of developing metabolic disease.
Conclusions
The FGF21 response to oral fructose in humans is dose dependent with peak FGF21 levels occurring 2 h following ingestion of a range of doses of fructose. FGF21 levels are variable both within and between lean, healthy individuals, but this variability is not statistically significant. For the first time, it is demonstrated that the ratio of intact to total FGF21 levels remain stable in the unstimulated and stimulated states. The underlying physiologic implications for the FGF21 response have yet to be elucidated; however, the response appears most robust and is reproducible, with a 75 g fructose challenge. We propose that larger studies confirm this dose as the standard for further investigation of an oral fructose tolerance test.
Funding
This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centres. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centres, or the National Institutes of Health. Statistical support was provided by Kush Kapur, PhD.
Author Contributions
J. D., M. H. and S. C. conceived and designed the experiments. J. D., S. C., A. M., M. R. and B. H. performed the experiments; A. M., S. C., J. D., M. H. and E. M. F. analysed the data; A. M., J. D., M. H. and E. M. F. wrote the paper. M. H. is currently at Duke University.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Migdal, A. , Comte, S. , Rodgers, M. , Heineman, B. , Flier, E. M. , Herman, M. , and Dushay, J. (2018) Fibroblast growth factor 21 and fructose dynamics in humans. Obesity Science & Practice, 4: 483–489. 10.1002/osp4.295.
This trial is registered on http://clinicaltrials.gov
Identifiers: NCT00968747, NCT2884791.
References
- 1. Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos‐Flier E. Fibroblast growth factor 21‐deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 2009; 150: 4931–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARalpha‐mediated induction of fibroblast growth factor 21. Cell Metab 2007; 5: 415–425. [DOI] [PubMed] [Google Scholar]
- 3. Kharitonenkov A, Adams AC. Inventing new medicines: the FGF21 story. Mol Metab 2014; 3: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher FM, Maratos‐Flier E. Understanding the physiology of FGF21. Annu Rev Physiol 2016; 78: 223–241. [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246–1253. [DOI] [PubMed] [Google Scholar]
- 6. Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010; 139: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen C, Cheung BM, Tso AW, et al. High plasma level of fibroblast growth factor 21 is an independent predictor of type 2 diabetes: a 5.4‐year population‐based prospective study in Chinese subjects. Diabetes Care 2011; 34: 2113–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chavez AO, Molina‐Carrion M, Abdul‐Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor‐21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009; 32: 1542–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li H, Fang Q, Gao F, et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol 2010; 53: 934–940. [DOI] [PubMed] [Google Scholar]
- 10. Gorar S, Culha C, Uc ZA, et al. Serum fibroblast growth factor 21 levels in polycystic ovary syndrome. Gynecol Endocrinol 2010; 26: 819–826. [DOI] [PubMed] [Google Scholar]
- 11. Gariani K, Drifte G, Dunn‐Siegrist I, Pugin J, Jornayvaz FR. Increased FGF21 plasma levels in humans with sepsis and SIRS. Endocr Connect 2013; 2: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shenoy VK, Beaver KM, Fisher FM, et al. Elevated serum fibroblast growth factor 21 in humans with acute pancreatitis. PLoS One 2016; 11: e0164351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos‐Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab 2015; 4: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soberg S, Sandholt CH, Jespersen NZ, et al. FGF21 Is a sugar‐induced hormone associated with sweet intake and preference in humans. Cell Metab 2017; 25: 1045–1053. e6. [DOI] [PubMed] [Google Scholar]
- 15. Lundsgaard AM, Fritzen AM, Sjoberg KA, et al. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol Metab 2017; 6: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bray GA. Soft drink consumption and obesity: it is all about fructose. Curr Opin Lipidol 2010; 21: 51–57. [DOI] [PubMed] [Google Scholar]
- 17. Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Nature 2012; 482: 27–29. [DOI] [PubMed] [Google Scholar]
- 18. Soberg S, Andersen ES, Dalgaard NB, et al. FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol Metab 2018; 11: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galman C, Lundasen T, Kharitonenkov A, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008; 8: 169–174. [DOI] [PubMed] [Google Scholar]
- 20. Dunshee DR, Bainbridge TW, Kljavin NM, et al. Fibroblast activation protein cleaves and inactivates fibroblast growth factor 21. J Biol Chem 2016; 291: 5986–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Umberger TS, Sloan JH, Chen J, et al. Novel sandwich immunoassays for the measurement of total and active FGF21. Bioanalysis 2014; 6: 3283–3293. [DOI] [PubMed] [Google Scholar]
- 22. Desai BN, Singhal G, Watanabe M, et al. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab 2017; 6: 1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agriculture USDo . Table 52‐High Fructose Corn Syrup: Estimated Number of per Capita Calories Consumed Daily, by Calendar Year. 2016. [Google Scholar]
- 24. Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009; 139: 1228S–1235S. [DOI] [PubMed] [Google Scholar]


