Summary
Background
Nonalcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease worldwide and is characterized by insulin resistance, hepatic steatosis and often prediabetes or diabetes. Canagliflozin, a selective sodium glucose cotransporter 2 inhibitor, is a new oral anti‐diabetic drug that reduces hyperglycaemia by promoting urinary glucose excretion. Glycosuria produced by canagliflozin is associated with weight loss, mainly due to reduced fat volume and improve insulin resistance. Reduced body weight and improvement of insulin resistance by canagliflozin may be an effective treatment for NAFLD.
Methods
Thirty‐five patients with NAFLD (17 men and 18 women) were enrolled and administered canagliflozin (100 mg). Body weight and serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma‐glutamyl transferase (γ‐GTP), low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides (TG), blood sugar (BS), glycated haemoglobin (HbA1C), uric acid (UA) and ferritin, and fibrosis‐4 (FIB‐4) index values were measured at baseline and at 3‐month and 6‐month follow‐up visits.
Results
Body weight and serum levels of AST, ALT, γ‐GTP, TG, UA, HbA1C, BS and ferritin decreased significantly after 3 and 6 months of canagliflozin treatment. Serum BS levels and FIB‐4 index values decreased slightly following 3 months of treatment; these results reached significance after 6 months. Reduced serum ALT levels at 6 months were significantly correlated with baseline HbA1C and ferritin levels. Moreover, a significant correlation between reduced body weight and serum ALT levels was observed at 6 months. Decreased serum ALT levels were significantly correlated with decreased serum ferritin at 6 months.
Conclusions
Canagliflozin significantly reduced the serum levels of BS, HbA1C, TG, UA and ferritin, as well as FIB‐4 index values and body weight, with improved liver function. Sodium glucose cotransporter 2 inhibitors may be an important therapeutic modality for improving liver injury in NAFLD patients.
Keywords: canagliflozin, NAFLD, SGLT2 inhibitor
Introduction
Nonalcoholic fatty liver disease (NAFLD) represents one of the most common causes of chronic liver disease worldwide and is characterized by chronic liver inflammation and fibrosis leading to cirrhosis, with an increased risk of liver cancer in some patients 1. NAFLD is the hepatic manifestation of metabolic syndrome and is strongly related to the features of this disorder 2. As such, most cases of NAFLD are closely related to obesity, impaired glucose tolerance, hyperlipidaemia and hypertension and are based on insulin tolerance and the hepatic manifestation of metabolic syndrome. The disease affects one‐third of the population and two‐thirds of patients with obesity or type 2 diabetes mellitus (T2DM).
Nonalcoholic steatohepatitis (NASH) is an aggressive form of NAFLD characterized by hepatocyte necrosis, inflammation and fibrosis; hence, the presence of fibrosis indicates a more aggressive course and may lead to cirrhosis. Premature mortality in NASH patients is related to both hepatic (cirrhosis and hepatocellular carcinoma) and extra‐hepatic complications, largely cardiovascular disease 3. NAFLD pathogenesis is multifactorial and includes excessive inappropriate dietary fat intake combined with peripheral insulin resistance and oxidative stress 4. Thus, it is likely that insulin resistance and cellular stress, resulting from hepatic steatosis, act collectively to promote NAFLD, a pro‐fibrotic state and progression to NASH 5. Canagliflozin is a sodium glucose cotransporter 2 (SGLT2) inhibitor developed for the treatment of adults with T2DM. It promotes urinary glucose excretion, resulting in decreased plasma glucose, mild osmotic diuresis and a net caloric loss 6. Canagliflozin is effective in improving glucose homeostasis, insulin sensitivity and beta cell function, as well as reducing body weight.
Since April 2014, ipragliflozin, dapagliflozin, luseogliflozin, canagliflozin, tofogliflozin and empagliflozin have been approved and are widely used in Japan 7. Improvement of insulin resistance by canagliflozin treatment may be effective in patients with NAFLD. Numerous previous studies using rodent demonstrated that SGLT2 inhibitors can ameliorate fatty liver with a significant loss in body weight, and the weight‐reducing effects of various SGLT2 inhibitors have been documented in humans 8. However, reports evaluating the effects of SGLT2 inhibitors in clinical practice are limited. The aim of this study is to examine the clinical efforts of administering canagliflozin in patients with NAFLD.
Methods
Study design
This study was a prospective, open‐label, uncontrolled pilot study between September 2015 and March 2017. All patients received 100 mg d−1 of canagliflozin for 6 months. Body weight was monitored throughout the study, and body mass indexes were calculated. Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma‐glutamyl transferase (γ‐GTP), low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), triglycerides (TG), blood glucose (BS), glycated haemoglobin (HbA1C), uric acid (UA) and ferritin, and fibrosis‐4 (FIB‐4) index values were measured at baseline, as well as at 3 and 6 months after commencing the study. FIB‐4 index values were calculated according to the following equation: FIB‐4 index = {[age (years) × AST (IU L−1)]/[platelet count(109 L−1) × ALT (IU L−1)]} 9.
Statistical analysis
Data are expressed as means ± standard deviation. Differences between the means of two variables were evaluated using an independent t‐test. Pearson's simple correlations were made between the data before the start of canagliflozin treatment and changes in variables after canagliflozin treatment. Statistical analyses were performed to compare the data before and after the study using an analysis of variance. p < 0.05 was regarded as statistically significant.
Patients
This study obtained informed consent from each patient and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Thirty‐five patients with NAFLD (17 men and 18 women) were enrolled in the present study. All patients were screened for metabolic syndrome, diabetes mellitus, dyslipidaemia, hypertension and NAFLD, with all current medications recorded, and underwent abdominal ultrasonography. The inclusion criteria required that all patients had a fatty liver, did not have other causes of chronic hepatitis (alcoholism, drug‐induced liver injury, viral hepatitis, hemochromatosis and autoimmune liver disease) and were older than 20 years. The main exclusion criteria were insulin use, current or potential pregnancy and an HbA1C value ≤ 5.5%. NAFLD was defined according to characteristic ultrasonographic findings, such as increased hepatorenal contrast or enhanced liver brightness 10. The mean patient age was 56.9 ± 14.3 years. The underlying disease was diabetes mellitus in 28 patients, hyperlipidaemia in 30 patients and hyperuricemia in five patients.
Results
All patients successfully completed the study protocol. Serum AST levels decreased significantly from 45.5 IU L−1 at baseline to 29.9 IU L−1 after 3 months and 28.6 IU L−1 after 6 months of canagliflozin treatment (p < 0.05). Serum ALT levels decreased significantly from 74.2 IU L−1 at baseline to 42.0 IU L−1 after 3 months and 40.4 IU L−1 after 6 months of canagliflozin treatment (p < 0.05).
Serum γ‐GTP levels decreased significantly from 80.6 IU L−1 at baseline to 57.5 IU L−1 after 3 months and 56.2 IU L−1 after 6 months of canagliflozin treatment (p < 0.05). Serum TG levels decreased significantly from 225.1 mg dL−1 at baseline to 172.1 mg dL−1 after 3 months and 180.7 mg dL−1 after 6 months of canagliflozin treatment (p < 0.05). Serum HbA1C levels significantly decreased from 7.45% at baseline to 6.58% after 3 months and 6.36% after 6 months of canagliflozin treatment (p < 0.05). Serum BS levels decreased slightly from 138.2 mg dL−1 at baseline to 134.5 mg dL−1 after 3 months; the decrease in serum BS levels reached significance after 6 months of canagliflozin treatment, with a value of 112.9 mg dL−1 (p < 0.05). Serum UA levels significantly decreased from 5.67 mg dL−1 at baseline to 5.02 mg dL−1 after 3 months and 5.11 mg dL−1 after 6 months of canagliflozin treatment (p < 0.05). Body weight significantly decreased from 73.3 kg at baseline to 70.7 kg after 3 months and 69.6 kg after 6 months of canagliflozin treatment (p < 0.05). Serum ferritin levels decreased significantly from 184.9 ng mL−1 at baseline to 143.8 ng mL−1 after 3 months and 117.3 ng mL−1 after 6 months of canagliflozin treatment (p < 0.05). FIB‐4 index values decreased slightly from 1.42 at baseline to 1.31 after 3 months; the FIB‐4 index value was 1.27 after 6 months of canagliflozin treatment (p < 0.05), which was a significant change. Serum LDL‐C and HDL‐C levels remained unchanged throughout the study. These data are shown in Table 1.
Table 1.
Changes in the examined variables after 3 and 6 months of canagliflozin treatment
| Pretreatment | 3 months | 6 months | |
|---|---|---|---|
| AST (IU L−1) | 45.5 ± 30.4 | 29.9 ± 15.6* | 28.6 ± 15.8* |
| ALT (IU L−1) | 74.2 ± 68.5 | 42.0 ± 31.7* | 40.4 ± 37.3* |
| γ‐GTP (IU L−1) | 80.6 ± 78.2 | 57.5 ± 54.9* | 56.2 ± 52.6* |
| LDL‐C (mg dL−1) | 126.3 ± 37.4 | 119.9 ± 32.3 | 120.3 ± 31.1 |
| HDL‐C (mg dL−1) | 53.0 ± 18.6 | 53.8 ± 17.9 | 54.5 ± 19.7 |
| TG (mg dL−1) | 225.1 ± 166.1 | 172.1 ± 103.3* | 180.7 ± 95.3* |
| UA (mg dL−1) | 5.67 ± 1.50 | 5.02 ± 1.15* | 5.11 ± 1.13* |
| BS (mg dL−1) | 138.2 ± 51.5 | 134.5 ± 52.7 | 112.9 ± 39.5* |
| HbA1C (%) | 7.45 ± 2.16 | 6.58 ± 1.25* | 6.36 ± 1.10* |
| Body weight (kg) | 73.3 ± 16.3 | 70.4 ± 16.7* | 69.6 ± 16.8* |
| Ferritin (ng mL−1) | 184.9 ± 149.1 | 143.8 ± 134.9* | 117.3 ± 107.7* |
| FIB‐4 index | 1.42 ± 0.76 | 1.31 ± 0.67 | 1.23 ± 0.63* |
Data are presented as mean ± standard deviation.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ‐GTP, gamma‐glutamyl transferase; LDL‐C, low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol; TG, triglycerides; UA, uric acid; BS, blood sugar; HbA1C, glycated haemoglobin; FIB‐4, fibrosis‐4.
p < 0.05 vs. before treatment.
The reduction in serum ALT levels after 6 months was negatively correlated with HbA1C levels at baseline (R = −0.323, p < 0.01) (Figure 1). Moreover, the decrease in serum ALT levels after 6 months was positively correlated with serum ferritin levels at baseline (R = 0.271, p < 0.01) (Figure 2). Furthermore, the reduction in serum ALT levels after 6 months was significant positively correlated with the decrease in serum ferritin levels after 6 months (R = 0.513, p < 0.01) (Figure 3). The decrease in serum ALT levels after 6 months was positively correlated with the reductions in body weight observed after 6 months (R = 0.397, p < 0.01) (Figure 4).
Figure 1.
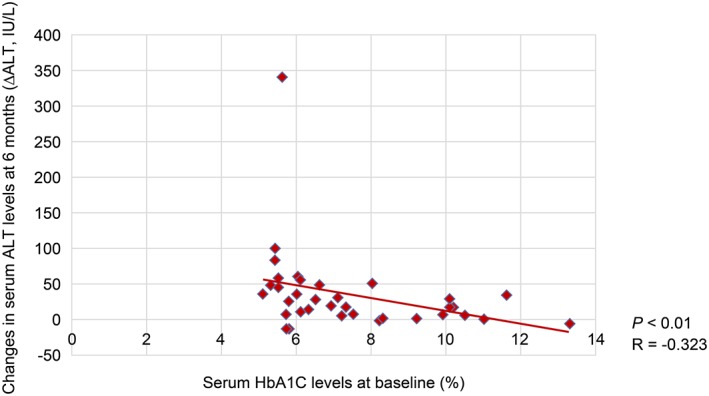
Correlation between decreased serum aminotransferase (ALT) levels and baseline serum glycated haemoglobin (HbA1C) levels.
Figure 2.
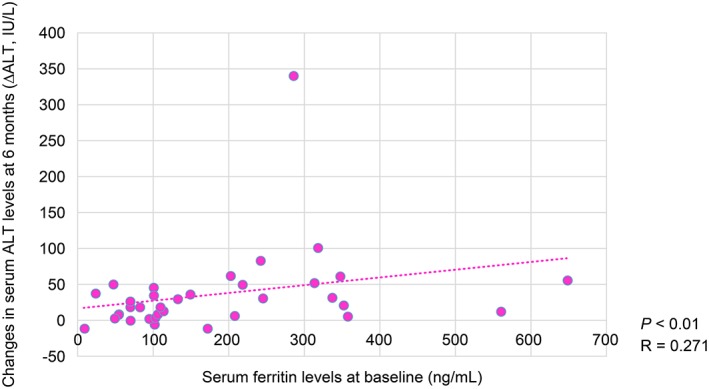
Correlation between decreased serum aminotransferase (ALT) levels and baseline serum ferritin levels at 6 months.
Figure 3.
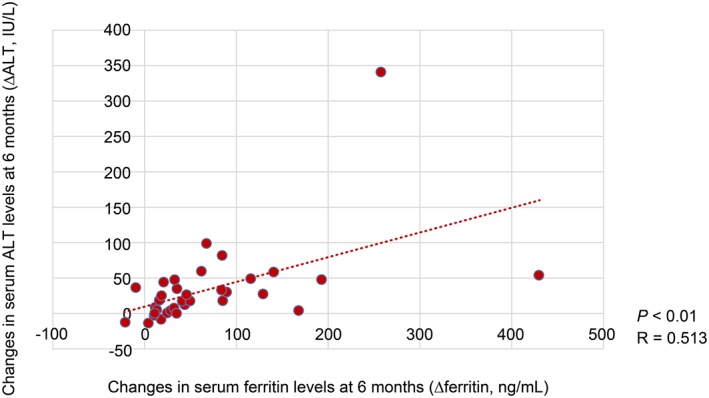
Correlation between decreased serum aminotransferase (ALT) levels and decreased serum ferritin levels.
Figure 4.
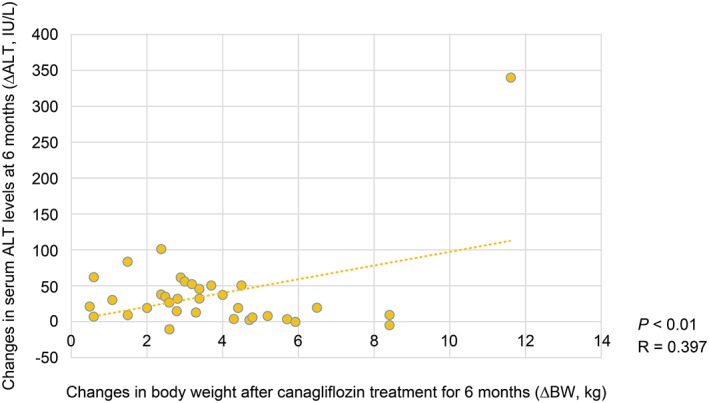
Correlation between reductions in body weight and serum aminotransferase (ALT) levels at 6 months.
Discussion
The aim of the present study was to investigate the efficacy of SGLT2 inhibitors for improving liver enzyme levels in patients with NAFLD. The results show that SGLT2 inhibitors improve glycaemic control and reduce body weight and serum AST, ALT and γ‐GTP levels. Previous studies have reported that serum HbA1C levels were approximately 0.5–0.7% lower in patients treated with SGLT2 inhibitors than in patients administered a placebo. In this study, serum HbA1C levels decreased by 0.9–1.1%, which was a greater change than that reported in previous studies 11. Further, blood sugar levels decreased significantly after 6 months of canagliflozin treatment. These results suggest that the reductions in casual blood glucose levels attributable to canagliflozin treatment improve insulin resistance and postprandial blood glucose levels over time. In this study, a decrease in body weight was observed after 3 months and was sustained over 6 months. Previous studies have reported that body weight was 2.5 kg lower in patients receiving SGLT2 inhibitors for 52 weeks than in patients administered placebo 12. Further, it has shown that SGLT2 inhibitors promote the renal excretion of glucose and decrease body fluid volume via osmotic diuretics. Consequently, the energy balance was negative, and a decrease in body weight was observed after 6 months. However, body weight reduction effects stagnate after approximately 3–6 months in patients receiving SGLT2 inhibitor treatment 13. These results suggest that long‐term investigations are necessary to fully understand the body weight reduction effects of canagliflozin.
Serum TG levels were significantly lower in patients receiving canagliflozin than in patients administered placebo; serum LDL‐C and HDL‐C levels were almost unchanged. The mechanisms underlying the decrease in serum TG levels associated with SGLT2 inhibitor treatment are unknown at present. Further studies are needed to investigate the effects of SGLT2 inhibitors on serum lipid metabolism.
In this study, serum UA levels significantly decreased after canagliflozin treatment. It has suggested that glucose transporter‐9 is involved in SGLT inhibitor‐induced reductions in serum UA levels 14. These results suggest that the reduction in serum UA levels affords a protective effect on renal function and has a positive impact on blood pressure and carbohydrate metabolism disorders.
In this study, serum AST, ALT and γ‐GTP levels significantly decreased after 6 months of canagliflozin treatment. Qing et al. reported that administration of the SGLT2 inhibitor luseogliflozin in a NASH rodent model resulted in weight loss and decreased serum ALT levels, as well as improved insulin resistance, liver fibrosis and fatty metamorphosis 15.
These results show that canagliflozin may improve hepatic steatosis and liver dysfunction in NAFLD patients. Moreover, the reductions in serum ALT levels were significantly correlated with baseline HbA1C levels at 6 months. The reductions in ALT levels were significantly correlated with reductions in body weight at 6 months. It further suggests that canagliflozin treatment may significantly improve liver dysfunction in NAFLD patients with mild carbohydrate metabolism disorders or in cases with significant weight loss after 6 months. The improvement in liver dysfunction observed in NAFLD patients may have been in conjunction with improvements in glycaemic control and insulin resistance in patients with T2DM and NAFLD 16. In studies of patients with NAFLD/NASH, weight loss is associated with improvements in liver dysfunction and other markers of NAFLD/NASH 17, 18. Further, there is a relationship between NAFLD and T2DM 19, and in patients with NAFLD, weight loss can improve serum insulin levels, liver function and quality of life 20.
Furthermore, the reductions in serum ALT levels significantly correlated with baseline serum ferritin levels and a decrease in serum ferritin levels at 6 months. Hyperferritinemia with mild hepatic iron accumulation is observed in 20–30% of patients with NAFLD and is commonly referred to as dysmetabolic iron overload syndrome. Besides directly inducing liver damage, excess iron is also involved in the pathogenesis of metabolic syndrome by inducing adipose tissue and insulin resistance and modifying the release of adipokines. Furthermore, hyperferritinemia and increased iron stores have been associated with greater severity of liver disease and hepatocellular carcinoma in patients with NAFLD 19. Abnormal iron metabolism in chronic liver disease is related to liver carcinogenesis. Hyperferritinemia has been observed in patients with NAFLD and is positively correlated with the severity of NAFLD 21. Further, hyperferritinemia is related to oxidative stress and mild hepatic iron overload. Increased iron stores are dependent on insulin resistance, which plays an important role in steatosis and steatosis hepatitis. Because iron plays a major role in liver damage and insulin resistance, phlebotomy, an iron‐depleting therapy, has been extensively studied in recent years. It appears to be a safe and promising intervention that could ameliorate the harmful effect of iron 22. Calculations for serum ferritin include a simple, low‐cost laboratory test that may be a good marker of hepatocellular aggression and liver fibrosis for the selection of patients who require hepatic biopsy 23. Moreover, ferritin may be an independent predictor of inflammation and liver fibrosis and may help in the decision of whether to perform a biopsy in individuals clinically diagnosed with NAFLD 24, 25, 26, 27, 28, 29. Thus, in NAFLD patients, hyperferritinemia, hepatic steatosis and insulin resistance are related to each other via oxidative stress, which is likely involved in the onset and progression of NAFLD; however, it is necessary to elucidate the detailed mechanisms in future studies to establish a treatment regimen.
In recent years, a need for non‐invasive diagnostic methods to assess liver damage has been noted, i.e. methods to identify symptoms of liver fibrosis that indicate possible development of advanced liver fibrosis or cirrhosis without the need for a liver biopsy 30. The FIB‐4 scoring system is a non‐invasive laboratory test to diagnose liver fibrosis in patients with NAFLD and is calculated using AST values, ALT values, platelet levels and age. The FIB‐4 index significantly decreased after canagliflozin treatment. Canagliflozin may contribute to decreased serum ferritin levels and FIB‐4 index values, as well as the suppression of hepatic fibrosis and development of liver cancer. This study showed a decrease in body weight, serum HbA1C, BS, AST, ALT, γ‐GTP, TG, UA and ferritin levels, as well as FIB‐4 index values in NAFLD patients administered canagliflozin for 6 months.
After administration of canagliflozin, excess glucose was excreted in the urine, which lowered blood glucose levels, reduced body weight and visceral fat and improved insulin resistance; further, impaired glucose tolerance and abnormal lipid metabolism were alleviated. These results suggest that liver dysfunction in NAFLD patients was improved. Decreased serum ferritin levels were also observed, likely attributable to improved glucose tolerance and liver dysfunction after administration of canagliflozin. Reduced iron liver stores attributable to administration of SGLT2 inhibitors decrease oxidative stress and improve insulin resistance, which can also lead to decreased steatosis in NAFLD patients.
The present study has several limitations. First, other hypoglycaemic, anti‐hypertensive, lipid‐lowering or UA‐lowering agents, as well as differences in food intake and/or exercise, may have influenced the study results. Second, the 6‐month observation period was short. A study examining the efficacy and safety of canagliflozin treatment for more than 1 year is necessary.
In conclusion, administration of canagliflozin was an effective clinical treatment for NAFLD. Canagliflozin treatment during the early stages of NAFLD likely suppresses the development of diabetes mellitus, liver fibrosis and liver cancer. In future studies, it will be necessary to examine the long‐term clinical efficacy of canagliflozin.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgement
We would like to thank Honyaku Center Inc. for English editing. This study did not receive any financial support.
Itani, T. , and Ishihara, T. (2018) Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obesity Science & Practice, 4: 477–482. 10.1002/osp4.294.
References
- 1. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346: 1221–1231. [DOI] [PubMed] [Google Scholar]
- 2. Beaton MD, Chakrabarti S, Adams PC. Inflammation is not the cause of an elevated serum ferritin in non‐alcoholic liver disease. Ann Hepatol. 2014; 13: 353–356. [PubMed] [Google Scholar]
- 3. Barb D, Portillo‐Sanchez P, Cusi K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism. 2016; 65: 1183–1195. [DOI] [PubMed] [Google Scholar]
- 4. Lizardi‐Cervera J, Aguilar‐Zapata D. Nonalcoholic fatty liver disease and its association with cardiovascular disease. Ann Hepatol. 2009; 8: S40–S43. [PubMed] [Google Scholar]
- 5. Nagano S, Katsuo K, Isaji M, et al. Remogliflozin etabonate improves fatty liver disease in diet‐induced obese male mice. J Clin Exp Hepatol. 2015; 5: 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016; 42: 25–32. [DOI] [PubMed] [Google Scholar]
- 7. Katsuyama H, Hasasaki H, Adachi H, et al. Effects of sodium‐glucose cotransporter 2 inhibitors on metabolic parameters in patients with type 2 diabetes: a chart‐based analysis. J Clin Med Res. 2016; 8: 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scheen AJ. Pharmacokinetics, phamacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. 2015; 54: 691–708. [DOI] [PubMed] [Google Scholar]
- 9. Vallet‐Pichhard A, Mallet V, Nalpas B, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007; 46: 32–36. [DOI] [PubMed] [Google Scholar]
- 10. Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007; 102: 2708–2715. [DOI] [PubMed] [Google Scholar]
- 11. Sun YN, Zhou Y, Chen X, Che WS, Leung SW. The efficacy of dapagliflozin combined with hypoglycaemic drugs in treating type 2 diabetes mellitus: meta‐analysis of randomized controlled trials. BMJ Open. 2014; 4: e004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012; 2: e001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium glucose cotransporter 2 inhibitors for type 2 diabetes. Ann Intern Med. 2013; 159: 262–274. [DOI] [PubMed] [Google Scholar]
- 14. Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014; 35: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qiang S, Nakatsu Y, Seno Y, et al. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr. 2015; 7: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris EH. Elevated liver function tests in type 2 diabetes. Clin Diabetes. 2005; 23: 115–119. [Google Scholar]
- 17. Ueno T, Sugawara H, Sujaku K, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997; 27: 103–107. [DOI] [PubMed] [Google Scholar]
- 18. Vilar‐Gonez E, Matinez‐Perez Y, Calzadilla‐Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015; 149: 367–378. [DOI] [PubMed] [Google Scholar]
- 19. Khodadoostan M, Zammanidoost M, Shavakhi A, et al. Effects of phlebotomy on liver enzyme and histology of patients with nonalcoholic fatty liver disease. Adv Biomed Res. 2017; 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004; 53: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kowdley KV, Belt P, Wilson LA, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012; 55: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Penkowa M, Dragnerva S, Marinova C, et al. Phlebotomy in the treatment of iron overload in patients with nonalcoholic and alcoholic fatty liver diseases. Int J Bus Hum Tech. 2012; 2: 48–51. [Google Scholar]
- 23. Barros RK, Cotrim HP, Daltro CH, Oliveira YA. Hyperferritinemia in patients with nonalcoholic fatty liver disease. Rev Assoc Med Bras. 2017; 63: 284–289. [DOI] [PubMed] [Google Scholar]
- 24. Ucar F, Sezer S, Erdogan S, Akyol S, Armutcu F, Akyol O. The relationship between oxidative stress and nonalcoholic fatty liver disease: its effects on the development of nonalcoholic steatohepatitis. Redox Rep. 2013; 18: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin‐6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008; 103: 1372–1379. [DOI] [PubMed] [Google Scholar]
- 26. Estrep JM, Baranowa A, Hossain N, et al. Expression of cytokine signaling genes in morbidly obese patients with nonalcoholic steatohepatitis and hepatic fibrosis. Obes Surg. 2009; 19: 617–624. [DOI] [PubMed] [Google Scholar]
- 27. Syn WK, Choi SS, Diehl AM, et al. Apoptosis and cytokines in nonalcoholic steatohepatitis. Clin Liver Dis. 2009; 13: 565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ibrahim MA, Kelleni M, Geddawy A. Nonalcoholic fatty liver disease: current and potential therapies. Life Sci. 2013; 9: 114–118. [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Zhou M, Lam KS, et al. Protective roles of adiponectin in obesity‐related fatty liver diseases: mechanisms and therapeutic implications. Arq Bras Endocrinol Metabol. 2009; 53: 201–212. [DOI] [PubMed] [Google Scholar]
- 30. Nones RB, Ivantes CP, Pedroso MLA. Can FIB4 and NAFLD fibrosis scores help endocrinologists refer patients with non‐alcoholic liver disease to a hepatologist? Arch Endocrinol Metab. 2017; 61: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]


