Abstract
Safety is the chief consideration in recipient preparation of spermatogonial stem cell (SSC) transplantation in mammals, especially humans. In this study, we compared the safety of the SSC transplantation recipients that were prepared both by testes heat shock plus testes busulfan injection (heat shock+busulfan(t)) and by busulfan intraperitoneal injection (busulfan i.p.) only. Our results showed that heat shock+busulfan(t) treatment significantly (p < 0.05) reduced mortality in mice and did not produce bone marrow cell toxicity. Furthermore, heat shock+busulfan(t) treatment directly damaged SSCs and exhausted almost all of the germ cells in the testis; the exhaustion of these cells is considered a key factor in the successful preparation of the recipients. Therefore, we used heat shock+busulfan(t) treatment to prepare recipients of SSC transplantation. Two months after SSC transplantation, the number and length of donor SSC-derived colonies in the testis of recipient in heat shock+busulfan(t) group was closed to that in busulfan i.p. group. Therefore, compared with busulfan i.p. treatment, heat shock+busulfan(t) treatment improved the safety of recipient preparation without reducing the efficiency of SSC transplantation. Two GFP-positive offspring were produced from 1 of the 20 recipients that had mated with female mice 72 days after SSC transplantation. In conclusion, heat shock with busulfan treatment is a safe method to prepare the recipient of SSC transplantation in mice.
Keywords: safe recipient preparation, SSCs transplantation, heat shock, busulfan
Introduction
Spermatogonial stem cell (SSC) autotransplantation and germline genomic editing have been reported as a potential cure for spermatogenic failure and may prevent the transmission of genomic diseases in humans1. First, SSC autotransplantation can be successful only if it is combined with a correction of the mutation which led to oligozoospermia and azoospermia. Recent advances in the CRISPR-Cas9 system allow for highly efficient genetic alterations of many cell types (including human cells) and organisms2. Therefore, if SSC genomic editing is combined with SSCs autotransplantation, it would allow patients who suffered from spermatogenic disorders to have their own biological children.
The safety of recipient preparation is very important for SSC autotransplantation, especially for humans. Recipients usually can be prepared by destroying endogenous germ cells and blocking spermatogenesis, so that donor cells could easily translocate from seminiferous tubule lumens to the basal compartment to undergo spermatogenesis after SSC transplantation3. W/WV mutant mice have been used as the earliest natural recipients for only SSC transplantation because SSCs from W/WV mutant mice cannot finish self-renewal4. Heat shock treatment (testicular hyperthermia) could create an empty space in the seminiferous tubules by inducing germ cell apoptosis5–11. Our previous study showed that heat shock treatment can be used safely to prepare recipients for SSC transplantation12. However, the window of the transplant period is shorter, because heat shock cannot eradicate the endogenous SSCs that could recover spermatogenesis later. Furthermore, the shortcoming of radiation therapy is the calcification of seminiferous tubules that could block the flow of transplanted SSCs in seminiferous tubules13.
Busulfan treatment is the most common method used to prepare recipients of SSC autotransplantation or allotransplantation3,4,12,14. Busulfan can eliminate almost all the endogenous germ cells in the recipient, creating an empty space in the SSCs niche. Therefore, busulfan has been used successfully to prepare recipients in mouse14,15, rat16,17, pig18, and monkey19. However, busulfan is a common alkylating chemotherapeutic agent and has preferential toxicity for proliferating cells. Busulfan produces systemic toxicity, and even death, resulting from myelosuppression in pig19, mouse20, and rat21. The side effects of busulfan limit its use in preparing SSC transplantation recipients in mammals, especially in humans.
Although heat shock did not eliminate SSCs that could recover endogenous spermatogenesis by one spermatogenic cycle, it could induce endogenous differentiated germ cells to undergo apoptosis, which creates a necessary empty space in the seminiferous tubules to inject busulfan solution into these tubules. The injected busulfan can eliminate almost all the endogenous SSCs in the tubules. Compared with 40 mg/kg body weight busulfan intraperitoneal (i.p.) injection, the dose of busulfan injected in the seminiferous tubules is lower. Hence testicular heat shock treatment with seminiferous tubule injection of busulfan perhaps is a safe way to prepared recipients for SSC transplantation in mice.
In this study, we examined the feasibility of using testicular heat shock treatment with seminiferous tubule injection of busulfan to prepare safe recipients for SSC transplantation in mice.
Materials and Methods
Animals
C57BL/6 J mice were purchased from Experimental Animal Center of Ningxia Medical University. EGFP transgenic C57BL/6 J mice were purchased from the Model Animal Research Center of Nanjing University. These animals were housed in a standard animal facility under controlled temperature (20°C) and photoperiod (12 h light, 12 h dark) with access to water and rodent food ad libitum. Animal care was in accordance with institutional guidelines and was approved by the Ningxia Medical University Animal Care and Use Committee. For heat shock treatment, 6-week-old male mice were anaesthetized with an i.p. injection of 1% napental (100 mg/kg body weight) (X-Y Biotechnology, Shanghai, China). Mice were hung above a water bath (HerryThec, Shanghai, China) and the lower half of the body was submerged in hot water at 43°C for 15 min (heat shock group). For busulfan treatment, busulfan (Sigma-Aldrich, St. Louis, MO, USA) solution at a concentration of 4 mg/ml was injected once into the 4-week-old mouse enterocoelia at a dose of 40 mg/kg body weight; the mice were then used as recipients after 4 weeks (busulfan i.p. group). For heat shock and busulfan combined treatment, mice were first treated with heat shock and then treated with low dose busulfan (1.5 mg/kg body weigh) (heat shock+busulfan (t) group). Approximately 10 μl of busulfan was injected once into the seminiferous tubules of each testis through the efferent duct 14 days after heat shock treatment. The mice were used as recipients 4 weeks after busulfan treatment.
Safety Assessment of Treatment
The mortality rates of mice, treated with busulfan i.p., heat shock, and heat shock+busulfan(t) were recorded. Red blood cell and white blood cell numbers were also counted to assess the level of myelosuppression induced by busulfan injection. Six mice were used in each group.
Histology and Immunohistochemistry
The mice were killed 4 weeks after busulfan treatment (in heat shock+busulfan(t) group and busulfan i.p. group) or heat shock treatment (in heat shock group). Testes were fixed in 4% paraformaldehyde (Sigma-Aldrich) at 4°C for 48 h, paraffin-embedded, sectioned (5 μm), and stained by haematoxylin and eosin (H&E; Sigma-Aldrich, St. Louis, MO, USA). The apoptotic germ cells were detected by TUNEL kit (Vazyme Biotech, Nanjing, China).
SSC Transplantation
A two-step enzymatic digestion protocol was used to obtain germ cell suspensions (2 × 107 cells/ml) from enhanced green fluorescent protein (EGFP)-transgenic C57BL/6 J donor mice at 6–8 days after birth. Recipients were anesthetized with an i.p. injection of 1% napental as described above. Approximately 10 μl of donor germ cell suspension was transplanted into the seminiferous tubules of each testis of the recipients by efferent duct injection 4 weeks after busulfan treatment. A total of 10 μl of donor germ cell suspension filled nearly 80–85% seminiferous tubules of each testis as determined by trypan blue (Sigma-Aldrich).
Analysis of Recipient Testes
Two months after transplantation, the testes of the recipients were collected. The tunica from the recipients’ testes were removed, and seminiferous tubules were dispersed. Donor-derived GFP-positive spermatogenic colony numbers and total length were counted under a fluorescence stereoscope with UV light (Nikon, Tokyo Prefecture, Japan).
Production of Offspring by the Recipient Mice
Each recipient was mated with two female mice 2 months after SSC transplantation. If the female mouse became pregnant, it was placed in another cage, and a new female mouse was put inside the cage in which the recipient mouse lived. The birth dates of offspring, litter sizes, and health conditions were checked and recorded. RT-PCR was used to detect the GFP gene in recipient offspring. The following GFP primers were used. Forward: tgaaccgcatcgagctgaaggg. Reverse: tccagcaggaccatgtgatcgc.
Statistical Analysis
Mortality rate, number of white and red blood cells, testicular weights, number of colonies, and average colony length per testis were compared using one-way ANOVA carried out in SAS software (SAS Institute Inc., Cary, NC, USA); p < 0.05 was considered statistically significant.
Results
Heat Shock with Busulfan Treatment has Few Side-Effects in Mice
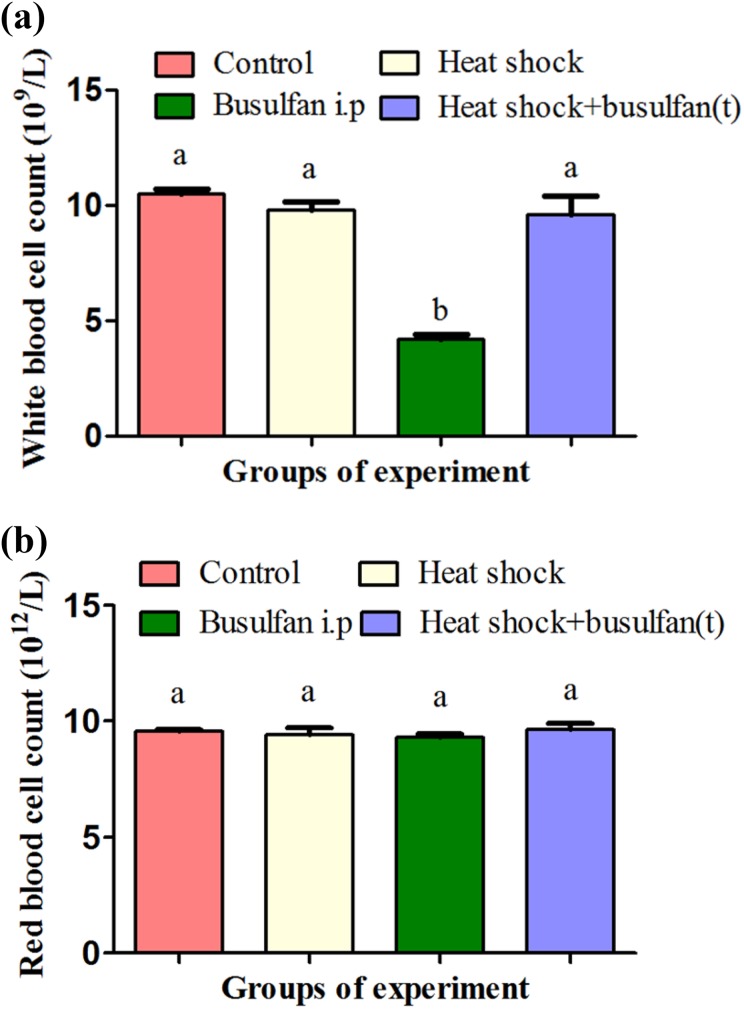
Our results showed that heat shock+busulfan(t) treatment decreased recipient mortality that was sharply induced using busulfan in previous studies during preparation of SSCs transplantation. No mice died in the heat shock+busulfan(t) group, while recipient mortality (30%) in the busulfan i.p. group was significantly higher (p < 0.05). In the heat shock group as well as in the DMSO-injected control group, no mice died (Table 1). As we know, busulfan is a chemotherapy drug that can kill haematopoietic stem cells. Therefore, we tested the effects of busulfan on the number of blood cells. Our results showed that, 28 days after treatment, the number of white blood cells was maintained stably in the heat shock+busulfan(t) group, but in the busulfan i.p. group, there was a significant decrease in the number of white blood cells (Fig. 1a). However, none of the treatments had an effect on the number of red blood cells (Fig. 1b).
Table 1.
The Mortality Rate of Recipient Mice After Various Treatments.
| Treatment | No. of mice | No. of deaths | Mortality rate (%) |
|---|---|---|---|
| Busulfan i.p. | 40 | 12 | 30 a |
| Heat shock | 40 | 0 | 0 b |
| Heat shock+busulfan(t) | 40 | 0 | 0 b |
| DMSO | 10 | 0 | 0 b |
Different letters indicate a statistically significant difference (p < 0.05).
Fig. 1.
Heat shock with busulfan treatment reduced bone marrow cell toxicity. (a) Two weeks after treatment, the number of white blood cells was maintained stably in the heat shock+busulfan(t) group; however, in the busulfan i.p. group, there was a significant decrease in the number of white blood cells. (b) No treatment had effects on the number of red blood cells.a,b Different letters indicate a statistically significant difference (p < 0.05).
Heat Shock with Busulfan Treatment Completely Induced Endogenous Germ Cell Depletion in Mice
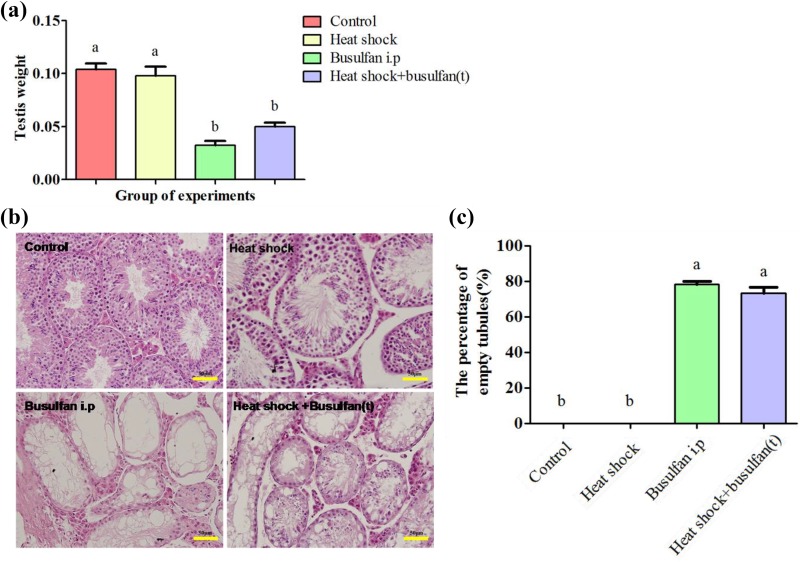
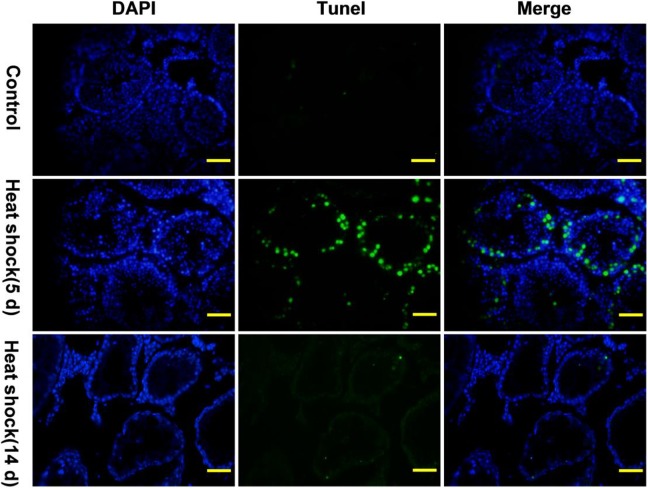
Our result showed that testicular weight was significantly lower (p < 0.05) in the heat shock+busulfan(t) group than in the control and heat shock groups 28 days after treatment, but there was no significant difference between heat shock+busulfan(t) group and busulfan i.p group (Fig. 2a). To further observe germ cell change in the seminiferous tubules after treatment, testicular histological section were prepared 4 weeks after busulfan treatment (in the heat shock+busulfan(t) and busulfan i.p. groups) or heat shock treatment (in the heat shock group), and we found that the number of germ cells in the shock+busulfan(t) group substantially decreased, similar to that of busulfan-treated mice (Fig. 2b). Many germ cells, especially primary and secondary spermatocytes, underwent apoptosis in the seminiferous tubules 10 days after heat shock treatment (Fig. 3). Busulfan treatment showed a more substantial reduction in germ cell number, and there were almost no germ cells in the testis 4 weeks after busulfan treatment (Fig. 2b). We observed many hollow seminiferous tubules in the shock+busulfan(t) and busulfan i.p. groups. There was substantial spermatogenesis in the heat shock treatment group, similar to the control group.
Fig. 2.
Heat shock with busulfan treatment induced testis weight reduction and germ cell loss. (a) Testicular weight was significantly lower in the heat shock+busulfan(t) group than in the control and heat shock groups 28 days after treatment, but there was no significant difference between the heat shock+busulfan(t) group and busulfan i.p. group. (b) The number of germ cells in the heat shock+busulfan(t) group substantially decreased, similar to that of busulfan-treated mice. (c) The percentage of empty tubules. Bar = 50 μm.
a, b Different letters indicate a statistically significant difference (p < 0.05).
Fig. 3.
Heat shock treatment induced germ cells, especially primary and secondary spermatocytes, apoptosis. The apoptosis of germ cells that were green detected by TUNEL kit 5 and 14 days after heat shock treatment. Fourteen days after heat shock treatment, there were many hollow seminiferous tubules in the testis. The nucleus of germ cells and somatic cells in testis tissue were stained by DAPI. Bar = 20 µm. n = 6.
The Recipient Prepared by Heat Shock With Busulfan Treatment had Similar Colony-Forming Efficiency as Busulfan-Treated Recipients
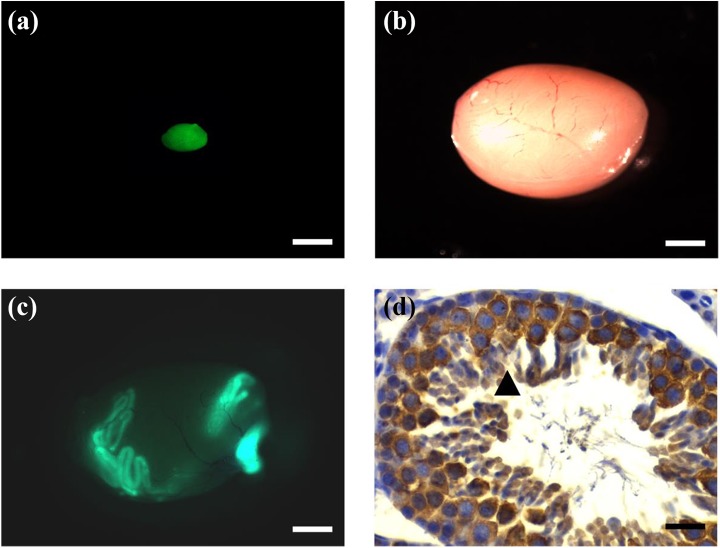
Germ cell suspensions from 6- to 8-day-old GFP-transgenic mice were transplanted into the testis of recipients, and GFP-positive colonies in all recipient testis could be found two months after transplantation. Germ cells, including spermatocytes and sperm, derived from GFP-positive colonies of donor SSCs, were detected in H&E-stained section of recipient testis (Fig. 4). The colony-forming efficiency of donor SSCs in heat shock+busulfan(t)-treated recipients were similar to that of busulfan-treated recipients. There was no significant difference in colony number and colony length between the heat shock+busulfan(t) and busulfan i.p. groups, indicating that heat shock+busulfan(t) treatment yielded a satisfactory colony-forming efficiency (Table 2).
Fig. 4.
Donor SSCs formed colonies and donor-derived spermatogenesis in the testes of mice in heat shock+busulfan(t) group. (a) Donor testis with ubiquitous expression of EGFP. Bar = 1 mm. (b) Recipient testis before SSC transplantation. Bar = 1 mm. (c) There are many donor-derived EGFP-positive colonies within recipient testis 2 months after transplantation. Bar = 1 mm. (d) Donor-derived SSCs formed spermatogenesis in the testes of recipients in the heat shock+busulfan(t) group 2 months after germ cell transplantation. ▴ Donor germ cell-derived spermatogenesis in recipient testis showed by GFP immuohistochemical staining with GPF antibody. Bar = 40 μm.
Table 2.
Donor Cell Colonization in the Testes of Recipient Mice*.
| Treatment | Testis weight (g) | Percent of testes with colonies | No. of colonies per testis | Total colony length per testis (mm) |
|---|---|---|---|---|
| Busulfan | 33 ± 4 a | 100% | 14 ± 4 a | 43.44 ± 3.16 a |
| Heat shock+ busulfan(t) | 50 ± 6 b | 100% | 13 ± 1 a | 40.58 ± 4.59 a |
*n = 10 animals per treatment (20 testes injected). Analysis was conducted 2 months after transplantation.
Different letters within a column indicate a statistically significant difference (p < 0.05).
GFP Positive Offspring Were Derived from the Recipient Prepared by Heat Shock with Busulfan Treatment
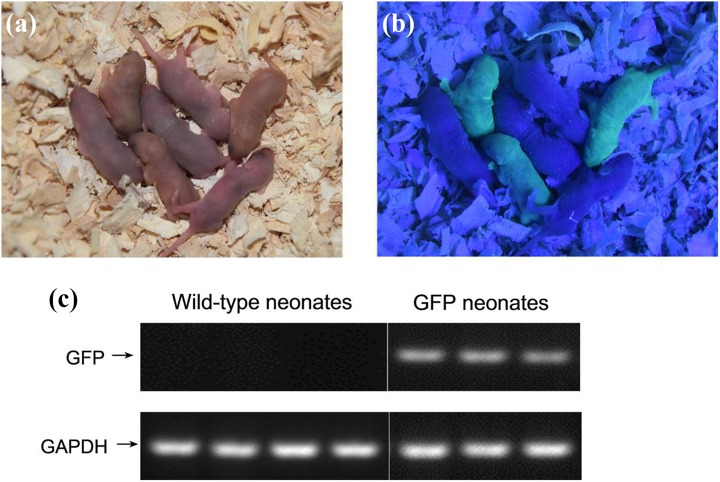
We successfully transplanted GPF-positive germ cell suspensions from donor mice to the testis of 20 recipients, and, 2 months after germ cell transplantation, each recipient was mated with two C57BL/6 J female mice to detect whether they could produce GFP-positive offspring. The earliest normal offspring were produced by one female mouse 23 days after mating with recipients, and the other mated female mice produced normal offspring subsequently. A nest of mice, including three GFP-positive and four GFP-negative offspring, was produced from one mated mouse 72 days after transplantation (Fig. 5b). The GFP-positive pups indicated that these three offspring originated from the sperm of donor SSCs, also confirmed by RT-PCR detection of GFP gene expression (Fig. 5c).
Fig. 5.
Offspring produced by donor cell-derived sperm. (a) Offspring from a wild-type C57 BL/6 J recipient that was transplanted with germ cell suspension of EGFP transgenic C57BL/6 J donor male mice and mated with a wild-type C57 BL/6 J female mouse 2 months after transplantation. (b) Three offspring were GFP-positive mice produced by GFP-positive donor SSCs derived sperm. C. RT-PCR further confirmed that three GFP -positive offspring expressed mRNA of the GFP gene.
Discussion
In the present study, we found that heat shock with busulfan treatment was a safe method to prepare recipients for SSC transplantation in mice. Unlike chemotherapy and radiotherapy, this treatment had few side-effects, such as systemic toxicity, myelosuppression, or even death. Therefore, heat shock with busulfan treatment could be a feasible method to prepare recipients of SSC transplantation.
Heat shock treatment with busulfan chemotherapy is a new way to prepare recipients of SSC transplantation in mice. Heat shock treatment only could induce endogenous differentiated germ cells, especially primary and secondary spermatocytes, apoptosis, but it did not eliminate SSCs that could recover endogenous spermatogenesis by one spermatogenic cycle12. At 10–15 days after heat shock treatment, almost all the spermatogenic cells in the inner compartment of seminiferous tubules disappeared through apoptosis, creating the necessary empty space in the seminiferous tubules to inject busulfan solution into these tubules. At 4 weeks after injection of 10 μl of busulfan solution, almost all the endogenous SSCs were eliminated.
Heat shock with busulfan treatment had little systemic toxicity because the doses of busulfan were very low. Busulfan is also called Myleran—a kind of sulfonate alkylating agent with cytotoxic effects similar to those of nitrogen mustard, belonging to the cell-cycle non-specific drug group. Busulfan generates carbenium ion, and that ion reacts with DNA molecules causing damage to DNA structure and function. Busulfan also has a selective inhibitory action on bone marrow20–24. Usually, high doses of busulfan given by i.p. injection cause serious side effects, inhibiting the production of bone marrow leukocytes and platelets and preventing the generation of erythrocytes and lymphocyte. Hence, busulfan is also a principal drug used for the treatment of chronic myelogenous leukaemia. In our study, we found that high doses (40 mg/kg body weight) of busulfan in the busulfan i.p. group substantially reduced the number of leukocytes in mice, but a low dose (1.5 mg/kg body weight) of busulfan in the heat shock+busulfan(t) group has no effect on leukocytes. The mortality rate of mice in the heat shock+busulfan(t) group was zero, significantly lower (p < 0.05) than that of the busulfan i.p. group.
Local irradiation has been used to prepare recipients of SSC transplantation in mice25, goats18, rams26, and bulls27. The efficiency of recipient preparation by irradiation is comparable to that of recipients prepared by busulfan13. However, this approach has many shortcomings, including calcification of seminiferous tubules13, inconvenience of application, and potential radiation leakage. Therefore, compared with busulfan and irradiation treatment, heat shock with busulfan treatment has few side effects, thereby providing a viable method to prepare recipients of SSC autotransplantation for humans.
The formation of donor-derived colonies in our study indicated that heat shock with busulfan treatment could be used to eliminate endogenous germ cells for donor SSCs transplantation. Two months after SSCs transplantation, colonies from donor-derived germ cells could be found in heat shock+busulfan(t)-treated recipient testes, indicating that the function of SSCs niche was not damaged by heat shock+busulfan(t) treatment. The efficiency of recipients prepared by heat shock with busulfan treatment was as high as that of busulfan-treated recipients. The numbers and total length of colonies in the heat shock+busulfan(t) group were similar to those of the busulfan-treated group, and GFP-positive donor SSC-derived offspring were produced from a recipient and the mated female mice. In conclusion, heat shock with busulfan treatment was a safe method to prepare recipients of SSC transplantation in mice.
Footnotes
Author Contribution: Wenzhi Ma and Jia wang performed the biological experiments, and write the article. Weijun Gao carry out the statistical analysis. Hua Jia and Wenzhi Ma conceived and designed the study. All authors read and proofed the final manuscript.
Ethical Approval: Animal care was in accordance with institutional guidelines and was approved by the Ningxia Medical University Animal Care and Use Committee.
Statement of Human and Animal Rights: C57BL/6 J mice were purchased from Experimental Animal Center of Ningxia Medical University. EGFP transgenic C57BL/6 J mice were purchased from the Model Animal Research Center of Nanjing University.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81460488, 81301683), the Key Research and Development Program of Ningxia Hui Autonomous Region (2016KJHM43) and Ningxia High School first-class Disciplines (West China first-class Disciplines Basic Medical Sciences at Ningxia Medical University) (NXYLXK2017B07).
References
- 1. Mulder CL, Zheng Y, Jan SZ, Struijk RB, Repping S, Hamer G, van Pelt AM. Spermatogonial stem cell autotransplantation and germline genomic editing: a future cure for spermatogenic failure and prevention of transmission of genomic diseases. Hum Reprod Update. 2016;22(5):561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32(4):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnston DS, Russell LD, Griswold MD. Advances in spermatogonial stem cell transplantation. Rev Reprod. 2000;5(3):183–188. [DOI] [PubMed] [Google Scholar]
- 4. Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yin Y, Hawkins KL, DeWolf WC, Morgentaler A. Heat stress causes testicular germ cell apoptosis in adult mice. J Androl. 1997;18(2):159–165. [PubMed] [Google Scholar]
- 6. Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. 2001;65(1):229–239. [DOI] [PubMed] [Google Scholar]
- 7. Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140(4):1709–1717. [DOI] [PubMed] [Google Scholar]
- 8. Bowler K. The effect of repeated applications of heat on spermatogenesis in the rat: a histological study. J Reprod Fertil. 1972;28(3):325–333. [DOI] [PubMed] [Google Scholar]
- 9. Zhang RD, Wen XH, Kong LS, Deng XZ, Peng B, Huang AP, Wan Y, Yang ZW. A quantitative (stereological) study of the effects of experimental unilateral cryptorchidism and subsequent orchiopexy on spermatogenesis in adult rabbit testis. Reproduction. 2002;124(1):95–105. [DOI] [PubMed] [Google Scholar]
- 10. Hansen PJ. Effects of heat stress on mammalian reproduction. Philos Trans R Soc Lond B Biol Sci. 2009;364(1534):3341–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. Int J Androl. 1995;18(4):169–184. [DOI] [PubMed] [Google Scholar]
- 12. Ma W, An L, Wu Z, Wang X, Guo M, Miao K, Tian J. Efficient and safe recipient preparation for transplantation of mouse spermatogonial stem cells: pretreating testes with heat shock. Biol Reprod. 2011;85(4):670–677. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Z, Shao S, Meistrich ML. Irradiated mouse testes efficiently support spermatogenesis derived from donor germ cells of mice and rats. J Androl. 2006;27(3):365–375. [DOI] [PubMed] [Google Scholar]
- 14. Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997; 41(1):111–122. [PubMed] [Google Scholar]
- 15. Ogawa T, Ohmura M, Yumura Y, Sawada H, Kubota Y. Expansion of murine spermatogonial stem cells through serial transplantation. Biol Reprod. 2003;68(1):316–322. [DOI] [PubMed] [Google Scholar]
- 16. Jiang FX, Short RV. Male germ cell transplantation in rats: apparent synchronization of spermatogenesis between host and donor seminiferous epithelia. Int J Androl. 1995;18(6):326–330. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Z, Renfree MB, Short RV. Successful intra- and interspecific male germ cell transplantation in the rat. Biol Reprod. 2003;68(3):961–967. [DOI] [PubMed] [Google Scholar]
- 18. Honaramooz A, Behboodi E, Hausler CL, Blash S, Ayres S, Azuma C, Echelard Y, Dobrinski I. Depletion of endogenous germ cells in male pigs and goats in preparation for germ cell transplantation. J Androl. 2005;26(6):698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, Schatten GP, Orwig KE. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25(9):2330–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hassan Z, Nilsson C, Hassan M. Liposomal busulphan: bioavailability and effect on bone marrow in mice. Bone Marrow Transplant. 1998;22(9):913–918. [DOI] [PubMed] [Google Scholar]
- 21. Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D, Zhang Y, Huang F, Zhou H, Fan Z, Tu S, Li Y, Guo X, Yu G, Liu Q. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol. 2013;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hassan M, Andersson BS. Role of pharmacogenetics in busulfan/cyclophosphamide conditioning therapy prior to hematopoietic stem cell transplantation. Pharmacogenomics. 2013;14(1):75–87. [DOI] [PubMed] [Google Scholar]
- 23. Radhakrishnan K, Bhatia M, Geyer MB, Del Toro G, Jin Z, Baker C, Harrison L, Morris E, Baxter-Lowe LA, Cairo MS. Busulfan, fludarabine, and alemtuzumab conditioning and unrelated cord blood transplantation in children with sickle cell disease. Biol Blood Marrow Transplant. 2013;19(4):676–677. [DOI] [PubMed] [Google Scholar]
- 24. Chen YB, Batchelor T, Li S, Hochberg E, Brezina M, Jones S, Del Rio C, Curtis M, Ballen KK, Barnes J, Chi AS, Dietrich J, Driscoll J, Gertsner ER, Hochberg F, LaCasce AS, McAfee SL, Spitzer TR, Nayak L, Armand P. Phase 2 trial of high-dose rituximab with high-dose cytarabine mobilization therapy and high-dose thiotepa, busulfan, and cyclophosphamide autologous stem cell transplantation in patients with central nervous system involvement by non-Hodgkin lymphoma. Cancer. 2015;121(2):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Creemers LB, Meng X, den Ouden K, van Pelt AM, Izadyar F, Santoro M, Sariola H, de Rooij DG. Transplantation of germ cells from glial cell line-derived neurotrophic factor-overexpressing mice to host testes depleted of endogenous spermatogenesis by fractionated irradiation. Biol Reprod. 2002;66(6):1579–1584. [DOI] [PubMed] [Google Scholar]
- 26. Oatley JM, Tibary A, de Avila DM, Wheaton JE, McLean DJ, Reeves JJ. Changes in spermatogenesis and endocrine function in the ram testis due to irradiation and active immunization against luteinizing hormone-releasing hormone. J Anim Sci. 2005;83(3):604–612. [DOI] [PubMed] [Google Scholar]
- 27. Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD, Woelders H, Kal HB, De Rooij DG. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126(6):765–774. [PubMed] [Google Scholar]