Abstract
Mesenchymal stem cells (MSCs) constitute an important repair system, but may be impaired by exposure to cardiovascular risk factors. Consequently, adipose tissue-derived MSCs from pigs with the metabolic syndrome (MetS) show decreased vitality. A growing number of microRNAs (miRNAs) are recognized as key modulators of senescence, but their role in regulating senescence in MSC in MetS is unclear. We tested the hypothesis that MetS upregulates in MSC expression of miRNAs that can serve as post-transcriptional regulators of senescence-associated (SA) genes. MSCs were collected from swine abdominal adipose tissue after 16 weeks of Lean or Obese diet (n = 6 each). Next-generation miRNA sequencing (miRNA-seq) was performed to identify miRNAs up-or down-regulated in MetS-MSCs compared with Lean-MSCs. Functional pathways of SA genes targeted by miRNAs were analyzed using gene ontology. MSC senescence was evaluated by p16 and p21 immunoreactivity, H2AX protein expression, and SA-β-Galactosidase activity. In addition, gene expression of p16, p21, MAPK3 (ERK1) and MAPK14, and MSC migration were studied after inhibition of SA-miR-27b. Senescence biomarkers were significantly elevated in MetS-MSCs. We found seven upregulated miRNAs, including miR-27b, and three downregulated miRNAs in MetS-MSCs, which regulate 35 SA genes, particularly MAPK signaling. Inhibition of miR-27b in cultured MSCs downregulated p16 and MARP3 genes, and increased MSC migration. MetS modulates MSC expression of SA-miRNAs that may regulate their senescence, and the p16 pathway seems to play an important role in MetS-induced MSC senescence.
Keywords: mesenchymal stem cells, senescence, microRNA, metabolic syndrome
Introduction
Mesenchymal stem cells (MSCs) constitute an important endogenous repair system1,2, which may be impaired by exposure to conditions constituting cardiovascular risk factors. In addition, exogenous delivery of MSCs has become an important regenerative tool, but the ability to administer autologous cell-based therapies has been impeded by a fall in MSC quantity and quality during disease states3. We previously observed impaired functionality and increased MSC senescence in pigs with metabolic syndrome (MetS), and altered cargo of the released extracellular vesicles4,5. Hence, MetS may impair the paracrine activity of MSC therein limiting the regenerative potential.
Cellular senescence results from environmental stimuli or damages such as in aging resulting in an irreversible cell cycle arrest6–8. The proliferation and differentiation potential of stem cells from patients with obesity or insulin resistance is negatively correlated with the number and viability of stem cells, and positively correlate with cell apoptosis and senescence9. However, the mechanisms by which MetS promotes MSC senescence remain unclear.
Senescent cells secrete a variety of proteins, collectively known as senescence-associated secretory phenotype (SASP), and protein patterns controlling senescence10. MicroRNAs (miRNAs) are small non-coding RNA fragments that act as central post-transcriptional regulators by repressing the expression of their messenger RNA (mRNA). A growing number of miRNAs are recognized as key modulators of senescence through the p53/p21 or pRB/p16 pathways. However, whether miRNAs are involved in MSC senescence secondary to MetS remains unknown.
The mitogen-activated protein kinase (MAPK) pathway, originally known as the extracellular signal-regulated kinases (ERK) pathway, encompasses a series of proteins involved in intracellular signaling that regulate cellular transcription and translation. The conventional MAPK includes the ERK1/2, c-Jun amino-terminal kinases (JNK), p38, and ERK5 families11. Each group is composed of a set of kinases, among which are MAPK3, a major component of the classic ERK1/2 pathway, and MAPK14, which encodes p38α, the prototypic member of the p38 MAPK family. MAPK is an important regulator of cell cycle progression12,13, but its role in MetS-induced MSC senescence remains unknown.
The current study tested the hypothesis that MetS modulates in MetS-MSCs the content of miRNA related to senescence. For this purpose, senescence biomarkers were evaluated and MSC miRNA expression characterized using high-throughput RNA sequencing analysis, as well as selective inhibition of miR-27b, a MAPK regulator14, which was found to be elevated in MetS-MSCs. Importantly, we characterize the miRNA spectrum that may be involved in regulation of MSC senescence in MetS. We found that p16INK4a expression, and the most two common markers of cellular senescence15, senescence-associated β-galactosidase (SA-ß-GAL) activity and H2A Histone Family Member X (H2AX), were all elevated. Inhibition of miR-27b downregulated expression of p16 and MAPK3, but not of MAPK14. These observations provide a better understanding of miRNAs’ action as post-transcriptional regulators of MetS-MSCs senescence.
Materials and Methods
Animals
Twelve female domestic pigs were randomized into two groups at three months of age. Control pigs (Lean, n = 6) were fed a standard chow, and MetS pigs (n = 6) a high-cholesterol/carbohydrate diet (5B4 L, protein 16.1%, ether extract fat 43.0%, and carbohydrates 40.8%; Purina Test Diet (Purina, Richmond, IN, USA) for 16 weeks16. Animal studies were approved by the Institutional Animal Care and Use Committee.
Systemic parameters including body weight, heart rate, arterial blood pressure, total cholesterol, low-density lipoprotein (LDL), triglycerides, and fasting glucose and insulin levels were measured by standard procedures17 (Hoffmann-La Roche, Basel, Switzerland, http://www.roche.com) after 16 weeks of feeding. Insulin resistance was assessed by the homeostasis model assessment of insulin resistance (HOMA-IR) index4,5. Animals were then euthanized with a lethal intravenous dose of 100 mg/kg of sodium pentobarbital (Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI, USA) and subcutaneous abdominal adipose tissue (5–10 g) collected.
MSCs Isolation, Characterization, and Culture
MSCs were isolated and cultured as described previously18. In brief, MSCs were harvested from abdominal subcutaneous fat tissue of pigs (10 g), which was then digested in collagenase-H, filtered (0.2-µm syringe filter), and cultured in advanced minimal essential medium (GIBCO/Invitrogen, Grand Island, NY, USA) supplemented with 5% platelet lysate for three weeks19. The third passage was collected and kept in GIBCO Cell Culture Freezing Medium at –80°C (GIBCO, Grand Island, NY, USA). MSCs phenotype was later examined with immunofluorescent staining as described previously20.
MiRNA Library Construction, Sequencing, and Data Analyses
MiRNA sequencing was performed as previously described5. Sequencing RNA libraries were prepared according to the manufacturer’s protocol (TruSeq RNA Sample Prep Kit v2, Illumina, San Diego, CA, USA). The concentration and size distribution of the libraries were determined on an Agilent Bioanalyzer DNA 1000 chip. A final sample concentration was performed using Qubit fluorometry (Invitrogen, Grand Island, NY, USA). Data was analyzed using the CAP-miRSeq-v1.1 workflow21, starting with unaligned FASTQs, aligning BAMs, and subsequently yielding Excel files containing raw and normalized known miRNA expression counts. EdgeR2.6.222 was employed to perform differential expression analysis between miRNAs from Lean- and MetS-MSCs.
miRNA Expression Analysis
We considered miRNAs with fold-change (MetS-MSCs/Lean-MSCs) >1.4 (p < 0.05) to be upregulated, and those with fold-change <0.7 (p < 0.05) as downregulated. TargetScan (Release 7.1, http://www.targetscan.org/vert_71) was used to predict the target genes of the MiRNA. Functional annotation clustering and pathway analysis was performed using DAVID 6.7 database (https://david-d.ncifcrf.gov/).
Validation of miRNASeq Analysis
For validation, representative miRNAs expression of miR-196a, miR-27b, miR-212-5p, and miR-let-7c in Lean-MSCs and MetS-MSCs was measured by quantitative polymerase chain reaction (qPCR) with GAPDH as reference gene. Total RNA was isolated from 5 × 105–1 × 106 MSC samples, as previously described5. All primers were from ThermoFisher Scientific, Minneapolis, MN, USA.
MSCs Senescence
MSCs senescence was evaluated by the expression of the DNA damage marker H2AX (1:200; Abcam, Cambridge, UK) using Western blot, while SA-ß-GAL activity of MSCs was measured using a Cellular Senescence Activity Assay kit (Enzo Life Sciences, Farmingdale, NY, USA) following the vendor’s protocol. p21 and p16 immunofluorescent staining was performed following standard protocols20, using primary antibodies: p16 (ab118459), mouse monoclonal (cell line JC8), Santa Cruz (SC-56330, Santa Cruz, CA, USA); p21, rabbit polyclonal (c19), Santa Cruz (SC-397). The percentage of the positively stained area was quantified using ZEN® 2012 blue edition (ZEISS, Munich, Germany).
Reagents and Transfections
The miR-27 inhibitor (anti-miR-27) and the control anti-miR-negative control (NC) were synthesized by Ambion (Life Technologies, Grand Island, NY, USA). MetS-MSCs were incubated with miR-27b inhibitor or anti-miR-NC, and a transfection agent (Lipofectamine RNAiMAX; Invitrogen; Life Technologies, Grand Island, NY, USA) for 48 h, following the manufacturer’s instructions. Gene expression of p16 (CDKN2A) and p21 was detected by qPCR in Lean-MSCs, MetS-MSCs+ anti-miR-NC, and MetS-MSCs + anti-miR-27. Because functional annotation clustering analysis attributed an important role for ERK2/MAPK3 in cellular senescence, we also measured the expression of MAPK3 and MAPK14. To establish the contribution of MetS-induced miRNA modulation to MSC function, we assessed cell migration in Lean- and MetS-MSCs untreated or incubated with anti-miR-27 using a chamber cell migration assay (QCM 24-well colorimetric cell migration assay ECM508, Millipore, Billerica, MA, USA)23. In brief, 300,000 pretreated cells with and without inhibitor were seeded into migration chambers utilizing an 8 μM polycarbonate membrane, and incubated for 16 h at 37°C and 5% CO2. Cells that migrated through the polycarbonate membrane were incubated with cell stain solution, and subsequently extracted and detected on a standard microplate reader (560 nm).
Statistical Analysis
Statistical analysis was performed using JMP 10.0 (SAS Institute, Cary, NC, USA). Data were expressed as mean ± standard deviation. Unpaired Student’s t-test was used to evaluate statistically significant differences between the Lean and MetS groups. Statistical significance was accepted if p < 0.05.
Results
Systemic Characteristics
After 16 weeks of diet, MetS pigs had greater body weight, hypertension, and elevated cholesterol levels (systemic total cholesterol, triglycerides, and LDL) compared with Lean pigs (Table 1). Fasting glucose levels were unchanged, yet fasting insulin levels and HOMA-IR score were higher in MetS compared with Lean pigs, indicating the development of insulin resistance.
Table 1.
Systemic Characteristics of Lean and Metabolic Syndrome (MetS) Pigs (n = 6 each).
| Parameter | Lean | MetS |
|---|---|---|
| Body weight (kg) | 68.0±9.8 | 92.1±2.3* |
| Mean blood pressure (mmHg) | 95.6±12.3 | 128.2±9.7* |
| Heart rate (beats/min) | 75.1±2.6 | 74.9±2.4 |
| Fasting glucose (mg/dl) | 119.4±18.5 | 105.5±10.5 |
| Fasting insulin (µU/ml) | 0.4±0.1 | 0.8±0.1* |
| HOMA-IR score | 0.6±0.1 | 1.9±0.1* |
| Total cholesterol (mg/dl) | 81.4±7.4 | 483.1±30.9* |
| LDL cholesterol (mg/dl) | 31.5±6.8 | 308.0±123.6* |
| Triglycerides (mg/dl) | 6.9±1.8 | 18.3±6.8* |
*p ≤ 0.05 vs. Lean.
HOMA-IR: homeostasis model assessment of insulin resistance; LDL: low-density lipoprotein.
MetS-MSCs miRNAs and Targeted Senescence-associated Gene Regulation
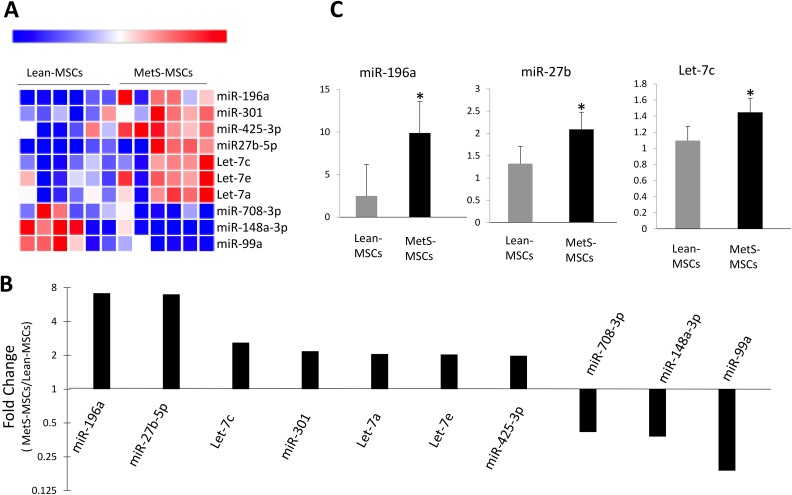
We identified with RNA-seq a total of 413 miRNAs in pig MSCs, of which seven miRNAs were upregulated and three downregulated in MetS-MSCs compared with Lean-MSCs (Fig. 1A). In order of fold-change (MetS- vs. Lean-MSCs), miR-196a(7.14), miR-27b(6.97), let-7c(2.59), miR-301(2.17), let-7a(2.03), let-7e(2.03), and miR-425-3p(1.98) were found to be upregulated in MetS-MSC, whereas miR708-3p(0.45), miR-148a-3p(0.37), and miR-99a(0.18) were downregulated (Fig. 1B). Expression patterns of miR-196a, miR-27b, and let-7c were subsequently also confirmed by qPCR (Fig. 1C).
Figure 1.
MicroRNAs (miRNAs) profile in Lean-MSCs and MetS-MSCs. (A) Heat map of miRNAs; seven were upregulated and three downregulated in MetS-MSCs compared with Lean-MSCs. (B) Fold-change (MetS- vs. Lean-MSCs) of the significantly different miRNA. (C) Quantitative polymerase chain reaction of candidate miRNAs followed the same patterns as miRNA-seq. miR-196a, miR-301, and miR-27ba were higher in MetS-MSCs compared with Lean-MSCs.
*p < 0.05 vs. Lean-MSCs.
Lean-MSC: Lean diet mesenchymal stem cell; MetS-MSC: metabolic syndrome mesenchymal stem cell.
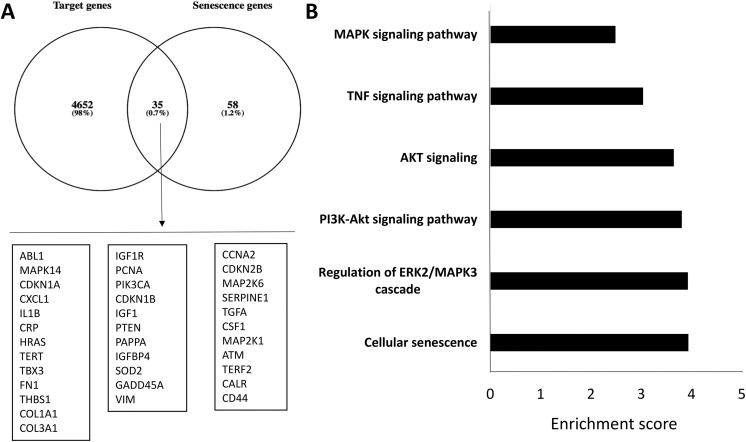
Altogether, these differentially expressed miRNAs in MetS-MSCs target 4687 genes, of which 35 are related to aging and/or senescence (Fig. 2A). Functional annotation clustering and pathway analysis of these 35 genes disclosed their association with cellular senescence, regulation of the ERK1/MAPK-3 cascade, as well as the P13K-Akt, AKT, tumor necrosis factor (TNF), and MAPK signaling pathways (Fig. 2B), which are clustered with SA genes. Table S1 in the Supplementary Material online details the 35 SA targeted genes. Interestingly, CDKN1A (encoding p21) and CDKN2B (encoding p15) were included among genes targeted by miRNAs.
Figure 2.
Targeted genes list of up- and downregulated microRNAs (miRNAs) in MetS-MSCs and functional pathway analysis. (A) Venn diagram showing distribution of miRNA target genes. Of 4687 target genes, 35 genes were related to senescence. (B) Enrichment of functional pathway of the 35 miRNA-targeted senescence genes using DAVID6.7.
MetS-MSC: metabolic syndrome mesenchymal stem cell.
Cellular Senescence in Lean- and MetS-MSCs
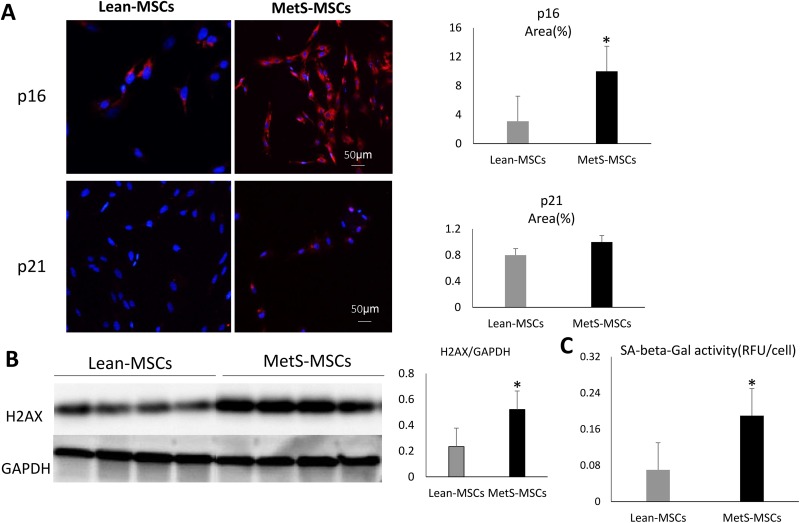
Compared with Lean-MSCs, MetS-MSCs showed significantly increased H2AX expression and SA-ß-GAL activity (Fig. 3), suggesting increased senescence. Immunofluorescent p16 staining was significantly elevated in MetS-MSCs (Fig. 3), whereas p21 was not, possibly because the upregulated let-7a in MetS-MSCs targets CDKN1A (encoding p21).
Figure 3.
Senescence in Lean- and MetS-MSCs. (A) Immunofluorescent staining of p16 and p21, showing significantly elevated p16 staining in MetS-MSCs, but not p21. (B) H2AX protein expression by Western-blot significantly increased in MetS-MSCs. (C) SA-ß-GAL activity was also higher in MetS-MSCs compared with Lean-MSCs.
*p < 0.05 vs. Lean-MSCs.
Lean-MSC: Lean diet mesenchymal stem cell; MetS-MSC: metabolic syndrome mesenchymal stem cell; SA-ß-GAL: senescence-associated β-galactosidase; H2AX: H2A Histone Family Member X; RFU: relative fluorescence unit; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
MiR-27b Inhibition in MetS-MSCs
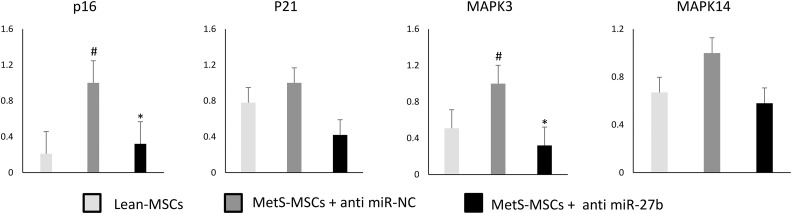
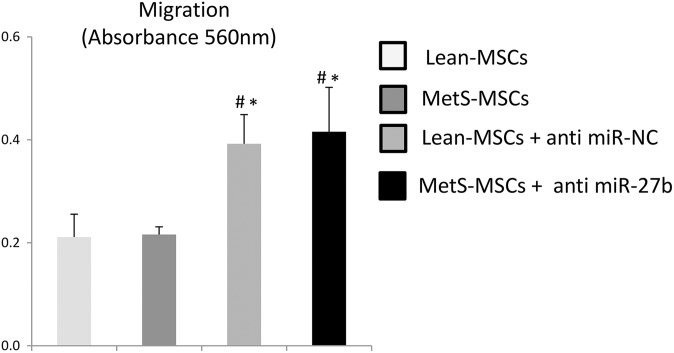
In order to validate the effect of miRNAs on cellular senescence, we used a miR-27b inhibitor to knock down its expression in MetS-MSCs. Compared with Lean-MSCs, MetS-MSCs+anti-miR-NC showed significantly higher p16 expression, which markedly decreased after inhibition of miR-27b; CDKN1A (p21) expression in the three groups remained unchanged. Notably, expression of MAPK3, but not MAPK14, was upregulated in MetS-MSCs+anti miR-NC vs. Lean-MSCs, and significantly decreased after miR-27b inhibition, suggesting that miR-27b may modulate cellular senescence through the ERK2/MAPK3 cascade (Fig. 4). Lastly, cell migration did not differ between Lean- and MetS-MSCs, but significantly increased in both groups after miR-27b inhibition (Fig. 5).
Figure 4.
MiR-27b inhibition and senescence. After inhibition of miR-27b in MetS-MSCs, the expression of p16 was significantly downregulated, whereas CDKN1A (p21) was not. MAPK3 was also downregulated after miR-27b inhibition.
#p < 0.05 vs. Lean-MSCs.
*p < 0.05 vs. MetS-MSCs+anti miR-NC.
Lean-MSC: Lean diet mesenchymal stem cell; MetS-MSC: metabolic syndrome mesenchymal stem cell; MAPK: mitogen-activated protein kinase.
Figure 5.
MiR-27b Inhibition and cell migration. Cell migration did not differ between Lean- and MetS-MSCs, but significantly increased in both groups after miR-27b inhibition.
#p < 0.05 vs. Lean-MSCs.
*p < 0.05 vs. MetS-MSCs.
Lean-MSC: Lean diet mesenchymal stem cell; MetS-MSC: metabolic syndrome mesenchymal stem cell.
Discussion
This study shows porcine distinct differences between miRNAs in adipose tissue-derived MSCs in MetS compared with Lean pigs. These differences include seven upregulated and three downregulated miRNAs in MetS which regulate 35 senescence-associated genes. Indeed, MetS MSCs showed increased p16-related senescence, which was attenuated after inhibition of miR-27b, in association with downregulation of MARP3 gene expression. These observations suggest that MetS dysregulates expression of miRNAs that target prominent senescence-associated signaling pathways, which might in turn impair MSC functionality and their impact on other cells.
The two major senescence-regulatory pathways, p53/p21 and pRB/p16, establish and maintain replicative and premature senescence. A hallmark feature of senescence is SASP, whereby senescent cells secrete cytokines and inflammatory mediators10. Accelerated senescence has been previously observed in kidneys of subjects with MetS or obesity. Kidney tubular p16INK4A is directly associated with body mass index, and its expression increases under high glucose condition24. Similarly, premature cardiac senescence in MetS rats is linked to p53 and p21 genes25. However, less is known about senescence in adipose tissue-derived MSCs in MetS.
The current study extends our previous observation4 of increased senescence in MetS-MSCs, and demonstrates a miRNA spectrum that may be involved in regulation of this process. Senescence biomarkers, especially p16, were elevated in MetS-MSCs, as were SA-ß-GAL activity and H2AX protein expression. Targeted genes identified by miRNAs pathway analysis highlight regulation of the ERK2/MAPK3 cascade, and signaling pathways of P13K-Akt, AKT, TNF, and MAPK as the major senescence pathways targeted by miRNAs in MetS-MSCs. To confirm their role in regulation of senescence, we inhibited one of these miRNAs, miR-27b. This maneuver elicited a fall in the expression of p16 and downregulation of MAPK3, which is involved in cellular processes including proliferation, differentiation, and cell cycle progression, implicating miRNAs in regulation of cellular senescence in MetS-MSCs.
Several miRNAs are implicated in promoting or suppressing senescence via specific pathways10, and by targeting various transcription factors. For example, miR-29 can reduce production of the transcription factor B-MYB, thereby enhancing senescence, and miR-24 prompts p16/INK4A mRNA translation, leading to a rise in p16 levels26. The miR-183 is related to oxidative stress-induced senescence by increasing production of the SASP factor and senescence regulator integrin-β127. Importantly, miR-27b regulates white and brown adipose tissue differentiation and function28,29, inhibits vascular formation in tumors30, and upregulates transforming growth factor-ß31, but its role in MSC senescence was unknown. This study shows that miR-27 is involved in MSCs senescence in MetS.
We observed in MetS-MSC a markedly elevated expression of p16, but not p21, suggesting diverse regulation of the SA pathway in MSC. Similar observations were reported in non-tumorigenic immortalized cell lines that lack functional p16 or p21 protein32,33. This might be partly due to differences in methylation levels of p16 and p21 encoding genes34. Furthermore, besides the association of upregulated p16 with evident MetS-MSC senescence, inhibition of the enriched miR-27b induced a threefold decrease in p16Ink4a expression. These observations support the postulated involvement of the Rb/p16 pathway in MetS-MSCs senescence.
ERK1 and ERK2 (also known as MAPK3 and MAPK1, respectively) are active in the MAPK cascade downstream to Ras-induced senescence35, and its activation is essential for both the initial phase of cellular proliferation as well as subsequent premature senescence36. Ras is the upstream factor to convey cell-surface signals to the nucleus via nuclear translocation of ERK1 and ERK2. In our pathway analysis, the SA genes HRAS (GTP Binding Protein H-Ras), Interleukin-1ß, MAP2K1 (MAPK/ERK Kinase-1), MAP2K6 (MAPK/ERK Kinase-6), and GADD45A (Growth Arrest and DNA Damage Inducible-Alpha), were targeted by miRNAs involved in regulation of the ERK2/ERK1cascade. The significant downregulation of MARP3 after miR-27b inhibition underscores its potential role in MSC senescence. Contrarily, other MAPK subgroups, such as p38 (MAPK14), were not modulated by miRNAs upregulated in MetS-MSCs, suggesting that MetS preferentially targets the classic ERK2/ERK1 pathway in porcine adipose tissue-derived MSCs. Cell migration did not differ between Lean- and MetS-MSCs, possibly due to the relatively early phase of MetS, but significantly increased in both groups after miR-27b inhibition. These observations suggest that miR-27b modulation of cellular senescence may interfere with MSC function, and potentially limit their therapeutic efficacy. Whether MSC preconditioning with miRNA modulation may improve the in vivo reparative capacity of these cells warrants further investigation.
Finally, we also observed activation of other pathways including: P13K-Akt, AKT, TNF, and MAPK signaling pathways. Both PI3K/Akt and AKT signaling serve an important role in cell aging, and their continuous activation induces growth arrest and senescence36. TNF signaling is related to reactive oxygen species production inducing aging cascades37. MAPK signaling is a common important element associated with all the other putative signaling pathways to induce cellular senescence. Taken together, our findings indicate that dysregulated miRNAs play a key role in modulating cellular senescence.
Our sample size was relatively small and the duration of MetS short, yet we observed clinical parameters consistent with MetS in our pig model. We used the third passage of MSCs for RNA-Seq, because at late passages MSC may become senescent38, whereas early-passage MSCs are genomically more stable in vitro39. We selected to inhibit a representative miRNA upregulated in MetS-MSCs, miR-27b, to highlight the potential role of miRNA in MetS-MSCs senescence. Similarly, we assessed the relationship between the ERK2/MAPK3 cascade and miR-27b by studying the representative MAPK3 and MAPK14. It would be worthwhile to explore in future studies the interaction of miR-27b with ERK2/MAPK3 to provoke MetS-MSCs senescence. Furthermore, the distinct roles of the p16 and p21 pathways in MetS-MSCs senescence also warrant future studies.
Using next-generation sequencing analysis, we identified differential miRNA expression signatures in adipose tissue-derived MSCs in porcine MetS- compared with Lean-MSC, and subsequently demonstrated their role in cell senescence. Elevated p16-dependent senescence in MetS-MSCs is associated with dysregulated expression of miRNAs that target prominent SA signaling pathways, particularly ERK2/MAPK3. Further studies are needed to explore in detail genes and molecules that regulate these pathways and contribute to the senescence burden in MetS-MSCs.
Supplemental Material
Supplemental Material, Table_S1 for Micro-RNAS Regulate Metabolic Syndrome-induced Senescence in Porcine Adipose Tissue-derived Mesenchymal Stem Cells through the P16/MAPK Pathway by Y. Meng, A. Eirin, X.-Y. Zhu, H. Tang, L.J. Hickson, A. Lerman, A.J. van Wijnen, and L.O. Lerman in Cell Transplantation
Acknowledgements
All authors have read the journal’s authorship agreement and that the manuscript has been reviewed by and approved by all named authors.
Ethical Approval: Animal studies were approved by the Institutional Animal Care and Use Committee.
Statement of Human and Animal Rights: Animal experiments conformed to the NIH guidelines (Guide for the Care and Use of Laboratory Animals).
Statement of Informed Consent: Statement of Informed Consent is not applicable for the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partly supported by NIH grant numbers DK104273, HL123160, DK102325, DK106427, and DK109134, the Central Society of Clinical and Translational Research, and Mayo Clinic: Mary Kathryn and Michael B. Panitch Career Development Award. We also acknowledge the generous philanthropic support of William and Karen Eby.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ahn SY, Chang YS, Sung DK, Sung SI, Ahn JY, Park WS. Pivotal role of brain-derived neurotrophic factor secreted by mesenchymal stem cells in severe intraventricular hemorrhage in newborn rats. Cell Transplant. 2017;26(1):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, Zhang ZG, Chopp M. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from MicroRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant. 2017;26(2):243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker N, Boyette LB, Tuan RS. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. 2015;70:37–47. [DOI] [PubMed] [Google Scholar]
- 4. Zhu XY, Ma S, Eirin A, Woollard JR, Hickson LJ, Sun D, Lerman A, Lerman LO. Functional plasticity of adipose-derived stromal cells during development of obesity. Stem Cells Transl Med. 2016;5(7):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meng Y, Eirin A, Zhu XY, Tang H, Chanana P, Lerman A, Van Wijnen AJ, Lerman LO. The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells. Cytometry A. 2017;93(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marycz K, Kornicka K, Basinska K, Czyrek A. Equine metabolic syndrome affects viability, senescence, and stress factors of equine adipose-derived mesenchymal stromal stem cells: new insight into EqASCs isolated from EMS horses in the context of their aging. Oxid Med Cell Longev. 2016;2016:4710326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu Y, Xiong K, Shi J, Cui Q, Xue L. A potential role of microRNAs in protein accumulation in cellular senescence analyzed by bioinformatics. PLoS One. 2017;12(6):e0179034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192(1):1–15. [DOI] [PubMed] [Google Scholar]
- 10. Abdelmohsen K, Gorospe M. Noncoding RNA control of cellular senescence. Wiley Interdiscip Rev RNA. 2015;6(6):615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keshet Y, Seger R. The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–38. [DOI] [PubMed] [Google Scholar]
- 12. Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773(8):1299–1310. [DOI] [PubMed] [Google Scholar]
- 13. Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26(22):3227–3239. [DOI] [PubMed] [Google Scholar]
- 14. Cao Z, Chen L. Inhibition of miR-27a suppresses the inflammatory response via the p38/MAPK pathway in intervertebral disc cells. Exp Ther Med. 2017;14(5):4572–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY). 2016;8(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pawar AS, Zhu XY, Eirin A, Tang H, Jordan KL, Woollard JR, Lerman A, Lerman LO. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity (Silver Spring). 2015;23(2):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24(10):1854–1859. [DOI] [PubMed] [Google Scholar]
- 18. Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res. 2014;103(4):461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eirin A, Zhang X, Zhu XY, Tang H, Jordan KL, Grande JP, Dietz AB, Lerman A, Textor SC, Lerman LO. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol Dial Transplant. 2014;29(2):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eirin A, Zhu XY, Ebrahimi B, Krier JD, Riester SM, van Wijnen AJ, Lerman A, Lerman LO. Intrarenal delivery of mesenchymal stem cells and endothelial progenitor cells attenuates hypertensive cardiomyopathy in experimental renovascular hypertension. Cell Transplant. 2015;24(10):2041–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun Z, Evans J, Bhagwate A, Middha S, Bockol M, Yan H, Kocher JP. CAP-miRSeq: a comprehensive analysis pipeline for microRNA sequencing data. BMC Genomics. 2014;15:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Z, Herrmann SM, Zhu X, Jordan KL, Gloviczki ML, Lerman A, Textor SC, Lerman LO. Preserved function of late-outgrowth endothelial cells in medically treated hypertensive patients under well-controlled conditions. Hypertension. 2014;64(4):808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F, Villaggio B, Gianiorio F, Tosetti F, Weiss U, Traverso P, Mji M, Deferrari G, Garibotto G. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295(5):F1563–F1573. [DOI] [PubMed] [Google Scholar]
- 25. Takahashi K, Takatsu M, Hattori T, Murase T, Ohura S, Takeshita Y, Watanabe S, Murohara T, Nagata K. Premature cardiac senescence in DahlS.Z-Lepr(fa)/Lepr(fa) rats as a new animal model of metabolic syndrome. Nagoya J Med Sci. 2014;76(1-2):35–49. [PMC free article] [PubMed] [Google Scholar]
- 26. Olivieri F, Rippo MR, Prattichizzo F, Babini L, Graciotti L, Recchioni R, Procopio AD. Toll like receptor signaling in “inflammaging”: microRNA as new players. Immun Ageing. 2013;10(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Targeting of integrin beta1 and kinesin 2alpha by microRNA 183. J Biol Chem. 2010;285(8):5461–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Price NL, Fernandez-Hernando C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim Biophys Acta. 2016;1861(12 Pt B):2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kong X, Yu J, Bi J, Qi H, Di W, Wu L, Wang L, Zha J, Lv S, Zhang F, Li Y, Hu F, Liu F, Zhou H, Liu J, Ding G. Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes. 2015;64(2):393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu H, Dowdle JA, Khurshid S, Sullivan NJ, Bertos N, Rambani K, Mair M, Daniel P, Wheeler E, Tang X, et al. Discovery of stromal regulatory networks that suppress ras-sensitized epithelial cell proliferation. Dev Cell. 2017;41(4):392–407e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki HI, Katsura A, Mihira H, Horie M, Saito A, Miyazono K. Regulation of TGF-beta-mediated endothelial-mesenchymal transition by microRNA-27. J Biochem. 2017;161(5):417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reznikoff CA, Yeager TR, Belair CD, Savelieva E, Puthenveettil JA, Stadler WM. Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res. 1996;56(13):2886–2890. [PubMed] [Google Scholar]
- 33. Boucher MJ, Jean D, Vezina A, Rivard N. Dual role of MEK/ERK signaling in senescence and transformation of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G736–G746. [DOI] [PubMed] [Google Scholar]
- 34. Guan RJ, Fu Y, Holt PR, Pardee AB. Association of K-ras mutations with p16 methylation in human colon cancer. Gastroenterology. 1999;116(5):1063–1071. [DOI] [PubMed] [Google Scholar]
- 35. Shin J, Yang J, Lee JC, Baek KH. Depletion of ERK2 but not ERK1 abrogates oncogenic Ras-induced senescence. Cell Signal. 2013;25(12):2540–2547. [DOI] [PubMed] [Google Scholar]
- 36. Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, McBryan T, Doyle B, McKay C, Oien KA, Enders GH, Zhang R, Sansom OJ, Adams PD. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell. 2011;42(1):36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi JW, Lee J, Park YI. 7,8-Dihydroxyflavone attenuates TNF-alpha-induced skin aging in Hs68 human dermal fibroblast cells via down-regulation of the MAPKs/Akt signaling pathways. Biomed Pharmacother. 2017;95:1580–1587. [DOI] [PubMed] [Google Scholar]
- 38. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noer A, Sorensen AL, Boquest AC, Collas P. Stable CpG hypomethylation of adipogenic promoters in freshly isolated, cultured, and differentiated mesenchymal stem cells from adipose tissue. Mol Biol Cell. 2006;17(8):3543–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Table_S1 for Micro-RNAS Regulate Metabolic Syndrome-induced Senescence in Porcine Adipose Tissue-derived Mesenchymal Stem Cells through the P16/MAPK Pathway by Y. Meng, A. Eirin, X.-Y. Zhu, H. Tang, L.J. Hickson, A. Lerman, A.J. van Wijnen, and L.O. Lerman in Cell Transplantation