Abstract
Brain death (BD) is associated with a systemic inflammation leading to worse graft outcomes. This study aimed to compare plasma cytokine values between brain-dead and critically ill patients, including septic and non-septic controls, and evaluate cytokine release kinetics in BD. Sixteen brain-dead and 32 control patients (16 with and 16 without sepsis) were included. Plasma cytokines were measured by magnetic bead assay after the first clinical exam consistent with BD and every 6 hours thereafter, and at the time of study entry in the control group. The values for IL-8 and IFN-γ were higher in brain-dead and septic patients than in non-septic patients [IL-8: 80.3 (18.7–169.6) vs. 68.2 (22.4–359.4) vs. 16.4 (9.2–42.7) pg/mL; P = 0.006; IFN-γ: 2.8 (1.6-6.1) vs. 3.4 (1.2–9.0) vs. 0.5 (0.5–1.8) pg/mL; P = 0.012]. TNF showed a clear tendency to increase in brain-dead patients [2.7 (1.0–4.8) vs. 1.0 (1.0–5.6) vs. 1.0 (1.0–1.0) pg/mL; P = 0.051], and IL-6 values were higher in brain-dead patients than in non-septic controls [174.5 (104.9–692.5) vs. 13.2 (7.3–38.6) pg/mL; P = 0.002]. These differences remained even after excluding brain-dead patients who also had sepsis (n = 3). IL-1β and IL-10 values increased from baseline to time point 2 (∼6 hours later) [IL-1β: 5.39 (1.93–16.89) vs. 7.11 (1.93–29.13) pg/mL; P = 0.012; IL-10: 8.78 (3.62–16.49) vs. 15.73 (5.49–23.98) pg/mL; P = 0.009]. BD-induced and sepsis-induced plasma cytokine values were similarly high, and both were higher than the observed in non-septic critically ill patients.
Keywords: brain death, inflammation; cytokines, sepsis, critical illness
Background
Brain-dead donors are the main source of organs for transplantation1,2. Brain death (BD) causes acute and massive catecholamine release, leading to an inflammatory state characterized by cytokine upregulation3. Accumulating evidence suggests that BD-induced inflammatory activity adversely affects graft survival4–8.
Experimentally, renal tubules and glomeruli from rat models of BD have demonstrated increased expression of tumor necrosis factor (TNF), interleukin-1β (IL-1β) and interleukin-6 (IL-6) compared with controls9. In line with this, renal biopsies from brain-dead donors have shown higher cytokine values than biopsies from living donors10,11. Moreover, kidney grafts from HLA-mismatched living donors have higher survival rates than kidneys from HLA-matched brain-dead donors12.
Primary lung graft dysfunction is a common complication of lung transplantation that results from ischemia-reperfusion injury, a process that might also be triggered by the systemic inflammatory state of the donor13. A significant increase in interleukin-8 (IL-8) values in bronchoalveolar lavage fluid from brain-dead lung donors has been demonstrated and correlates with early graft dysfunction after lung transplant7. In addition, patients who died within 30 days of lung transplant had elevated IL-6 gene expression in biopsies prior to implantation14. Interestingly, myocardial TNF mRNA expression during organ retrieval predicted right ventricular dysfunction in heart recipients15,16. Furthermore, liver biopsies from brain-dead donors have shown higher CD4 and CD8 infiltration than biopsies from living donors17. In a previous study by our group, we observed an increase in IL-6 and TNF plasma values and upregulation of TNF mRNA expression in pancreatic tissue from brain-dead donors compared with control patients undergoing pancreatectomy due to malignant tumors, but the kinetics of cytokine release during BD was not evaluated18.
Brain-dead patients suffer a variety of insults, ranging from the lesion that has led to BD to shock, mechanical ventilation, cardiac arrest, and sepsis. All these injuries are frequently observed in other critically ill patients and may also trigger the inflammatory cascade19–21. To date, the contribution of critical illness to BD-associated inflammation has not been investigated. The present study was therefore designed to compare plasma cytokine values from brain-dead patients with those from critically ill patients, including septic and non-septic individuals, and evaluate the cytokine profile following BD in a time-dependent manner.
Materials and Methods
Brain-Dead Patients and Controls
The study protocol was approved by the ethics committee at Hospital de Clínicas de Porto Alegre. Informed consent was obtained from patients or their legal representatives. BD was assessed independently by two physicians and was based on the following criteria: coma with complete unresponsiveness, absence of brainstem reflexes, apnea test, and confirmatory test with absence of cerebral blood flow, according to Brazilian law22. From June 2013 to June 2015, brain-dead patients older than 18 years admitted to the intensive care unit (ICU) were prospectively included in the study after the first clinical examination consistent with BD. Controls were defined as critically ill patients without suspected BD admitted to the same ICU. For each brain-dead patient, two control patients were included: the first septic patient to the right and the first non-septic patient to the left of the case’s bed. Sepsis was defined as the presence of infection and organ dysfunction23. Blood samples were collected at study entry for all groups. For the BD group, additional blood samples were collected at 6 and 12 hours (h), for a total of three samples per patient. Control patients had blood samples collected only once, at study entry, as we considered that controls would have a steady inflammatory profile. Clinical and laboratory data were recorded for brain-dead and control patients.
Plasma Cytokine Quantification
Blood samples were immediately centrifuged at 1260 units of gravity (g) for 10 min at 4 degrees Celsius (°C) and plasma was stored at −80°C until analysis. All samples were analyzed at the same time after being thawed at room temperature and centrifuged at 1000 g for 10 min. Plasma values of TNF, IL-1β, IL-6, IL-8, interleukin-10 (IL-10), and interferon-γ (IFN-γ) were assessed by magnetic bead assay using the Human Magnetic Custom Luminex® Kit (Invitrogen Life Technologies, Carlsbad, USA) and the Luminex® 200™ magnetic bead plate reader (Luminex, Austin, USA) following the manufacturers’ instructions. A standard curve was generated by serial dilutions of the reconstituted standard. Samples and standards were incubated with mixed beads overnight at room temperature on an orbital shaker. Beads were washed and then incubated with a detection antibody at room temperature for 1 h and with streptavidin for 30 min. Beads were washed and resuspended, and the plate was subsequently analyzed on the Luminex® 200™ reader. The results were plotted as a function of fluorescence intensity. Mean fluorescence intensity (MFI) takes into account the number of fluorescent pixels within the scanned area. MFI values below the detection limit were assumed to be equal to the lowest value detected (0.91 pg/mL for TNF, 1.93 pg/mL for IL-1β, 0.36 pg/mL for IL-6, 2.87 pg/mL for IL-8, 1.17 pg/mL for IL-10, and 0.48 pg/mL for IFN-γ). MFI was then converted to picograms (pg)/mL based on the standard curve. All samples were analyzed in duplicate.
Statistical Analysis
Categorical variables were expressed as percentages. Data were expressed as mean and standard deviation (SD) if normally distributed. Variables with skewed distribution were expressed as median and interquartile range. Groups were compared using one-way analysis of variance with Tukey’s post-hoc test, Kruskal–Wallis test, or chi-square test as appropriate. Plasma cytokine levels from brain-dead patients were compared at different time points using generalized linear models. Sample size for this study was calculated considering a power of 80% and an α-error of 5% to detect a difference of at least one SD in TNF log18. Values were considered statistically significant if P<0.05. Statistical analyses were performed using SPSS, version 18.0 (Chicago, IL).
Results
Sixteen brain-dead patients and 32 controls (16 septic and 16 non-septic patients) were included in the study. The baseline characteristics of all patients are shown in Table 1. Stroke was the leading cause of BD (62.5%), followed by anoxic encephalopathy (12.5%). The most common sites of infection in septic patients were the lung (40.0%) and the abdomen (26.6%). Non-septic patients were admitted to the ICU due to pulmonary embolism (20.0%), hemorrhagic stroke (20.0%), cardiac arrest survivors (13.3%), elective cardiac surgery (13.3%), or other causes (33.4%). Age, sex, body mass index and illness severity, assessed by APACHE II score, did not differ significantly between groups. Length of ICU stay and duration of mechanical ventilation were similar between groups. As expected, brain-dead and septic patients required more vasopressor support than non-septic patients, and hypernatremia and hypothermia were more common in brain-dead patients than in both control groups.
Table 1.
Baseline Characteristics of Brain-Dead Patients and Controls.
| Brain dead (n = 16) |
Septic controls (n = 16) |
Non-septic controls (n = 16) |
P | |
|---|---|---|---|---|
| Age (yr) | 55 ± 9 | 48 ± 17 | 52 ± 21 | 0.515 |
| Men, n (%) | 6 (37.5) | 8 (50) | 5 (31.3) | 0.543 |
| APACHE II score | 21.8 ± 8 | 23.9 ± 8 | 19.8 ± 10 | 0.481 |
| BMI (kg/m2) † | 24 ± 2 | 26 ± 8 | 29 ± 8 | 0.192 |
| Time from ICU admission, (days) | 2 (1–7.7) | 4 (1–10) | 4 (2–10) | 0.762 |
| Ventilation support, (days) | 3.5 (1–7) | 2.5 (1–7) | 1 (0–10) | 0.388 |
| Vasopressor support, n (%) | 11 (68.8) | 9 (56.3) | 4 (25) | 0.039§ |
| Episode of cardiac arrest, n (%) | 4 (25) | 0 | 3 (18.8) | 0.132 |
| Use of steroids, n (%) | 6 (37.5) | 9 (56.3) | 3 (18.8) | 0.091 |
| Plasma sodium (mEq/L) | 152 ±6.7 | 140 ± 6.5 | 142 ± 4.3 | <0.001 |
| Body temperature (°C) | 35 (34.4–36.1) | 36.1 (35.1–36.7) | 36.2 (36–36.6) | 0.031* |
| Hemoglobin (g/dL) | 10 ± 2.2 | 9 ± 2 | 8.9 ± 1.8 | 0.291 |
| White blood count (per mm3 × 1000) | 11.8 (7–15) | 12.3 (6.5–17.6) | 9.8 (7–14.9) | 0.916 |
| Creatinine (mg/dL) | 1.13 (0.8–1.5) | 1.23 (0.6–1.7) | 0.76 (0.5–1.7) | 0.596 |
†BMI: body mass index. §Non-septic control patients are different from brain-dead and septic control patients. ¶Brain-dead and septic control patients and brain-dead and non-septic control patients are different. * Brain-dead and non-septic control patients are different.
BD Induces Inflammation Similar to that Observed in Sepsis
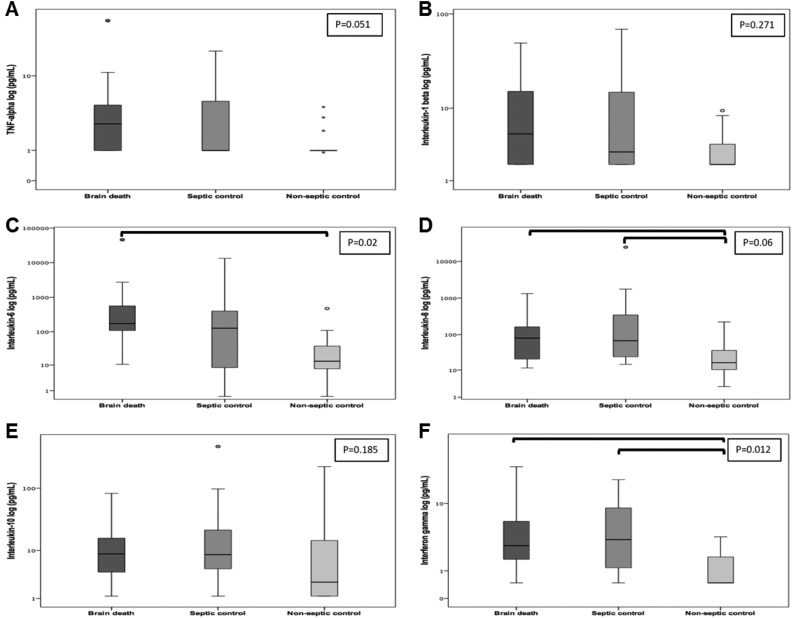
First, we examined the plasma cytokine profile of brain-dead and control patients at study entry (1.4 ± 1.3 h after the first clinical examination consistent with BD) and data are shown in Fig 1. The values of IL-8 were significantly higher in brain-dead and septic patients than in non-septic patients [80.3 (18.7–169.6) vs. 68.2 (22.4–359.4) vs. 16.4 (9.2–42.7) pg/mL; P = 0.006]. Similar findings were obtained for IFN-γ values, which were also higher in brain-dead and septic patients than in non-septic controls [2.8 (1.6–6.1) vs. 3.4 (1.2–9.0) vs. 0.5 (0.5–1.8) pg/mL; P = 0.012]. Regarding IL-6, plasma values were upregulated in brain-dead patients compared with non-septic control patients [174.5 (104.9–692.5) vs. 13.2 (7.3–38.6) pg/mL; P = 0.002], but not compared with septic patients [174.5 (104.9–692.5) vs. 134.7 (7.2–413.6) pg/mL; P = 0.524]. Conversely, there were no differences in IL-1β [5.4 (1.9–16.9) vs. 3.1 (1.9–15.0) vs. 1.9 (1.9–4.0) pg/mL; P = 0.271] and IL-10 values [8.8 (3.6–16.5) vs. 8.5 (4.3–22.9) vs. 3.0 (1.2–16.1) pg/mL; P = 0.185] between brain-dead, septic, and non-septic patients. TNF showed a clear tendency to increase in brain-dead patients compared with the other two groups [2.7 (1.0–4.8) vs. 1.0 (1.0–5.6) vs. 1.0 (1.0–1.0) pg/mL; P = 0.051].
Fig 1.
Plasma cytokine values determined by magnetic bead assay in brain-dead patients and controls. A. Tumor necrosis factor (pg/mL). B. Interleukin-1β (pg/mL). C. Interleukin-6 (pg/mL). D. Interleukin-8 (pg/mL). E. Interleukin-10 (pg/mL). F. Interferon-γ (pg/mL). Kruskal–Wallis with pairwise comparison. Statistically significant differences as indicated by the bars (IL-6: P = 0.01 for BD vs. non-septic controls; IL-8: P = 0.029 for BD vs. non-septic controls and P = 0.01 for septic vs. non-septic controls; IFN-γ: P = 0.028 for BD vs. non-septic controls and P = 0.031 for septic vs. non-septic controls). Graphs are plotted on a logarithmic scale, representing median and interquartile range. Dots and asterisks represent outliers.
Influence of Superimposed Sepsis on Cytokine Levels in Brain-Dead Patients
Three brain-dead patients (18.7%) had sepsis, all of pulmonary origin. In order to eliminate this potential confounder, septic brain-dead patients were excluded from the analysis. IL-6 values remained significantly higher in brain-dead patients than in non-septic control patients (P = 0.003), while IFN-γ levels remained higher in brain-dead and septic patients than in non-septic patients (P = 0.011). There were no differences in IL-1β (P = 0.281) and IL-10 (P = 0.213) values between groups. IL-8 values differed only between brain-dead and non-septic patients (P = 0.008). Remarkably, TNF values became significantly higher in brain-dead patients than in non-septic patients (P = 0.025).
Plasma Cytokine Release Kinetics over Time in Brain-Dead Patients
The time course of plasma cytokine levels during BD was then characterized, showing variation over time (Table 2). The levels of IL-1β and IL-10 increased from baseline (1.4 ± 1.3 h; first clinical examination consistent with BD; n = 16) to time point 2 (7 ± 1.2 h; second clinical examination consistent with BD; n = 15), but no significant differences were observed at time point 3 (14.7 ± 2.1 h; at the time of organ retrieval; n = 6). IFN-γ showed a non-significant increase in post-hoc analysis from time point 2 to time point 3 (P = 1.0), probably due to the reduced sample size.
Table 2.
Plasma Cytokines Kinetics in Brain-Dead Patients.
| Baseline (n = 16) |
Time point 2 (n = 15) |
Time point 3 (n = 6) |
P | |
|---|---|---|---|---|
| TNF (pg/mL) | 2.70 (0.99–4.76) | 3.28 (0.99–6.59) | 0.99 (0.99–5.95) | 0.278 |
| IL-1β (pg/mL) | 5.39 (1.93–16.89) | 7.11 (1.93–29.13) | 6.48 (1.93–19.21) | 0.012§ |
| IFN- γ (pg/mL) | 2.79 (1.6–6.06) | 2.79 (1.6–6.06) | 4.04 (0.47–8.57) | 0.045* |
| IL-6 (pg/mL) | 174.48 (104.9–692.51) | 223.74 (142.79–2664.93) | 395.77 (22.93–5594.31) | 0.096 |
| IL-8 (pg/mL) | 80.30 (18.72–169.61) | 68.24 (39.84–222.37) | 90.71 (26.43–241.29) | 0.391 |
| IL-10 (pg/mL) | 8.78 (3.62–16.49) | 15.73 (5.49–23.98) | 11.1 (1.17–15.23) | 0.009§ |
§Baseline and time point 2 are different. * P is non-significant in post-hoc analysis (P = 1.0).
Baseline refers to the first clinical examination consistent with BD (1.4 ± 1.3 h after study entry); time point 2 refers to the second clinical examination consistent with BD (7 ± 1.2 h after study entry); time point 3 refers to the time of organ retrieval (14.7 ± 2.1 h after study entry).
Because cytokines varied during the observation period, we therefore compared plasma cytokine levels between brain-dead patients at time point 2 and the control patients sample at baseline. The results showed that IL-8 and IFN-γ plasma values were significantly higher in brain-dead and septic patients than in non-septic control patients [IL-8: 68.2 (39.8–222.4) vs. 68.2 (22.4–359.4) vs. 16.4 (9.2–42.7) pg/mL; P = 0.003 and IFN-γ: 4.0 (1.8–14.3) vs. 3.4 (1.2–9.0) vs. 0.5 (0.5 -1.8) pg/mL; P = 0.001], similar to the results obtained at the baseline analysis, reported above. IL-6 also showed the same pattern observed at the baseline analysis, with higher values in brain-dead patients at time point 2 than in non-septic control patients [223.7 (142.8–2664.9) vs. 13.2 (7.3–38.6) pg/mL; P = 0.001], but both groups did not differ from the septic group. Notably, IL-10 and TNF values, which were not significantly different at baseline, became higher in brain-dead patients than in non-septic control patients at time point 2 [IL-10: 15.7 (5.5–24.0) vs. 3.0 (1.2–16.1) pg/mL; P = 0.047 and TNF: 3.3 (1.0–6.6) vs. 1.0 (1.0–1.0) pg/mL; P = 0.01]. Plasma IL-1β values were similar in all groups regardless of the time point of measurement.
Discussion
We presently show that BD is associated with higher systemic inflammation than that induced by critical illness without associated sepsis. Interestingly, cytokine values in BD were similar to those observed in sepsis. This is evidenced by upregulation of IL-6, IL-8, IL-10, IFN-γ, and TNF in brain-dead patients. In addition, we demonstrated an elevation in IL-1β and IL-10 values during the course of BD diagnosis.
Sepsis is a clinical syndrome classically associated with increased inflammation, with both pro- and anti-inflammatory cytokine upregulation24,25. Increased cytokine release is partially responsible for the clinical manifestations of sepsis and for organ dysfunction26. In addition, TNF, IL-6, and IL-10 are associated with organ failure and mortality27–30. Notably, in our study, the systemic inflammation in brain-dead patients was similar to that observed in septic patients. Moreover, even after excluding brain-dead patients with sepsis from the analysis, the two groups remained similar.
Other acute critical illnesses, such as trauma21, hemorrhagic shock31, and cardiac arrest32 may manifest as a systemic inflammatory response that contributes to multiple organ dysfunction syndrome. Adrie et al. investigated the inflammatory response of 61 successfully resuscitated out-of-hospital cardiac arrest patients compared with septic patients and healthy controls. Plasma values of IL-6, IL-8, and IL-10 were elevated on admission, with values as high as in patients with severe sepsis, and vasopressor use was associated with increased cytokine release in non-survivors33. Mechanical ventilation has also been shown to trigger pulmonary and systemic inflammation with enhanced values of TNF, IL-1β, and IL-6 in plasma and bronchoalveolar lavage even in normal lungs34–38. In our study, cytokines, except for IL-1β, were higher in brain-dead patients than in non-septic control patients, although these two groups of patients did not differ in terms of occurrence of cardiac arrest, disease severity (as assessed by APACHE II score), or duration of mechanical ventilation; however, as expected, brain-dead patients more frequently required vasopressor support. Taken together, these findings suggest that systemic inflammatory response in BD is triggered mainly by BD itself rather than by other injuries that develop during critical illness.
Inflammation secondary to BD is partially responsible for primary graft dysfunction and induced by the innate and adaptive immune systems39. Cytokines participate in both responses and are released in reaction to injury and infection, orchestrating the inflammatory response by acting on immune cell differentiation, proliferation, and activity. Increased values of IL-6 have been consistently found in brain-dead patients6,8,18,40–42. Our findings are consistent with the presence of systemic inflammation in BD and shed light on the cytokine profile and kinetics during BD. Interestingly, we identified increased plasma values not only of IL-6 but also of IL-8, IFN-γ, IL-10, and TNF in brain-dead patients compared with critically ill patients without sepsis. IL-10 and TNF values were elevated at time point 2 (around the time of confirmation of BD), which is consistent with the period in which TNF was upregulated in our previous study18. However, these results should be interpreted with caution, since these cytokines were similar among groups when baseline results were considered and were statistically elevated in BD patients only at time point 2. The importance of inflammation in transplant outcomes has been demonstrated in a recent study by our group43. In an experimental model of BD, rats treated with exendin-4, a glucagon-like peptide-1 (GLP-1) analog that has anti-inflammatory properties, showed reduced inflammation and increased pancreatic islet viability compared with brain-dead control animals43. In that study, pancreatic gene expression of IL-1β was elevated in brain-dead rats, but decreased with exendin-4 treatment. Thus, therapies to reduce inflammation in organ donors, such as pro-inflammatory cytokine blockade, appear to be a promising intervention in donors in order to improve recipient outcomes.
Studies performing a time-course analysis of cytokine expression have shown increased cytokine levels at the time of BD diagnosis13,39,40,44. In humans, serial assessment of plasma IL-6 levels in patients with severe head injury showed values above the normal range on admission, but patients who developed BD had a marked increase in IL-6 at the time of BD confirmation compared with those who did not40. Supporting the hypothesis that inflammatory mediators peak before organ retrieval, Lopau et al. showed increased soluble interleukin-2 (IL-2) and TNF receptor values and IL-6 serum values at the time of BD diagnosis compared with controls undergoing neurosurgical intervention, followed by a drop after 6 h44. In the present study, cytokines increased from baseline to 6 h, which was approximately the time of BD confirmation.
This is the first study to compare plasma cytokine levels between brain-dead patients and critically ill patients, aiming to evaluate the role of sepsis and other intensive care situations in the inflammation promoted by BD. The main strengths of our study are the exclusion of septic brain-dead patients from analysis, eliminating a major confounder, and the collection of samples at the first clinical examination for BD, and then before BD confirmation. This approach allowed us to describe the kinetics of cytokine release from an early period, when we believed that the inflammatory response would be pronounced, until organ retrieval or circulatory arrest, when cytokine values may be decreasing.
This study has some limitations. First, the sample size was based on TNF values from a previous study18 and may be underpowered to detect smaller differences. Second, we did not collect samples for time-course analysis in controls, so the results for IL-10 and TNF should be interpreted with caution, warranting further studies to clarify this issue. Third, we had a significant loss to follow-up at time point 3 (due to the fact that only a few patients became effective organ donors), limiting a better evaluation of the kinetics of cytokine release later after BD diagnosis. In line with this, a time course with more time points of measurement, such as at 1 and 2 h in addition to 6 and 12 h, might have more consistently demonstrated plasma cytokine behavior.
Conclusions
In conclusion, our data suggest that BD-induced systemic inflammation is as high as that induced by sepsis, and both BD and sepsis are associated with higher inflammation than the observed in critical illness without associated sepsis. These findings support the hypothesis that BD itself triggers the inflammatory cascade and prompt clinical evaluation of therapeutic approaches to attenuate this response and improve graft outcomes.
Acknowledgments
We thank Patricia Koehler Dos Santos for her expert assistance in the cytokine analysis and Vania Naomi Hirakata for her expert statistical counseling. We would like to thank the FIPE (Fundo de Incentivo à Pesquisa) of Hospital de Clínicas de Porto Alegre for their support in manuscript publication.
Footnotes
Authors’ Contribution: PS participated in the study conception and design, data acquisition, analysis and interpretation of data, statistical analysis, drafting and revision of the manuscript. GC participated in data acquisition and analysis. JR participated in data acquisition and analysis. DC participated in the study conception and revision of the manuscript. CBL participated in the study conception and design, interpretation of data, statistical analysis and revision of the manuscript. THR participated in the study conception and design, data acquisition, interpretation of data and revision of the manuscript.
THR is the guarantor of this work and, as such, had full access to all data and takes responsibility for the integrity of the data and the accuracy of data analysis.
Ethical Approval: The study protocol was approved by the ethics committee at Hospital de Clínicas de Porto Alegre. Informed consent was obtained from patients or their legal representatives.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the ethics committee at Hospital de Clínicas de Porto Alegre (130142) approved protocols and according to the Declaration of Helsinki of 1975.
Statement of Informed Consent: Written informed consent was obtained from the patients or their legal representatives for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Fundo de Incentivo à Pesquisa e Ensino (FIPE) of Hospital de Clínicas de Porto Alegre and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS). CBL and DC receives a scholarship from Conselho Nacional de Desenvolvimento Cinetífico e Tecnológico (CNPq-PQ).
References
- 1. Nagata H, Matsumoto S, Okitsu T, Iwanaga Y, Noguchi H, Yonekawa Y, Kinukawa T, Shimizu T, Miyakawa S, Shiraki R, Hoshinaga K, Tanaka K. Procurement of the human pancreas for pancreatic islet transplantation from marginal cadaver donors. Transplantation. 2006;82(3):327–331. [DOI] [PubMed] [Google Scholar]
- 2. Kotloff RM, Blosser S, Fulda GJ, et al. Management of the potential organ donor in the ICU: society of critical care medicine/American college of chest physicians/association of organ procurement organizations consensus statement. Crit Care Med. 2015;43(6):1291–1325. [DOI] [PubMed] [Google Scholar]
- 3. Tjernberg J, Ekdahl KN, Lambris JD, Korsgren O, Nilsson B. Acute antibody-mediated complement activation mediates lysis of pancreatic islets cells and may cause tissue loss in clinical islet transplantation. Transplantation. 2008;85(8):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pratschke J, Wilhelm MJ, Kusaka M, Basker M, Cooper DK, Hancock WW, Tilney NL. Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation. 1999;67(3):343–348. [DOI] [PubMed] [Google Scholar]
- 5. Murugan R, Venkataraman R, Wahed AS, Elder M, Hergenroeder G, Carter M, Madden NJ, Powner D, Kellum JA; HIDonOR Study Investigators. Increased plasma interleukin-6 in donors is associated with lower recipient hospital-free survival after cadaveric organ transplantation. Crit Care Med. 2008;36(6):1810–1816. [DOI] [PubMed] [Google Scholar]
- 6. Plenz G, Eschert H, Erren M, Wichter T, Böhm M, Flesch M, Scheld HH, Deng MC. The interleukin-6/interleukin-6-receptor system is activated in donor hearts. J Am Coll Cardiol. 2002;39(9):1508–1512. [DOI] [PubMed] [Google Scholar]
- 7. Fisher AJ, Donnelly SC, Hirani N, Haslett C, Strieter RM, Dark JH, Corris PA. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med. 2001;163(1):259–265. [DOI] [PubMed] [Google Scholar]
- 8. Weiss S, Kotsch K, Francuski M, Reutzel-Selke A, Mantouvalou L, Klemz R, Kuecuek O, Jonas S, Wesslau C, Ulrich F, Pascher A, Volk HD, Tullius SG, Neuhaus P, Pratschke J. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7(6):1584–1593. [DOI] [PubMed] [Google Scholar]
- 9. Kusaka M, Pratschke J, Wilhelm MJ, Ziai F, Zandi-Nejad K, Mackenzie HS, Hancock WW, Tilney NL. Activation of inflammatory mediators in rat renal isografts by donor brain death. Transplantation. 2000;69(3):405–410. [DOI] [PubMed] [Google Scholar]
- 10. Schwarz C, Regele H, Steininger R, Hansmann C, Mayer G, Oberbauer R. The contribution of adhesion molecule expression in donor kidney biopsies to early allograft dysfunction. Transplantation. 2001;71(11):1666–1670. [DOI] [PubMed] [Google Scholar]
- 11. Nijboer WN, Schuurs TA, van der Hoeven JA, Leuvenink HG, van der Heide JJ, van Goor H, Ploeg RJ. Effects of brain death on stress and inflammatory response in the human donor kidney. Transplant Proc. 2005;37(1):367–369. [DOI] [PubMed] [Google Scholar]
- 12. Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333(6):333–336. [DOI] [PubMed] [Google Scholar]
- 13. Avlonitis VS, Wigfield CH, Golledge HD, Kirby JA, Dark JH. Early hemodynamic injury during donor brain death determines the severity of primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(1):83–90. [DOI] [PubMed] [Google Scholar]
- 14. Kaneda H, Gutierrez C, de Perrot M, et al. Pre-implantation multiple cytokine mRNA expression analysis in donor lung grafts predicts survival after lung transplantation in humans. J Heart Lung Transplant. 2004;23:S49–S50. [DOI] [PubMed] [Google Scholar]
- 15. Birks EJ, Burton PB, Owen V, Mullen AJ, Hunt D, Banner NR, Barton PJ, Yacoub MH. Elevated tumor necrosis factor-alpha and interleukin-6 in myocardium and serum of malfunctioning donor hearts. Circulation. 2000;102(19 suppl 3):III352–III358. [DOI] [PubMed] [Google Scholar]
- 16. Birks EJ, Owen VJ, Burton PB, Bishop AE, Banner NR, Khaghani A, Polak JM, Yacoub MH. Tumor necrosis factor-alpha is expressed in donor heart and predicts right ventricular failure after human heart transplantation. Circulation. 2000;102(3):326–331. [DOI] [PubMed] [Google Scholar]
- 17. Jassem W, Koo DD, Cerundolo L, Rela M, Heaton ND, Fuggle SV. Leukocyte infiltration and inflammatory antigen expression in cadaveric and living-donor livers before transplant. Transplantation. 2003;75(12):2001–2007. [DOI] [PubMed] [Google Scholar]
- 18. Rech TH, Crispim D, Rheinheimer J, Barkan SS, Osvaldt AB, Grezzana Filho TJ, Kruel CR, Martini J, Gross JL, Leitão CB. Brain death-induced inflammatory activity in human pancreatic tissue: a case-control study. Transplantation. 2014;97(2):212–219. [DOI] [PubMed] [Google Scholar]
- 19. van Wessem KJP, Heeres M, Leliefeld PHC, Koenderman L, Leenen LPH. Lipopolysaccharide and hemorrhagic shock cause systemic inflammation by different mechanisms. J Trauma Acute Care Surg. 2013;74(1):37–44. [DOI] [PubMed] [Google Scholar]
- 20. van Wessem KJ, Hennus MP, Heeres M, Koenderman L, Leenen LP. Mechanical ventilation is the determining factor in inducing an inflammatory response in a hemorrhagic shock model. J Surg Res. 2013;180(1):125–132. [DOI] [PubMed] [Google Scholar]
- 21. van Wessem KJ, Hennus MP, van Wagenberg L, Koenderman L, Leenen LP. Mechanical ventilation increases the inflammatory response induced by lung contusion. J Surg Res. 2013;183(1):377–384. [DOI] [PubMed] [Google Scholar]
- 22. Lei nº 9.434. Dispõe sobre a Remoção de Órgãos, Tecidos e Partes do Corpo Humano Para Fins de Transplante e Tratamento e dá Outras Providências. Lei nº 9.434; 1997. Available from: http://www.planalto.gov.br/Ccivil_03/LEIS/L9434.htm [Google Scholar]
- 23. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bernard AM, Bernard GR. The immune response: targets for the treatment of severe sepsis. Int J Inflam. 2012;2012:697592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown KA, Brown GA, Lewis SM, Beale R, Treacher DF. Targeting cytokines as a treatment for patients with sepsis: a lost cause or a strategy still worthy of pursuit? Int Immunopharmacol. 2016;36:291–299. [DOI] [PubMed] [Google Scholar]
- 26. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(21):840–851. [DOI] [PubMed] [Google Scholar]
- 27. Damas P, Reuter A, Gysen P, Demonty J, Lamy M, Franchimont P. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit Care Med. 1989;17(10):975–978. [DOI] [PubMed] [Google Scholar]
- 28. Pettila V, Hynninen M, Takkunen O, Kuusela P, Valtonen M. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002;28(9):1220–1225. [DOI] [PubMed] [Google Scholar]
- 29. Monneret G, Finck ME, Venet F, Debard AL, Bohé J, Bienvenu J, Lepape A. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett. 2004;95(2):193–198. [DOI] [PubMed] [Google Scholar]
- 30. Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(2):R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonnier DI, Makley AT, Friend LA, Bailey SR, Lentsch AB, Pritts TA. Hemorrhagic shock induces a proinflammatory milieu in the gut lumen. J Surg Res. 2011;170(2):272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10(3):208–212. [DOI] [PubMed] [Google Scholar]
- 33. Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106(5):562–568. [DOI] [PubMed] [Google Scholar]
- 34. Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):109–116. [DOI] [PubMed] [Google Scholar]
- 35. Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54–61. [DOI] [PubMed] [Google Scholar]
- 36. Uhlig U, Uhlig S. Ventilation-induced lung injury. Compr Physiol. 2011;1(2):635–661. [DOI] [PubMed] [Google Scholar]
- 37. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136. [DOI] [PubMed] [Google Scholar]
- 38. Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watts RP, Thom O, Fraser JF. Inflammatory signalling associated with brain dead organ donation: from brain injury to brain stem death and posttransplant ischaemia reperfusion injury. J Transplant. 2013;2013:521369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amado JA, López-Espadas F, Vázquez-Barquero A, Salas E, Riancho JA, López-Cordovilla JJ, García-Unzueta MT. Blood levels of cytokines in brain-dead patients: relationship with circulating hormones and acute-phase reactants. Metabolism. 1995;44(6):812–816. [DOI] [PubMed] [Google Scholar]
- 41. Kuecuek O, Mantouvalou L, Klemz R, Kotsch K, Volk HD, Jonas S, Wesslau C, Tullius S, Neuhaus P, Pratschke J. Significant reduction of proinflammatory cytokines by treatment of the brain-dead donor. Transplant Proc. 2005;37(1):387–388. [DOI] [PubMed] [Google Scholar]
- 42. Kaminska D, Tyran B, Mazanowska O, Rabczynski J, Szyber P, Patrzalek D, Chudoba P, Polak WG, Klinger M. Cytokine gene expression in kidney allograft biopsies after donor brain death and ischemia-reperfusion injury using in situ reverse-transcription polymerase chain reaction analysis. Transplantation. 2007;84(9):1118–1124. [DOI] [PubMed] [Google Scholar]
- 43. Carlessi R, Lemos NE, Dias AL, Oliveira FS, Brondani LA, Canani LH, Bauer AC, Leitão CB, Crispim D. Exendin-4 protects rat islets against loss of viability and function induced by brain death. Mol Cell Endocrinol. 2015;412:239–250. [DOI] [PubMed] [Google Scholar]
- 44. Lopau K, Mark J, Schramm L, Heidbreder E, Wanner C. Hormonal changes in brain death and immune activation in the donor. Transpl Int. 2000;13(suppl 1):S282–S285. [DOI] [PubMed] [Google Scholar]