Abstract
As a featured ocular inflammatory disease, autoimmune uveitis is the major cause of blindness in the clinic. Although current immunosuppressive regimens can alleviate the progression of autoimmune uveitis, they have serious side effects. Therefore, an alternative therapeutic strategy is urgently required. The present study investigated the therapeutic efficacy of human amniotic epithelial cells (hAECs) on autoimmune uveitis in a rat model. Herein, experimental autoimmune uveitis (EAU) was induced in rats via a subcutaneous injection of interphotoreceptor retinoid-binding protein. EAU rats were treated with hAECs or the vehicle solution via a subretinal injection on day 0 and day 6 after immunization, and rats were sacrificed on day 12 and day 18 for further analysis. The pathological development of EAU was evaluated by slit lamp microscopy. Immune cell infiltration and retinal structure damage were examined by histological examination of hematoxylin and eosin (H&E) and immunofluorescence staining. T-cell subsets were detected by flow cytometry, and the levels of inflammatory cytokines were quantified by enzyme-linked immunosorbent assay (ELISA). hAEC treatment ameliorated the pathological progression of EAU and preserved the retinal structure organization and thickness, especially in the preventive group that received a subretinal injection on day 0. Moreover, hAECs inhibited the retinal infiltration of macrophages and T-cells. Mechanistically, hAECs modulated the balance of T-cell subsets by downregulating T helper (Th)17 cells and upregulating T regulatory (Treg) cells, as confirmed by decreased interleukin (IL)-17 and increased IL-10 levels in the spleens and lymph nodes of EAU rats. Furthermore, hAECs improved the local cytokine environment in EAU rats by suppressing the monocyte chemoattractant protein (MCP)-1, IL-17 and interferon (IFN)-γ levels and enhancing the IL-10 in the aqueous humor. Therefore, subretinal transplantation of hAECs in EAU rats ameliorated ocular inflammation, preserved the retinal structure and coordinated the immune balance. The current study provides a novel therapeutic strategy for autoimmune uveitis and related ocular inflammatory diseases in the clinic.
Keywords: experimental autoimmune uveitis, human amniotic epithelial cells, cell therapy, immunomodulation
Introduction
Uveitis is an intraocular inflammatory disorder occurring in the uvea layer (iris, ciliary body, and choroid), resulting in visual impairment due to retinal damage. It is estimated that uveitis accounts for 2.8–10.0% of all cases of blindness or 30,000 new cases of blindness in the western world each year1–3. As the major type of noninfectious uveitis, autoimmune uveitis (AU) affects different age groups either as a local disease in the eye or as part of a systemic immune disorder with key pathological event of retinal structure damage and inflammation4–6. It usually has clinical features of eye redness, pain to light sensitivity, blurred vision or declining visual acuity according to the involvement of different uvea parts at different stages of disease progression4. In the clinic, AU is one of the common origins of numerous ocular inflammatory diseases, including sarcoidosis, sympathetic ophthalmia, birdshot retinochoroidopathy, and Vogt–Koyanagi–Harada’s disease7.
To date, immunosuppressive regimens are the mainstay of AU therapy, such as corticosteroids, a common first-line drug. Although alleviation of AU progression has been observed, patients receiving long-term use of corticosteroids suffer serious side effects, including cushingoid syndrome, diabetes, osteoporotic bones, and metabolic disturbances8. Recently, biologic agents targeting tumor necrosis factor (TNF)-α (infliximab, etanercept, and adalimumab), IL-2 receptors (daclizumab) and lymphocytes (rituximab and alemtuzumab) have been developed against the corticosteroid burden on patients during AU treatment. However, these drugs have also demonstrated obvious side effects, such as reactivation of latent tuberculosis and malignant diseases9. Therefore, the study of alternative therapeutic strategy is urgently required.
Human amniotic epithelial cells (hAECs) have been isolated from human amniotic membrane and have been drawing attention as a potential cell source for cell therapy, based on their several characterized features10,11. During embryonic development, hAECs are derived from the inner cell mass within the blastocyst, which ensures their plasticity comparable with stem cells10–12. Moreover, hAECs demonstrate low immunogenicity due to a low expression of major histocompatibility complex (MHC) II antigens (human leukocyte antigen (HLA)-DQ and HLA-DR) and co-stimulatory markers (CD80 and CD86) as well as a high expression of HLA-E and HLA-G13–15. hAECs show no tumorigenicity due to the lack of telomerase10,11. In addition, harvesting an abundant number of hAECs from amniotic membranes to fulfill clinical use is relatively easy. More importantly, recent studies have indicated anti-inflammation properties of hAECs in inhibiting immune cell activation, which may expand their therapeutic potential in inflammatory diseases14.
In the present study, we investigated the therapeutic effect of hAECs in the experimental autoimmune uveitis (EAU) model, which mimicked AU-mediated ocular inflammation in the clinic16. Subretinal injection of hAECs inhibited the pathological process of EAU and ameliorated ocular inflammation via regulating local and systemic immune responses.
Materials and Methods
Animals
Male Lewis rats (6−8 weeks old) were obtained from Vital River (Beijing, China) and housed under pathogen-free conditions with 12 h day-night cycle. All procedures involving rats were approved by the Laboratory Animal Care and the Use Committee of Zhejiang University.
EAU Induction and hAEC Treatment
The EAU model was established in rats by subcutaneous injection into two hind footpads with interphotoreceptor retinoid-binding protein (IRBP) 1169–1191 peptide (PTARSVGAADGSSWEGVGVVPDV; Sangon, Shanghai, China) at a dose of 35 µg per rat. The IRBP 1169–1191 peptide was emulsified at 1:1 (v/v) with complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO, USA) containing 2.5 mg/ml Mycobacterium tuberculosis H37RA (Sigma-Aldrich). To evaluate the therapeutic effect of hAECs on EAU, EAU rats were injected with hAECs on day 0 and day 6 after immunization (termed as preventive group and therapeutic group, respectively). EAU rats injected with a vehicle solution of balanced salt solution (BSS) at the same time points were set as control groups. 3×105 hAECs in 2 µl BSS or equal volume of BSS were injected into EAU rats by subretinal injection. Rats were sacrificed on day 12 and day 18 after immunization in different groups for further analysis.
hAEC Isolation and Culture
Human amniotic membranes were obtained with written and informed consent from healthy mothers undergoing Cesarean section. Human placentas were obtained from healthy mothers who provided written informed consent after uncomplicated elective Cesarean section. The procedure was approved by the Institutional Patients and Ethics Committee of the International Peace Maternity and Child Health Hospital, Shanghai Jiaotong University School of Medicine. All donors were negative for hepatitis A, B, C, and D as well as human immunodeficiency virus (HIV)-I and Treponema pallidum antibody (TPAB). hAECs were isolated from the collected placenta. In brief, the amniotic membrane was peeled from the placental chorion and washed in Hank’s balanced salt solution (HBSS, Thermo Scientific, MA, USA) to discard blood cells. The amniotic membrane was digested with 0.25% trypsin (ethylenediaminetetraacetic acid) for 30 min at 37°C in a water bath. Two volumes of complete culture medium (F12/Dulbecco’s modified Eagle’s medium containing 10% KnockOut Serum Replacement (KSR), 2 mM L-glutamine, 1% nonessential amino acid, 55 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 1% antibiotic-antimycotic (all from Thermo Scientific, Waltham, MA, USA) and 10 ng/ml epidermal growth factor (Peprotech, Rocky Hill, NJ, USA)) were added to the trypsin digestion medium, and the cell suspension was centrifuged for 10 min at 300×g. The cell pellet was then suspended in complete culture medium for cell culture. When cells reached 80–90% confluence, they were harvested with 0.25% trypsin and then resuspended in HBSS at a concentration of 1.5×105 cells/µl for injection.
Clinical Assessment of EAU
The inflammation and severity of EAU were examined with a slit lamp microscope starting from day 2 after immunization and daily thereafter to assess disease development. Clinical signs of inflammation were scored in a masked fashion as grades 0 to 4 as previously described in detail17. In brief, the grades were characterized as follows: grade 0 represents no disease (eyes reflect light and are translucent); grade 1 represents enlargement of the iris vessel and abnormal pupil contraction; grade 2 represents cellular infiltrates and hazy anterior chamber with decreased red reflex; grade 3 represents moderately opaque anterior chambers, still visible pupils, and dull red reflex; and grade 4 represents opaque anterior chambers, obscured pupils, and absence of red reflex. Rats were followed up until 20 days after immunization.
Histology
After aqueous humor collection, the eyeballs were fixed in 10% formaldehyde for 24 hours followed by dehydrating with 70% alcohol and embedding in paraffin according to a standard protocol. Sections (5 µm) were cut and stained with hematoxylin and eosin (H&E). For evaluating inflammatory cell invasion and retina damage, histopathological disease scores were assigned on a scale of 0 to 4 as previously described in detail18.
Half of the eyeballs were freshly embedded under an optimal cutting temperature and cut into 5 µm sections at the same time point for histological assessment to evaluate the severity of retinal infiltration of inflammatory cells. Sections were fixed in acetone for 10 min at −20°C and then washed with phosphate-buffered saline (PBS) followed by incubating with blocking buffer (1% bovine serum albumin and 5% horse serum (HS) in PBS) for 1 hour at room temperature. After blocking, sections were incubated with anti-CD3 (1:200 dilution, Abcam, Cambridge, MA, USA) and anti-CD68 (1:200 dilution, Abcam) primary antibodies overnight at 4°C. Sections were then washed with PBS and incubated with secondary antibodies for 1 hour at room temperature. Lastly, slides were mounted with mounting medium containing 4’,6-diamidino-2-phenylindole (DAPI) and then examined using a fluorescence microscope (EPI-800; Nikon, Tokyo, Japan).
Flow Cytometry
To measure the suppressive effect of hAECs on T-cell proportions in EAU, spleens and lymph nodes were collected. Lymphocytes were prepared using red blood lysis buffer and 200 µm filters. Cells were washed twice with PBS and resuspended in medium (Complete RPMI 1640) containing 2 µM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 10% fetal bovine serum (FBS) and 50 µM β-mercaptoethanol (all from Thermo Scientific, MA, USA). Cells were stained with fluorescein isothiocyanate (FITC)-anti-CD4, phycoerythrin (PE)-anti-CD25, PE-anti-interleukin (IL)-17, and allophycocyain (APC)-anti-FOXP3 followed by flow cytometric analysis (FACS Calibur; BD Biosciences, Franklin Lakes, NJ, USA). The ratios of IL-17+ T-cells/CD4+ T-cells and FOXP3+ T regulatory (Treg) cells/CD4+CD25+ cells were calculated in each group.
Cytokine Measurement
Single cell suspensions from spleens and lymph nodes of EAU rats were seeded into flat bottom 12-well microtiter plates (Corning, Corning, NY, USA) at a concentration of 2×106 cells/well in a final volume of 1000 µl/well. Cells were treated with 30 µg/ml IRBP peptide in medium supplemented with 10% FBS. Plates were incubated in a humidified atmosphere of 5.5% CO2 at 37°C for 3 days. IL-10 and IL-17 production levels were measured in supernatants by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. Moreover, interferon (IFN)-γ, IL-10, monocyte chemoattractant protein (MCP)-1 and IL-7 levels in the aqueous humor of EAU rats in different groups were measured using Luminex (Laizi Biotech, Shanghai, China).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad software, CA, USA). Data are presented as the mean ± standard error of the mean (SEM). Comparisons were performed using unpaired Student’s t test or two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. P-values were two-tailed, and values <0.05 were considered statistically significant.
Results
hAECs Restrain the Pathological Development of EAU
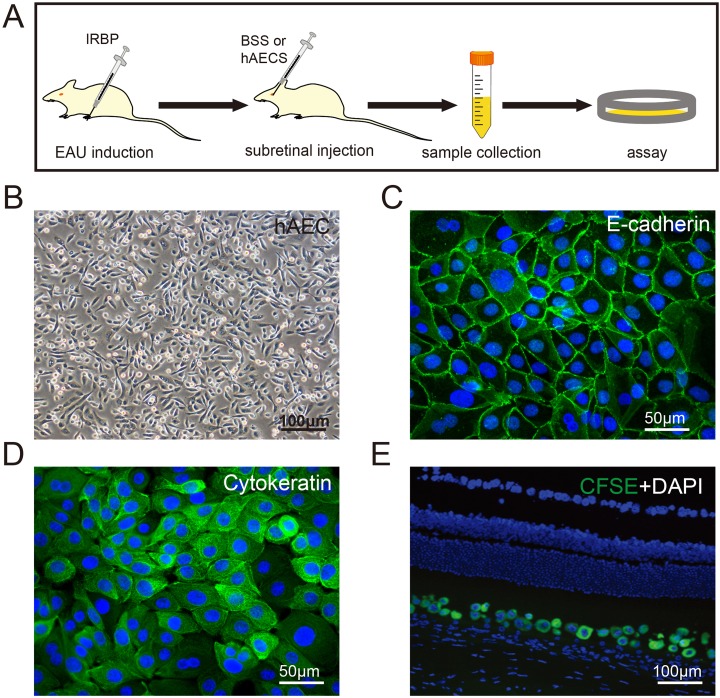
Corresponding to the symptom diagnosis in the clinic, the pathological development of EAU was evaluated by slit lamp microscopy (Fig. Supplement 1A). Early clinical signs were recorded from day 5 after immunization, including dilated blood vessels in the iris, hazy anterior chamber, and decreased red reflect. The pathological development reached a peak around day 12 as indicated by severe hypopyon, occlusion of pupil, and the loss of red reflect, and it then went into a stable progressive stage after day 16 (Fig. Supplement 1B). To investigate the role of hAEC treatment in both the onset and progression of EAU, subretinal injection of hAECs or the BSS vehicle was performed on day 0 or day 6 in rats followed by sacrifice for further analysis on day 12 and day 18, respectively, at two representative time points during the course of disease (Fig. 1A). The hAECs used in the present study were freshly isolated and cultured (Fig. 1B) with specific markers (Fig. 1C, D), and they were clearly tracked after subretinal injection (Fig. 1E).
Fig. 1.
Schematic of hAEC treatment in the EAU model. (A) EAU was induced by injection of interphotoreceptor retinoid-binding protein in Lewis rats. EAU rats were treated with hAECs by subretinal injection on day 0 and day 6 after immunization (termed as preventive group and therapeutic group, respectively). The vehicle solution (BSS) was injected in control groups. EAU rats in all the groups were sacrificed on day 12 and day 18 after immunization (the peak and stable stage of the disease, respectively), and tissues were harvested for further analysis. (B) Morphology of hAECs employed in the current study. Isolated hAECs showed a homogeneous population of cuboidal cells with diameters of 5–10 µm. (C, D) Immunostaining images displaying the high expression of epithelial markers of E-cadherin and cytokeratin in hAECs respectively. (E) Identification of GFP-labeled hAECs (green) in the subretinal area 7 days after injection.
Scale bar=100 µm in (B, E), 50 µm in (C, D).
BSS: balanced salt solution; EAU: experimental autoimmune uveitis; GFP: green fluorescent protein; hAEC: human amniotic epithelial cells.
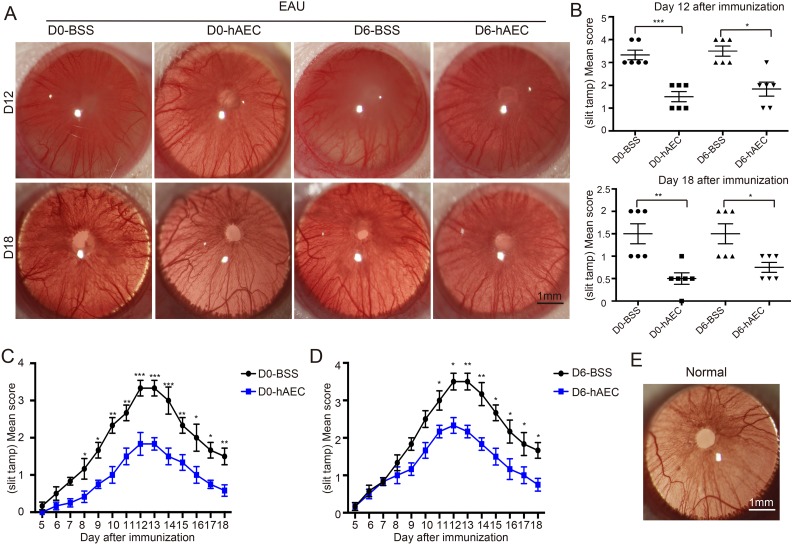
Compared with the BSS control group, hAEC treatment in the preventive group (hAEC injection on day 0) rescued ocular pathological symptoms. hAEC treatment resulted in only a few engorged blood vessels in the iris and mild turbidity of the aqueous humor on day 12, and it resulted in a normal pupil and clear aqueous humor on day 18 as a normal control (Fig. 2E). Significant alleviation occurred in the therapeutic group (hAEC injection on day 6) with several engorged blood vessels in the iris, decreased red reflex and moderate hypopyon on day 12, and slight turbidity of the aqueous humor on day 18 was observed (representative images in Fig. 2A with pathological quantification in 2B). The pathological evaluation of the EAU progression provided further evidential support (Fig. 2C, D).
Fig. 2.
hAEC treatment suppresses the pathological development in EAU rats. EAU rats were treated with hAECs or vehicle solution (BSS) by subretinal injection on day 0 and day 6 after immunization, and the pathological development of EAU was evaluated by slit lamp microscopy on day 12 and day 18 after immunization (the peak and stable stage of the disease, respectively). Representative slit lamp images are shown in (A), and corresponding pathological scores for individual rats are indicated by dots in (B). To further evaluate the effect of hAECs on the entire process of EAU, clinical signs in EAU rats were recorded daily starting on day 5 after immunization. The corresponding quantifications of pathological scores are shown in (C) for the groups with treatment on day 0 and in (D) for the groups with treatment on day 6 after immunization. Data are presented as mean ± SEM. n = 6 in each group. *p < 0.05; **p < 0.01; ***p < 0.001. Statistical analysis was performed using an unpaired Student’s t test (B) as well as a two-way ANOVA and Tukey’s multiple comparison test (C, D). A representative slit lamp image of a normal control is shown in (E).
Scale bar=1 mm.
ANOVA: analysis of variance; BSS: balanced salt solution; EAU: experimental autoimmune uveitis; hAEC: human amniotic epithelial cells; SEM: standard error of the mean.
hAECs Ameliorate Retinal Structure Damage
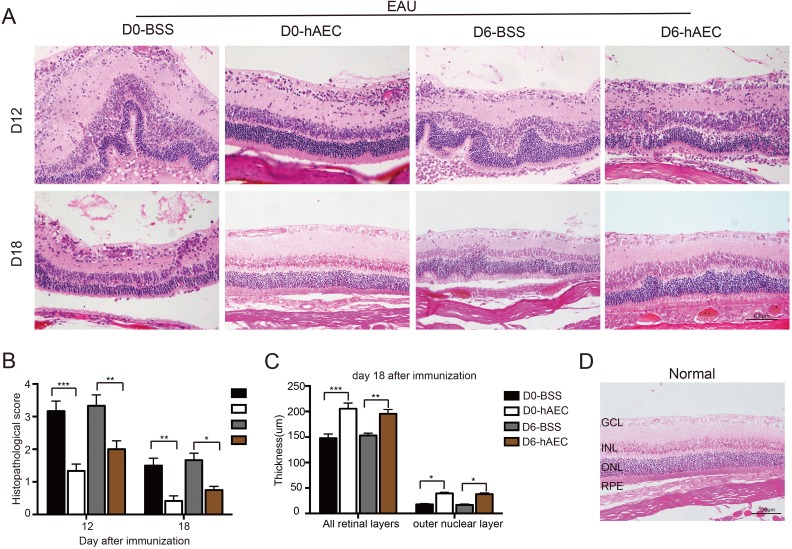
To investigate the effect of hAEC treatment on tissue injuries, retinal structure changes were analyzed by histological examination. Consistent with the pathological observation, the retinal structure in the control groups was severely damaged and disorganized with massive inflammatory cell infiltration into the vitreous cavity and retina around day 12, which corresponded to the peak of ocular inflammation of EAU (Fig. 3A with quantification in 3B). The progressive retinal injury further led to decreased retinal thickness around day 18 in control groups (Fig. 3C). In contrast, less severe retinal damage was observed in the therapeutic group, and even better improvement in the preventive group on day 12 (Fig. 3A with quantification in 3B). More importantly, hAEC injection preserved the retinal structure organization (Fig. 3A) and outer nuclear layer (ONL) thickness (Fig. 3C) comparable with normal level (Fig. 3D) during the late stage of EAU.
Fig. 3.
hAEC treatment ameliorates retinal structure damage. EAU rats were treated with hAECs or vehicle solution (BSS) by subretinal injection on day 0 and day 6 after immunization and sacrificed for tissue harvest on day 12 and day 18 after immunization (the peak and stable stage of the disease, respectively). Histological examination of the retinal structure in different EAU rat groups was performed based on H&E staining. Representative images are shown in (A), and the damage to the retinal structure is quantified in (B). The thicknesses of the retina and ONL are quantified in (C). Data are presented as the mean ± SEM from six separate specimens per group from three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. Statistical analysis was performed using a two-way ANOVA and Tukey’s multiple comparison test. Representative retinal structure image of normal control is shown in (D).
Scale bar=100 µm.
ANOVA: analysis of variance; BSS: balanced salt solution; EAU: experimental autoimmune uveitis; GCL: ganglion cell layer, hAEC: human amniotic epithelial cells; INL: inner nuclear layer, ONL: outer nuclear layer, RPE: retinal pigment epithelium; SEM: standard error of the mean.
hAECs Inhibit Inflammatory Cell Infiltration
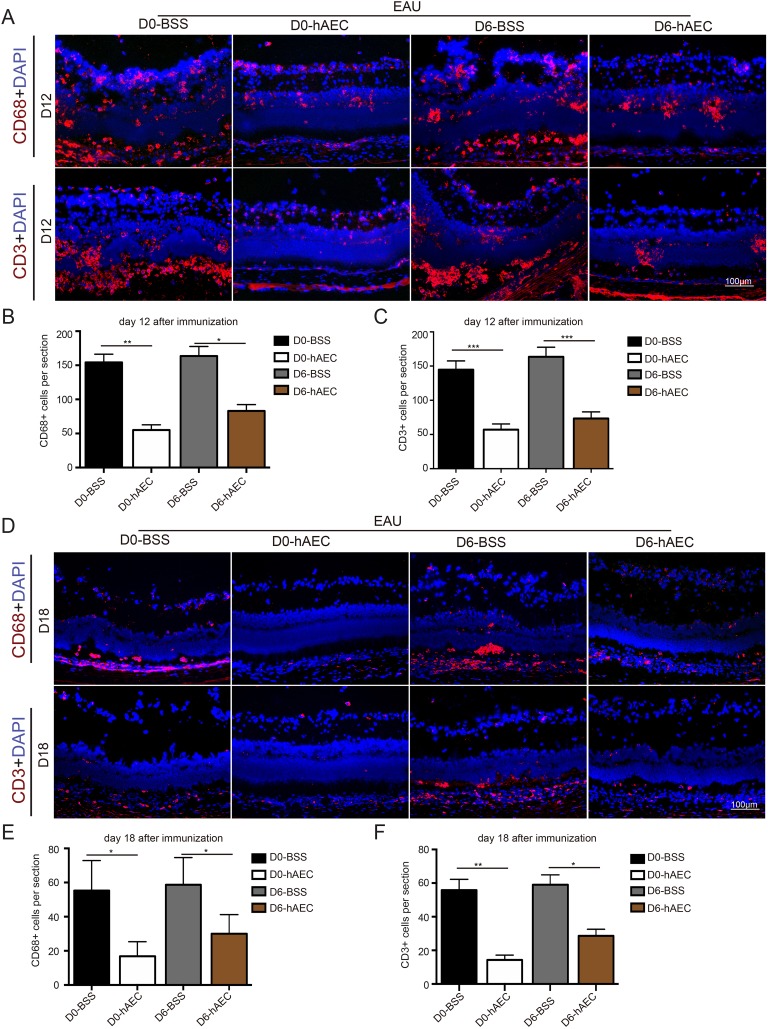
The histological examination above indicated that the infiltration of inflammatory cells is the key event to induce ocular inflammation and resultant retinal damage during EAU. Indeed, it has been well established that EAU is an immune disorder mediated by T-cells. To this end, retinal infiltration of macrophages and T-cells was determined by immunofluorescence staining. Consistent with the pathological phenotype, the numbers of CD68-positive cells and CD3-positive cells were sharply increased in the control groups on day 12, corresponding to the peak of ocular inflammation (Fig. 4A with quantifications in Fig. 4B, 4C). These results suggested that large amounts of macrophages and T-cells were activated and migrated into retinas. In contrast, much less infiltration of macrophages and T-cells was detected in retinas in the preventive group and therapeutic group (Fig. 4A with quantifications in Fig. 4B, 4C). On day 18, the inflammatory cells were significantly decreased in the retinas of the two hAEC-treated groups compared with the control groups (Fig. 4D with quantifications in Fig. 4E, 4F). But the suppressive effect seemed better in the preventive group.
Fig. 4.
hAEC treatment inhibits macrophage and T-cell infiltration of retina. EAU rats were treated with hAECs or vehicle solution (BSS) by subretinal injection on day 0 and day 6 after immunization and sacrificed for tissue harvest on day 12 and day 18 after immunization (the peak and stable stage of the disease, respectively). The retinal infiltration of macrophages and T-cells was determined by immunofluorescence staining of CD68 and CD3, respectively. Representative immunofluorescence images in retinas harvested on day 12 and day 18 are shown in (A) and (D), respectively, with quantification of infiltrated immune cells in (B, C) and (E, F), respectively.
Data are presented as the mean ± SEM. n = 6 in each group. *p < 0.05; **p < 0.01; ***p < 0.001. Statistical analysis was performed using an unpaired Student’s t test. Scale bar=100 µm.
BSS: balanced salt solution; EAU: experimental autoimmune uveitis; hAEC: human amniotic epithelial cells; SEM: standard error of the mean.
hAECs Preserve Immune Cell Balance in EAU Rats
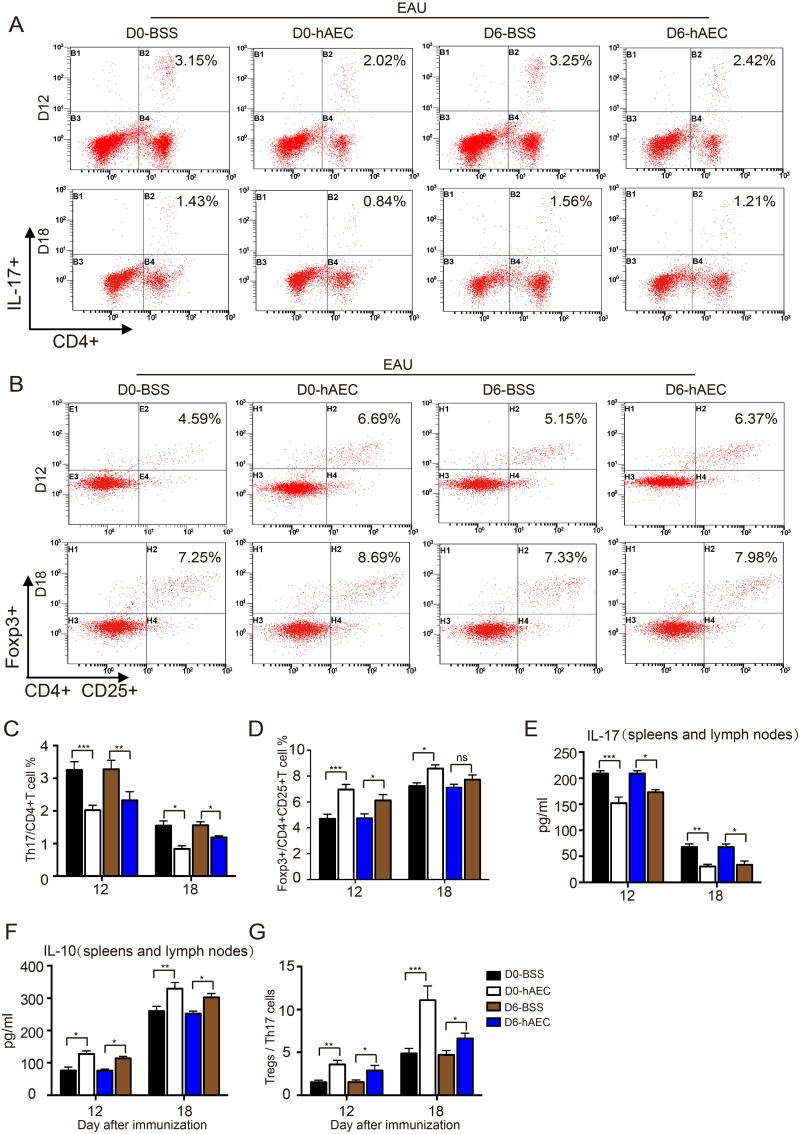
To explore the immunomodulatory function of hAECs in EAU, lymphocytes in spleens and lymph nodes were isolated from rats in all the groups, and the proportion of Th17 cells and Treg cells in different groups were examined by flow cytometry. In control groups, the proportion of Th17 cells remarkably increased around day 12 and thereafter reached a certain level around day 18 in EAU rats. In contrast, the augmentation of Th17 cells was suppressed by hAEC injection in both preventive and therapeutic groups, especially at the peak of ocular inflammation around day 12 (Fig. 5A with quantification in Fig. 5C). Moreover, the reduced proportion of Treg cells in EAU rats was restored upon hAEC treatment during disease development (Fig. 5B with quantification in Fig. 5D). The hAEC-induced T-cell proportion changes were confirmed by decreased IL-17 and increased IL-10 levels detected in the spleens and lymph nodes of EAU rats (Fig. 5E, 5F). Importantly, the ratio of Treg/Th17 cells was significantly increased in hAEC-treated groups on both day 12 and day 18 (Fig. 5G). Taken together, these findings demonstrated that hAEC treatment alters the Th17/Treg cell balance by downregulating Th17 cells and upregulating Treg cells.
Fig. 5.
The effect of hAEC treatment on proportions of T-cells in EAU rats. EAU rats were treated with hAECs or vehicle solution (BSS) by subretinal injection on day 0 and day 6 after immunization, and spleens and lymph nodes were harvested on day 12 and day 18 after immunization (the peak and stable stage of the disease, respectively). Th17 and Treg cell proportions were determined by flow cytometry. Representative histograms are shown in (A, B) with quantification of mean intensity in (C, D). The T-cell subtype IL-17 (E) and IL-10 (F) cytokine levels in the spleens and lymph nodes were determined by ELISA. The ratios of Treg cells/ Th17 cells are shown in (G). Data are presented as the mean ± SEM. n = 6 in each group. *p < 0.05; **p < 0.01; ***p < 0.001. Statistical analysis was performed using a two-way ANOVA and Tukey’s multiple comparison test.
ANOVA: analysis of variance; BSS: balanced salt solution; EAU: experimental autoimmune uveitis; ELISA: enzyme-linked immunosorbent assay; hAEC: human amniotic epithelial cells; IL: interleukin; ns: no significance; SEM: standard error of the mean; Th: T helper; Treg: T regulatory.
hAECs Improve the Cytokine Environment in EAU Rats
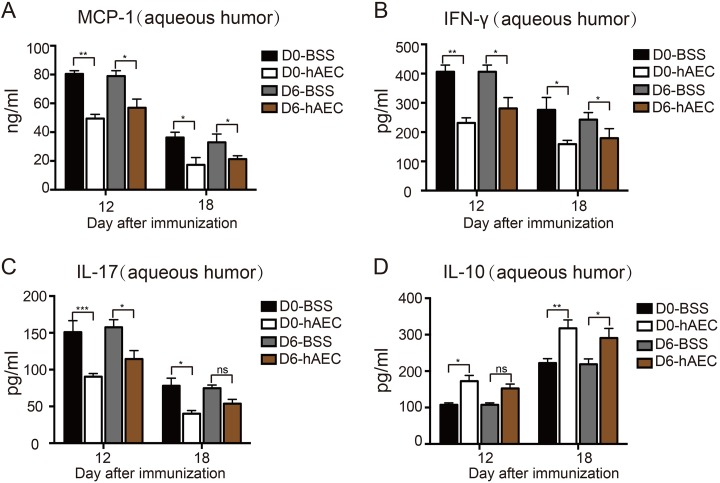
The levels of cytokines in the aqueous humor from all groups were analyzed by Luminex to determine the effect of hAEC treatment on the ocular cytokine environment in EAU. On day 12 (peak of ocular inflammation), EAU rats treated with hAECs exhibited significantly downregulated production of the MCP-1, IL-17 and IFN-γ proinflammatory cytokines (Fig. 6A–6C) but significantly upregulated production of the IL-10 anti-inflammatory cytokine (Fig. 6D). The levels of IL-4 and IL-6 were not affected. These findings indicated that hAECs coordinated the cytokine environment against ocular inflammation during EAU.
Fig. 6.
The effect of hAEC treatment on cytokine environment in aqueous humor of EAU rats. EAU rats were treated with hAECs or vehicle solution (BSS) by subretinal injection on day 0 and day 6 after immunization and sacrificed on day 12 and day 18 after immunization (the peak and stable stage of the disease, respectively). The MCP-1 (A), IFN-γ (B), IL-17 (C) and IL-10 (D) cytokine levels in the aqueous humor were then determined by ELISA in different groups. *P < 0.05, **P < 0.01, ***P < 0.001; ns, no significance. Data are presented as the mean ± SEM and were compared using a two-way ANOVA and Tukey’s multiple comparison test.
ANOVA: analysis of variance; BSS: balanced salt solution; EAU: experimental autoimmune uveitis; ELISA: enzyme-linked immunosorbent assay; hAEC: human amniotic epithelial cells; IFN: interferon; IL: interleukin; MCP: monocyte chemoattractant protein; ns: no significance; SEM: standard error of the mean; Th: T helper; Treg: T regulatory.
Discussion
In the present study, we investigated the therapeutic potential of hAECs in EAU. Subretinal transplantation of hAECs in EAU rats ameliorated ocular inflammation, preserved the retinal structure and coordinated the immune balance. Although hAEC treatment at the initial stage of EAU demonstrated a better suppressive effect on ocular inflammation than treatment during disease development, the treatment at both time points resulted in a similar rescue of ocular symptoms, retinal structure disorganization and photoreceptor loss. Thus, hAECs may ameliorate both the incidence and progression of EAU. More importantly, these results indicate that hAECs may be ideal candidates for EAU in the clinic with application in patients with various symptoms. To our knowledge, this is the first study to report the therapeutic benefit of hAECs in ocular inflammatory diseases. The key function of hAECs in EAU is the immunomodulatory effect. Accordingly, the regulatory mechanism of hAECs in the immune response was further investigated.
Studies have reported that the pathogenesis of EAU is mediated by Th1 and Th17 cell types. In addition, Th2 and Treg cells play an important role in the regression of EAU19,20. In the current study, a well-established posterior uveitis rat model was employed, which mimicked clinical ocular inflammation in AU and retinal injury21. In the EAU model, hAECs treatment improved disease symptoms by systemic modulation of the immune balance, referring to both Th cells and Treg cells. The present results demonstrated that hAECs selectively downregulate Th17 cells and upregulate Treg cells, thereby restoring the ratio of Treg/Th17. Of note, an increase of anti-inflammatory cytokines and a decrease of proinflammatory cytokines were detected in the aqueous humor, spleen and lymph nodes. These findings indicated that hAEC treatment improves the local cytokine microenvironment by inhibiting the ocular infiltration of immune cells. hAECs may work in a cell-to-cell pattern for suppressing the activity and inducing apoptosis of immune cells based on the high expression of complement inhibitory proteins and Fas/Fas ligand, respectively14. On the other hand, hAECs may secrete different groups of cytokines due to specific paracrine activity as reported by others and our studies11,22,23. In this context, the hAECs-derived TNF-α, transforming growth factor-beta (TGF-β) and macrophage migration-inhibitory factor (MIF) were released into serum, thereby modulating the systemic immune response in lymph nodes and spleen. Such regulation inhibited effector T-cell proliferation and promoted Treg cell maturation. Moreover, MIF and TGF-β also inhibited the migration of macrophages and polarized macrophages toward an M2 phenotype. Consequently, fewer macrophages, Th1 T-cells and Th17 T-cells could escape the peripheral tolerance and traffic to the eyes.
Previous reports have shown that mesenchymal stem cells (MSCs) play inhibitory roles during the development of EAU by regulating the balance of various subtypes of T-cells18,24–26. However, several studies have revealed that in pathological circumstances, such as inflammation and diabetic mellitus, proinflammatory cytokines and reactive oxygen species (ROS)-induced deactivation and apoptosis in MSCs as well as immunogenicity and even tumorigenicity in some cases27,28. As compared with MSCs, the lack of telomerase and high expression of HLA-G and HLA-E guarantees no tumorigenicity and immunogenicity in hAECs. Given that hAECs are routinely isolated from different donors, it is important to assess donor-specific variations for their clinical application. Previous studies have reported that hAECs had low expression of MHC class II antigens. In the present study, no expression of HLA-DR and HLA-DQ was detected with or without stimulation of IFN-γ in hAECs from different donors (data not shown). Our previous study demonstrated consistent expression of signature markers on the cell membrane and in the cytoplasm in different batches of hAECs11. The present study demonstrated comprehensive function of hAECs on the polarization of effector CD4+ T-cells during AU. Particularly, the inflammatory microenvironment augmented the production of TGF-β, IL-10 and other anti-inflammatory cytokines in hAECs29. Therefore, hAECs may be a candidate for cell therapy for AU and related ocular inflammatory diseases in the clinic.
In the present study, hAECs were administered by subretinal injection according to the characteristics of AU, which offered the benefit of targeted delivery of hAECs into the posterior segment without obvious invasive injuries, indicating the possibility of multiple injections. The subretinal cavity allowed a high-dose injection at one time. In addition, local immunological privilege based on the blood–retinal barrier helped the survival and activity of the injected hAECs. Taken together, these characteristics favored the surgical strategy for hAEC implantation in AU patients.
In conclusion, the present study provided a potential new therapeutic strategy for AU and related ocular inflammatory diseases. Subretinal hAEC transplantation remarkably inhibited EAU progression, in which hAECs exerted their immunoregulatory function for inducing immune tolerance. Future preclinical studies are required to elucidate the optimal cell number for treatment, long-term therapeutic efficacy and strategy of multi-site injection.
Supplemental Material
supplemental_figure for Subretinal Transplantation of Human Amniotic Epithelial Cells in the Treatment of Autoimmune Uveitis in Rats by Jinying Li, Chen Qiu, Zheng Zhang, Weixin Yuan, Zhen Ge, Bing Tan, Pengjie Yang, Jia Liu, Xiaolong Zhu, Cong Qiu, Dongmei Lai, Lihe Guo, and Luyang Yu in Cell Transplantation
Footnotes
Ethical Approval: All animal experiments were carried out with ethical approval from the Laboratory Animal Care and the Use Committee of Zhejiang University.
Statement of Human and Animal Rights: Animals used in this study were treated according to the Laboratory Animal Care and the Use Committee of Zhejiang University. And human subjects were approved by the Institutional Patients and Ethics Committee of the International Peace Maternity and Child Health Hospital, Shanghai Jiaotong University School of Medicine.
Statement of Informed Consent: The human subjects used in this article have informed and obtained informed consent.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Project of Health Collaborative Innovation of Guangzhou City (Grant No. 201704020214), the National Natural Science Foundation of China (Grant No. 81770444 and 81600354) and Fundamental Research Funds for the Central Universities of China.
ORCID iD: Luyang Yu  http://orcid.org/0000-0002-1301-6285
http://orcid.org/0000-0002-1301-6285
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14(5–6):303–308. [DOI] [PubMed] [Google Scholar]
- 2. Darrell RW, Wagener HP, Kurland LT. Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch Ophthalmol. 1962;68:502–514. [DOI] [PubMed] [Google Scholar]
- 3. Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California epidemiology of uveitis study. Ophthalmology. 2004;111(3):491–500; discussion 500. [DOI] [PubMed] [Google Scholar]
- 4. Prete M, Dammacco R, Fatone MC, Racanelli V. Autoimmune uveitis: clinical, pathogenetic, and therapeutic features. Clin Exp Med. 2016;16(2):125–136. [DOI] [PubMed] [Google Scholar]
- 5. Caspi RR. Mechanisms underlying autoimmune uveitis. Drug Discovery Today. 2006;3(2):199–206. [Google Scholar]
- 6. Nguyen QD, Callanan D, Dugel P, Godfrey DG, Goldstein DA, Wilensky JT. Treating chronic noninfectious posterior segment uveitis: the impact of cumulative damage - Proceedings of an expert panel roundtable discussion. Retina. 2006;suppl:1–16. [DOI] [PubMed] [Google Scholar]
- 7. Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120(9):3073–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LeHoang P. The gold standard of noninfectious uveitis: corticosteroids. Dev Ophthalmol 2012;51:7–28. [DOI] [PubMed] [Google Scholar]
- 9. Gomes Bittencourt M, Sepah YJ, Do DV, Agbedia O, Akhtar A, Liu H, Akhlaq A, Annam R, Ibrahim M, Nguyen QD. New treatment options for noninfectious uveitis. Dev Ophthalmol. 2012;51:134–161. [DOI] [PubMed] [Google Scholar]
- 10. Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23(10):1549–1559. [DOI] [PubMed] [Google Scholar]
- 11. Yang PJ, Yuan WX, Liu J, Li JY, Tan B, Qiu C, Zhu XL, Qiu C, Lai DM, Guo LH, Yu LY. Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation. Acta Pharmacol Sin. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Izumi M, Pazin BJ, Minervini CF, Gerlach J, Ross MA, Stolz DB, Turner ME, Thompson RL, Miki T. Quantitative comparison of stem cell marker-positive cells in fetal and term human amnion. J Reprod Immunol. 2009;81(1):39–43. [DOI] [PubMed] [Google Scholar]
- 13. Akle CA, Welsh KI, Adinolfi M, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981;2(8254):1003–1005. [DOI] [PubMed] [Google Scholar]
- 14. Li HC, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900–907. [DOI] [PubMed] [Google Scholar]
- 15. Strom SC, Gramignoli R. Human amnion epithelial cells expressing HLA-G as novel cell-based treatment for liver disease. Hum Immunol. 2016;77(9):734–739. [DOI] [PubMed] [Google Scholar]
- 16. Commodaro AG, Bueno V, Belfort R, Rizzo LV. Autoimmune uveitis: the associated proinflammatory molecules and the search for immunoregulation. Autoimmun Rev. 2011;10(4):205–209. [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Ren X, Li G, Jiao C, Zhang L, Zhao S, Wang J, Han ZC, Li X. Mesenchymal stem cells ameliorate experimental autoimmune uveoretinitis by comprehensive modulation of systemic autoimmunity. Invest Ophthalmol Vis Sci. 2011;52(6):3143–3152. [DOI] [PubMed] [Google Scholar]
- 18. Li G, Yuan L, Ren X, Nian H, Zhang L, Han ZC, Li X, Zhang X. The effect of mesenchymal stem cells on dynamic changes of T-cell subsets in experimental autoimmune uveoretinitis. Clin Exp Immunol. 2013;173(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakoda Y, Nagai T, Murata S, Mizuno Y, Kurosawa H, Shoda H, Morishige N, Yanai R, Sonoda KH, Tamada K. Pathogenic function of herpesvirus entry mediator in experimental autoimmune uveitis by induction of Th1-and Th17-Type T-cell responses. J Immunol. 2016;196(7):2947–2954. [DOI] [PubMed] [Google Scholar]
- 20. Lin P, Suhler EB, Rosenbaum JT. The future of uveitis treatment. Ophthalmology 2014;121(1):365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agarwal RK, Silver PB, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Biol. 2012;900:443–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao X, Guo Y, Wang Q, Xu M, Zhang Q, Li T, Lai D. The paracrine effect of transplanted human amniotic epithelial cells on ovarian function improvement in a mouse model of chemotherapy-induced primary ovarian insufficiency. Stem Cells Int. 2016;2016:4148923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banas RA, Trumpower C, Bentlejewski C, Marshall V, Sing G, Zeevi A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol. 2008;69(6):321–328. [DOI] [PubMed] [Google Scholar]
- 24. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. [DOI] [PubMed] [Google Scholar]
- 25. Oh JY, Kim TW, Jeong HJ, Lee HJ, Ryu JS, Wee WR, Heo JW, Kim MK. Intraperitoneal Infusion of Mesenchymal Stem/Stromal Cells Prevents Experimental Autoimmune Uveitis in Mice. Mediators Inflamm. 2014;2014:624640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR, Yoon SO, Choi H, Prockop DJ, Oh JY. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci U S A 2016;113(1):158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Veceric-Haler Z, Cerar A, Perse M. (Mesenchymal) stem cell-based therapy in cisplatin-induced acute kidney injury animal model: risk of immunogenicity and tumorigenicity. Stem Cells Int. 2017;2017:7304643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Denu RA, Hematti P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid Med Cell Longev. 2016;2016:2989076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Insausti CL, Blanquer M, Garcia-Hernandez AM, Castellanos G, Moraleda JM. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014;7:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplemental_figure for Subretinal Transplantation of Human Amniotic Epithelial Cells in the Treatment of Autoimmune Uveitis in Rats by Jinying Li, Chen Qiu, Zheng Zhang, Weixin Yuan, Zhen Ge, Bing Tan, Pengjie Yang, Jia Liu, Xiaolong Zhu, Cong Qiu, Dongmei Lai, Lihe Guo, and Luyang Yu in Cell Transplantation